We found that most infant golden snub-nosed monkeys were nursed by females other than their mothers during the first 3 months.
Abstract
While regular allomaternal nursing (suckling) has been documented in a number of rodent and carnivore species, as well as in some prosimians, New World monkeys, and humans, it is not common in Old World monkeys and apes. Here, we present a detailed field study of allomaternal nursing in golden snub-nosed monkeys (Rhinopithecus roxellana, Colobinae). We found that more than 87% of infants were nursed by females other than their mothers. Allomaternal nursing was largely confined to the first 3 months of an infant’s life and occurred predominantly between related females who nursed each other’s offspring in a reciprocal manner. Allomaternal nursing enhanced infant survivorship and did not have a negative impact on the future reproductive success of allonursers. Our findings expand the taxonomic distribution of allomaternal nursing and provide fresh insight into the possible factors driving evolution of allomaternal nursing behavior in primates, including humans.
INTRODUCTION
The evolution of lactation in metatherian and eutherian mammals has resulted in a large degree of nutritional and developmental dependency between a female and her offspring. Milk production is energetically costly for mothers who need to synthesize and provide nutrients, hormones, vitamins, and immune compounds to support the growth and development of their young infants (1). Under conditions of limited access to resources, nursing may negatively affect maternal health through physiological and nutritional stress, decreasing survivorship, and future reproductive success (2). In this regard, lactating females should be reluctant to invest time or energy into nursing others’ offspring. Other forms of allomaternal care, such as handling, grooming, protecting, socializing, carrying, and playing with infants, are almost ubiquitous in primates (3), but regular allomaternal nursing occurs only in some prosimians (4, 5), New World monkeys (6–9), and some human societies (10). Among Old World monkeys and apes, regular allomaternal nursing is generally absent, although a few species have been seen to exhibit allomaternal nursing (3). The few anecdotes include one chimpanzee (Pan troglodytes) grandmother who adopted and nursed her grandchild (11), the temporary adoption and suckling of an infant by an unrelated female savanna baboon (Papio cynocephalus) (12), and a nulliparous pregnant Japanese macaque (Macaca fuscata) who suckled her yearling sister in the weeks before her own parturition (13).
Allomaternal nursing may enhance infant survivorship by providing additional nutrients for accelerating growth (14) or supporting the development of neural tissue during a critical period of development (15). Allomaternal nursing also provides a more diverse set of immune compounds for infants that may improve their resistance against pathogens and parasites (16) and may alter the infant gut microbiome and increase digestive efficiency (17). Moreover, allomaternal nursing may provide benefits to an infant’s mother by allowing her to reduce postnatal reproductive costs or to reinvest energy and resources into future offspring (14). However, mothers may also incur high fitness costs if allomothers fail to properly handle, feed, or protect vulnerable infants or act aggressively leading to infant injury or death (18). Last, allonursers may incur costs if milk given to another female’s infant reduces milk available for the allonurser’s infant and if other females do not reciprocate (2).
Several hypotheses have been proposed to explain the evolution of allomaternal nursing behavior (14). These include kin selection, in cases in which the allonurser is closely related to the infant she nursed; reciprocity, under conditions in which pairs of females experience momentary milk depletion and nurse each other’s offspring during periods of excess milk production regardless of relatedness; misdirected maternal care, in which lactating females lack the capacity to discriminate against nursing others’ offspring; and acquisition of parenting skills, in which nulliparous females benefit by improving their maternal skills through nursing others’ offspring in the weeks immediately before giving birth. Although allomaternal nursing is argued to have evolved through kin selection or reciprocity in several species of group-living mammals (19), given limited systematic data, its evolutionary pathway in individual primate species remains unclear.
Here, we provide the first documented account of widespread allomaternal nursing behavior in an Old World monkey, the golden snub-nosed monkey (Rhinopithecus roxellana, Colobinae). These monkeys inhabit high-elevation (1000 to 4100 m above sea level) temperate forests in northwest and southwest China (28°26′ to 33°48′) characterized by extremely cold winters (nighttime temperatures may drop to −14°C) and strong seasonal shifts in resource availability (20, 21). They live in a multilevel society in which several one-male multifemale units (OMUs) form a stable and cohesive large band of 50 to >400 individuals that feed, forage, travel, and rest together (22). Also, related (matrilines) females are reported to form relatively stable social bonds (23, 24) and exhibit widespread allomaternal care such as handling and grooming of young infants (including neonates) (24), and there is one published report of allomaternal nursing in the closely related black-and-white snub-nosed monkeys (Rhinopithecus bieti) (25). R. roxellana is a strictly seasonal breeder with more than 90% of births occurring from March to May (early to late spring) (26), resulting in several adult females nursing their young offspring at the same time. Mean interbirth interval is approximately 2 years, although a small percentage of females may experience an interbirth interval of more than 3 years or less than 1 year (27). Females continue to nurse infants for a period of 12 to 14 months postpartum (28) and, therefore, during the birth season, lactating females include both females with unweaned infants (approximately 1 year of age) during the previous breeding season and females with neonates (<6 months of age) during the current breeding season.
