Case #1
A 67 year old woman with a 50 pack year smoking history was diagnosed with KRAS G12C mutant metastatic lung adenocarcinoma (Figure 1). The patient responded to first line carboplatin and paclitaxel, but then progressed 19 months after diagnosis. At first progression she declined a biopsy and was started on nivolumab. Two weeks after her 36th cycle of nivolumab she experienced widespread progression including the development of a pericardial effusion with tamponade requiring pericardiocentesis. The pericardial fluid and pleural fluid from a new effusion revealed small cell lung carcinoma (SCLC). Next generation sequencing from pleural and pericardial fluid at the time of transformation revealed no KRAS mutation, a TP53 S315S frameshift mutation in both the pleural and pericardial fluid, and an RB1 splice site mutation in the pleural fluid. The patient was treated with carboplatin and etoposide followed by paclitaxel, and she died 11 months after SCLC and 4 years after her initial diagnosis.
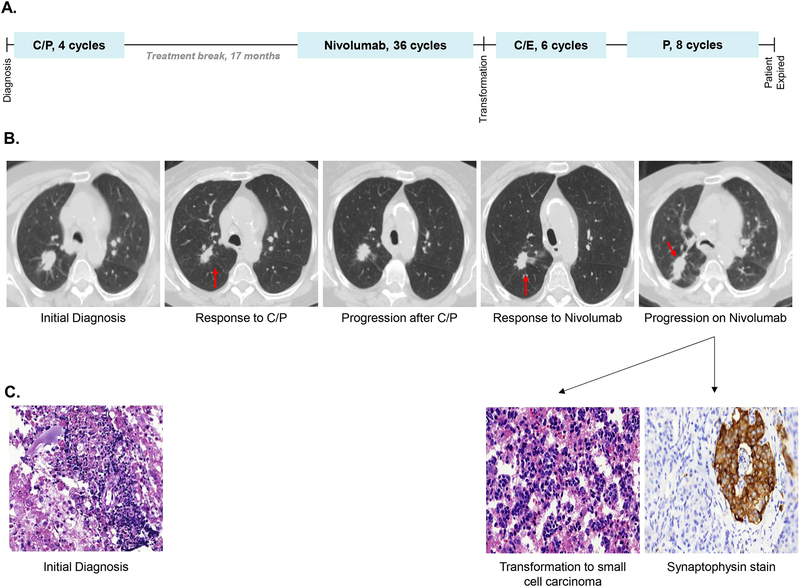
Figure 1. Case 1, Chronological summary of therapies, imaging studies, and available pathology from diagnosis of adenocarcinoma to transformation to small cell carcinoma.
(A) The patient received four cycles of carboplatin and paclitaxel (C/P) followed by a 17 month therapy holiday. She then received 36 cycles of nivolumab every two weeks with an initial response, and two weeks after her 36th dose of nivolumab she had widespread disease progression with biopsy revealing transformation to small cell carcinoma. She went on to receive six cycles of carboplatin and etoposide (C/E) and had a partial response. Two months after completion of C/E she had disease progression and received eight cycles of paclitaxel (P) with a response in her thoracic disease, but she had progression in the central nervous system prior to her death 11 months after small cell transformation.
(B) Treatment responses were observed in the primary right upper lobe nodule (red arrow) to both carboplatin and paclitaxel and nivolumab, followed by widespread progression, including in the right upper lobe nodule (red arrow), at the time of transformation to small cell carcinoma.
(C) Pathology from a level 4R lymph node at the time of diagnosis demonstrated poorly differentiated adenocarcinoma. Cytology from pericardial fluid at the time of transformation demonstrated small cell carcinoma, confirmed by synaptophysin immunohistochemistry.
Case #2
A 75 year old woman with a 30 pack year smoking history was diagnosed with KRAS G12C mutant metastatic lung adenocarcinoma (Figure 2). The patient responded to first line carboplatin, paclitaxel, and bevacizumab, and progressed 24 months after diagnosis. A repeat clinical trial related biopsy revealed lung adenocarcinoma, and she received 33 cycles of nivolumab with stable disease by RECIST 1.1. After an 11 month treatment holiday, she experienced asymptomatic progression with biopsy proven transformation to SCLC. Peripheral blood cell free DNA analysis at the time of SCLC transformation revealed conservation of the KRAS G12C mutation at an allele frequency (AF) of 19.47%, a TP53 R273C mutation with AF 0.55%, and no RB1 mutation was detected. The patient was treated in sequence with carboplatin and etoposide, nivolumab and ipilimumab, and irinotecan monotherapy. She died 16 months after SCLC transformation and 5.5 years after her initial diagnosis.
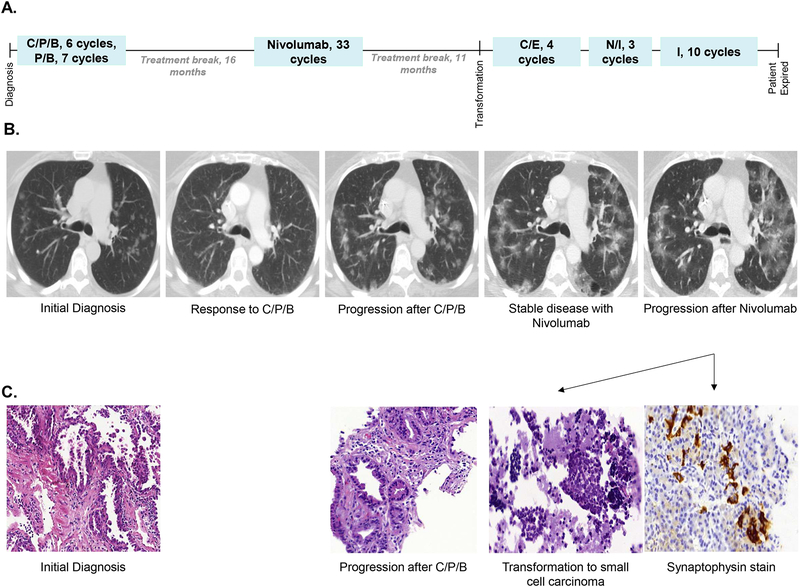
Figure 2. Case 2, Chronological summary of therapies, imaging studies, and available pathology from diagnosis of adenocarcinoma to transformation to small cell carcinoma.
