Abstract
Background:
Children with congenital gastrointestinal anomalies (CGIAs) experience multiple stressors while hospitalized in neonatal intensive care units during an essential time of growth and development. Early stress and inadequate nutrition are linked to altered growth patterns and later neurodevelopmental delays. In other at-risk populations, improved fat-free mass (FFM) accretion is associated with improved cognitive outcomes.
Objective:
To determine if body composition is associated with cognitive function in preschool-age children with CGIAs.
Study design:
An observational study examined body composition and cognition in 34 preschool-age children with CGIAs. Anthropometric measurements and body composition testing via air displacement plethysmography were obtained. Measurements were compared with a reference group of healthy, term-born children. Cognition was measured with the NIH Toolbox Early Childhood Cognition Battery. Linear regression was used to test the association of body composition with cognitive function.
Results:
Compared with the reference group, children with CGIAs had similar anthropometric measurements (weight, height, and body mass index z-scores) and body composition at preschool-age. Processing speed scores were lower than standardized means (p=0.001). Increased FFM was associated with higher receptive vocabulary scores (p=0.001), cognitive flexibility scores (p=0.005), and general cognitive function scores (p=0.05).
Conclusions:
At preschool-age, children with CGIAs have similar growth and body composition to their peers. In children with CGIAs, higher FFM was associated with higher cognitive scores. Closer tracking of body composition and interventions aimed at increasing FFM may improve long-term outcomes in this population.
Keywords: Body composition, Neonatal surgery, Neurodevelopment, Outcomes, Inflammation, Fat-free mass
1. Introduction
Children with congenital gastrointestinal anomalies (CGIAs) (e.g. gastroschisis, omphalocele, Hirschsprung’s disease, esophageal and bowel atresia, and diaphragmatic hernia) are often exposed to multiple stressors early in life during a vital time of growth and development. These stressors include hospitalization in the neonatal intensive care unit (NICU), anesthesia exposure, and major surgery. Additionally, these children are at significant risk for prolonged periods of inadequate nutrition related to enteral feeding intolerance and increased catabolic stress [1]. Exposure to physiologic stressors and inflammation during the neonatal period may have harmful effects on the developing infant, and they have been implicated as risk factors for future growth failure [2,3] and neurodevelopmental delays [4–7].
Like children with CGIAs, children born prematurely are often exposed to physiologic stressors (e.g. NICU hospitalization, inadequate nutrition, infection) early in life. It is well known that premature infants are at risk for growth failure [8,9] and future neurodevelopmental delays [10–12], potentially related to critically timed nutrient deficiencies [4,13,14]. Improved early growth is associated with improved neurodevelopmental outcomes amongst preterm infants [15–17]. Specifically, improved fat-free mass (FFM) gains in children born prematurely have been associated with improved neurodevelopment at 12 months of age and faster speed of processing at 4 months and 4 years of age [18–20]. To our knowledge, no published study has reported associations between body composition and neurodevelopmental outcomes in children with CGIAs.
In this study, we aimed to describe growth and neurodevelopment in this unique group of children and identify areas in which targeted interventions may be applied to promote optimal growth and development. We hypothesized that children with CGIAs demonstrating higher FFM accretion would show higher performance scores on cognitive tests. Conversely, those demonstrating higher fat-mass (FM) and higher percentage body fat (%BF) would show lower performance scores on cognitive tests.
2. Methods
2.1. Participants
Preschool-age children (4 to 5 years-old) born between September 2011 and December 2013 admitted to one of three, large, level 4 neonatal intensive care units in a metropolitan Midwest area with a surgical diagnosis affecting the gastrointestinal system (i.e. gastroschisis, omphalocele, esophageal and bowel atresia, imperforate anus, Hirschsprung’s disease, and diaphragmatic hernia) were recruited to participate. Children born prior to 35 weeks gestation and those with chromosomal anomalies known to affect growth or cognition were excluded. Clinical data regarding the child’s diagnosis, birth history, surgical history, and initial hospitalization were collected from the electronic medical record.
2.2. Study design
A prospective, observational study design was used to evaluate growth, body composition, inflammation, and cognition in preschool-aged children with CGIAs. Eligible children whose parents consented to be contacted for research studies were identified by ICD-9 and ICD-10 codes for the desired diagnoses and recruited via mail and/or phone calls. Each preschool-age child participated in one study visit. At time of the study visit, the parent gave informed written consent for their child’s participation in the study. Ethical approval was acquired from both participating hospital systems through their respective Institutional Review Boards. The study was conducted from November 2016 to March 2018.
