Abstract
Human onchocerciasis, caused by infection by the filarial nematode Onchocerca volvulus, is a major neglected public health problem that affects millions of people in the endemic regions of sub-Saharan Africa and Latin America. Onchocerciasis is known to be associated with skin and eye disease and more recently, neurological features have been recognized as a major manifestation. Especially the latter poses a severe burden on affected individuals and their families. Although definite studies are awaited, preliminary evidence suggests that neurological disease may include the nodding syndrome, Nakalanga syndrome and epilepsy but to date, the exact pathophysiological mechanisms remain unclear. Currently, the only way to prevent Onchocera volvulus associated disease is through interventions that target the elimination of onchocerciasis through community distribution of ivermectin and larviciding the breeding sites of the Similium or blackfly vector in rivers. In this review, we discuss the epidemiology, potential pathological mechanisms as well as prevention and treatment strategies of onchocerciasis, focusing on the neurological disease.
Keywords: Onchocerca volvulus, Epilepsy, Neurological features
1. Introduction
Onchocerciasis (river blindness) is a leading neglected tropical disease that affects millions of people in Africa, Latin America and Asia and causes morbidity, disability, low productivity and poverty in endemic areas (Boatin, 2008). This disease is caused by the filarial nematode Onchocerca volvulus, which is transmitted by female blackflies of the genus Simulium (Boussinesq, 1997).
Female blackflies inject infective L3-stage larvae into a human during a blood meal. In the human host, the worms reach maturity after 1–3 years and adult worms (macrofilariae) reside in nodules under the skin where the female worm can live for up to 15 years. During this period they are capable of producing millions of motile microfilariae which migrate under the skin of infected persons until they are ingested by another female blackfly during a blood meal (Sato et al., 2017). In the blackfly, microfilariae migrate to the thoracic muscles of the fly where they develop into the third stage larvae (L3), which move to the head and mouth part of the fly in 6–12 days and are transferred to another human host during the next blood meal.
Over 90% of infections occur in sub Saharan Africa. In infected persons, the main clinical features are skin lesions, including pruritic dermatitis and nodules. Many patients suffer visual impairment (river blindness) and more recently, the disease has been associated with epilepsy – Onchocerciasis Associated Epilepsy (OAE) and nodding syndrome. In this paper, we describe the epidemiology and provide an overview of the main clinical features and the neurological manifestations of onchocerciasis. We also explore possible pathogenic mechanisms in the development of brain disease and propose potential treatment strategies.
2. Epidemiology
2.1. Burden and distribution
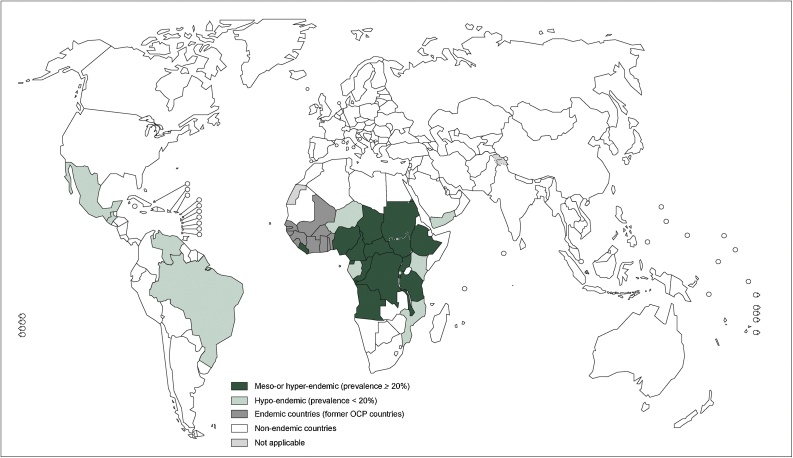
Approximately 37 million people in tropical Africa and 140,000 in Latin America are infected with O. volvulus (Boussinesq, 1997; JHF, 2004a). Although the disease is endemic in Central- and South America and Yemen, 99% of the infections occur in sub Saharan Africa, where a favorable ecology for the blackfly prevails, Fig. 1. Onchocerciasis transmission and disease burden are determined by the presence of the blackflies, with the highest infection loads as well as blindness and epilepsy prevalence close to blackfly breeding sites in fast flowing rivers (Boussinesq, 1997; Katabarwa et al., 2018). Furthermore, several Simulium species exist, each with specific ecological preferences and flying distance from the breeding site, which should be taken into account when estimating the disease burden and implementing control strategies (Katabarwa et al., 2018).
Fig. 1.
This figure is adapted from the WHO and show the worldwide distribution of Onchocerciasis and the countries with ongoing transmission in 2013, http://www.who.int/onchocerciasis/distribution/en/.
Most infected patients suffer from variable skin lesions and approximately one million individuals suffer from visual impairment, with at least 340,000 cases of blindness attributable to the disease. More than 46,000 lose their vision every year as a result of onchocerciasis (JHF, 2004b). Both river blindness and OAE can be prevented by community distribution of ivermectin (CDTI), which kills the microfilaria, in combination with vector control. It has been estimated that approximately 400 000 cases of epilepsy in Africa could be prevented by appropriate onchocerciasis control (Colebunders et al., 2018a; Hotterbeekx et al., 2018a).
