Abstract
Epidemiologic and histopathologic associations between endometriosis and epithelial ovarian cancer have been reported; however, the underlying molecular and cellular mechanisms are not well understood. A possible genetic link has been suggested in recent publications. Driver mutations in PIK3CA, KRAS, ARID1A, and other genes have been found in the epithelium of intrauterine endometrial tissue, ovarian and extraovarian pelvic endometriosis tissue, ovarian cancers associated with endometriosis (i.e., clear cell and endometrioid type), and other epithelial ovarian cancers. This makes sense because pelvic endometriosis occurs primarily as a result of retrograde menstruation and implantation of endometrial tissue fragments in ovarian inclusion cysts or extraovarian peritoneal or subperitoneal sites. Unlike epithelial cells, endometriotic stromal cells are mutation free but contain widespread epigenetic defects that alter gene expression and induce a progesterone-resistant and intensely inflammatory environment, driven by estrogen via estrogen receptor-β. The resulting increased estrogenic action in the stroma drives inflammation and sends paracrine signals to neighboring epithelial cells to enhance proliferation. In addition, massively high concentrations of estrogen in the ovary may exert an additional and direct genotoxic effect on DNA and cause accumulation of additional mutations and malignant transformation in initially mutated endometriotic epithelial cells in an ovarian endometrioma, which may initiate epithelial ovarian cancer. The same epithelial mutations and inflammatory processes in stroma are seen in extraovarian deep-infiltrating endometriosis, but carcinogenesis does not occur. We provide a focused review of the literature and discuss the implications of recent genetic breakthroughs linking endometriosis and ovarian cancer.
Histologically, pelvic endometriosis is defined as the presence of endometrium-like tissue in the ovary or pelvic peritoneum. In contrast to endometrial tissue, which consists of a substantial epithelial component that lines deep invaginations into the stroma, endometriotic tissue is composed primarily of stromal cells with scant and superficially located epithelial cells (1). Because ovarian cancer originates from epithelial cells, it is important to consider this histological distinction. Three independent groups using next-generation sequencing have identified mutations occurring in the epithelial cells of ovarian and extraovarian (deep-infiltrating) pelvic endometriosis samples from premenopausal women (2–4). No mutations were detected in the stromal cell component of endometriosis. Intriguingly, the epithelial cells of histologically normal intrauterine endometrial tissue from women with or without endometriosis also displayed a similar mutational profile (4). The epithelial mutations in endometriosis or endometrium included distinct driver mutations for ovarian cancer [e.g., in the PIK3CA, KRAS and ARID1A genes (2–4)]. At first glance, these findings were puzzling because the direct observation of malignant transformation of endometriotic lesions, particularly in the extraovarian locations, has rarely been reported in the literature (2–4). By the same token, intrauterine endometrium does not turn malignant unless it is associated with atypical hyperplasia. It is plausible that ovarian endometriomas may transform into invasive epithelial ovarian cancer over an extended time, measured in years (2–4). On the basis of these new, intriguing findings and other literature, we discuss in this review the significance of mutations in epithelial cells in endometriosis in general and a plausible link between epithelial mutations in endometriosis and ovarian cancer initiation.
A substantial body of epidemiologic evidence suggests a link between endometriosis and epithelial ovarian cancer (2, 3), but a plausible underlying mechanism has remained elusive. The risk factors and pathogenic processes for these diseases remained controversial or poorly understood until the 21st century, when two mechanisms gained general acceptance. Sampson (5) and others have long postulated that fragments of menstrual endometrium pass retrograde through the fallopian tubes, then implant and persist on the surface of the ovary, within a recently ruptured follicle, on pelvic peritoneal surfaces, or in the space between the rectum and vagina (Fig. 1). This mechanism has more recently been demonstrated in primate models and observed naturally in humans, and is also supported by the observation that spontaneous endometriosis occurs exclusively in species that regularly menstruate (6). Cellular and molecular data generated using human tissues of pelvic endometriosis strongly support this mechanism (7–9).
Figure 1.
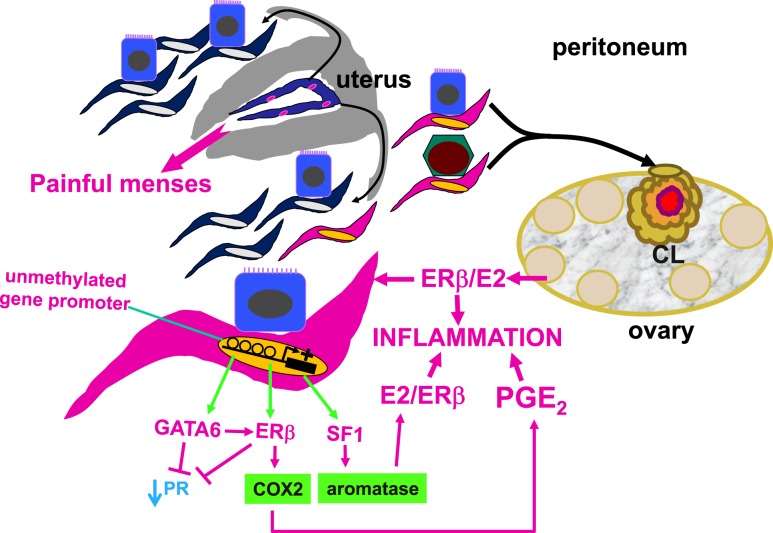
Central roles of the endometrial stromal cell and epigenetic regulation in endometriosis. In a nonpregnant ovulatory woman, endometrium is shed and renewed every month. Although most of the menstrual material composed of endometrial tissue fragments is expelled through the cervix into the vagina, a portion of it travels retrograde into the lower abdominal cavity and spills on pelvic tissues, including the ovaries. Although retrograde menstruation is observed in most women, only ∼10% of premenopausal women develop the histologic evidence or symptoms of endometriosis. Histologically, the majority of pelvic endometriotic lesions contain endometrial stromal cells, whereas fewer lesions harbor epithelial cells that are relatively scanty. If these endometrial tissue fragments implant on the peritoneum that covers the pelvic portions of the rectovaginal pouch, bowel, uterus, or pelvic side walls, these lesions are usually referred to as peritoneal endometriosis. If they line up along an ovarian inclusion cyst wall, this arrangement eventually evolves into an ovarian endometrioma, which, over time, accumulates remarkable amounts of blood and inflammatory products. Results of molecular and cellular studies suggest the eutopic endometrium of women with endometriosis contains stem-like stromal cell types that travel to the pelvis via retrograde menstruation. These stromal cells exhibit widespread epigenetic defects, such as inappropriately unmethylated DNA at gene promoters. This leads to inappropriate expression of the transcription factors, GATA6, ERβ, and SF1. These critical factors initiate a vicious cascade that activates the expression of cyclooxygenase-2 and aromatase, which leads to the production of large quantities of E2 and PGE2 fueling inflammation. GATA6 and ERβ suppress PR expression, causing progesterone resistance. If the endometrial tissue fragments become trapped in a CL cyst that has recently ruptured, they may survive and give rise to an ovarian endometrioma. CL, corpus luteum; E2, estradiol; ERβ, estrogen receptor-β; GATA6, GATA-binding factor-6; PGE2, prostaglandin E2; PR, progesterone receptor; SF1, steroidogenic factor-1.
A second mechanism that has recently gained acceptance pertains to the cellular origins of ovarian cancer (10). Many researchers originally believed that fragments of the damaged ovarian surface epithelium, the tunica albuginea, become trapped in ovarian follicles after ovulation and are exposed to intense inflammation; years later, these cells serve as progenitors for epithelial ovarian cancer (10). This mechanism is predicated on the observation that ovarian cancer risk increases with the number of ovulatory cycles (7–9). Based on accumulating epidemiologic and experimental data, there is the suggestion that the progenitor cell type does not arise from the ovarian surface epithelium but from the tubal or uterine epithelium (10–12). Surgical removal of the fallopian tubes and/or uterus radically decreases the risk for ovarian cancer (11, 12). Moreover, the majority of epithelial ovarian cancers mimic the cellular properties of the cervical, endometrial, or tubal epithelium (10). Results of studies using genetically engineered mouse models indicate the uterine horns are required for the formation of peritoneal endometriosis or epithelial ovarian cancer (13).