2.3. Growth assessment
Standard anthropometric measures (weight, height, and body mass index (BMI)) were obtained at the time of the study visit. Weight was measured with an electronic scale to the nearest 0.1 gram, and height was measured using a stadiometer (Seca; Hamburg, Germany). Body composition (total body FM and FFM) was determined using air displacement plethysmography (Bod Pod with Pediatric Option, Cosmed, Ltd.; Concord, CA) as described by Fields and Allison [21]. Body measurements were compared with de-identified measures obtained from a reference group of 51 appropriate for gestational age children born at term who were enrolled in a previous study [18].
2.4. Neurodevelopmental assessment
Cognitive function was evaluated using the National Institutes of Health (NIH) Toolbox. This assessment tool was chosen due to its ability to directly target multiple aspects of cognition, and its ease of use via an iPad application. For this study, the Early Childhood Cognition Battery was utilized, which has been validated in children ages 3–6 years [22]. This battery evaluates several subdomains of cognitive function including receptive language (Picture Vocabulary test, PV), attention (Flanker Inhibitory Control and Attention test, FL), episodic memory (Picture Sequence Memory test, PSM), cognitive flexibility (Dimensional Change Card Sort test, DCCS), and processing speed (Pattern Comparison Processing Speed test, PS). In addition, it generates a Cognition Early Childhood Composite score (CECC), which is a measure of general cognitive function based on the successful completion of the first four tests within the battery (PV, FL, PSM, DCCS). For our analysis, fully corrected t-scores normed for age, gender, race, ethnicity, and parent’s education were utilized [23].
2.5. Statistical analysis
Descriptive statistics were expressed as mean (standard deviation) or median (interquartile range) for continuous variables, or count (percentage) for categorical variables. Comparisons of demographics and body measurements between the group of children with CGIAs and the healthy reference group were performed using two-sample t-test or Fisher’s exact test, as appropriate. Cognitive test scores (i.e. NIH toolbox fully corrected t-scores) were compared with their corresponding standardized population value normed for age, gender, race, ethnicity and parent’s education value using a two-sample t-test.
Associations between body measurements and cognitive test scores were assessed by simple linear regression or multivariate linear regression for adjusted analysis. Pearson correlation was used to assess the associations between the initial hospital course characteristics (mechanical ventilator days, total parenteral nutrition (TPN) days, length of stay) and body composition/cognitive test scores. All analyses were performed using SAS (v9.4; SAS Institute, Cary, NC). Statistical significance was defined as p≤0.05.
3. Results
3.1. Patient characteristics
A total of 34 children were enrolled in this study. 186 eligible children were identified from a search within the electronic medical record. Successful contact was made with 105 families of eligible children, and 34 families consented to participation. Table 1 describes the characteristics of the participating children with CGIAs. The majority of participating children were diagnosed with gastroschisis (23%) or Hirschsprung’s disease (21%). NICU hospital courses varied in severity. All children underwent at least one abdominal surgery, with 19 (55%) requiring additional surgeries. 11 (32%) children required mechanical ventilation beyond the perioperative period (>2 days). All children received TPN (range 3–116 days) with 12 children (35%) receiving TPN for less than 7 days, and 6 (18%) receiving TPN for more than 30 days. Four children (12%) had gastrostomy tubes placed prior to their discharge from the NICU, and all but one gastrostomy tube had been removed by the time of their study visit at preschool-age. Only one child had been diagnosed with short bowel syndrome.
Table 1.