We conducted a comprehensive examination of the frequency and adaptive function of allomaternal nursing in a free-ranging and well-habituated band of R. roxellana and tested four hypotheses: (i) kin selection, which predicts that females will preferentially nurse offspring of close relatives; (ii) reciprocity, which predicts that a female whose infant is nursed by a given female will preferentially allonurse that female’s infant during the same birth season or during the following birth season; (iii) misdirected maternal care, which predicts that lactating females with neonates allonurse a greater number of different infants than females with yearling infants who more effectively discriminate their offspring from neonates; and (iv) acquisition of parenting skills, which predicts that primiparous mothers will devote more time to allomaternal nursing than multiparous mothers or primiparous mothers will preferentially nurse others’ infant.
RESULTS
General description of allomaternal nursing
Over the course of five birth seasons, 87% (40 of 46) of infants were observed to suckle from one or more females other than their mothers, with 48% (22 of 46) suckling from at least two additional females. The mean number of allonursers per infant was 1.41 ± 0.84. In all cases, allonursers were members of an infant’s OMU. The mean age at which an infant was first allonursed was 2.6 ± 2.5 days (median, 2; range, 1 to 10 days), and the mean age that allomaternal nursing ended was 77.1 ± 25.6 days (median, 85 days; range, 43 to 96). The mean duration of allomaternal nursing bouts was longer than maternal nursing bouts (141.4 ± 23.9 s versus 130.3 ± 66.7 s, t = 3.58, P < 0.001). Data on the proportion of allomaternal daytime nursing behavior among all potential allonursers and lactating female dyads (mother and allonurser) are presented in table S1.
Allonursers provided milk to infants principally during their first 3 months of life; this contrasted with mothers, who nursed their biological infants for more than 1 year. The average proportion of nursing bouts infants received from females other than their mothers during their first 3 months of life ranged from 0 to 84.3% (mean, 6.5 ± 12.4%). This included an adoption event in which a 2-day-old infant was abandoned by its mother (the latter had a serious eye infection) and was adopted and allonursed by its older sister whose own infant had died several days earlier. Given that the inclusion of data from this infant strongly influenced the overall proportion of allonursing events presented earlier, we then excluded the adopted infant, resulting in revised proportions ranging from 0 to 19.1% (mean, 4.8 ± 4.2%). We also excluded this infant from all further analyses.
Influence of allomaternal nursing on infant mortality and interbirth interval
Four of the six infants who did not receive allomaternal nursing from another OMU member died during winter (before weaning). In contrast, only 6 of 40 allonursed infants died before the age of weaning; this included two cases of male infanticide that occurred in late summer and early fall, respectively, after an OMU takeover by a new resident adult male (27). All of the remaining 34 infants who were allonursed and 2 non-allonursed infants survived for more than 1 year. Overall, the survivorship of non-allonursed infants was significantly lower than that of allonursed infants (2 of 6 versus 34 of 40, Z score = −2.86, P = 0.004).
In an attempt to examine the potential costs of allomaternal nursing to lactating females, we compared the interbirth interval of females who served as allonursers (n = 29) and females who did not allonurse (n = 13). The results indicated no significant difference (686.2 ± 126.4 days for females who did not allonurse compared to 696.4 ± 99.0 days for allonursers, t12,28 = −0.257, P = 0.798).
Factors affecting the likelihood of female allomaternal nursing
Relatedness was an important factor in explaining the variation in allomaternal nursing behavior according to model averaging, the best model, and in all potential models (Table 1 and table S2). Specifically, females who provided allomaternal nursing were related to the infant at the level of grandmother-mother dyads or aunt-niece/nephew dyads (Mann-Whitney test: daughter-mother versus sibling-sister, U = 10.00, P < 0.001; daughter-mother versus unrelated, U = 40.00, P < 0.001; sibling-sister versus unrelated, U = 178.5, P = 0.770; Fig. 1A).
Table 1. Model-averaged coefficients of generalized linear mixed models for factors potentially affecting the likelihood of a female acting as an allonurser and the result of the best model.
| Fixed effect | Estimate coefficient | SE | Z value | P | Relative variable importance |
| (A) Model averaging | |||||
| Intercept | 3.515 | 1.242 | 2.866 | 0.0016 | |
| Relatedness | 1.579 | 0.625 | 2.491 | 0.0127 | 1.00 |
| Reciprocity | 6.963 | 1.650 | 4.175 | 0.00002 | 1.00 |
| Age of offspring | −1.766 | 1.142 | 1.535 | 0.1246 | 0.90 |
| Reproductive history | 2.171 | 1.3157 | 1.637 | 0.1016 | 0.86 |
| (B) Best model | |||||
| Intercept | −3.7192 | 1.1413 | −3.259 | 0.0011 | |
| Relatedness | 1.6725 | 0.6164 | 2.718 | 0.0065 | |
| Reciprocity | 7.2261 | 1.6407 | 4.404 | 0.0001 | |
| Age of offspring | −2.0913 | 0.9650 | −2.167 | 0.0302 | |
| Reproductive history | 2.4982 | 1.1539 | 2.165 | 0.0304 |
Fig. 1. Factors affecting the extent of allomaternal nursing (allonursing).