It may be intuitive that endometriosis is associated primarily with endometrioid ovarian cancer, but it is also associated with increased risk for other ovarian cancer types such as clear cell and serous (7–9). Ovulation not followed by pregnancy is a widely accepted risk factor for ovarian cancer and frequently associated with retrograde travel of blood and cellular material that probably contain and carry epithelial cells of the cervix, endometrium, or the fallopian tube to the ovarian surface or into the lumen of a recently ovulated follicle (14). Thus, it is plausible that endometriosis involves excessive retrograde menstruation that carries cancer progenitor cells into the ovarian parenchyma (Fig. 1) (4, 14).
Endometriosis
Many clinicians, patients, and researchers now recognize pelvic endometriosis as a complex clinical syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including the ovaries, and is strongly linked to recurrent pelvic pain and persistent episodes of ovulation, menstruation, and cycling steroid hormones (1). Pelvic endometriosis affects an estimated one in 10 women during their reproductive years (i.e., between 12 and 52 years of age), or ∼200 million women worldwide. The risk of pelvic endometriosis increases with a greater lifetime number of ovulatory cycles (1).
The aforementioned mechanism originally proposed by Sampson accounts for the majority of all forms of pelvic endometriosis, including peritoneal, ovarian, and rectovaginal (Fig. 1) (7, 8). Eutopic endometrial and ectopic endometriotic tissues of women contain epigenetically defective stromal cells with certain stem cell features and share similar molecular abnormalities, including abnormal expression of nuclear receptors [e.g., steroidogenic factor-1 (SF1) and estrogen receptor (ER)–β] and key enzymes (e.g., aromatase and cyclo-oxygenase-2) (7, 8).
Distinct cellular and molecular abnormalities involving inflammation and steroid responsiveness have been well described at least in two types of tissues: eutopic (intrauterine) endometrium and ectopic endometriotic tissue. Histologically, most endometriotic implants are composed primarily of stromal cells and contain a small epithelial component (1). The endometriotic stromal cell is epigenetically abnormal and demonstrates partial phenotypes of ovarian theca-granulosa cells [estradiol (E2) biosynthesis] and macrophages (cytokine production) (9). E2, primarily acting via its receptor ERβ, is a master regulator of all key pathological processes in endometriosis and enhances lesion survival and inflammation leading to pain (15). Endometriosis is resistant to the effects of progesterone because of progesterone receptor (PR) deficiency in this tissue, leading to differentiation defects both in endometrium and endometriosis (16). Thus, the key underlying mechanisms affect the intrauterine endometrium and extrauterine endometriosis tissue and include poorly differentiated endometrial mesenchymal progenitor/stem cells, widespread epigenetic defects, ERβ-mediated activation of inflammation, and progesterone resistance (9).
Epithelial Ovarian Cancer
Epithelial ovarian cancer comprises ∼3% of women’s cancers in the United States, but it is the most fatal gynecologic cancer and the fifth most fatal of all cancers in women. The National Cancer Institute estimates that 22,240 new cases of ovarian cancer will be diagnosed and 14,070 women will die of the disease in the United States in 2018 (https://www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdq). In the United States, the lifetime risk of invasive ovarian cancer is ∼1.3% (one in 77 women), and the lifetime risk of dying from invasive ovarian cancer is ∼1 in 100.
The median age at diagnosis of ovarian cancer is 63 years. A family history of ovarian or breast cancer in a first-degree relative approximately triples the risk. Approximately 10% of ovarian cancer cases could be traced to an inheritable risk factor such as a germ-line mutation in BRCA1 or BRCA2 DNA mismatch repair genes (17, 18). It is estimated that a large portion of ovarian cancers develop because of somatic driver mutations. Thus, environmental factors possibly play a major role. Suppression of ovulation, induced by pregnancy, use of combined oral contraceptives, or breastfeeding, is protective against ovarian cancer (19). Longer periods of breastfeeding correlate with a larger protective effect. Women who give birth to more children have a larger decrease in ovarian cancer risk, an effect observed with up to five births (19). The use of oral contraceptives reduces the risk of ovarian cancer by up to 50% and this protective effect can persist for up to three decades, even after their discontinuation (19).
Ovarian cancer is rarely diagnosed before widespread intra-abdominal metastases develop (20). Treatment usually involves surgery, chemotherapy, and newer biological agents (21). Factors that influence prognosis include stage and histologic grade, and the presence or absence of residual disease at the completion of initial surgery (22). The average 5-year survival rate for all ovarian cancer stages is 46%; the 10-year survival rate is ∼35% (23).
Link Between Endometriosis and Epithelial Ovarian Cancer
Epidemiologic evidence
A recent meta-analysis of data from 13 epidemiologic studies reporting prevalence of endometriosis in women with ovarian cancer found significant associations in three categories: clear cell carcinoma, endometrioid ovarian carcinoma, and general epithelial ovarian carcinoma (subgroup unspecified) (24). Endometriosis, therefore, may be a precursor of any epithelial ovarian cancer, with a particularly increased risk for clear cell and endometrioid carcinomas (25). Whether this association is causative is unclear, but endometriosis may progress to atypical endometriosis and endometriosis-associated ovarian cancer (EAOC). EAOC becomes manifest at an earlier age and often presents with earlier-stage disease; thus, these patients tend to experience better survival outcomes.
Histological analyses indicate that EAOC is most likely to coexist with endometrioid and clear cell carcinomas of the ovary. The clear cell and endometrioid subtypes are relatively rare and represent 5% to 10% of all epithelial ovarian carcinomas (26). In a series of patients with ovarian cancer undergoing surgery, however, 26% of patients with endometrioid malignancies and 21% of patients with clear cell malignancies had concomitant endometriosis, whereas the frequency of endometriosis in patients with other tumors remained <6% (25). In a pooled group of case-control studies involving >23,000 patients (7911 with ovarian cancer), a history of endometriosis was associated with a 1.5% lifetime risk of developing endometrioid, clear cell, or low-grade serous ovarian cancer (27). Taken together, when compared with women who do not have a history of endometriosis, women with endometriosis seem to have a two- to threefold increased risk of developing epithelial ovarian cancer (25).
Experimental evidence
It has been proposed that somatic mutations of the tumor suppressors, AT-rich interactive domain-containing protein 1A (ARID1A), phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA), and phosphatase and tensin homolog (PTEN) play critical roles in the progression of endometriosis to an atypical histology and EAOC (28–30). In a mouse model, local activation of an oncogenic Kirsten rat sarcoma (K-ras) allele (via injection of adenoviral Cre) induced ovarian or peritoneal endometriosis (13). Interestingly, peritoneal endometriosis occurred only when the needle for Cre injection was passed through the uterus/oviduct, suggesting the indispensable involvement of the uterotubal cells in peritoneal endometriosis (13). Conditional deletion of the tumor suppressor Pten within the ovarian surface epithelium also gave rise to ovarian lesions with an endometrioid epithelial morphology (13). The combination of K-ras activation and Pten deletion in the ovary induces growth of invasive and widely metastatic endometrioid ovarian adenocarcinomas (13). This ovarian cancer model recapitulates the specific tumor histomorphology and metastatic potential of the human disease, with complete penetrance and a latency of several weeks (13). In a separate study in which a murine model of endometriosis was used, activation of K-ras in donor endometrial tissue promoted endometriotic lesion development (31). These experimental observations in murine models were supported by a human study demonstrating that K-ras variant alleles were more frequently detected in endometriosis cases vs controls (31% vs 5%) (32). Thus, somatic activation of oncogenes or silencing of tumor suppressors related to endometriosis etiology seems to play a role in the development of EAOCs.