(A) The patient received six cycles of carboplatin, pemetrexed, and bevacizumab (C/P/B), followed by seven cycles of pemetrexed and bevacizumab maintenance. She experienced disease progression after a 16 month therapy holiday. She received 33 cycles of second line nivolumab with disease stabilization, and after an 11 month therapy holiday she progressed with transformation to small cell carcinoma. She received four cycles of carboplatin and etoposide (C/E) with disease stabilization, and then experienced disease progression four months after completion of C/E. She received three cycles of nivolumab and ipilimumab (N/I) with disease progression, then received 10 cycles of irinotecan (I) with initial disease stabilization. After 10 cycles of irinotecan she had further disease progression, and then transitioned to hospice care dying 16 months after small cell transformation.
(B) The patient’s disease was primarily measured by upper lobe ground glass opacities (GGOs). She experienced a partial response by RECIST criteria with first line therapy, then disease stabilization on second line nivolumab. She had asymptomatic diffuse progression of her bilateral GGOs at the time of transformation to small cell carcinoma.
(C) Pathology from a left lower lobe lesion at the time of diagnosis demonstrated lung adenocarcinoma. At the time of first progression pathology from a right lower lobe lesion revealed lung adenocarcinoma consistent with the prior diagnosis. Pathology from a station 7 lymph node at the time of second progression demonstrated small cell carcinoma, confirmed by synaptophysin immunohistochemistry.
Discussion
The transformation of lung adenocarcinoma to SCLC has been observed in 5–15% of patients treated with epidermal growth factor receptor (EGFR) inhibitors1,2. In contrast, the transformation of lung adenocarcinoma to SCLC during nivolumab therapy has been reported only once3. Many cases have been felt to represent SCLC transformation rather than mixed histology at diagnosis as a result of their response to initial therapy and less aggressive clinical course than SCLC2; both of these attributes are recapitulated in our cases. In our first case, loss of the initial KRAS G12C driver mutation at SCLC diagnosis raises the possibility that SCLC was a second primary malignancy, but the initial KRAS G12C mutation was conserved in our second case, supporting the transformation hypothesis.
Our cases suggest that dedifferentiation from lung adenocarcinoma to SCLC may be a mechanism of resistance to PD-1 blockade, an intriguing finding that links the potential shared cell-of-origin theory (alveolar type II cells4) of lung adenocarcinoma and SCLC2,5. In these two cases we do not have information about TP53 and RB1 mutations at diagnosis, but it may be speculated that the presence of these mutations at diagnosis increases the risk of SCLC transformation in patients with lung adenocarcinoma treated with PD-1 blockade, similar to the recently published observation in patients with EGFR mutant lung adenocarcinoma1.
As the clinical applications of checkpoint inhibitors in patients with lung adenocarcinoma expand all clinicians must be aware of the potential for transformation to SCLC as a potential mechanism of resistance.
Acknowledgements
We would like to thank Kelli Boyd, Sherry Smith, and Yingjun Yan for their help with project coordination and slide review. We would also like to thank Seyed Ali Hosseini, Samantha Henderson, Kristy Potts for their help with sequencing.
Financial Support: WTI was supported by the National Institutes of Health (NIH) and National Cancer Institute (NCI) Vanderbilt Clinical Oncology Research Career Development Award (VCORCDP) 2K12CA090625–17 and an American Society of Clinical Oncology / Conquer Cancer Foundation Young Investigator Award (YIA). CML was supported by a VICC Young Ambassadors Award, a Damon Runyon Clinical Investigator Award, a LUNGevity Career Development Award, a V Foundation Scholar-in-Training Award, a Lung Cancer Foundation of America / International Association for the Study of Lung Cancer Lori Monroe Scholarship, and by the NIH and NCI R01CA121210, U10CA180864, P01CA129243, and U54 CA217450. KEB was supported by a Merck- Cancer Research Institute Irivington Post-doctoral Fellowship and a Lung Cancer Foundation of America/International Association for the Study of Lung Cancer with Bristol-Myers Squibb Joint Young Investigator Award for Translational Immuno-oncology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures: CML has served as a consultant for Pfizer, Novartis, Astra Zeneca, Genoptix, Sequenom, Ariad, Takeda, Foundation Medicine, Cepheid, and BluePrints Medicine. CML has received research funding (to her institution) from Xcovery, Astra Zeneca, and Novartis. LH is a consultant for Astra Zeneca, Abbvie, Bristol Myers Squibb, EMD Serono, Incyte, Merck, Roche, Tessaro, and Xcovery. JH, LPL, and CKR have an ownership stake and are employees of Resolution Biosciences. WTI, KEB, and KA report no conflicts of interest.
References
- 1.Lee JK, Lee J, Kim S, et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 2017;35:3065–74. [DOI] [PubMed] [Google Scholar]
- 2.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir Med Case Rep 2017;21:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754–64. [DOI] [PubMed] [Google Scholar]
- 5.Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013;8:1265–71. [DOI] [PubMed] [Google Scholar]