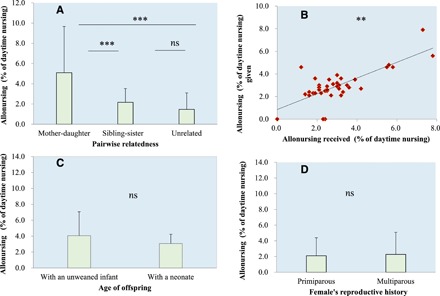
(A) Increased allomaternal nursing within mother-daughter dyads. (B) Positive relationship between mother’s and allonurser’s pattern of reciprocal allomaternal nursing. (C) No difference in allomaternal nursing proportions between females with an unweaned infant (approximately 1 year of age) and females with a neonate (<6 months of age). (D) No difference in allomaternal nursing proportions between primiparous and multiparous mothers (**P < 0.01; ns, no significance, P > 0.05).
Reciprocity was also an important factor in explaining the allomaternal nursing behavior in all models (Table 1 and table S2). The proportion of an infant’s allomaternal nursing received from a given female was positively correlated with its mother’s allomaternal nursing of that female’s infant (rs = 0.577, P < 0.01; Fig. 1B). Approximately 90% of mothers (25 of 28), whose offspring were nursed by another female, reciprocated and allonursed the infant of that female during the current or following year (see table S1). In the case of six infants, their mothers rejected all attempts by other infants to nurse. Our observations indicate that these infants, in turn, were actively rejected by other lactating females when they tried to nurse.
The age of female’s offspring, i.e., whether a female had given birth during the current breeding season (and had a neonate <6 months of age) or during the previous breeding season (and had an unweaned infant of approximately 1 year of age), had no influence on the likelihood that she engaged in allomaternal nursing (P = 0.3926) (Table 1). More than half of all allonursing females (37 of 62) had an unweaned infant of approximately 1 year of age. The remaining allonursers (n = 25) had a neonate of less than 6 months of age. During our study, the proportion of time the females with neonates engaged in allomaternal nursing (3.07 ± 1.16%) did not differ from the proportion of time the females who had an unweaned infant of approximately 1 year of age engaged in allonursing (4.06 ± 3.01%) (U = 409.5, P = 0.472) (Fig. 1C).
Last, a female’s reproductive history had no influence on the likelihood of allomaternal nursing according to model averaging (P = 0.1016) (Table 1). Our results indicate that the proportion of time of allomaternal nursing by primiparous females (2.10 ± 2.29%) did not differ from that of multiparous females (2.28 ± 2.80%) (U = 439.50, P = 0.95; Fig. 1D). We found that multiparous females preferentially nursed other females’ offspring more frequently than did primiparous mothers (36 of 41 versus 6 of 13, Z score = −3.15, P = 0.0016).
DISCUSSION
On the basis of more than 5 years of field observations of 46 infants and their mothers, our results provide the first evidence of regular reciprocal allomaternal nursing in a catarrhine nonhuman primate. We found that, in a band of golden snub-nosed monkeys, more than 87% of infants were allonursed by one or two additional adult females who were not their mother. In addition, although our population of golden snub-nosed monkeys lived in a multilevel society composed of four to six OMUs during the study period, mothers were found to only allonurse the offspring of females residing in the same OMU. Allomaternal nursing was largely confined to the first 3 months of an infant’s life and principally occurred between related females who reciprocally nursed each other’s offspring.
Allonursers in R. roxellana were related to the infants they nursed at the level of grandmother or aunt. In addition, the best model for the likelihood of allomaternal nursing indicated a strong positive correlation between the proportion of time that female A’s infant was allonursed by female B and the proportion of time that female B’s infant was allonursed by female A. Therefore, the results of our study support the predictions of both the kin selection and reciprocity hypotheses and provide an evolutionary explanation for allomaternal nursing in R. roxellana. This is consistent with studies of allomaternal nursing in several other mammalian taxa (14). In contrast, although it has been argued previously to explain allomaternal nursing in R. bieti (25), our current results provide no support for the misdirected maternal care hypothesis, as there was no significant difference in the proportion of allomaternal nursing between mothers with neonates and mothers with unweaned yearling infants, which suggests that mothers can effectively identify their offspring. In addition, primiparous female R. roxellana did not engage in more frequent allomaternal nursing than multiparous females, which suggests that the parenting skills hypothesis is not applicable to our study subject, although it has been proposed to explain allomaternal nursing in M. fuscata (13).