Characteristics of children with CGIAs
| Variable | CGIA (n=34) |
|---|---|
| Primary diagnosis | |
| Gastroschisis | 8(23) |
| Hirschsprung’s disease | 7(21) |
| Imperforate anus | 4(12) |
| Tracheoesophageal fistula esophageal atresia | 4(12) |
| MalrotationVolvulus | 3(9) |
| Congenital diaphragmatic hernia | 3(9) |
| Jejunal atresia | 2(6) |
| Duodenal stenosis | 2(6) |
| Omphalocele | 1(2) |
| Delivery mode (n=33) | |
| C-section | 17(52) |
| Vaginal | 16 (49) |
| Maternal age at delivery (years) | 31 (28. 32) |
| Birth weight category | |
| SGA | 7(21) |
| LGA | 5(15) |
| Apgar score. 5 minute (n=31) | 9 (8. 9) |
| TPN days | 14 (5. 26) |
| Gastrostomy tube placed | 4(12) |
| Cholestasis present | 7(21) |
| Ventilator days | 2(1.10) |
| Number of operations | |
| One | 15 (45) |
| Multiple | 19(55) |
| Antibiotic days | 2 (1.10) |
| Length of stay days | 19.5 (10. 44) |
| Health problems following NICU discharge | |
| 0: No problem | 9(27) |
| 1: Mild (eczema, asthma. UTL ear infection) | 3(9) |
| 2: Moderate (outpatient investigations. <2 admissions) | 14(41) |
| 3: Severe (>2 admissions, major surgery) | 8(23) |
| Any preschool attendance | 24 (76) |
Data expressed as median (IQR) for continuous variables or n (%) for categorical variables, SGA=small for gestational age, LGA=large for gestational age, TPN=total parenteral nutrition, NICU=neonatal intensive care unit, UTI=urinary tract infection
3.2. Anthropometric measurements and body composition
All children completed anthropometric measurement and body composition testing. Table 2 describes these measurements as compared with a reference group. Children with CGIAs had lower gestational age (37.9 vs. 39.8 weeks, p<0.001), lower birth weight (3.2 vs. 3.5 kg, p=0.02), and were older at the time of their preschool visit (4.7 vs. 4.4 years, p=0.005) than children in the reference group. Despite these differences, anthropometric measurements (weight, height, and BMI z-scores) and body composition (FFM, FM, and %BF) were similar between the groups. After adjusting for age and sex, body composition measurements remained similar between the groups.
Table 2.
Anthropometric measurements and body composition of children with CGIAs compared with a reference group of term-born, healthy children at preschool-age
| Variable | CGIA (n=34) | Reference (n=51) | p-value |
|---|---|---|---|
| Demographics | |||
| Male sex | 22 (65) | 26 (51) | 0.27 |
| White Race | 31 (91) | 38 (75) | 0.09 |
| Mother’s education (college & above) | 29 (85.3) | 45 (88.3) | 0.75 |
| Gestational age (wks) | 37.9 (1.71) | 39.8 (1.03) | <0.001 |
| Birth weight (kg) | 3.22 (0.69) | 3.53 (0.46) | 0.02 |
| Age at preschool visit (yrs) | 4.7 (0.63) | 4.37 (0.23) | 0.005 |
| Anthropometric measurements | |||
| Weight z-score | −0.16 (1.05) | 0.02 (1.03) | 0.40 |
| Height z-score | 0.04 (1.00) | −0.08 (0.84) | 0.56 |
| BMI z-score | −0.18 (0.91) | 0.16 (0.77) | 0.07 |
| Body composition | |||
| Fat-free mass (kg) | 14.0 (2.57) | 13.72 (1.40) | 0.56 |
| Fat mass (kg) | 3.59 (1.23) | 3.46 (1.13) | 0.62 |
| Percent body fat | 20.38 (6.38) | 19.85 (5.00) | 0.68 |
p-values calculated from two-sample t-test, data expressed as mean (standard deviation) for continuous variables or n (%) for categorical variables
3.3. NIH Toolbox Early Childhood Cognition Scores
Figure 1 depicts the NIH Toolbox Early Childhood Cognition test scores for children with CGIAs in relation to standardized values (mean 50, SD 10). Cooperation with the testing was variable, as reflected in the varying n for each test. Overall, the children with CGIAs achieved scores similar to the standardized mean for receptive language (PV), attention (FL), cognitive flexibility (DCCS), and episodic memory (PSM). General cognitive function scores were higher than the standardized mean (CECC, p=0.04) while processing speed scores were lower (PS, p=0.001).
Figure 1.
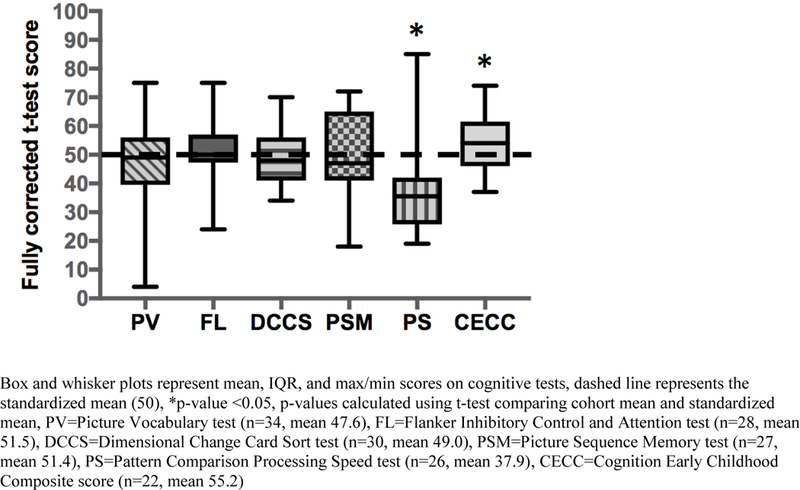
NIH Toolbox Early Childhood Cognition test scores in children with CGIAs.