Inheritance and genomics
As observed in other chronic inflammatory conditions, endometriosis risk is transmitted in families in a complex and polygenic fashion (33). Results of an early study suggested the mothers and sisters of women with severe endometriosis had sevenfold higher risk for endometriosis (33). In addition, familial cases of endometriosis have an earlier onset of symptoms that are more severe compared with nonfamilial disease (34). In an analysis of a large number of twins, it was estimated that 52% of disease variance was due to genetic factors (35). Thus, endometriosis as a trait seems to be inherited in approximately one-half of cases.
Germline mutations in ovarian cancer
The majority (90%) of ovarian cancer cases do not seem to be inherited. These cancers are linked to somatic mutations that are acquired during a woman’s lifetime and do not cluster in families (17, 18). The remaining 10% of women with ovarian cancer have inherited germline mutations that predispose them to ovarian cancer (17, 18). Hereditary breast-ovarian cancer syndrome due to mutations in the tumor suppressor genes BRCA1 and BRCA2 account for the majority of inherited ovarian cancer (17, 18).
A much smaller proportion of inheritable ovarian cancer is associated with hereditary nonpolyposis colorectal cancer (a.k.a., Lynch syndrome) (36). Lynch syndrome is associated with mutations in a number of genes involved in the DNA mismatch repair pathway. The risk of endometrial (uterine) cancer associated with Lynch syndrome is ∼40%, whereas the risk of ovarian cancer is 10% (36).
Germline genetic variants in endometriosis
A genetic variant may be a mutation—a rare genetic variant that usually affects the structure or function of a protein—or it may be a single-nucleotide polymorphism (SNP), which is a common genetic variant that does not alter a protein. Since the 1990s, an intense effort was put toward discovering a germline mutation for familial endometriosis (37). Many “off-the-shelf,” candidate gene loci have been interrogated in familial or nonfamilial disease, but no causative mutations were found (38, 39). In an analysis of >1000 affected sister-pair families, genetic linkage was found between chromosome 10q26 and endometriosis (40). Follow-up efforts to map chromosome 10q26 suggested a possible association with the cytochrome P4502C19 (CYP2C19) gene, but no mutations that affect this gene could be demonstrated in patients with endometriosis (41, 42). Interestingly, CYP2C19 encodes an enzyme involved in the metabolism of proton pump inhibitors and antidepressants (43). E2 inhibits CYP2C19 expression via its receptor ERα (43). A subanalysis of 248 families with more than three affected members identified a key linkage to chromosome 7p13-15; however, no germline mutations were found (44, 45). Thus far, no family-based or case-control genetic association studies have revealed germ-line mutations associated with endometriosis (45–47).
Genome-wide association studies
Genome-wide association studies (GWAS) are observational studies of genetic variants, such as SNPs, across the entire genomes of different individuals to identify any variant associated with a trait or human disease. Over the last decade, several endometriosis GWAS have looked at thousands of endometriosis cases and control subjects, primarily women of Japanese or European ancestry (41, 42, 48). Together, these studies have identified endometriosis-associated loci near genes that transcribe noncoding RNAs or mRNAs translated into proteins. Some of these include cyclin-dependent kinase inhibitor 2B antisense RNA (CDKN2B-AS1), wingless-type MMTV integration site family member 4 (WNT4), vezatin (VEZT), fibronectin (FN1), and inhibitor of DNA binding 4 (ID4) (49, 50). Metanalyses of endometriosis GWAS data revealed several gene loci involved in ovarian steroid hormone signaling, including growth regulation by estrogen in breast cancer-1 (GREB1), coiled-coil domain containing 170 (CCDC170), ERα (ESR1), spectrin repeat containing nuclear envelope 1 (SYNE1), and FSH β-subunit (FSHB) (49, 50). Although much is known about the ovarian FSHB function (51), GREB1 encodes a protein target of E2/ERα. GREB1 is expressed in ≤85% of serous, endometrioid, mucinous, and clear cell carcinomas and increases proliferation and extracellular matrix formation by ovarian cancer cells (52). The majority of serous, endometrioid, and mucinous ovarian cancers are positive for either ESR1 or GREB1 (52). Recurrent rearrangements between ESR1 and the nearby gene CCDC170 have been observed in aggressive and endocrine-resistant luminal-B breast malignancies, suggesting a link between CCDC170 and estrogen action (53).
A critical missense SNP 19 kb downstream of ESR1 in the SYNE1 gene also was identified in endometriosis GWAS (54), which encodes a protein important for nuclear structure and organization, Golgi function, and cytokinesis (54). A SYNE1 isoform was downregulated in patients with serous and mucinous ovarian cancers (54). Thus, some of these loci may be involved in the development of moderate to severe ovarian disease, with stronger effect sizes in stage III and IV cancers (55). Until recently, no germline mutations affecting these genes in familial cases of endometriosis have been reported. Taken together, genes identified in endometriosis GWAS are linked to uterine development and stem cell function (WNT4), ovulatory function (FSHB, ESR1), and estrogen action (ESR1, GREB1, CCDC170, SYNE1, CYP2C19) (43, 52, 53) and also have associations with breast or ovarian cancer (52–54).
Somatic mutations in ovarian cancer
Frequent somatic mutations in TP53, KRAS, BRAF, PIK3CA, PTEN, and CTNNB1 have been reported in major types of epithelial ovarian carcinomas (56). Large-scale whole-exome sequencing analyses of >400 high-grade serous ovarian cancers in the Cancer Genome Atlas network demonstrated TP53 mutations in 96% of tumors and low prevalence but statistically recurrent somatic mutations in other genes, including NF1, BRCA1, BRCA2, RB1, and CRKRS (57, 58). Pathway analyses suggested that homologous recombination is defective in approximately half the tumors analyzed, and that NOTCH and FOXM1 signaling are involved in serous ovarian cancer pathophysiology (57). The mutation spectrum marks high-grade serous tumors as entirely distinct from other ovarian cancer subtypes. For instance, clear cell ovarian cancers have few TP53 mutations but have recurrent ARID1A and PIK3CA mutations (28, 56). Endometrioid ovarian cancers, on the other hand, have frequent CTTNB1, ARID1A, and PIK3CA mutations and a lower rate of TP53 mutations (28, 56). TP53 mutations occur frequently not only in high-grade serous tumors but also in mucinous and clear cell tumors (59). KRAS mutations are primarily seen in mucinous ovarian cancers (59).
Ovarian clear cell and endometrioid carcinomas are the most closely linked subtypes to endometriosis and are speculated to arise from endometriotic lesions (28). In one study, ARID1A mutations were uniquely seen in 46% of ovarian clear cell carcinomas, 30% of endometrioid carcinomas, and in no high-grade serous ovarian carcinomas. Seventeen carcinomas had two somatic mutations each (28). Loss of ARID1A protein correlated strongly with ovarian clear cell carcinoma and endometrioid carcinoma subtypes in the presence of ARID1A mutations (28). ARID1A mutations and loss of protein expression were detected in clear cell tumors and contiguous atypical endometriosis, but not in distant endometriotic lesions, suggestive of a progressive transformation of a benign endometriotic lesion to ovarian cancer (28). Another sequencing analysis of clear cell cancers showed that ARID1A (62%) and PIK3CA (51%) were frequently mutated. Other less frequently mutated genes included KRAS (10%), PPP2R1A (10%), PTEN (5%), MLL3 (15%), ARID1B (10%), and PIK3R1 (8%) (60). In addition to ARID1A, CNNTB1 gene mutations were present in high frequency in endometrioid ovarian cancer (61). Thus, mutations in ARID1A, PIK3CA, and CTNNB1 are uniquely overrepresented mutations in EAOC.