2.2. Clinical features and pathology of onchocerciasis
Onchocerciasis is responsible for disabilities, significant morbidity, psychosocial problems and reduced ability to work and agricultural productivity in the affected populations. During infection, the adult worms release millions of microfilariae, which migrate into different parts of the body through the lymphatic system, especially the connective tissue in the dermis, to the eye, and potentially to the brain. When these microfilariae die, they elicit an inflammatory process, which is the key feature of the clinical manifestation of onchocerciasis (Sato et al., 2017; Colebunders et al., 2018a).
2.2.1. Skin manifestations
The adult worms reside under the skin for several years, encapsulated by host tissue, forming nodules located around muscles, especially on bony prominences, the onchocercoma (Okulicz, 2015). These onchocercomas are formed by continuous stimulation of an inflammatory response by foreign proteins, including proteins derived from the endosymbiotic bacterium, Wolbachia (Brattig, 2004).
Onchodermatitis is another skin manifestation of onchocerciasis which usually starts with itchy skin rash referred to as acute papular onchodermatitis, and is caused by an immune response against dying microfilariae. The rash may be intermittent with small, sparse papular lesions or closely packed papules of about 1 mm radius (Sejvar et al., 2013). If left untreated, the infection progresses into a chronic papular onchodermatitis which is described as pruritic, hyper pigmented, flat-topped papulomacular rash of about 3 mm which may or may not be associated with excoriation. Incomplete pigment loss combined with islands of normal skin pigmentation is also known as leopard skin and usually occurs in the lower limbs. Lichenified onchodermatitis will occur in the chronic phase and is associated with the formation of raised, discrete, pruritic hyperpigmented papular nodules. The nodules or onchocercomas are usually found around bony prominences, especially of the iliac crest, ischial tuberosity, elbows and scapula. In some areas, a more severe form of this phase has been described (Sowda), which is associated with skin hyperpigmentation, pruritic swollen limbs (usually unilateral) surrounded by enlarged lymph nodes (ME et al., 1993).
During chronic onchocerciasis, atrophy of the skin, with wrinkled dry skin, is also reported due to recurrent inflammation, which leads to thinning and loss of elasticity of the skin (cigarette skin). A hanging groin usually occurs in older individuals due to lymphatic obstruction in the groin area combined with cigarette skin and is a key feature of onchocerciasis (Okulicz, 2015).
2.2.2. Eye manifestations (River blindness)
Eye manifestations of onchocerciasis present as progressive visual impairment with increasing age and is causes by microfilariae migrating to the cornea and posterior regions of the eye, where they elicit an immune response when they die (Brattig, 2004; Gillette-Ferguson et al., 2004; Newland et al., 1991). This immune response is induced by surface proteins of the endosymbiontic bacteria Wolbachia, which are released by the degenerating microfilariae, thereby recruiting neutrophils and stimulating a pro-inflammatory response (Gillette-Ferguson et al., 2004). Neutrophils release nitric oxide, oxygen free radicals and matrix metalloproteinases, which are cytotoxic to resident corneal cells (Gillette-Ferguson et al., 2004). Repeated inflammation over time leads to chronic keratitis and sclerosis and is associated with progressive loss of corneal clarity and peripheral vision (Pearlman and Hall, 2000).
Chorioretinal changes including intra retinal pigment changes, retinal pigment epithelium atrophy, sub retinal fibrosis, optic neuropathy, retinitis and white intra retinal deposits were reported in a study as commonly occurring features of onchocerciasis (Newland et al., 1991). Advanced and repetitive inflammation may result in corneal fibrosis and or opacification that progresses to blindness. Choroioretinal lesions commonly occur around the optic disc and outer portions of the macula. While blindness is the most damning feature, bilateral blindness is relatively rare with some studies reporting as low as 0.4% of the affected population. The eye manifestations of onchocerciasis may be complicated with secondary glaucoma of the anterior and posterior segment lesions and optic atrophy (Newland et al., 1991).
2.2.3. Neurological and psychiatric manifestations of onchocerciasis
Onchocerciasis has been linked to several neurological and psychiatric syndromes, including but not limited to the Nakalanga syndrome, nodding syndrome and epilepsy.
The Nakalanga syndrome was first described in Uganda in 1962 in an area highly endemic for onchocerciasis (Jelliffe et al., 1962). This syndrome is characterized by dwarfism and mainly affects children aged 3–10 years that were previously developing normally. Patients present with severe growth retardation, delayed sexual development, mental retardation, facial deformation including a small mandible, protruding teeth, kyphoscoliosis and often but not always, generalized epileptic seizures (Kipp et al., 1996). In the areas around Mabira forest, Uganda, new cases ceased with the implementation of onchocerciasis control (Katabarwa et al., 2018).
Several studies in O. volvulus endemic areas have also linked this parasitic infection with the high population prevalence of epilepsy in these areas (Boussinesq et al., 2002). This epilepsy is mostly characterized by generalized tonic-clonic convulsions and has also been associated with impaired cognitive function, dysmorphic features, arrested puberty or delayed growth and malnutrition as in Nakalanga syndrome.