Allomaternal nursing behavior in R. roxellana also enhances infant survivorship and might be crucial for offspring survival during winter in high-elevation temperate forests. Infant mortality in wild snub-nosed monkeys during their first winter can be very high (more than 55%) (29, 30). The transition to winter is characterized by a decrease in resource availability coupled with an increase in thermoregulatory energy costs as a result of subzero temperatures. If infants have not reached a threshold level of development before the onset of winter, then they may be unlikely to survive winter (29, 30). Therefore, energy requirements for mothers to support rapid postnatal development might be highest in the months immediately following birth (31) and gradually reach peak around mid-lactation before infants begin to feed themselves (32). Milk received from allonursers during the first 3 months of life might provide the additional energy intake required to facilitate high rates of early infant growth and development before the time infants begin to feed on solid food at approximately 4 months of age (28). Furthermore, rapid postnatal development might enable infants to take advantage of an increase in leaves and ripe fruits that become available in summer and autumn. As a result, when winter begins, allonursed infants have the benefit of an expedited level of maturation and development compared to non-allonursed infants and have an increased chance of surviving through the extreme winter.
Allomaternal nursing behavior in R. roxellana could enhance current or future reproductive success of allonursers with both neonates and yearlings. On several occasions during the study, we observed mothers rejecting their own neonates’ attempts at suckling when the interval between feedings was too short, which might indicate that milk was temporarily depleted or there was a shortage of milk. Regular allomaternal nursing would obviously address this momentary milk shortfall and benefit allonursers with neonates when other females reciprocate. Furthermore, given differences in the nutritional requirements of lactation for mothers with rapidly growing infants, who require large and regular quantities of milk, compared to mothers whose infants are 1 year old, relatively independent, toward the end of the process of weaning, and obtain virtually all of their nutrients from the consumption of solid food, the cost of allomaternal nursing to mothers with yearling infants is expected to be very low and the benefits are expected to be potentially high if infants born to them during the next birth season are reciprocally allonursed by other female residents of their OMU.
Two critical issues in the evolution of allonursing behavior are the degree to which milk is best regarded as a limited resource and the cost to mothers of sharing milk with the offspring of another female. However, there is evidence that, at least in some mammals, an increase in the frequency of nursing has a positive effect on maternal milk production. A study of domestic pigs found that increased frequency of nursing across a 2-day period resulted in a positive increase in maternal milk output. Relatedly, sows who nursed at shorter intervals (35 min versus 70 min) provided their offspring more milk across the nursing period, and their offspring gained more weight than sows who nursed over longer intervals (33). Thus, increased nursing frequency can result in increased milk production (34). In addition, in the case of human and nonhuman primates, milk production is reported to increase with female parity (35). Assuming similar processes occur in golden snub-nosed monkey, then by increasing nursing frequency (e.g., nursing one’s own offspring plus the offspring of another female’s infant), allonursers may experience an increased milk production that benefits their offspring as well as the offspring of close relatives. Moreover, if first time mothers produce less milk than experienced mothers, then this may help to explain our results indicating that primiparous mothers were notably less likely to engage in allonursing than multiparous mothers.
Our study expands the taxonomic distribution of regular allomaternal nursing in primates from prosimians and New World monkeys to catarrhines and potentially offers an opportunity for examining the evolutionary mechanisms promoting allomaternal nursing in primates and other mammals. A recent comparative analysis showed that allomaternal nursing in mammals was most common in species where females produce multiple infants in litters (polytocy) (36); this implies that energetic requirements associated with a large litter size might favor allolactation. Energetic requirements might also vary as a result of brain size (3) or environmental harshness (37). The conditions leading to the evolution of allonursing may also include a pattern of seasonal reproduction resulting in several females nursing infants during the same period of time as well as cohesive and socially supportive relationships among females. Golden snub-nosed monkeys inhabit highly seasonal temperate forests at high elevations with long winters in which nighttime temperatures commonly drop below 0°C (20–22), and resource scarcity may jeopardize survival (21). Approximately 90% of births occur over a relatively short birth season in spring (26), and related females co-reside in small and relatively stable OMUs and form tolerant relationships with each other (23, 24); golden snub-nosed monkeys also have the largest adult brain volume of all colobine primates (38). These social and ecological traits are conducive to the evolution of allomaternal nursing, and some of these also occur in all other primates in which regular allomaternal nursing has been reported (table S3). For example, Malagasy lemurs live in small groups and have relatively larger litter sizes, and their breeding is restricted to the short wet season (36), while capuchins have highly encephalized brains, an extended period of nursing, and infant and juvenile development, form matrilineal groups, and live in seasonal habitats (6, 8, 9). Human mothers have relatively high energetic requirements due to a protracted period of infancy and juvenility and extended postnatal brain growth (15). Therefore, considering that similar social and ecological traits typify all primate species with allomaternal nursing (including humans), we propose that allomaternal nursing may have arisen through natural selection when heavy postnatal energetic requirements and when harsh or unpredictable environmental conditions placed a premium on shared provisioning. However, while the variables we identified (availability of female kin, environmental harshness, etc.) may make allolactation more likely in some species, there are primates that have a similar constellation of socio-ecological factors but do not exhibit allolactation. Therefore, as allonursing in primates evolved independently in a small number of distantly related taxa that vary in behavior, life history traits, and ecology, it is unlikely that any single set of conditions best explains these multiple origins. Future research should address this through a broader phylogenetically controlled comparative analysis with a more extensive dataset.