Somatic mutations in endometriosis
Whole-exome sequencing studies have recently reported somatic alterations in ovarian endometriomas and nonovarian deep-infiltrating endometriosis (2–4). Li et al. (3) were the first to describe single nucleotide variants in laser microdissected epithelial cells collected from healthy endometrial epithelium, ovarian endometriotic cells, and matched eutopic endometrial epithelial cells. Most gene variants were associated with cell adhesion and chromatin remodeling and were present in ectopic and matched eutopic endometrial cells (3). They found alterations in ARID1A in eutopic endometrial and ovarian endometriotic epithelial cells (3). Surprisingly, they also found thousands of nonsynonymous base-pair changes predicted to alter protein structure in eutopic endometrial epithelial cells from disease-free women (3). These investigators did not study any endometrial or endometriotic stromal cells and did not demonstrate any functional, pathologic, or clinical consequences of these exomic variations (3).
In a second and more detailed study, 107 ovarian endometriomas and 82 histologically normal intrauterine endometrial epithelium samples isolated by laser microdissection were sequenced (4). Somatic mutations were seen in several of the same genes that are mutated in EAOC (Fig. 2). In ovarian endometriotic epithelium, PIK3CA and KRAS were the most frequently mutated genes, followed by ARID1A (Fig. 2). In a separate cohort of 109 intrauterine endometrial glands, which include epithelial cells that are invaginated into the endometrium, cancer-associated gene mutations were seen in a heterogenous distribution (4). Interestingly, endometrial epithelium rarely showed mutated ARID1A (4). The authors speculated that endometrial cells containing a high frequency of cancer-associated gene mutations are delivered by retrograde flow and have a selective advantage to attach and grow on ectopic sites, thus increasing the risk of endometriosis and malignant transformation (4). The most stunning and consistent finding in these two independent reports was that histologically normal, eutopic endometrial epithelium in disease-free women with regular predictable menses harbored a substantial number of mutations, some of which are driver mutations found in ovarian cancer tissue (Fig. 2) (57, 58).
Figure 2.
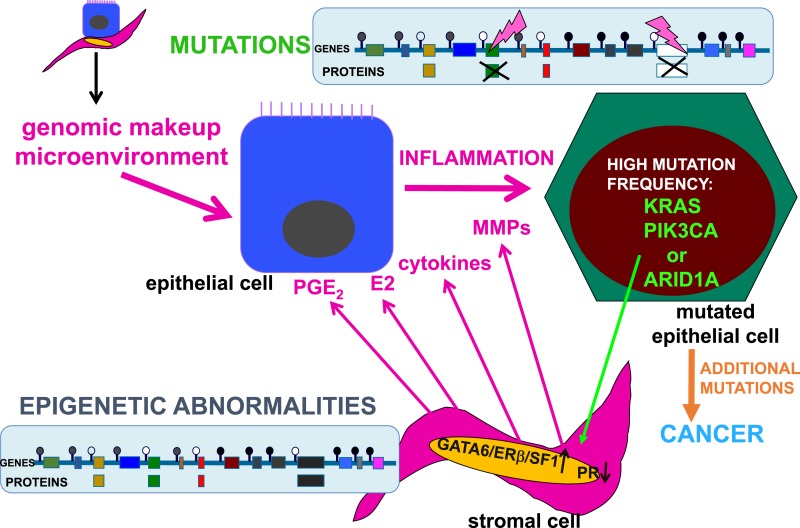
Interactions between the genomic processes in endometriotic stromal and epithelial cells. The genomic composition and microenvironment (intraovarian vs extraovarian) determine the malignant transformation of endometriotic lesions. The stromal cells do not contain mutations but do show abnormal epigenetic marks that modulate gene expression. For example, DNA methylation (closed circles) or unmethylation (open circles) silences or permits transcription of specific genes. In endometriotic stromal cells, progesterone resistance is due to suppression of PR and overexpression of GATA6, ERβ, and SF1 proteins. Inflammatory factors and proteins that remodel endometrial tissues, such as PGE2, E2, cytokines, and MMPs, accumulate in the stroma. In endometriotic epithelial cells, tumor driver mutations disrupt critical protein function, including PIK3CA, KRAS, ARID1A, and many others. The highly inflammatory and estrogenic, as well as progesterone-resistant, microenvironment in ovarian endometriomas may enhance the accumulation of additional mutations and proliferation of epithelial cells, which eventually become malignant and invasive. The specific effects of these epithelial mutations of stromal cell function in endometrial or endometriotic tissue are currently unknown. MMP, matrix metalloproteinase; PGE2, prostaglandin E2.
A third whole-exome sequencing study assessed epithelial and stromal components of extraovarian deep-infiltrating endometriotic tissue (2). The stromal cells of extraovarian deep-infiltrating endometriotic tissue did not reveal any mutations (Fig. 2) (2). However, this study reported a number of somatic mutations in epithelial cells of extraovarian endometriosis in 19 of 24 patients. The mutant-allele frequencies were generally low (<20%), suggesting that only a subset of endometriotic epithelial cells had mutations (2). Although only five endometriotic tissue samples (26%) carried ovarian cancer driver mutations (in ARID1A, PIK3CA, KRAS, or PPP2R1A), the probability of these driver genes being present at this rate only by chance was inferred to be extremely low (P = 0.001) (Fig. 2) (2). Droplet digital PCR analysis of an additional cohort of extraovarian endometriotic lesions showed that a minority of epithelial cells (4% to 38%) in each lesion harbored a KRAS mutation (2).
Because the risk of developing adenocarcinoma in patients with extraovarian endometriosis is near zero, it is not clear whether these sporadic epithelial mutations in deep-infiltrating endometriosis are clinically significant (2). This observation in extraovarian endometriosis is nevertheless quite important. When considered together with similar ovarian cancer driver mutations found in eutopic endometrium and ovarian endometriomas, retrograde travel of mutated endometrial epithelial cells seems to be a fairly common event (28, 56). In case these mutated cells are deposited in extraovarian sites, they do not seem to initiate a malignant process. On the other hand, if they populate an ovarian inclusion cyst, these mutations may drive the development of various types of ovarian cancers, including clear cell and endometrioid cell cancers (28, 56).
Epigenetic defects in endometriosis
Stromal cells compose the majority of endometriotic lesions and account for steroid-related inflammatory processes but lack any somatic mutations (Fig, 2) (2). However, endometriotic stromal cells do contain widespread epigenetic defects compared with eutopic endometrial stromal cells (Figs. 1 and 2). Epigenetic regulation of gene expression refers to alterations to the chromatin that do not involve a change in the DNA sequence (62). These processes include DNA methylation and histone modification, each of which regulates whether DNA is “open” or “closed” for gene transcription in a specific cell type (Fig. 1). These epigenetic alterations may persist through cell divisions for the duration of the cell’s life and may also be passed on to future generations (62). Epigenetic regulation of gene expression and protein production is the hallmark of stem cell function and drives the differentiation of progenitor/stem cells to more mature cell populations giving rise to specialized organ function. The proposed key roles of endometrial tissue stem cells in the development of endometriosis underscore the indispensable function of epigenetic mechanisms in the pathogenesis of endometriosis (Fig. 2) (63).
The failure to demonstrate causative germ cell or somatic mutations in endometriosis to date has led researchers to turn their attention to epigenetic mechanisms. Endometriotic cells express variable levels of the DNA methyltransferase enzymes, which maintain DNA methylation on the C5 position of cytosine in CpG dinucleotides (15). Several human and murine studies have explored pathologic gene regulation in endometriosis related to differential DNA methylation between the eutopic endometrium and endometriosis, as well as between the eutopic tissues from women with or without endometriosis (64, 65).
Several studies have compared the genome-wide differences in DNA methylation in healthy human endometrial and endometriotic stromal cells, using interaction analysis to correlate the findings to gene expression (66). Substantial differences in methylation were mapped to ∼400 genes; a large proportion were found to be transcription factors involved in endometriosis pathoprogression and the process of decidualization (66). Key differentially methylated genes included the HOX clusters, nuclear receptor genes, and GATA family transcription factors (66). Interestingly, promoter methylation of HOXA10 and A11 genes was also identified in advanced, high-grade, serous ovarian cancer and linked to responsiveness to platinum (67).