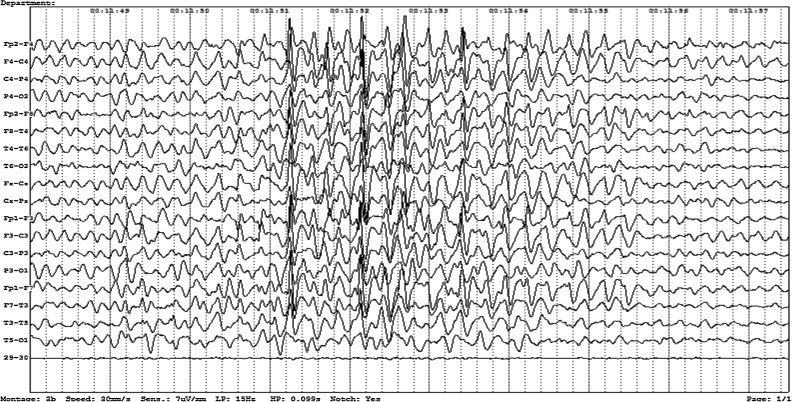
While currently no concrete experimental evidence exists to prove that onchocerciasis causes epilepsy, there is a very strong epidemiological association between the high incidence and prevalence of epilepsy in endemic areas and the population burden of onchocerciasis. In western Uganda, for example, the incidence of epilepsy increased from 156 cases per 100,000/year in areas with lower O. volvulus endemicity to 231 cases per 100,000/year in areas with higher endemicity (Kaddumukasa et al., 2013). In a meta-analysis of African population-based surveys, the prevalence of epilepsy increased, on average, by 0.4% for every 10% increase in onchocerciasis prevalence (Pion et al., 2009; Druet-Cabanac et al., 1999). The main difference between OAE and other forms of epilepsy is the age of onset: between the ages of 3–18 years old, with a mean age around 8–14 years old and the fact that the epilepsy occurs mostly in villages with high O. volvulus transmission and that there are often other siblings in the family affected by epilepsy (Colebunders et al., 2016a, b; Levick et al., 2017). EEGs performed to date show generalized epileptiform discharges and episodes of slowing, Fig. 2.
Fig. 2.
This is an electroencephalographic recording from scalp electrodes of a 15-year-old male Ugandan adolescent with Onchocerca associated epilepsy. He has had symptoms for at least 3 years. The recording shows generalized epileptiform discharges. Recording by Dr Richard Idro.
The other neurological disease associated with O. volvulus, the nodding syndrome, is characterized by a unique form of epileptic seizures – head nodding - in which patients have loss of neck muscle tonus, resulting in repeated forward dropping of the head (nodding). Symptoms usually occur in a children previously free of perinatal trauma, epilepsy, or mental retardation, and showing normal growth and development (Idro et al., 2013a). Like other forms of OAE, the onset of head nodding is between the ages of 3–18 years. The other physical manifestations and complications include stunted growth, peripheral muscle wasting and progressive generalized wasting, lip changes and gross physical deformities. The bone age is delayed by 1–6 years (Sejvar et al., 2013). Other seizure types include myoclonic-, absence- and/or generalized tonic–clonic seizures and these develop 1–3 years after the onset of the illness. Patients also develop other motor abnormalities such as deformities of the chest, trunk and limbs. Psychiatric manifestations are especially reported in nodding syndrome, including wandering behavior, aggression, depression and disordered perception with marked cognitive impairment (Idro et al., 2013a). The EEG pattern suggests a symptomatic generalized epilepsy. Different degrees of cortical and cerebellar atrophy are seen on brain MRI and a few cases of hippocampal changes have been reported. Five stages with worsening physical, EEG and brain imaging features have been described: a prodromal stage, the development of head nodding and cognitive decline, other seizure types, multiple complications and severe disability (Sejvar et al., 2013; Winkler et al., 2008).
2.3. The pathogenesis of brain disease in O. Volvulus infection
The biological mechanisms behind the pathogenesis of the neurological manifestations of O. volvulus remain unclear and it is debated whether microfilariae can cross the blood-brain barrier. In a study in 1976, microfilariae were detected in the CSF of heavily infected individuals but these results have not be replicated in other studies (Winkler et al., 2008; Duke et al., 1976; Konig et al., 2010). The wide spectrum of clinical manifestations of O. volvulus infection however makes it very difficult to elucidate the exact biological mechanisms behind the disease, which is most likely multifactorial. First, other than direct entry, the pathological mechanisms of epilepsy associated with onchocerciasis might be immune mediated, like posterior eye disease (Johnson et al., 2017). There is cross-reactivity between the Ov39 antigen of O. volvulus and the retinal hr44 antigen, which is suggested to have a potential role in the progression of chorio-retinitis (Leypoldt et al., 2015). Recent evidence also points towards cross-reacting antibodies in nodding syndrome. Johnson et al., identified antibodies to leiomodin-1 (LM-1) to be more abundant in the serum and CSF of patients with nodding syndrome compared to unaffected village controls (Johnson et al., 2017). LM-1 is a protein present in neurons, muscle tissue and the thyroid gland of healthy individuals and LM-1 antibodies show cross-reactivity with O. volvulus lysate and structural homology with the tropomyosin protein of O. volvulus (Johnson et al., 2017). Furthermore, the serum of patients that where positive for LM-1 antibodies was also shown to be neurotoxic. However, not all patients with nodding syndrome in this study tested positive for LM-1 antibodies, suggesting that this may not the only mechanism involved. On the other hand, the disease progression of onchocerciasis associated epilepsy also shows remarkable similarity to other autoimmune epilepsy disorders, such as limbic encephalitis, which are also characterized by seizures, psychiatric and behavioral changes and progressive encephalopathy (Korff and Dale, 2017). However, auto-antibodies commonly involved in autoimmune limbic encephalitis, such as anti-NMDA or anti-VGKC receptor antibodies are not universally positive in nodding syndrome, although anti-VGKC receptor antibodies were found in some cases and more research is necessary (Dietmann et al., 2014; Idro et al., 2013b).