Last, in our study, allomaternal nursing usually occurred between related females who reciprocally nursed each other’s offspring. Mothers also permitted other females to take their infants as early as their first day of life and let them carry and groom their infants (23). These female bonds may be mediated through kinship and common residence in the same OMU, as well as other forms of social behaviors (i.e., grooming) that promote a set of affiliative and permissive relationships, which are required to develop infant-mother–allomaternal caregiver relationship (39). These relationships are also crucial in human social interactions (16). Therefore, our findings generate previously unknown insights into the causes and consequences of the evolution of allomaternal nursing in primates and may prove valuable for an understanding of its role in human evolution.
MATERIALS AND METHODS
Study site
We conducted the study at Dalongtan (31°29′N, 110°18′E; elevation, 2200 m) in Shennongjia National Nature Reserve, central China. This area maintains a highly seasonal temperate forest characterized by 5 months of winter (November to March) during which snow covers the ground (21, 22). Temperature varies throughout the year, with the highest mean monthly temperature occurring in July (27.7°C) and the lowest mean monthly temperature occurring in January (−2.5°C).
Study subjects
The focal group was habituated for tourism with all events of deaths and births recorded since 2006; as a result of successful habituation, observations were made on a daily basis at distances between 5 and 50 m (22, 23). The focal population contained four OMUs of 62 to 82 individuals, 23 of which were fertile females, and one all-male unit composed of 5 to 7 adult and juvenile males. The four OMUs (XX, DD, BT, and NN) were stable in 2012 and 2013, but all resident males were replaced by new males (WY, XB, HH4, and XZ) in September of 2013. Two OMUs (XZ and WY) separated in 2015 and 2016, increasing the number of OMUs from four to six. These two new OMUs were named XZ and WY (after the name of their male leader). We observed 47 infants (except one stillborn), which were born between March and May in the years 2012–2016 (for details, see Table 2 and table S1). Thirty-one adult females (including 13 primiparous females) acted as allonursers. The number and identifiers of lactating females in the different years are shown in Table 2 and table S4.
Table 2. Birth seasonality and number of infants and lactating females observed during the study period.
| Year |
Birth seasonality |
Number of infants |
Number of lactating females |
| 2012 | 1 April to 21 May |
7* | 18 |
| 2013 | 20 March to 1 June |
12 | 16 |
| 2014 | 25 March to 27 May |
7 | 17 |
| 2015 | 1 March to 8 May |
17** | 20 |
| 2016 | 18 March to 26 April |
4 | 10 |
*Including one stillborn.
**Including one born on 25 August.
All adult OMU members were identifiable based on a unique set of physical features (23), such as body size, pelage color, evidence of injuries or scars, the shape and size of a female’s nipples, and the shapes of granulomatous flanges, which are present as fleshy nodules on both sides of the upper lip in adult males; adult males are easily distinguished from adult females by body size. Infants were identifiable based on distinct physical features of their body and face and OMU residence.
Behavioral data
We began daily observations following the birth of infants. Data were collected from 0800 to 1800 when the weather permitted. We used a 10-min focal animal sampling protocol and continuous recording to document the duration of all infant-caring behaviors (including nursing, carrying, or guarding) displayed by a given female, whether the female was the infant’s mother, and whether the female was from the infant’s OMU or from a different OMU in the band. A nursing bout was recorded when an infant contacted a female’s nipple and began suckling. We also recorded all incidents in which an infant attempted to suckle but was rejected by the lactating female.
We collected focal samples of infants via a randomized method, and for each infant, we attempted to obtain an equal amount of observations during the study period. If visual contact was lost with the focal subject or if suckling was not visible (i.e., if the focal infant was obscured by arboreal vegetation), we ended the sample collection. We collected allomaternal nursing data from April to November 2012, March to November 2013, and March to August in 2014–2016. The mean observation time per infant was 119.97 ± 18.05 hours, with a total observation time of 5518.65 hours.
Pairwise relatedness
We plucked hair bulbs directly by hand (with gloves) from all adult females in the focal band and extracted DNA from the hair follicles for relatedness analysis (24). DNA amplification and microsatellite genotyping methods were described in detail in a previous study (22, 24). For those allomaternal nursing female dyads (allonurser and recipient’s mother) in the group between 2012 and 2016 that we were unable to estimate relatedness, we used a pairwise relatedness estimator (r) of the females to identify kinship and performed the analysis using KINGROUP v2.0 (40).