GATA2 transcriptionally regulates genes involved in hormone-driven differentiation of stromal cells. In endometriotic cells, GATA2 is hypermethylated and repressed, and GATA6 is hypomethylated and activated. GATA6 blocks hormone sensitivity; represses GATA2; induces markers of endometriosis such as aromatase, SF1, and ERβ; and represses ERα and PR when ectopically expressed in healthy endometrial cells (Fig. 1) (66). This unique epigenetic fingerprint in endometriosis suggests that DNA methylation contributes to disease status and members of the GATA family are key regulators of uterine physiology: Aberrant DNA methylation in endometriotic cells correlates with a shift in GATA isoform expression that facilitates progesterone resistance and disease progression (Fig. 1) (63, 66, 68).
According to findings of a recent study,, in contrast to endometrial mesenchymal stem cells from disease-free patients, eutopic endometrial mesenchymal stem cells isolated from patients with endometriosis do not differentiate in vitro to stromal cells that decidualize properly (63). This suggested that the proinflammatory and progesterone-resistant gene expression signature in eutopic endometrial stromal tissue from patients with endometriosis originates from defective endometrial mesenchymal stem cells. Together with the DNA methylation defects observed in the eutopic endometrium of patients with endometriosis, these findings substantiate the extraordinarily important and interactive roles of epigenetics and stem cells in the pathophysiology of endometriosis (68).
Expression patterns of specific genes in endometriotic or patient-matched eutopic endometrial stromal cells are suggestive of an ovarian granulosa cell-like differentiation (69). The expression levels of several nuclear receptors, including SF1, ERα, ERβ, and PR, are significantly different in endometriotic tissue compared with endometrium. Compared with healthy endometrium, there is a 12,000-fold greater expression of the orphan nuclear receptor SF1 in endometriotic tissue. SF1 is epigenetically regulated via methylation of a dense cluster of CpGs (an “island”) within its promoter. In normal endometrial stromal cells, the SF1 CpG island is heavily methylated, leading to the recruitment of methyl-CpG-binding domain protein 2 and transcriptional repression that results in low levels of SF1 and aromatase (7, 9, 70). In contrast, in endometriotic stromal cells, which contain abundant SF1 and its target aromatase, the SF1 promoter CpG island is not methylated and, therefore, not repressed. The unmethylated SF1 promoter allows binding of the transcription factor upstream stimulatory factor-2 (USF2) and activation of SF1 expression (71). Upon stimulation with prostaglandin E2 or cAMP, SF1 binds to the promoters of key steroidogenic genes in a coordinated fashion (70), catalyzing the local conversion of cholesterol to progesterone and E2 in a stepwise fashion in endometriosis (Fig. 1) (7, 9, 72). Thus, SF1 expression is determined epigenetically in endometriotic and endometrial tissue, through differential binding of activator vs inhibitor complexes to the SF1 promoter (70, 71).
In endometriotic stromal cells, ERβ levels are 142-fold higher and ERα levels are ninefold lower than in endometrial stromal cells (73). A CpG island in the promoter of the ERβ gene (ESR2) is hypomethylated in endometriotic stromal cells (permitting high expression) and hypermethylated in endometrial stromal cells (repressing expression). The opposite is seen for the ESR1 promoter, which is unmethylated in eutopic endometrium and heavily methylated in endometriotic tissues (66). ERα levels are strikingly lower in endometriotic vs endometrial stromal cells (73). It is thus conceivable that the pathologically high ERβ-to-ERα ratio in endometriotic stromal cells perturbs E2 induction of the PR gene, giving rise to low PR expression in endometriosis and ultimately progesterone resistance (Fig. 1) (16).
Plausible mechanisms that link endometriosis to ovarian cancer
There is considerable epidemiologic and circumstantial evidence that links endometriosis to ovarian cancer. Three recent exome-wide sequencing studies demonstrated commonly occurring epithelial mutations in PIK3CA and ARID1A in endometriosis that are uniquely shared with clear cell and endometrioid ovarian epithelial cancers (2, 3, 28, 56). In addition, mutations in KRAS that are commonly observed in low-grade serous ovarian cancers are uniquely observed in the epithelial cells of extraovarian endometriotic lesions (2, 56). As a further twist, the epithelial cells (a.k.a., glandular cells) in histologically and clinically normal endometrial tissue harbors many driver mutations (e.g.,PIK3CA, KRAS) with comparable mutant allele frequencies to those found in ovarian endometriotic epithelium (3, 4).
In contrast to epithelial cells, the stromal cells of extraovarian or ovarian endometriosis seem to lack any classical mutations that alter protein function. Endometriotic stromal cells contain numerous specific epigenetic defects that favor overproduction of E2 and overexpression of the steroid receptor ERβ that mediates an intense and E2-induced inflammatory process involving overproduction of cytokines and prostaglandins (7, 9, 15, 66). In parallel, pathologic epigenetic alterations give rise PR deficiency and progesterone resistance (Fig. 2) (16).
Endometrial tissue fragments of stroma and epithelium that menstruate retrograde onto ovarian and pelvic peritoneal surfaces survive and persist in these new locations in 10% of women, which is clinically defined as pelvic endometriosis (Fig. 1). We speculate that intense inflammation, progesterone resistance, and high levels of E2 (unopposed by progesterone action) in the stromal component lead to a high proliferative activity and enrichment of driver mutations (e.g.,PIK3CA, KRAS, ARID1A) in attached endometriotic epithelial cells. If the mutated epithelial cells happen to be in an extraovarian location, epithelial proliferation and mutagenesis levels remain sufficiently low and cancer very rarely develops. However, if endometriotic tissue is located in an ovarian cyst (endometrioma), then the accumulated products of inflammation, high estrogenic environment, and possibly other aspects of a procarcinogenic microenvironment in the ovary increase the likelihood of high critical mutation frequency and carcinogenic transformation. Even serous or mucinous tumors may originate from tubal or cervical epithelial cells that are mixed and carried along with retrograde menstrual tissue (Figs. 1 and 2). These unexpected epithelial mutations in endometrial and endometriotic tissues raise provocative questions that remain unanswered. We attempted to provide plausible answers to some of these questions.
What prevents the carcinogenic transformation of histologically normal eutopic endometrial epithelium that harbors remarkable levels of somatic driver mutations?
It is likely that each of these single mutations per se is not sufficient to initiate cancer. The accumulation of additional mutations and chronic estrogenic influence originating from the neighboring stromal cells seems to be necessary for the development of endometrial cancer (74). On the other hand, progesterone or a progestin, which is present in sufficient quantities during the luteal phase, pregnancy or combination oral contraceptive treatment, induces paracrine signals from the stromal cells to provide robust protection against the possible carcinogenic potential of these sporadic epithelial mutations (74). An additional protective mechanism may simply be anatomic: Substantial portions of these mutated cells must be shed and cleared periodically via ovulatory menses.
If ovarian endometriosis significantly increases the risk of ovarian cancer, then why is cancer arising from a clinically detectable ovarian endometrioma such a rare event?
The current clinical practice is long-term observation of most ovarian cysts presumed to be endometriomas by ultrasound evaluation (75). Interestingly, it is extremely rare that these endometriomas turn malignant (retrospectively estimated to be 0.14%) (75). Several factors may be responsible for this seemingly surprising observation. (i) EAOC may arise from smaller and clinically occult inclusion cysts harboring endometrial tissue. (ii) The lag period between a clinically detectable endometrioma and ovarian cancer seems to be at least several decades, which makes it difficult to link them epidemiologically. (iii) The incidence and prevalence of ovarian endometrioma are estimated to be 40- to 100-fold higher than the incidence of ovarian cancer (16, 76). Prospective clinical studies are needed to answer this important question.
How do the mutations in epithelial cells affect mutation-free endometrial or endometriotic stromal cells with respect to the development of endometriosis or cancer?