O. volvulus contains the endosymbiont bacterium Wolbachia, which is needed for the survival and fertility of the worm and are known to modulate the host immune system (Brattig, 2004). Wolbachia surface antigens are recognized by the immune system and stimulate a Th1-mediated immune response and mediate an inflammatory response in the cornea and may be associated with the visual decline (Brattig, 2004). These antigens are released once microfilariae start dying and have been shown to induce keratitis in mice (Saint Andre et al., 2002). It is unknown whether these bacteria may be involved in disease pathogenesis.
If at all they do, microfilariae could enter the central nervous system by two potential points of entry: the optical system or the bloodstream. Since microfilariae were found in the anterior and posterior sections of the eye, they may have access to the brain along the optic nerves and inflammation in the eye might contribute to brain disease (Rodger, 1960). On the other hand, since microfilariae are also present in the lymphatic system and even in the bloodstream of heavily infected individuals, they might cross the blood brain barrier when circulating in the subarachnoid space (Fuglsang and Anderson, 1974).
Magnetic resonance imaging (MRI) of the brains of children suffering from nodding syndrome show generalized atrophy in the cerebral cortex as well as atrophy in specific regions in some individuals, including the hippocampus and Purkinje cells (Sejvar et al., 2013; Idro et al., 2013a). Remarkably, not all patients show the same abnormalities on MRI but most abnormalities were found in the group of patients with both head nodding and other type of seizures (Winkler et al., 2013). Severe brain atrophy has been confirmed in a post-mortem study of Ugandan children by investigators from the US Centers of Disease Control and the National Institutes of Health. In this study, polarizable, crystal-like structures that dissolved when the brains were stored in 70% ethanol were also observed (Colebunders et al., 2018b). However, these changes have not been seen in an ongoing post mortem study (Hotterbeekx et al., 2018b).
When epilepsy remains untreated, repeated seizures lead to cumulative brain injury, cognitive decline and worsening of the seizures, creating a vicious circle. It is possible that in addition to the primary pathological processes, some of the brain injury seen in OAE may be a consequence of uncontrolled seizures.
2.4. Treatment recommendations
Currently, the main treatment for onchocerciasis is ivermectin (Mectizan®). Ivermectin is a highly lipophilic 16-membered macrocyclic lactone from Streptomyces avermitilis. It paralyses and kills the microfilariae, relieving the intense itching of the skin and halting the progression towards blindness (Wolstenholme et al., 2016). A single-dose of ivermectin effectively kills microfilariae by blocking postsynaptic, glutamate-gated chloride ion channels, and paralyzing the nematode. It also appears to enhance immune responses against O. volvulus in the treated host (Udall, 2007). A 15-month study showed that ivermectin is more effective at preventing further reactive onchocerca skin lesions than at clearing existing lesions. Effective ivermectin treatment apparently requires a robust immune response (Udall, 2007). Administration of single doses of 150 μg/kg every 3 months is recommended based on evidence of decreased rates of post treatment reactions (e.g., edema, pruritis, and backache) over time, compared with yearly dosing (Hunyadi et al., 2017).
Ivermectin has not been shown to kill the adult worm but reduces the fertility of the females by paralyzing the microfilariae in utero, thereby reducing the production of new microfilariae. This effect is, however temporary and skin repopulation with microfilariae is resumed after 3–4 months (Basanez et al., 2008). Therefore, ivermectin controls but does not cure onchocerciasis and there is need for repeated treatment multiple times a year. Presently, the only approved medication with a significant effect against the adult worms is suramin, but toxicity, inconvenience (twice-daily injections administered for several weeks), and availability only through the US Centers for Disease Control virtually limits its clinical utility for treatment of onchocerciasis in Africa (Udall, 2007). A promising new drug, moxidectin, has been shown to have significant macrofilaridical activity in animal studies and is safe for use in humans as demonstrated in phase II trials (Van Laethem and Lopes, 1996).
Another treatment strategy with doxycycline, targets the endosymbiotic Wolbachia bacteria of O. volvulus and has been shown to significantly reduce the lifespan of the adult worm. In 2000, a landmark study first showed that doxycycline cleared Wolbachia from the endodermis and uteri of adult female worms, leading to unusually extensive worm sterility not seen with other anti-filarial treatments. In a placebo-controlled trial, treatment with doxycycline 100 mg per day for 6 weeks, followed by a single dose 150 μg/kg of ivermectin resulted in up to 19 months of amicrofilaridermia, as well as 100% elimination of Wolbachia species from worms that were isolated and tested immune-histologically (Udall, 2007; Abegunde et al., 2016). The authors suggested that infected patients who permanently leave endemic areas should be offered, in addition to ivermectin, a 4– 6-week course of doxycycline (100–200 mg per day) to achieve long-term amicrofilaridermia. Hoerauf et al. recommend the concurrent administration of ivermectin with doxycycline therapy, as well as administration of another ivermectin dose 6–8 months later to eradicate microfilariae too immature to be sensitive to the initial treatment. However, caution should accompany the concurrent use of ivermectin and doxycycline, because these agents have not been formally studied for drug interactions (Udall, 2007).