The relatedness of female dyads involved in allomaternal nursing was divided into three categories (table S1): mother-daughter (n = 10), sister-sister (n = 8), and an unrelated or distantly related pair (n = 36). All “mother-daughter” dyads and six full “sister-sister” dyads were determined by observing infants born into OMUs since 2006, and two half sister-sister dyads were identified on the basis of the genetic data. All other dyads were allocated as unrelated or distantly related pair. We combined full and half sister-sister dyads into a single category because there were no significant differences between these two allonursing dyads (Mann-Whitney test, U = 26.00, P = 0.913).
Statistical analysis
As short contact does not allow for milk transfer (4, 8), suckling bouts lasting less than 30 s were excluded from data analysis. We described the proportion of allomaternal nursing during the first 14 weeks (3 months) of each infant’s life. We limited our analysis to this 3-month period because more than 98.0% of allomaternal nursing was confined to this period (based on data of 19 infants in 2012–2013). The mean proportion of allomaternal nursing from each 2-week interval for each donor was used as the total allomaternal nursing score from that female. The total proportion of allomaternal nursing for a particular infant was summed from all donors.
To calculate infant mortality and the interbirth interval of allonursing females, we used the records of births and deaths between 2011 and 2018. A Mann-Whitney test was used to identify differences in allomaternal nursing based on relatedness and whether the allonurser had a neonate (<6 months of age) or an unweaned infant (approximately 1 year of age). A two-sample t test was used to identify differences in the duration of nursing bouts between allonursers and biological mothers as well as differences in the interbirth interval of females who acted as allonursers and females who did not act as allonursers. A Z score test was used to identify whether mortality of infants who did not allonurse was higher than infants who were allonursed and whether primiparous females exhibited a greater preference to allonurse compared to multiparous females.
A generalized linear mixed model was used to determine the set of factors affecting the likelihood that a female would allonurse. The response variable was whether a female nursed another’s offspring (binary response variable, yes = 1, no = 0) across the entire study period, and the independent variables were relatedness, reciprocity, the age of offspring, and the female’s reproductive history. Relatedness referred to the degree of kinship between female dyads that engaged in allomaternal nursing (allonurser and recipient’s mother). We assigned a value of “3” for a mother-daughter dyad, “2” for a sister-sister dyad, and “1” for an unrelated or distantly related pair according to the paternity and relatedness analysis. Reciprocity referred to whether the female nursed that mother’s infant in the previous year or in the current breeding season. If the mother did nurse that female’s infant, reciprocity was set to 1; otherwise, it was set to “0”. The age of offspring referred to whether a mother had an unweaned infant of approximately 1 year of age (0) or a mother had a neonate <6 months of age (1). The female’s reproductive history referred to whether the female was “primiparous” (0) or “multiparous” (1).
For statistical analysis, we used R version 3.5.1 with the lme4 package (41). First, a global model was set for all factors with both a logit link function and a binomial error distribution. Then, using the AICc (the second-order Akaike information criterion), we determined the best model with the lowest AICc value (42) from a set of candidate models. We generated a subset of models by calculating the difference between the AICc value of the best-fitting model and all other models and then using a cutoff of 2AICc as the criterion for inclusion in the subset. Last, we averaged those models using the MuMIn package (43) and estimated the relative weight of all models by combining the parameters’ weight (44). All means are reported with SDs.
Supplementary Material
Acknowledgments
We thank L. Wei and L. Lu for help in collecting data in the field. We thank T. H. Clutton-Brock, H. Elise, K. MacLeod, and other members of LARG at the Department of Zoology in Cambridge University for constructive suggestion of this paper. Z.X. wishes to thank L. Chen for the suggestion of data analysis. P.A.G. wishes to thank M. Chrissie, G. Sara, and G. Jenni for support. Funding: The study was partially funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31020000) and the National Key Technology R&D Program of China (2016YFC0503200 to M.L.), as well as the National Natural Science Foundation of China (31821001 to M.L. and 31870509 and 31670397 to Z.X.). Ethics statement: All research methods adhered to the Chinese legal requirements and were evaluated and approved by Central South University of Forestry and Technology’s Institutional Animal Care and Use Committee (protocol no. 2012-018). Author contributions: Z.X. and M.L. designed the study. Z.X., P.F., H.C., R.L., B.Z., W.Y., and H.Y. collected data. Z.X., P.F., H.C., C.C.G., P.A.G., and M.L. contributed to the data interpretation. Z.X. wrote the first draft of the manuscript. Z.X., H.C., C.C.G., P.A.G., and M.L. contributed to its final form. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaav0499/DC1
Table S1. The infant’s mother, (potential) allonurser, proportion of allomaternal nursing (based on time spent nursing), and kin relationship between the allonurser and the infant’s mother in the social units of a free-ranging group of golden snub-nosed monkeys (R. roxellana).