Nothing is known about this possible phenomenon at this time. It is plausible that altered paracrine signals resulting from these epithelial mutations may affect the epigenetic makeup of the neighboring stromal cells and enhance their inflammatory phenotype (Figs. 2 and 3). Inflammation then would contribute to the disease process in endometriosis in general or promote the development of ovarian cancers arising from ovarian endometriosis (Figs. 2 and 3).
Figure 3.
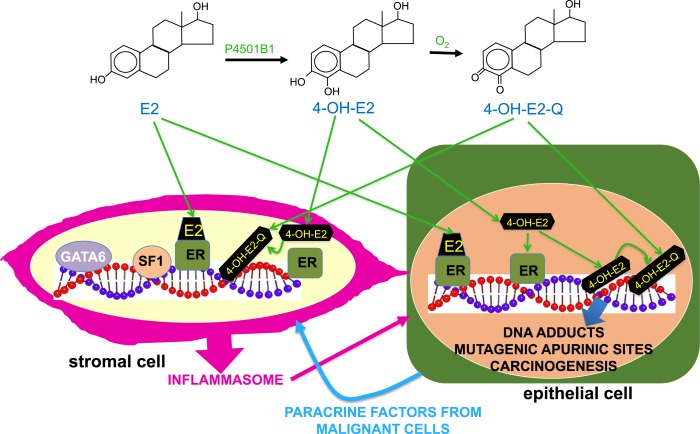
Ovarian microenvironment, mutagenesis, and carcinogenesis. Endometriotic epithelial cells with driver mutations in extraovarian sites do not seem to become malignant, whereas identical driver mutations are found in endometriosis-associated ovarian cancer, suggesting that the ovarian microenvironment is unique for enabling initially mutated endometriotic epithelial cells to acquire additional mutations and become malignant. In ovarian tissue, E2 and other estrogens are produced at levels that are >10,000 times those present in peripheral blood. We hypothesize that the genotoxic metabolites of estrogens cause DNA adducts resulting in mutagenic apurinic sites and accumulation of additional mutations during cell division. In fact, the levels of the CYP1B1 enzyme in endometriotic stromal cells are ∼100 times those found in normal endometrial stromal cells. This enzyme catalyzes the conversion of E2 to its 4-hydroxy-catechol metabolite, 4-OH-E2, which is further converted to redox-active quinones (4-OH-E2-Q). 4-OH-E2-Q may cause DNA damage by alkylation or oxidation leading to mutagenesis. Accumulation of more mutations in addition to an original driver mutation (see Fig. 2) likely facilitates the malignant transformation of an endometriotic epithelial cell located in an ovarian inclusion cyst. These epithelial cells lie adjacent to mutation-free but epigenetically defective stromal cells that produce inflammatory substances under the control of several transcription factors, including GATA6, SF1, and ERβ. In addition to regulating gene transcription, both ERα and ERβ in epithelial cells may concentrate toxic estrogen metabolites at DNA sites to intensify DNA damage and mutagenesis.
What are the characteristics of the eutopic endometrium in patients with endometriosis who are prone to developing ovarian cancer?
The answer to this question could be extraordinarily useful from a clinical perspective. We are not aware of any studies regarding the cellular and molecular characteristics of the eutopic endometrium in women who developed EAOC. However, if we would understand the molecular characteristics of the eutopic endometrium of the women predisposed to develop EAOC, then preventive measures against ovarian cancer could be used, such as long-term ovarian suppression.
Do the stem-like features leading to progesterone resistance in endometrial stromal cells play a central role in endometriosis and associated cancer?
In eutopic endometrial tissue, chronic estrogen exposure is carcinogenic, whereas progesterone is protective (74). Intriguingly, these carcinogenic or protective effects are mediated via stromal estrogen and progesterone receptors that send paracrine signals to endometrial epithelial cells (74). It is reasonable to hypothesize that similar mechanisms may regulate, in part, the carcinogenic processes in endometriotic tissue and its malignant counterpart EAOC. Thus, progesterone resistance present in ovarian endometriotic stromal cells may impair the progesterone-dependent protective effects against epithelial carcinogenesis, giving rise to EAOC that arises from ectopic endometrial epithelial cells.
What are the unique elements in the ovarian microenvironment that permit transformation of endometriotic epithelial cells?
On the basis of a considerable body of evidence, it is conceivable that massive concentrations of estrogen in the ovary may exert a direct genotoxic effect on DNA of ectopic endometrial (endometriotic) epithelial cells located in an ovarian endometrioma (51, 77, 78). This, in turn, would cause the accumulation of additional mutations in endometriotic epithelial cells (Fig. 3). In fact, the cytochrome P450 enzyme CYP1B1 convert estrogens to 4-hydroxy-catechol estrogens and, eventually, their depurinating quinone metabolites that cause DNA adducts leading to mutagenic apurinic sites (Fig. 3) (79).This would lead to accumulation of additional mutations during epithelial cell division. Although this mechanism has previously been viewed as ER independent, it was more recently proposed that ER might shuttle or concentrate the highly redox-active catechol estrogen–quinones at estrogen-target genes, where large amounts of reactive oxygen species may cause selective and intense DNA damage (Fig. 3) (78).
This direct genotoxic effect of estrogen traditionally has been studied in breast cancer (78). Estrogen concentrations in ovarian tissue, however, may be >10,000-fold higher than those found in breast or other peripheral tissues (51, 80). It was recently reported that the mRNA levels of CYP1B1, the enzyme that produces genotoxic quinone metabolites from estrogen, were ∼100-fold higher in ovarian endometrioma stromal cells compared with eutopic endometrial stromal cells (81). Thus, the intraovarian production of genotoxic quinones from estrone or E2 may be five to seven orders of magnitude higher compared with extraovarian body sites. Moreover, massive concentrations of estrogen in the ovary may also stimulate the inflammatory process via ERβ in endometriotic stromal cells, which may contribute to the carcinogenic process in neighboring epithelial cells (Figs. 2 and 3) (82, 83). Therefore, these unique properties of the intraovarian environment may explain why the risk for malignant transformation of ectopic endometrial lesions in the ovary is exponentially higher compared with the extraovarian sites (Fig. 3).