Currently, a phase II placebo controlled trial is testing if doxycycline may be used for the treatment of nodding syndrome in Uganda. The trial that started in 2016 is recruiting 230 participants and randomizing these to either doxycycline 100 mg daily for 6 weeks or placebo. Participants will be followed up for 24 months. Study results are expected at the end of 2019 (Anguzu et al., 2018).
2.5. Vector control to eliminate onchocerciasis
Prevention of onchocerciasis is the most cost effective and efficient measure to reduce the burden of the public health problem. Vector control is one of the most effective strategies for the elimination of onchocerciasis (Boussinesq, 1997). This strategy works by blocking the transmission chain - destroying the vector at its most vulnerable stage, the larval stage (World Health Organization. Twenty Years of Onchocerciasis control., 1994). It was implemented in six Kenyan foci in 1946 (McMahon, 1967) using DDT to eliminate the local vector Simulium neavei. Follow-up studies conducted in 1964 confirmed that the parasite had been eliminated from these foci (Roberts et al., 1967). Overall, the control operations were very effective and protected a population of approximately 225,000 people living in an area of 4144 km2 (McMahon, 1967). During the same period, an attempt to control onchocerciasis in the Victoria Nile focus was also underway in Uganda. This control effort that used DDT from 1951 to 1973 was successful in eliminating Simulium damnosum s.s. in central Uganda (Barnley, 1956).
The Kenyan and Ugandan successes was used as a model for the first international onchocerciasis control program in Africa. The Onchocerciasis Control Program of West Africa, or OCP, was a large-scale vertically implemented program which aimed to eliminate blindness due to onchocerciasis in eleven countries in West Africa where the disease was a major public health problem (Katabarwa et al., 2012). The OCP began in 1975 and relied primarily upon a strategy of aerial spraying of DDT in Simulium damnosum sensu lato larval breeding sites. A great deal of public health value was accomplished by this landmark effort; skin disease was significantly reduced, more than 200,000 cases of blindness were prevented and the size and distribution of the O. volvulus population in the region was substantially decreased (Davies, 1994).
More recently, Uganda demonstrated the power of utilizing a combination of vector control and semi-annual ivermectin mass drug administration in control efforts. Since the start of these measures in 2007, this resulted in the apparent interruption of transmission of onchocerciasis in 15 of the 17 foci in Uganda, and has been confirmed in the Wadelai, Itwara and Mt. Elgon foci (Kaddumukasa et al., 2013; Lakwo et al., 2013). For example, in one region of Uganda, comparison of total fly catches before and after ground larviciding revealed a drop from 5334 flies in 2007 to 0 flies in 2009 (Lakwo et al., 2017). Thus, in the nodding syndrome affected areas of northern Uganda, onchocerciasis control has heavily used vector control methods with ground larviciding largely with temephos (Abate®, EC500) and biannual treatment with ivermectin. Aerial spraying has also been applied (Idro et al., 2016). These data and other studies have proven that vector control in combination with ivermectin mass drug administration is a powerful strategy to eliminate onchocerciasis (Ndyomugyenyi et al., 2004). Similar success has been reported in Bioko islands, which is part of the Republic of Equatorial Guinea and is the only island in the World to have endemic onchocerciasis (Traore et al., 2009). These achievements have stimulated the adoption of vector control as additional strategy to accelerate onchocerciasis elimination in several countries. With more efficient application, hundreds of thousands of incident cases of onchocerca associated neurological disease in Africa will be prevented.
2.6. Conclusions and future perspectives
We are still in early days in understanding onchocerca associated brain disease. An OAE alliance was recently established following the first international workshop on OAE in October 2017 in Antwerp (Colebunders et al., 2018b). This consortium aims to combine the efforts of many experts in a wide variety of disciplines to take the OAE research to the next level and to eliminate onchocerciasis. There is a current need to estimate the burden of OAE in many countries to provide additional arguments to strengthen international onchocerciasis elimination programs. Furthermore, understanding the pathogenesis of OAE will help to develop better prevention and treatment strategies, such as immunotherapy in combination with appropriate antiepileptic treatment. In addition, providing a clear explanation how this epilepsy is caused will help to reduce the stigma and misperceptions that are associated with epilepsy in many remote villages, like that the person suffering from epilepsy is cursed or that it is contagious. Moreover, people with epilepsy require specialized care and current health systems should be improved to reach those in the most remote areas, where most OAE cases exist.
Competing interests
The authors declare that they have no competing interests.
Funding
The research activities of Dr Idro are funded by an African Research Leadership Award (MR/M025489/1) provided by the Medical Research Council (UK) and the UK Department for International Development under the MRC/DFID Concordat agreement. This award is also part of the EDCTP2 programme supported by the European Union.