Table S2. AICc-ranked candidate model set showing relative importance of the following parameters on whether a female acted as allonurser.
Table S3. Social and ecological traits of primate species for which regular allomaternal nursing has been reported.
Table S4. Adult females’ reproductive history in focal group that is composed of four to six OMUs.
REFERENCES AND NOTES
- 1.Gittleman J. L., Thompson S. D., Energy allocation in mammalian reproduction. Am. Zool. 28, 863–875 (1988). [Google Scholar]
- 2.Clutton-Brock T. H., Albon S. D., Guinness F. E., Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Isler K., van Schaik C. P., Allomaternal care, life history and brain size evolution in mammals. J. Hum. Evol. 63, 52–63 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Eberle M., Kappeler P. M., Family insurance: Kin selection and cooperative breeding in a solitary primate (Microcebus murinus). Behav. Ecol. Sociobiol. 60, 582–588 (2006). [Google Scholar]
- 5.Vasey N., The breeding system of wild red ruffed lemurs (Varecia rubra): A preliminary report. Primates 48, 41–54 (2007). [DOI] [PubMed] [Google Scholar]
- 6.O’Brien T. G., Robinson J. G., Allomaternal care by female wedge-capped capuchin monkeys: Effects of age, rank and relatedness. Behaviour 119, 30–50 (1991). [Google Scholar]
- 7.Williams L., Gibson S., McDaniel M., Bazzel J., Barnes S., Abee C., Allomaternal interactions in the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Am. J. Primatol. 34, 145–156 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Baldovino M. C., Di Bitetti M. S., Allonursing in tufted capuchin monkeys (Cebus nigritus): Milk or pacifier? Folia Primatol. 79, 79–92 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Sargeant E. J., Wikberg E. C., Kawamura S., Fedigan L. M., Allonursing in white-faced capuchins (Cebus capucinus) provides evidence for cooperative care of infants. Behaviour 152, 1841–1869 (2015). [Google Scholar]
- 10.Hewlett B. S., Winn S., Allomaternal nursing in humans. Curr. Anthropol. 55, 200–229 (2014). [PubMed] [Google Scholar]
- 11.Wroblewski E. E., An unusual incident of adoption in a wild chimpanzee (Pan troglodytes) population at Gombe National Park. Am. J. Primatol. 70, 995–998 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Alberts S. C., The challenge of survival for wild infant baboons. Am. Sci. 104, 366–373 (2016). [Google Scholar]
- 13.Tanaka I., Non-offspring nursing by a nulliparous pregnant female just before first parturition in free-ranging Japanese macaques. Primates 45, 205–206 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Roulin A., Why do lactating females nurse alien offspring? A review of hypotheses and empirical evidence. Anim. Behav. 63, 201–208 (2002). [Google Scholar]
- 15.Hrdy S. B., Variable postpartum responsiveness among humans and other primates with “cooperative breeding”: A comparative and evolutionary perspective. Horm. Behav. 77, 272–283 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Garnier R., Gandon S., Chaval Y., Charbonnel N., Boulinier T., Evidence of cross-transfer of maternal antibodies through allosuckling in a mammal: Potential importance for behavioral ecology. Mamm. Biol. 78, 361–364 (2013). [Google Scholar]
- 17.Pannaraj P. S., Li F., Cerini C., Bender J. M., Yang S., Rollie A., Adisetiyo H., Zabih S., Lincez P. J., Bittinger K., Bailey A., Bushman F. D., Sleasman J. W., Aldrovandi G. M., Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 171, 647–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod K. J., Nielsen J. F., Clutton-Brock T. H., Factors predicting the frequency, likelihood and duration of allonursing in the cooperatively breeding meerkat. Anim. Behav. 86, 1059–1067 (2013). [Google Scholar]
- 19.Lukas D., Clutton-Brock T., Cooperative breeding and monogamy in mammalian societies. Proc. Biol. Sci. 279, 2151–2156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkpatrick R. C., Grueter C. C., Snub-nosed monkeys: Multilevel societies across varied environments. Evol. Anthropol. 19, 98–113 (2010). [Google Scholar]
- 21.Yiming L., Seasonal variation of diet and food availability in a group of Sichuan snub-nosed monkeys in Shennongjia Nature Reserve, China. Am. J. Primatol. 68, 217–233 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Xiang Z.-F., Yang B.-H., Yu Y., Yao H., Grueter C. C., Garber P. A., Li M., Males collectively defend their one-male units against bachelor males in a multi-level primate society. Am. J. Primatol. 76, 609–617 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Yu Y., Xiang Z.-F., Yao H., Grueter C. C., Li M., Female snub-nosed monkeys exchange grooming for sex and infant handling. PLOS ONE 8, e74822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo S., Huang K., Ji W., Garber P. A., Li B., The role of kinship in the formation of a primate multilevel society. Am. J. Phys. Anthropol. 156, 606–613 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Ren B., Li D., Garber P. A., Li M., Evidence of allomaternal nursing across one-male units in the Yunnan snub-nosed monkey (Rhinopithecus bieti). PLOS ONE 7, e30041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Z., Yang W., Qi X., Yao H., Grueter C. C., Garber P. A., Li B., Li M., An examination of factors potentially influencing birth distributions in golden snub-nosed monkeys (Rhinopithecus roxellana). PeerJ 5, e2892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao H., Yu H., Yang B., Yang W., Xu H., Grueter C. C., Li M., Xiang Z., Male infanticide in the golden snub-nosed Monkey (Rhinopithecus roxellana), a seasonally breeding primate. Int. J. Primatol. 37, 175–184 (2016). [Google Scholar]