3.4. Cognition in relation to body composition
Table 3 shows the associations between body measurements and cognitive test scores in children with CGIAs. Increased FFM was associated with higher receptive language scores (PV, β=3.1, SE=0.9, p=0.002), higher cognitive flexibility scores (DCCS, β=1.7, SE=0.6, p=0.005), and higher general cognitive function scores (CECC, β=1.5, SE=0.7, p=0.05) on unadjusted analysis. When adjusted for age and sex, FFM was no longer significantly associated with receptive language scores or general cognitive function scores at the 0.05 level. However, these associations continued to reach significance at the 0.1 level.
Table 3.
Associations between body composition and NIH Toolbox Early Childhood Cognition Battery test scores in children with CGIAs
| Test | Cognitive Subdomain |
BMI z-score | Fat-Free Mass | Fat Mass | Percent Body Fat | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | p-value | β (SE) | p-value | β(SE) | p-value | β (SE) | p-value | ||
| PV | Receptive | 3.5 (2.9) | 0.23 | 3.1 (0.9) | 0.001 | −1.5 (2.2) | 0.51 | −0.8 (0.4) | 0.06 |
| Language | 1.9 (1.0)a | 0.08a | −0.6 (1.9)a | 0.76a | −0.3 (0.4)a | 0.41a | |||
| FL | Inhibitory Control | 1.4 (2.3) | 0.54 | 0.7 (0.8) | 0.35 | −2.7 (1.8) | 0.14 | −0.6 (0.3) | 0.06 |
| and Attention | 0.7 (0.9)a | 0.46a | −3.7 (1.6)a | 0.03a | −0.9 (0.3)a | 0.009a | |||
| DCCS | Cognitive | 4.2 (1.6) | 0.01 | 1.7 (0.6) | 0.005 | 0.7 (1.4) | 0.63 | −0.2 (0.3) | 0.47 |
| Flexibility | 1.8 (0.7)a | 0.01a | 0.9 (1.4)a | 0.51a | −0.1 (0.3)a | 0.77a | |||
| PSM | Episodic Memory | 2.4 (3.0) | 0.43 | 1.3 (1.0) | 0.23 | 0.3 (2.2) | 0.89 | −0.1 (0.3) | 0.76 |
| 0.6 (1.1)a | 0.58a | 0.1 (2.0)a | 0.97a | −0.04 (0.5)a | 0.93a | ||||
| PS | Processing Speed | 3.3 (3.7) | 0.38 | −0.1 (1.3) | 0.96 | 2.1 (2.7) | 0.45 | 0.5 (0.6) | 0.37 |
| −0.2 (1.4)a | 0.89a | 1.2 (2.5)a | 0.62a | 0.4 (0.5)a | 0.47a | ||||
| CECC | General Cognitive | 4.9 (2.1) | 0.03 | 1.5 (0.7) | 0.05 | 1.9 (1.8) | 0.33 | 0.04 (0.4) | 0.92 |
| Function | 1.5 (0.8)a | 0.08a | 1.2 (1.8)a | 0.49a | −0.01 (0.4)a | 0.98a | |||
Linear regression analysis utilizing NIH Toolbox fully corrected t-scores normed for age, gender, race, ethnicity, and parent’s education
Adjusted for age and sex, PV=Picture Vocabulary test (n=34), FL=Flanker Inhibitory Control and Attention test (n=28), DCCS=Dimensional Change Card Sort test (n=30), PSM=Picture Sequence Memory test (n=27), PS=Pattern Comparison Processing Speed test (n=26), CECC=Cognition Early Childhood Composite score (n=22)
Increased FM and %BF were both negatively associated with attention scores (FL) after adjusting for age and sex (FM, β=−3.7, SE=1.6, p=0.03; and %BF, β=−0.9, SE=0.3, p=0.009). Increased BMI was associated with higher cognitive flexibility scores (DCCS, β=4.2, SE=1.6, p=0.01) and higher general cognitive functioning scores (CECC, β=4.9, SE=2.1, p=0.03). Weight and height z-scores were not significantly associated with any cognitive test scores.