Concluding Remarks
Here, we highlighted and discussed the stunningly unexpected findings that came from three recent whole-exome sequencing studies of endometrial and endometriotic epithelial cells. We also attempted to provide some initial thoughts about the provocative questions these new findings raise. Developing novel disease models and paradigm-shifting approaches will be essential to providing definitive answers to these challenging questions.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant R37-HD38691 to S.E.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ARID1A
AT-rich interactive domain-containing protein 1A
- CCDC170
coiled-coil domain containing 170
- E2
estradiol
- EAOC
endometriosis-associated ovarian cancer
- ESR1
estrogen receptor-α
- ER
estrogen receptor
- FSHB
FSH β-subunit
- GREB1
growth regulation by estrogen in breast cancer-1
- GWAS
genome-wide association studies
- K-ras
Kirsten rat sarcoma
- PIK3CA
phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit α
- PR
progesterone receptor
- PTEN
phosphatase and tensin homolog
- SF1
steroidogenic factor-1
- SNP
single-nucleotide polymorphism
- SYNE1
spectrin repeat containing nuclear envelope 1
References
- 1. Bulun SE. Endometriosis In: Strauss J, Narnieri R, eds. Yen & Jaffe’s Reproductive Endocrinology. Philadelphia, PA: Elsevier; 2018:609–642. [Google Scholar]
- 2. Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376(19):1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Zhang Y, Zhao L, Wang L, Wu Z, Mei Q, Nie J, Li X, Li Y, Fu X, Wang X, Meng Y, Han W. Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum Mol Genet. 2014;23(22):6008–6021. [DOI] [PubMed] [Google Scholar]
- 4. Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, Tanaka K, Yamamoto T, Motoyama T, Inoue I, Enomoto T. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Reports. 2018;24(7):1777–1789. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. [Google Scholar]
- 6. D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8(1):84–88. [DOI] [PubMed] [Google Scholar]
- 7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 8. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–224. [DOI] [PubMed] [Google Scholar]
- 10. Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cadish LA, Shepherd JP, Barber EL, Ridgeway B. Risks and benefits of opportunistic salpingectomy during vaginal hysterectomy: a decision analysis. Am J Obstet Gynecol 2017;217(5):603.e1–603.e6. [DOI] [PubMed] [Google Scholar]
- 12. Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11(1):63–70. [DOI] [PubMed] [Google Scholar]
- 14. Vercellini P, Crosignani P, Somigliana E, Viganò P, Buggio L, Bolis G, Fedele L. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26(9):2262–2273. [DOI] [PubMed] [Google Scholar]
- 15. Dyson MT, Kakinuma T, Pavone ME, Monsivais D, Navarro A, Malpani SS, Ono M, Bulun SE Aberrant expression and localization of deoxyribonucleic acid methyltransferase 3B in endometriotic stromal cells. Fertil Steril. 2015;104:953–963.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 17. Lu HM, Li S, Black MH, Lee S, Hoiness R, Wu S, Mu W, Huether R, Chen J, Sridhar S, Tian Y, McFarland R, Dolinsky J, Tippin Davis B, Mexal S, Dunlop C, Elliott A. Association of breast and ovarian cancers with predisposition genes identified by large-scale sequencing [published online ahead of print 16 August 2018]. JAMA Oncol. doi: 10.1001/jamaoncol.2018.2956. [DOI] [PMC free article] [PubMed]
- 18. Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, Beeri R, Gal M, Grinshpun-Cohen J, Djemal K, Mandell JB, Lee MK, Beller U, Catane R, King MC, Levy-Lahad E. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA. 2014;111(39):14205–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am J Obstet Gynecol. 2004;191(3):733–740. [DOI] [PubMed] [Google Scholar]
- 20. Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102(4):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marchetti C, Pisano C, Facchini G, Bruni GS, Magazzino FP, Losito S, Pignata S. First-line treatment of advanced ovarian cancer: current research and perspectives. Expert Rev Anticancer Ther. 2010;10(1):47–60. [DOI] [PubMed] [Google Scholar]
- 22. Chan JK, Tian C, Monk BJ, Herzog T, Kapp DS, Bell J, Young RC; Gynecologic Oncology Group . Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008;112(10):2202–2210. [DOI] [PubMed] [Google Scholar]
- 23. Bell J, Brady MF, Young RC, Lage J, Walker JL, Look KY, Rose GS, Spirtos NM; Gynecologic Oncology Group . Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;102(3):432–439. [DOI] [PubMed] [Google Scholar]
- 24. Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer - a review. Acta Obstet Gynecol Scand. 2014;93(1):20–31. [DOI] [PubMed] [Google Scholar]
- 25. Wilbur MS, Shih IM, Segars JH, Fader AN.Cancer implications for patients with endometriosis. Semin Reprod Med. 2017;35(1):110–116. [DOI] [PubMed] [Google Scholar]
- 26. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–2529. [DOI] [PubMed] [Google Scholar]
- 27. Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A; Ovarian Cancer Association Consortium . Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D, Xiong J, Darr DB, Pardo-Manuel de Villena F, Kim WY, Magnuson T. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6(1):6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60(24):7052–7056. [PubMed] [Google Scholar]
- 31. Cheng CW, Licence D, Cook E, Luo F, Arends MJ, Smith SK, Print CG, Charnock-Jones DS. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J Pathol. 2011;224(2):261–269. [DOI] [PubMed] [Google Scholar]
- 32. Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E, Flores I, Weidhaas JB, Taylor HS. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4(3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson JL, Elias S, Malinak LR, Buttram VC Jr. Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137(3):327–331. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy S, Mardon H, Barlow D. Familial endometriosis. J Assist Reprod Genet. 1995;12(1):32–34. [DOI] [PubMed] [Google Scholar]
- 35. Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71(4):701–710. [DOI] [PubMed] [Google Scholar]
- 36. Jönsson JM, Bartuma K, Dominguez-Valentin M, Harbst K, Ketabi Z, Malander S, Jönsson M, Carneiro A, Måsbäck A, Jönsson G, Nilbert M. Distinct gene expression profiles in ovarian cancer linked to Lynch syndrome. Fam Cancer. 2014;13(4):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kennedy S. The genetics of endometriosis. Eur J Obstet Gynecol Reprod Biol. 1999;82(2):129–133. [DOI] [PubMed] [Google Scholar]
- 38. Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahmioglu N, Missmer SA, Montgomery GW, Zondervan KT. Insights into assessing the genetics of endometriosis. Curr Obstet Gynecol Rep. 2012;1(3):124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Treloar SA, Wicks J, Nyholt DR, Montgomery GW, Bahlo M, Smith V, Dawson G, Mackay IJ, Weeks DE, Bennett ST, Carey A, Ewen-White KR, Duffy DL, O’connor DT, Barlow DH, Martin NG, Kennedy SH. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77(3):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, Gordon SD, Wallace L, Henders AK, Visscher PM, Kraft P, Martin NG, Morris AP, Treloar SA, Kennedy SH, Missmer SA, Montgomery GW, Zondervan KT. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43(1):51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Painter JN, Nyholt DR, Morris A, Zhao ZZ, Henders AK, Lambert A, Wallace L, Martin NG, Kennedy SH, Treloar SA, Zondervan KT, Montgomery GW. High-density fine-mapping of a chromosome 10q26 linkage peak suggests association between endometriosis and variants close to CYP2C19. Fertil Steril. 2011;95(7):2236–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mwinyi J, Cavaco I, Pedersen RS, Persson A, Burkhardt S, Mkrtchian S, Ingelman-Sundberg M. Regulation of CYP2C19 expression by estrogen receptor α: implications for estrogen-dependent inhibition of drug metabolism. Mol Pharmacol. 2010;78(5):886–894. [DOI] [PubMed] [Google Scholar]
- 44. Zondervan KT, Treloar SA, Lin J, Weeks DE, Nyholt DR, Mangion J, MacKay IJ, Cardon LR, Martin NG, Kennedy SH, Montgomery GW. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13-15. Hum Reprod. 2007;22(3):717–728. [DOI] [PubMed] [Google Scholar]