References
- Boatin B. The onchocerciasis control programme in West Africa (OCP) Ann. Trop. Med. Parasitol. 2008;102(Suppl 1):13–17. doi: 10.1179/136485908X337427. Epub 2008/09/26. [DOI] [PubMed] [Google Scholar]
- Boussinesq M. Human onchocerciasis in Africa. Med. Trop. (Mars) 1997;57(4):389–400. Epub 1997/01/01. L’onchocercose humaine en Afrique. [PubMed] [Google Scholar]
- Sato Y.T.Y., Sakai K., Suzuki S., Kato S., Nishi M. International OAE Workshop. 2017. Household burdens of and community response to nodding syndrome in northern Uganda. Abstract 3. [Google Scholar]
- JHF R. World Health Organization; Geneva: 2004. The Global Burden of Onchocerciasis in 1990; p. 2004. world health organization. [Google Scholar]
- Katabarwa M.N., Lakwo T., Habomugisha P., Unnasch T.R., Garms R., Hudson-Davis L. After 70 years of fighting an age-old scourge, onchocerciasis in Uganda, the end is in sight. Int. Health. 2018;10(suppl_1):i79–i88. doi: 10.1093/inthealth/ihx044. Epub 2018/02/23. [DOI] [PubMed] [Google Scholar]
- JHF R. World Health Organization; Geneva: 2004. The Global Burden of Onchocerciasis in 1990.http://wwwwhoint/healthinfo/global_burden_disease/Onchocerciasis%201990pdf (Accessed on 25 April 2011) [Google Scholar]
- Colebunders R., Nelson Siewe F.J., Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2018;34(3):208–216. doi: 10.1016/j.pt.2017.11.009. Epub 2017/12/31. [DOI] [PubMed] [Google Scholar]
- Hotterbeekx A., Menon S., Siewe J.F.N., Colebunders R. Onchocerciasis associated epilepsy: an important neglected public health problem. Seizure. 2018 doi: 10.1016/j.seizure.2018.01.006. Epub 2018/01/13. [DOI] [PubMed] [Google Scholar]
- Okulicz Jason F. 2015. Dermatologic Manifestations of Onchocerciasis (River Blindness) Clinical Presentation. medscape. [Google Scholar]
- Brattig N.W. Pathogenesis and host responses in human onchocerciasis: impact of Onchocerca filariae and Wolbachia endobacteria. Microbes Infect. 2004;6(1):113–128. doi: 10.1016/j.micinf.2003.11.003. Epub 2004/01/24. [DOI] [PubMed] [Google Scholar]
- Sejvar J.J., Kakooza A.M., Foltz J.L., Makumbi I., Atai-Omoruto A.D., Malimbo M. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12(2):166–174. doi: 10.1016/S1474-4422(12)70321-6. Epub 2013/01/12. [DOI] [PubMed] [Google Scholar]
- ME M., RJ H., CD M., JF W., HW G S.C. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br. J. Dermatol. 1993;129(3):260–269. doi: 10.1111/j.1365-2133.1993.tb11844.x. [DOI] [PubMed] [Google Scholar]
- Gillette-Ferguson I., Hise A.G., McGarry H.F., Turner J., Esposito A., Sun Y. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness) Infect. Immun. 2004;72(10):5687–5692. doi: 10.1128/IAI.72.10.5687-5692.2004. Epub 2004/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland H.S., White A.T., Greene B.M., Murphy R.P., Taylor H.R. Ocular manifestations of onchocerciasis in a Rain-Forest Area of West Africa. Br. J. Ophthalmol. 1991;75(3):163–169. doi: 10.1136/bjo.75.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Hall L.R. Immune mechanisms in Onchocerca volvulus-mediated corneal disease (river blindness) Parasite Immunol. 2000;22(12):625–631. doi: 10.1046/j.1365-3024.2000.00345.x. Epub 2000/12/21. [DOI] [PubMed] [Google Scholar]
- Jelliffe D.B., Jones P.R., Stroud C.E. Nakalanga notes on the endemic dwarfism of Uganda. Trop. Geogr. Med. 1962;14:97–104. Epub 1962/06/01. [PubMed] [Google Scholar]
- Kipp W., Burnham G., Bamuhiiga J., Leichsenring M. The Nakalanga syndrome in Kabarole District, Western Uganda. Am. J. Trop. Med. Hyg. 1996;54(1):80–83. doi: 10.4269/ajtmh.1996.54.80. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- Boussinesq M., Pion S.D.S., Demanga-Ngangue KamgnoJ. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2002;96(5):537–541. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- Kaddumukasa M., Kaddumukasa M., Matovu S., Katabira E. The frequency and precipitating factors for breakthrough seizures among patients with epilepsy in Uganda. BMC Neurol. 2013;13(182) doi: 10.1186/1471-2377-13-182. Epub 2013/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion S.D., Kaiser C., Boutros-Toni F., Cournil A., Taylor M.M., Meredith S.E. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl. Trop. Dis. 2009;3(6) doi: 10.1371/journal.pntd.0000461. Epub 2009/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druet-Cabanac M., Preux P.M., Bouteille B., Bernet-Bernady P., Dunand J., Hopkins A. Onchocerciasis and epilepsy: a matched case-control study in the Central African Republic. Am. J. Epidemiol. 1999;149(6):565–570. doi: 10.1093/oxfordjournals.aje.a009853. Epub 1999/03/20. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Hendy A., van Oijen M. Nodding syndrome in onchocerciasis endemic areas. Trends Parasitol. 2016;32(8):581–583. doi: 10.1016/j.pt.2016.05.013. Epub 2016/06/13. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Mandro M., Mokili J.L., Mucinya G., Mambandu G., Pfarr K. Risk factors for epilepsy in Bas-Uele Province, Democratic Republic of the Congo: a case-control study. Int. J. Infect. Dis. 2016;49:1–8. doi: 10.1016/j.ijid.2016.05.018. Epub 2016/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick B., Laudisoit A., Tepage F., Ensoy-Musoro C., Mandro M., Bonareri Osoro C. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005732. Epub 2017/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R., Opoka R.O., Aanyu H.T., Kakooza-Mwesige A., Piloya-Were T., Namusoke H. Nodding syndrome in Ugandan children—clinical features, brain imaging and complications: a case series. BMJ Open. 2013;3(5) doi: 10.1136/bmjopen-2012-002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.S., Friedrich K., König R., Meindl M., Helbok R., Unterberger I. The head nodding syndrome—clinical classification and possible causes. Epilepsia. 2008;49(12) doi: 10.1111/j.1528-1167.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Duke B.O., Vincelette J., Moore P.J. Microfilariae in the cerebrospinal fluid, and neurological complications, during treatment of onchocerciasis with diethylcarbamazine. Tropenmed. Parasitol. 1976;27(2):123–132. Epub 1976/06/01. [PubMed] [Google Scholar]
- Konig R., Nassri A., Meindl M., Matuja W., Kidunda A.R., Siegmund V. The role of Onchocerca volvulus in the development of epilepsy in a rural area of Tanzania. Parasitology. 2010;137(10):1559–1568. doi: 10.1017/S0031182010000338. Epub 2010/04/15. [DOI] [PubMed] [Google Scholar]
- Johnson T.P., Tyagi R., Lee P.R., Lee M.H., Johnson K.R., Kowalak J. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci. Transl. Med. 2017;9(377) doi: 10.1126/scitranslmed.aaf6953. Epub 2017/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F., Armangue T., Dalmau J. Autoimmune encephalopathies. Ann. N. Y. Acad. Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. Epub 2014/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff C.M., Dale R.C. The immune system in pediatric seizures and epilepsies. Pediatrics. 2017;140(3) doi: 10.1542/peds.2016-3534. Epub 2017/08/11. [DOI] [PubMed] [Google Scholar]
- Dietmann A., Wallner B., Konig R., Friedrich K., Pfausler B., Deisenhammer F. Nodding syndrome in Tanzania may not be associated with circulating anti-NMDA-and anti-VGKC receptor antibodies or decreased pyridoxal phosphate serum levels-a pilot study. Afr. Health Sci. 2014;14(2):434–438. doi: 10.4314/ahs.v14i2.20. Epub 2014/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R., Opoka R.O., Aanyu H.T., Kakooza-Mwesige A., Piloya-Were T., Namusoke H. Nodding syndrome in Ugandan children--clinical features, brain imaging and complications: a case series. BMJ Open. 2013;3(5) doi: 10.1136/bmjopen-2012-002540. Epub 2013/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Andre A., Blackwell N.M., Hall L.R., Hoerauf A., Brattig N.W., Volkmann L. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295(5561):1892–1895. doi: 10.1126/science.1068732. Epub 2002/03/09. [DOI] [PubMed] [Google Scholar]
- Rodger F.C. The pathogenesis and pathology of ocular onchocerciasis. Am. J. Ophthalmol. 1960;49:104–135. Epub 1960/01/01. [PubMed] [Google Scholar]
- Fuglsang H., Anderson J. Microfilariae of Onchocerca volvulus in blood and urine before, during, and after treatment with diethylcarbamazine. J. Helminthol. 1974;48(2):93–97. doi: 10.1017/s0022149x00022653. Epub 1974/06/01. [DOI] [PubMed] [Google Scholar]
- Winkler A.S., Friedrich K., Velicheti S., Dharsee J., Konig R., Nassri A. MRI findings in people with epilepsy and nodding syndrome in an area endemic for onchocerciasis: an observational study. Afr. Health Sci. 2013;13(2):529–540. doi: 10.4314/ahs.v13i2.51. Epub 2013/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R., Mandro M., Njamnshi A.K., Boussinesq M., Hotterbeekx A., Kamgno J. Report of the first international workshop on onchocerciasis-associated epilepsy. Infect. Dis. Poverty. 2018;7(1):23. doi: 10.1186/s40249-018-0400-0. Epub 2018/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotterbeekx A., Onzivua S., Menon S., Colebunders R. Histological examination of post-mortem brains of children with nodding syndrome. Ann. Transl. Med. 2018;6(7):134. doi: 10.21037/atm.2018.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invert. Neurosci. 2016;16(3):7. doi: 10.1007/s10158-016-0190-7. Epub 2016/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall D.N. Recent updates on onchocerciasis: diagnosis and treatment. Clin. Infect. Dis. 2007;44(1):53–60. doi: 10.1086/509325. Epub 2006/12/05. [DOI] [PubMed] [Google Scholar]