When evaluating associations between the initial hospital course and body measurements/cognitive tests, increased length of stay and increased number of mechanical ventilator days were associated with lower FFM (r=−0.34, p=0.04 and r=−0.40, p=0.01 respectively). Both length of stay and ventilator days were not associated with cognitive test scores. The number of TPN days (a marker of overall nutritional status) was not significantly associated with body measurements or cognitive test scores.
4. Discussion
Children with CGIAs experience multiple stressors early in life during a time of rapid growth and brain development. These early exposures to suboptimal nutrition and growth as well as exposures to multiple surgeries and inflammation may impact future growth and neurodevelopmental potential. Reassuringly, this cohort of at-risk children with CGIAs were similar in size and body composition to a group of healthy, term-born children, and they generally performed well on cognitive testing. However, similar to other at-risk populations [18], processing speed scores were significantly lower than standardized means. When analyzing the relationship between growth and neurodevelopmental outcomes, we found that increased FFM in children with CGIAs was associated with higher receptive language scores, higher cognitive flexibility scores, and higher composite cognitive function scores.
Growth failure has been described in children with CGIAs prior to preschool-age. Neonates requiring surgery for GI diagnoses have been described as shorter, lighter, and with lower FM than matched controls at 43–44 weeks corrected gestational age [2]. Children with gastroschisis have been described as having suboptimal weight gain at 1 year [24] with continued suboptimal weight and linear growth at 2 years [25], and children with Hirschsprung’s disease have been described as having decreased length at 1 year [26]. In our cohort of preschool-age children with CGIAs, growth and body composition were similar to a reference group of healthy children. This suggests that any potential differences in growth are corrected, and that these children are able to catch-up to the growth of their peers by preschool-age. A similar phenomenon is seen in preterm infants whose growth and body composition differ early in life from healthy, term-born peers but largely catch-up and are similar by preschool-age [9,27,28].
Although the potential early differences in growth were no longer present at preschool-age, brain growth and resulting brain function may still be impacted by early stress, inflammation, and inadequate nutrition. Several studies have described lasting neurodevelopmental delays in children requiring neonatal surgery including deficits in cognition [29,30], receptive language/verbal intelligence [29–31] executive function [32], working memory [33], attention [31,34], gross motor skills [29,34], and fine motor skills [30,31]. Perhaps one of the most thorough longitudinal studies involving a patient population similar to our cohort was conducted by Ludman et al. [7,35,36]. This prospective observational study evaluated neurodevelopmental outcomes in children requiring major neonatal surgery. At 3 years of age, these children had cognitive abilities that were similar to matched controls [36]. At school-age (11–13 years), they were significantly behind in all measures of educational attainment (English, Math, Science, academic performance) [7]. The authors hypothesize that the increased presence of neurodevelopmental delays later in life may be related to early neural damage that only becomes apparent when cognitive tasks require more complex skills.
In our study, children with CGIAs completed a series of cognitive tests to closely examine several subdomains of cognition and potentially detect subtle differences in cognition that may be present at preschool-age and impact later school performance. Overall, children with CGIAs performed well on these tests and achieved the mean standardized scores in the cognitive subdomains of receptive language, attention, episodic memory, and cognitive flexibility. General cognitive function scores were in fact higher than the standardized mean, which potentially reflects the high motivation and educational status of the families participating in this study.
Although children with CGIAs generally performed well on cognitive testing, processing speed scores were significantly lower than standardized means. Deficits in speed of processing have also been described in children born prematurely [18–20]. Myelination and the structural integrity of white matter tracts are important factors in speed of processing [37,38]. The formation of these structures may be particularly vulnerable to the physiologic stressors and inflammation likely experienced by both preterm-born children and children with CGIAs during the newborn period. Early damage to these structures and subsequent deficits in speed of processing may not be noticed until school-age when children are required to work under pressure to complete more rigorous tasks.
Although the brain may be more vulnerable to physiologic stressors early in life, it is also a time when the brain is most plastic and amenable to interventions [4,39,40]. Optimizing nutrition with the goal of increasing FFM gains may be one intervention targeted to improve brain growth and cognitive function, specifically speed of processing. In preterm infants, increased growth during and following NICU hospitalization has been associated with improved neurodevelopmental outcomes [16,41–43]. Scheurer et al. showed that increased FFM was associated with faster speed of processing and higher IQ scores in preschool-age children born prematurely [18]. Similarly in our study, higher FFM was associated with higher receptive language, cognitive flexibility, and general cognitive function scores in preschool-age children with CGIAs. However, the associations between FFM and receptive language and general cognitive function scores lost some significance when adjusting for age and sex in this small cohort.