- 45. Lin J, Zong L, Kennedy SH, Zondervan KT. Coding regions of INHBA, SFRP4 and HOXA10 are not implicated in familial endometriosis linked to chromosome 7p13-15. Mol Hum Reprod. 2011;17(10):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Treloar SA, Zhao ZZ, Le L, Zondervan KT, Martin NG, Kennedy S, Nyholt DR, Montgomery GW. Variants in EMX2 and PTEN do not contribute to risk of endometriosis. Mol Hum Reprod. 2007;13(8):587–594. [DOI] [PubMed] [Google Scholar]
- 47. Zhao ZZ, Nyholt DR, Le L, Treloar SA, Montgomery GW. Common variation in the CYP17A1 and IFIT1 genes on chromosome 10 does not contribute to the risk of endometriosis. Open Reprod Sci J. 2008;1(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 2010;42(8):707–710. [DOI] [PubMed] [Google Scholar]
- 49. Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG, Attia J, Holliday EG, McEvoy M, Scott RJ, Kennedy SH, Treloar SA, Missmer SA, Adachi S, Tanaka K, Nakamura Y, Zondervan KT, Zembutsu H, Montgomery GW. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, O D, Peterse D, Rexrode KM, Ridker PM, Schork AJ, MacGregor S, Martin NG, Becker CM, Adachi S, Yoshihara K, Enomoto T, Takahashi A, Kamatani Y, Matsuda K, Kubo M, Thorleifsson G, Geirsson RT, Thorsteinsdottir U, Wallace LM, Yang J, Velez Edwards DR, Nyegaard M, Low SK, Zondervan KT, Missmer SA, D’Hooghe T, Montgomery GW, Chasman DI, Stefansson K, Tung JY, Nyholt DR; iPSYCH-SSI-Broad Group . Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bulun SE. Physiology and pathology of the female reproductive axis In: Melmed S, Polonsky K, Larsen P, and Kronenberg H, eds. Williams Textbook of Endocrinology. 13th ed.Philadelphia, PA: Elsevier; 2016:627–663. [Google Scholar]
- 52. Hodgkinson K, Forrest LA, Vuong N, Garson K, Djordjevic B, Vanderhyden BC. GREB1 is an estrogen receptor-regulated tumour promoter that is frequently expressed in ovarian cancer. Oncogene. 2018;37(44):5873–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Veeraraghavan J, Tan Y, Cao XX, Kim JA, Wang X, Chamness GC, Maiti SN, Cooper LJ, Edwards DP, Contreras A, Hilsenbeck SG, Chang EC, Schiff R, Wang XS. Recurrent ESR1-CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat Commun. 2014;5(1):4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, Pike MC, Ness RB, Moysich K, Chenevix-Trench G, Beesley J, Webb PM, Chang-Claude J, Wang-Gohrke S, Goodman MT, Lurie G, Thompson PJ, Carney ME, Hogdall E, Kjaer SK, Hogdall C, Goode EL, Cunningham JM, Fridley BL, Vierkant RA, Berchuck A, Moorman PG, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Yang HP, Garcia-Closas M, Chanock S, Lissowska J, Song H, Pharoah PD, Shah M, Perkins B, McGuire V, Whittemore AS, Di Cioccio RA, Gentry-Maharaj A, Menon U, Gayther SA, Ramus SJ, Ziogas A, Brewster W, Anton-Culver H, Pearce CL; Australian Ovarian Cancer Study Management Group; Australian Cancer Study (Ovarian Cancer); Ovarian Cancer Association Consortium (OCAC) . ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20(5):702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ, Shih IeM. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma [published correction appears in Nature. 2012;490(7419):292]. Nature. 2011;474(7353):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, Zhang Q, Koboldt DC, Xie M, Kandoth C, McMichael JF, Wyczalkowski MA, Larson DE, Schmidt HK, Miller CA, Fulton RS, Spellman PT, Mardis ER, Druley TE, Graubert TA, Goodfellow PJ, Raphael BJ, Wilson RK, Ding L. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Commun. 2014;5(1):3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rechsteiner M, Zimmermann AK, Wild PJ, Caduff R, von Teichman A, Fink D, Moch H, Noske A. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol. 2013;95(2):235–241. [DOI] [PubMed] [Google Scholar]
- 60. Murakami R, Matsumura N, Brown JB, Higasa K, Tsutsumi T, Kamada M, Abou-Taleb H, Hosoe Y, Kitamura S, Yamaguchi K, Abiko K, Hamanishi J, Baba T, Koshiyama M, Okuno Y, Yamada R, Matsuda F, Konishi I, Mandai M. Exome sequencing landscape analysis in ovarian clear cell carcinoma shed light on key chromosomal regions and mutation gene networks. Am J Pathol. 2017;187(10):2246–2258. [DOI] [PubMed] [Google Scholar]
- 61. Lu Y, Cuellar-Partida G, Painter JN, Nyholt DR, Morris AP, Fasching PA, Hein A, Burghaus S, Beckmann MW, Lambrechts D, Van Nieuwenhuysen E, Vergote I, Vanderstichele A, Doherty JA, Rossing MA, Wicklund KG, Chang-Claude J, Eilber U, Rudolph A, Wang-Gohrke S, Goodman MT, Bogdanova N, Dörk T, Dürst M, Hillemanns P, Runnebaum IB, Antonenkova N, Butzow R, Leminen A, Nevanlinna H, Pelttari LM, Edwards RP, Kelley JL, Modugno F, Moysich KB, Ness RB, Cannioto R, Høgdall E, Jensen A, Giles GG, Bruinsma F, Kjaer SK, Hildebrandt MA, Liang D, Lu KH, Wu X, Bisogna M, Dao F, Levine DA, Cramer DW, Terry KL, Tworoger SS, Missmer S, Bjorge L, Salvesen HB, Kopperud RK, Bischof K, Aben KK, Kiemeney LA, Massuger LF, Brooks-Wilson A, Olson SH, McGuire V, Rothstein JH, Sieh W, Whittemore AS, Cook LS, Le ND, Gilks CB, Gronwald J, Jakubowska A, Lubiński J, Gawełko J, Song H, Tyrer JP, Wentzensen N, Brinton L, Trabert B, Lissowska J, Mclaughlin JR, Narod SA, Phelan C, Anton-Culver H, Ziogas A, Eccles D, Gayther SA, Gentry-Maharaj A, Menon U, Ramus SJ, Wu AH, Dansonka-Mieszkowska A, Kupryjanczyk J, Timorek A, Szafron L, Cunningham JM, Fridley BL, Winham SJ, Bandera EV, Poole EM, Morgan TK, Risch HA, Goode EL, Schildkraut JM, Webb PM, Pearce CL, Berchuck A, Pharoah PD, Montgomery GW, Zondervan KT, Chenevix-Trench G, MacGregor S; Australian Ovarian Cancer Study; International Endogene Consortium (IEC) . Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum Mol Genet. 2015;24(20):5955–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. [DOI] [PubMed] [Google Scholar]
- 63. Barragan F, Irwin JC, Balayan S, Erikson DW, Chen JC, Houshdaran S, Piltonen TT, Spitzer TL, George A, Rabban JT, Nezhat C, Giudice LC. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94(5):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193(2):371–380. [DOI] [PubMed] [Google Scholar]
- 65. Borghese B, Barbaux S, Mondon F, Santulli P, Pierre G, Vinci G, Chapron C, Vaiman D. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24(9):1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, Kakinuma T, Ono M, Jafari N, Dai Y, Bulun SE. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, Berry WA, Huang T, Nephew KP. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72(9):2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC. Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol Reprod. 2016;95(5):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fouquet B, Santulli P, Noel JC, Misrahi M. Ovarian-like differentiation in eutopic and ectopic endometrioses with aberrant FSH receptor, INSL3 and GATA4/6 expression. BBA Clin. 2016;6:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92(8):3261–3267. [DOI] [PubMed] [Google Scholar]
- 71. Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol. 2008;22(4):904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94(2):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681–687. [DOI] [PubMed] [Google Scholar]
- 74. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kuo HH, Huang CY, Ueng SH, Huang KG, Lee CL, Yen CF. Unexpected epithelial ovarian cancers arising from presumed endometrioma: a 10-year retrospective analysis. Taiwan J Obstet Gynecol. 2017;56(1):55–61. [DOI] [PubMed] [Google Scholar]
- 76. Kavoussi SK, Odenwald KC, As-Sanie S, Lebovic DI. Incidence of ovarian endometrioma among women with peritoneal endometriosis with and without a history of hormonal contraceptive use. Eur J Obstet Gynecol Reprod Biol. 2017;215:220–223. [DOI] [PubMed] [Google Scholar]
- 77. Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. [DOI] [PubMed] [Google Scholar]
- 78. Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6(12):947–960. [DOI] [PubMed] [Google Scholar]
- 80. diZerega GS, Marrs RP, Lobo R, Ujita EL, Brown J, Campeau JD. Correlation of inhibin and follicle regulatory protein activities with follicular fluid steroid levels in anovulatory patients. Fertil Steril. 1984;41(6):849–855. [DOI] [PubMed] [Google Scholar]
- 81. Piccinato CA, Neme RM, Torres N, Sanches LR, Cruz Derogis PB, Brudniewski HF, E Silva JC, Ferriani RA. Increased expression of CYP1A1 and CYP1B1 in ovarian/peritoneal endometriotic lesions. Reproduction. 2016;151(6):683–692. [DOI] [PubMed] [Google Scholar]
- 82. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Monsivais D, Dyson MT, Yin P, Coon JS, Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, Pavone ME, Su E, Bulun SE. ERβ- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol Endocrinol. 2014;28(8):1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]