- Hunyadi B., Siekierska A., Sourbron J., Copmans D., de Witte P.A.M. Automated analysis of brain activity for seizure detection in zebrafish models of epilepsy. J. Neurosci. Methods. 2017;287:13–24. doi: 10.1016/j.jneumeth.2017.05.024. Epub 2017/06/05. [DOI] [PubMed] [Google Scholar]
- Basanez M.G., Pion S.D., Boakes E., Filipe J.A., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect. Dis. 2008;8(5):310–322. doi: 10.1016/S1473-3099(08)70099-9. Epub 2008/05/13. [DOI] [PubMed] [Google Scholar]
- Van Laethem Y., Lopes C. Treatment of onchocerciasis. Drugs. 1996;52(6):861–869. doi: 10.2165/00003495-199652060-00007. Epub 1996/12/01. [DOI] [PubMed] [Google Scholar]
- Abegunde A.T., Ahuja R.M., Okafor N.J. Doxycycline plus ivermectin versus ivermectin alone for treatment of patients with onchocerciasis. Cochrane Database Syst. Rev. 2016;(1):CD011146. doi: 10.1002/14651858.CD011146.pub2. Epub 2016/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguzu R., Akun P.R., Ogwang R., Shour A.R., Sekibira R., Ningwa A. Setting up a clinical trial for a novel disease: a case study of the Doxycycline for the Treatment of Nodding Syndrome Trial - challenges, enablers and lessons learned. Glob. Health Action. 2018;11(1):1431362. doi: 10.1080/16549716.2018.1431362. Epub 2018/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Twenty Years of Onchocerciasis control . World Bank/FAO/WHO/UNDP; 1994. A Review of OCP in West Africa From 1974-1994. WHO Document. [Google Scholar]
- McMahon J.P. A review of the control of Simulium vectors of onchocerciasis. Bull. World Health Organ. 1967;37(3):415–430. Epub 1967/01/01. [PMC free article] [PubMed] [Google Scholar]
- Roberts J.M., Neumann E., Gockel C.W., Highton R.B. Onchocerciasis in Kenya 9, 11 and 18 years after elimination of the vector. Bull. World Health Organ. 1967;37(2):195–212. Epub 1967/01/01. [PMC free article] [PubMed] [Google Scholar]
- Barnley G.R. Control of Simulium vectors of onchocerciasis in Uganda. Proceedings of the Tenth International Congress of Entomology. 1956;3:535. [Google Scholar]
- Katabarwa M.N., Walsh F., Habomugisha P., Lakwo T.L., Agunyo S., Oguttu D.W. Transmission of onchocerciasis in wadelai focus of northwestern Uganda has been interrupted and the disease eliminated. J. Parasitol. Res. 2012;2012:748540. doi: 10.1155/2012/748540. Epub 2012/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.B. Sixty years of onchocerciasis vector control: a chronological summary with comments on eradication, reinvasion, and insecticide resistance. Annu. Rev. Entomol. 1994;39:23–45. doi: 10.1146/annurev.en.39.010194.000323. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- Lakwo T.L., Garms R., Rubaale T., Katabarwa M., Walsh F., Habomugisha P. The disappearance of onchocerciasis from the Itwara focus, western Uganda after elimination of the vector Simulium neavei and 19 years of annual ivermectin treatments. Acta Trop. 2013;126(3):218–221. doi: 10.1016/j.actatropica.2013.02.016. Epub 2013/03/06. [DOI] [PubMed] [Google Scholar]
- Lakwo T., Garms R., Wamani J., Tukahebwa E.M., Byamukama E., Onapa A.W. Interruption of the transmission of Onchocerca volvulus in the Kashoya-Kitomi focus, western Uganda by long-term ivermectin treatment and elimination of the vector Simulium neavei by larviciding. Acta Trop. 2017;167:128–136. doi: 10.1016/j.actatropica.2016.12.029. Epub 2016/12/31. [DOI] [PubMed] [Google Scholar]
- Idro R., Opar B., Wamala J., Abbo C., Onzivua S., Mwaka D.A. Is nodding syndrome an Onchocerca volvulus-induced neuroinflammatory disorder? Uganda’s story of research in understanding the disease. Int. J. Infect. Dis. 2016;45:112–117. doi: 10.1016/j.ijid.2016.03.002. Epub 2016/03/19. [DOI] [PubMed] [Google Scholar]
- Ndyomugyenyi R., Tukesiga E., Buttner D.W., Garms R. The impact of ivermectin treatment alone and when in parallel with Simulium neavei elimination on onchocerciasis in Uganda. Trop. Med. Int. Health. 2004;9(8):882–886. doi: 10.1111/j.1365-3156.2004.01283.x. Epub 2004/08/12. [DOI] [PubMed] [Google Scholar]
- Traore S., Wilson M.D., Sima A., Barro T., Diallo A., Ake A. The elimination of the onchocerciasis vector from the island of Bioko as a result of larviciding by the WHO African Programme for Onchocerciasis Control. Acta Trop. 2009;111(3):211–218. doi: 10.1016/j.actatropica.2009.03.007. Epub 2009/07/22. [DOI] [PubMed] [Google Scholar]