Similar to the associations seen with increased FFM, increased BMI was associated with increased cognitive flexibility scores and general cognitive function scores in children with CGIAs. BMI is a traditional measurement of body fat based on adult height and weight. In children, BMI does not reliably predict body fat percentage [44]. Furthermore in non-obese children, increased BMI may more closely reflect increased FFM [45], which potentially explains the similar associations seen with increasing FFM and increasing BMI in our cohort of non-obese children.
Conversely, increased FM and %BF were associated with decreased attention scores. Increased FM gains during toddlerhood have also been associated with lower full scale IQ and lower processing speed scores in healthy, term-born children [18]. Additional studies have likewise shown associations with obesity and poor academic performance [46,47]. Increased FM and obesity have been linked with increased inflammation [48,49], and the presence of chronic inflammation has been shown to adversely affect brain development [50,51].
This study has some limitations in addition to the small sample size. As with other studies, it is possible that we are over-estimating cognitive abilities in our cohort due to selection biases. Families that have the resources and the motivation to participate in research studies are also likely to have exposure to educational and nutritional resources that may improve their child’s growth and developmental outcomes [52]. Additionally, only those children willing to participate in the given tests could be scored making it difficult to distinguish between those who are unmotivated versus those who are cognitively unable to complete the tests. Therefore, this study may not be capturing the true extent of cognitive deficiencies present in the population of children with CGIAs.
5. Conclusion
This is the first study to examine body composition in relation to cognition in preschool-age children with CGIAs. Similar to preterm-born children, we found that children with CGIAs had slower processing speed and that increased FFM was associated with higher scores on cognitive testing. Increased FFM may be a marker of improved nutritional status, organogenesis, and brain growth, which ultimately results in improved cognitive function. Chronic inflammation and higher FM may also negatively impact later cognition. Results from this study suggest that early interventions aimed at optimizing FFM gains through improved nutrition may lead to more optimal long-term outcomes for this population.
Highlights:
Children with congenital gastrointestinal anomalies are at significant risk for growth failure and neurodevelopmental delays given their early exposures to physiologic stressors such as major surgery and inadequate nutrition.
At preschool-age, children with congenital gastrointestinal anomalies had similar body composition to a reference group of peers, and they generally performed well on cognitive testing. However, speed of processing scores were significantly lower than standardized means.
Like children born prematurely, higher fat-free mass was associated with higher cognitive test scores in children with congenital gastrointestinal anomalies.
Acknowledgements:
The authors wish to acknowledge the significant contributions of Lei Zhang, ScM and Michael Ehrhardt, BSc. The Center for Neurobehavioral Development at the University of Minnesota was integral in providing the staff and space that enabled successful study completion.
Sources of support: Funding for the project was from the Benjamin Walker Hanson Fund of the University of Minnesota Foundation and the Center for Neurobehavioral Development Seed Grant. The Center for Neurobehavioral Development at the University of Minnesota provided research space. Statistical analysis was performed through The Biostatistical Design and Analysis Center of The Clinical and Translational Science Institute of the University of Minnesota.
Abbreviations:
- CGIA
congenital gastrointestinal anomaly
- FFM
fat-free mass
- FM
fat mass
- %BF
percent body fat
- BMI
body mass index
- NICU
Neonatal Intensive Care Unit
- TPN
total parenteral nutrition
- NIH
National Institutes of Health
- PV
Picture Vocabulary test
- FL
Flanker Inhibitory Control and Attention test
- DCCS
Dimensional Change Card Sort test
- PSM
Picture Sequence Memory test
- PS
Pattern Comparison Processing Speed test
- CECC
Cognition Early Childhood Composite score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to report.