- 28.Liu R., Yao H., Yang W., Xiang Z., Mother-infant interaction in a provisioned group of golden snub-nosed monkeys Rhinopithecus roxellana in Shennongjia, China. Acta Theriol. Sinica 36, 158–168 (2016). [Google Scholar]
- 29.Kirkpatrick R. C., Long Y. C., Zhong T., Xiao L., Social organization and range use in the Yunnan snub-nosed monkey Rhinopithecus bieti. Int. J. Primatol. 19, 13–51 (1998). [Google Scholar]
- 30.Li Y., Liu X., Liao M., Yang J., Stanford C. B., Characteristics of a group of Hubei golden snub-nosed monkeys (Rhinopithecus roxellana hubeiensis) before and after major snow storms. Am. J. Primatol. 71, 523–526 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Pontzer H., Raichlen D. A., Gordon A. D., Schroepfer-Walker K. K., Hare B., O’Neill M. C., Muldoon K. M., Dunsworth H. M., Wood B. M., Isler K., Burkart J., Irwin M., Shumaker R. W., Lonsdorf E. V., Ross S. R., Primate energy expenditure and life history. Proc. Nat. Acad. Sci. U.S.A. 111, 1433–1437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Noordwijk M. A., Kuzawa C. W., van Schaik C. P., The evolution of the patterning of human lactation: A comparative perspective. Evol. Anthropol. 22, 202–212 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Spinka M., Illmann G., Algers B., Stétkova Z., The role of nursing frequency in milk production in domestic pigs. J. Anim. Sci. 75, 1223–1228 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Auldist D. E., Carlson D., Morrish L., Wakeford C. M., King R. H., The influence of suckling interval on milk production of sows. J. Anim. Sci. 78, 2026–2031 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Hinde K., Power M. L., Oftedal O. T., Rhesus macaque milk: Magnitude, sources, and consequences of individual variation over lactation. Am. J. Phys. Anthropol. 138, 148–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macleod K. J., Lukas D., Revisiting non-offspring nursing: Allonursing evolves when the costs are low. Biol. Lett. 10, 20140378 (2014). [Google Scholar]
- 37.Emlen S. T., The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39 (1982). [Google Scholar]
- 38.van Woerden J. T., Willems E. P., van Schaik C. P., Isler K., Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Silk J. B., House B. R., The evolution of altruistic social preferences in human groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodnight K. F., Queller D. C., Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol. Ecol. 8, 1231–1234 (1999). [DOI] [PubMed] [Google Scholar]
- 41.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018); www.R-project.org/.
- 42.Akaike H., A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723 (1974). [Google Scholar]
- 43.K. Bartón, MuMIn: Multi-model inference, R package, version 1.7.2, 2009; http://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf.
- 44.Grueber C. E., Nakagawa S., Laws R. J., Jamieson I. G., Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 24, 699–711 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Robinson J. G., Demography and group structure in Wedgecapped capuchin monkeys, Cebus olivaceus. Behaviour 104, 202–232 (1988). [Google Scholar]
- 46.Jack K. M., Fedigan L., Male dispersal patterns in white-faced capuchins, Cebus capucinus: Part 1: Patterns and causes of natal emigration. Anim. Behav. 67, 761–769 (2004). [Google Scholar]
- 47.Roth G., Dicke U., Evolution of the brain and Intelligence. Trends Cogn. Sci. 9, 250–257 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Di Bitetti M. S., Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. J. Zool. 253, 33–45 (2001). [Google Scholar]
- 49.Hill K. R., Walker R. S., Božičević M., Eder J., Headland T., Hewlett B., Hurtado A. M., Marlowe F., Wiessner P., Wood B., Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–1289 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaav0499/DC1
Table S1. The infant’s mother, (potential) allonurser, proportion of allomaternal nursing (based on time spent nursing), and kin relationship between the allonurser and the infant’s mother in the social units of a free-ranging group of golden snub-nosed monkeys (R. roxellana).
Table S2. AICc-ranked candidate model set showing relative importance of the following parameters on whether a female acted as allonurser.
Table S3. Social and ecological traits of primate species for which regular allomaternal nursing has been reported.
Table S4. Adult females’ reproductive history in focal group that is composed of four to six OMUs.