REFERENCES:
- 1.Pierro A, Eaton S Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg 2008;17(4):276–84. [DOI] [PubMed] [Google Scholar]
- 2.De Cunto A, Paviotti G, Travan L, Bua J, Cont G, Demarini S Impact of surgery for neonatal gastrointestinal diseases on weight and fat mass. J Pediatr 2015;167(3):568–71. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzenberg SJ, Georgieff MK Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018;141(2):e20173716. [DOI] [PubMed] [Google Scholar]
- 4.Prado EL, Dewey KG Nutrition and brain development in early life. Nutr Rev 2014;72(4):267–84. [DOI] [PubMed] [Google Scholar]
- 5.Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 2017;127(2):227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker K, Badawi N, Holland AJ, Halliday R Developmental outcomes following major surgery: what does the literature say? J Paediatr Child Health 2011;47(11):766–70. [DOI] [PubMed] [Google Scholar]
- 7.Ludman L, Spitz L, Wade A Educational attainments in early adolescence of infants who required major neonatal surgery. J Pediatr Surg 2001;36(6):858–62. [DOI] [PubMed] [Google Scholar]
- 8.Ramel SE, Brown LD, Georgeiff MK The impact of neonatal illness on nutritional requirements - one size does not fit all. Curr Pediatr Rep 2014;2(4):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramel SE, Gray HL, Ode KL, Younge N, Georgieff MK, Demerath EW Body composition changes in preterm infants following hospital discharge. J Pediatr Gastroenterol Nutr 2011;53(3):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos IS, Matijasevich A, Domingues MR, Barros AJ, Victora CG, Barros FC Late preterm birth is a risk factor for growth faltering in early childhood: a cohort study. BMC Pediatr 2009;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alyward GP Neurodevelopmental outcomes of infants born prematurely. Dev Behav Pediatr 2005;26(6):427–40. [DOI] [PubMed] [Google Scholar]
- 12.Aarnoudse-Moens CS, Weisglas-Kuperus N, Van Goudoever JB, Oosterlaan J Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124(2):717–28. [DOI] [PubMed] [Google Scholar]
- 13.Ramel SE, Georgieff MK Preterm nutrition and the brain. World Rev Nutr Diet 2014;110(July):190–200. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda S, Rabinovitz S, Mostofsky D Nutritional deficiencies in learning and cognition. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 3(December):S22–5. [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishnan U, DiGirolamo AM, Winichagoon P, Flores R, Singkhornard J, et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch Pediatr Adolesc Med 2012;166(5):411. [DOI] [PubMed] [Google Scholar]
- 16.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012;102(1):19–24. [DOI] [PubMed] [Google Scholar]
- 17.Shah PS, Wong KY, Merko S, Bishara R, Dunn M, Asztalos E, et al. Postnatal growth failure in preterm infants: ascertainment and relation to long-term outcome. J Perinat Med 2006;34(6):484–9. [DOI] [PubMed] [Google Scholar]
- 18.Scheurer J, Zhang L, Plummer E, Hultgren S, Demerath EW, Ramel SE Body composition changes in preterm children from infancy to 4 years are associated with early childhood cognition. In press at Neonatology [DOI] [PMC free article] [PubMed]
- 19.Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res 2013;74(5):576–83. [DOI] [PubMed] [Google Scholar]
- 20.Pfister KM, Zhang L, Miller NC, Ingolfsland EC, Demerath EW, Ramel SE Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. In press at Pediatric Research [DOI] [PMC free article] [PubMed]
- 21.Fields DA, Allison DB Air-displacement plethysmography pediatric option in 2–6 years old using the four-compartment model as a criterion method. Obesity 2012;20(8):1732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer PJ, Zelazo PD NIH toolbox cognition battery (CB): summary, conclusions, and implications for cognitive development. Monogr Soc Res Child Dev 2013;78(4):133–46. [DOI] [PubMed] [Google Scholar]
- 23.Beaumont JL, Havlik R, Cook KF, Hays RD, Wallner-Allen K, Korper SP, et al. Norming plans for the NIH Toolbox. Neurology 2013;80(Issue 11, Supplement 3):S87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minutillo C, Rao SC, Pirie S, McMichael J, Dickinson JE Growth and developmental outcomes of infants with gastroschisis at one year of age: a retrospective study. J Pediatr Surg 2013;48(8):1688–96. [DOI] [PubMed] [Google Scholar]
- 25.South AP, Marshall DD, Bose CL, Laughon MM Growth and neurodevelopment at 16 to 24 months of age for infants born with gastroschisis. J Perinatol 2008;28(10):702–6. [DOI] [PubMed] [Google Scholar]
- 26.More K, Rao S, McMichael J, Minutillo C Growth and developmental outcomes of infants with Hirschsprung disease presenting in the neonatal period: a retrospective study. J Pediatr 2014;165(1):73–77.e2. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MJ, Wootton SA, Leaf AA, Jackson AA Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 2012;130(3):E640–9. [DOI] [PubMed] [Google Scholar]
- 28.Scheurer JM, Zhang L, Gray HL, Weir K, Demerath EW, Ramel SE Body composition trajectories from infancy to preschool in children born premature versus full-term. J Pediatr Gastroenterol Nutr 2017;64(6):e147–53. [DOI] [PubMed] [Google Scholar]
- 29.Walker K, Badawi N, Halliday R, Stewart J, Sholler GF, Winlaw DS, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. J Pediatr 2012;161(4):748–752.e1. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer GM, Walker K, Baur L, Badawi N Developmental outcomes and physical activity behaviour in children post major surgery: an observational study. BMC Pediatr 2016;16(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lap CC, Brizot ML, Pistorius LR, Kramer WL, Teeuwen IB, Eijkemans MJ, et al. Outcome of isolated gastroschisis; an international study, systematic review and meta-analysis. Early Hum Dev 2016;103:209–18. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AC, Gunn JK, Hutchinson EA, Moran MM, Kelly LM, Sevil UC, et al. Cognition and behaviour in children with congenital abdominal wall defects. Early Hum Dev 2018;116(August 2017):47–52. [DOI] [PubMed] [Google Scholar]
- 33.Harris EL, Hart SJ, Minutillo C, Ravikumara M, Warner TM, Williams Y, et al. The long-term neurodevelopmental and psychological outcomes of gastroschisis: a cohort study. J Pediatr Surg 2016;51(4):549–53. [DOI] [PubMed] [Google Scholar]
- 34.Elsinga RM, Roze E, Van Braeckel KN, Hulscher JB, Bos AF Motor and cognitive outcome at school age of children with surgically treated intestinal obstructions in the neonatal period. Early Hum Dev 2013;89(3):181–5. [DOI] [PubMed] [Google Scholar]
- 35.Ludman L, Spitz L, Landsdown R Developmental progress of newborns undergoing neonatal surgery. J Pediatr Surg 1990;25(5):469–71. [DOI] [PubMed] [Google Scholar]
- 36.Ludman L, Spitz L, Lansdown R Intellectual development at 3 years of age of children who underwent major neonatal surgery. J Pediatr Surg 1993;28(2):130–4. [DOI] [PubMed] [Google Scholar]
- 37.Turken U, Whitfield-Gabrieli S, Bammer R, Baldo J, Nina F, Gabrieli JD Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage 2008;42(2):1032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields RD White matter in learning, cognition and psychiatric disorders. Trends Neurosci 2008;31(7):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wachs TD, Georgieff M, Cusick S, Mcewen BS Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci 2014;1308(1):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgieff MK Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85(2):614–20. [DOI] [PubMed] [Google Scholar]
- 41.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr 2013;163(6):1564–1569.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr 2016;173:108–15. [DOI] [PubMed] [Google Scholar]
- 43.Duerden EG, Guo T, Schneider J, Graz MB, Hagmann P, Chakravarty MM, et al. Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics 2018;141(3). [DOI] [PubMed] [Google Scholar]
- 44.Forsum E, Flinke Carlsson E., Henriksson H, Henriksson P, Löf M Total body fat content versus BMI in 4-year-old healthy Swedish children. J Obes 2013;2013, art.no.206715. [DOI] [PMC free article] [PubMed]
- 45.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes 2005;29(1):1–8. [DOI] [PubMed] [Google Scholar]
- 46.Carey FR, Singh GK, Brown H III, Wilkinson AV Educational outcomes associated with childhood obesity in the United States: cross-sectional results from the 2011–2012 National Survey of Children’s Health. Int J Behav Nutr Phys Act 2015;12(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu N, Chen Y, Yang J, Li F Childhood obesity and academic performance: the role of working memory. Front Psychol 2017;8(APR):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer K, Eng DS, Lumeng CN, Gebremariam A, Lee JM The relationship between body fat mass percentiles and inflammation in children. Obesity (Silver Spring) 2014;22(5):1332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dedoussis GV, Kapiri A, Samara A, Dimitriadis D, Lambert D, Pfister M, et al. Expression of inflammatory molecules and associations with BMI in children. Eur J Clin Invest 2010;40(5):388–92. [DOI] [PubMed] [Google Scholar]
- 50.Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res 2005;57(2):282–6. [DOI] [PubMed] [Google Scholar]
- 51.Bennet L, Dhillon S, Lear CA, van den Heuij L, King V, Dean JM, et al. Chronic inflammation and impaired development of the preterm brain. J Reprod Immunol 2018;125(November 2016):45–55. [DOI] [PubMed] [Google Scholar]
- 52.Doyle LW, Anderson PJ, Burnett A, Callanan C, McDonald M, Hayes M, et al. Developmental disability at school age and difficulty obtaining follow-up data. Pediatrics 2018;141(2). [DOI] [PubMed] [Google Scholar]


