Abstract
Background.
Aortic valve replacement (AVR) for calcific aortic stenosis is associated with high rates of perioperative stroke and “silent” cerebral infarcts on diffusion-weighted magnetic resonance imaging (MRI), but cognitive outcomes in elderly AVR patients compared with individuals with cardiac disease who do not undergo surgery are uncertain.
Methods.
One hundred ninety AVR patients (mean age=76±6y) and 198 non-surgical participants with cardiovascular disease (mean age=74±6y) completed comprehensive cognitive testing at baseline (pre-surgery), and 4–6 weeks and 1-year postoperatively. Surgical participants also completed perioperative stroke evaluations, including postoperative brain MRI. Mixed model analyses and reliable change scores examined cognitive outcomes. Stroke outcomes were evaluated in participants with and without postoperative cognitive dysfunction.
Results.
Based on reliable change scores, only 12.4% of the surgical group demonstrated postoperative cognitive dysfunction at 4–6 weeks and 7.5% at 1-year. Although the surgical group had significantly lower scores in working memory/inhibition 4–6 weeks after surgery, the groups did not differ at 1-year. In surgical participants, postoperative cognitive dysfunction was associated with more acute cerebral infarct number (p<0.01) and total volume (p<0.01) on MRI.
Conclusions.
In high-risk, aged participants undergoing surgical AVR for aortic stenosis, postoperative cognitive dysfunction was surprisingly limited and was resolved by 1-year in most. Post-operative cognitive dysfunction at 4–6 weeks was associated with more and larger acute cerebral infarcts.
Older age is a leading risk factor for cardiac postoperative cognitive dysfunction (POCD) (1), with contributors including embolic and ischemic brain injury (2), inflammatory processes (3), increased time on cardiopulmonary bypass (4), and prolonged anesthetic exposure (5). POCD is most frequently reported within 3 months after cardiac surgery. Non-surgical, comparison participants also show mild cognitive decline in longer-term studies (6, 7), and findings are mixed regarding increased risk for dementia following coronary artery bypass graft (CABG) (8, 9). Thus, longer-term cognitive outcomes remain unclear, especially for older patients.
Large studies of cardiac POCD with non-surgery peers have focused on patients <65 years of age (10, 11) undergoing CABG (6, 7). Young CABG patients face lower rates of stroke and other risk factors than older patients requiring aortic valve replacement (AVR) for aortic stenosis (12–15) who have been studied in small samples with abbreviated cognitive examinations. Collectively, this literature may underestimate the impact of other types of cardiac surgery on cognition for older adults at risk for stroke.
Recent studies of our surgical cohort documented a 19% incidence of clinical stroke and transient ischemic attack (TIA) and a 61% incidence of “silent” acute cerebral infarcts (16) (78% classified as embolic infarcts (17)). Herein we tested the hypothesis that surgical AVR for calcific aortic stenosis in this aged cohort, because of a high rate of acute cerebral infarcts and clinical stroke, would be associated with cognitive decline and a high incidence of POCD.
Patients and Methods
This research was approved by the University of Pennsylvania Institutional Review Board. All participants provided informed consent and were compensated for their time.
Participants
Fluent English speakers ≥65 years were recruited from April 2008 to September 2012 at two University of Pennsylvania Health System hospitals. Exclusion criteria included carotid stenting or endarterectomy within the previous 6 weeks, active psychiatric disorder, severe sensory/motor or cognitive impairment (18), and/or significant neurological disease/disorder.
Surgery participants were screened from 721 referrals. One hundred ninety-six individuals enrolled and received open, surgical AVR for calcific moderate to severe aortic stenosis; 190 agreed to participate in the cognitive testing at baseline. The anesthetic and surgical procedures were determined by the clinical team (16, 17).
Non-surgery peers were recruited from 2,317 individuals who had undergone a nonsurgical cardiac procedure (e.g., echocardiogram). From this pool, 198 medically stable participants with mild or mild to moderate aortic stenosis, with or without coronary artery disease, and who had not undergone a percutaneous cardiac procedure within the past 6 weeks were recruited.
Sample size was based on obtaining a minimum precision of 7% in the incidence rate estimates of stroke, new lesions, and POCD at 4–6 weeks in the surgery cohort (10% minimum expected incidence rate). The estimate of 160 participants was inflated by 20% to account for dropout. Thus, the target sample for the surgical group was 200 participants. A comparable-sized non-surgical comparison group resulted in a total sample target of 400 participants. Comparisons between the surgical and non-surgical group had >90% power under this design.
Demographic and Clinical Data
Basic demographic and clinical data were obtained with questionnaires (18, 19) at baseline (Supplemental Table 1).
Cognitive Protocol
Thirteen cognitive variables sampling five domains were collected at each time point (Supplemental Table 1). Test selection was informed by consensus guidelines (20) and psychometric data (21, 22). All participants were tested by three trained research assistants who were supervised by a licensed clinical neuropsychologist. Tests were scored by the administrator and a second coder, who was blinded to group status. Discrepancies were resolved by a third coder blinded to group status.
Cognitive scores were standardized using z-score transformation based on the mean and standard deviation (SD) of the entire sample at baseline. Five composite scores at each time point were computed by averaging the z-scores from each cognitive domain: episodic memory, visuoconstruction, language, working memory/inhibition, and attention (6, 23). All composite scores were normally distributed.
There are no universally accepted criteria for POCD. We used reliable change index (RCI) scores (24) to operationalize POCD as a meaningful change in cognitive functioning in surgical participants relative to the non-surgical group. RCIs for 13 cognitive variables were calculated by computing the change score (difference between baseline and 4–6 weeks and 1-year) for each participant and the calculating the following formula: [(change score) – (mean change scorenon-surgical)]/(SD of change scorenon-surgical)]. Composite RCI scores were formed by averaging the RCIs for the tests in each domain (episodic memory, etc.). We defined POCD as a decline of one or more of the composite RCI scores that was 1.67 SD or greater than the mean RCI of the non-surgical participants.
Postoperative MRI Protocol
MRI of the brain occurred within one week of surgery on a 1.5 Tesla Siemens Magnetom Avanto (Siemens, Erlangen, Germany) or GE Signa Excite (GE Medical Systems, Milwaukee, WI) scanner. Acute cerebral lesions were defined as hyperintensities on diffusion-weighted imaging (DWI) sequences, with matching hypointensities on apparent diffusion coefficient maps. T2-fluid attenuate inversion recovery MRI images were reviewed to rule out artifacts. Two trained readers blinded to background data independently read the scans. A radiologist resolved discrepancies. Acute cerebral lesions were manually segmented using a viewing and segmenting tool (MRIcron, http://www.nitrc.org/projects/mricron). Acute cerebral lesion number and total volume and the maximum single acute cerebral lesion volume are reported. Imaging procedures have been published (16).
Neurologic Evaluation and Postoperative Stroke Status
Trained stroke neurologists performed the National Institute of Health (NIH) Stroke Scale [(25)] and neurological examinations preoperatively and on postoperative days 1, 3, and 7. Postoperative status was classified as follows: 1) Stroke/TIA: new focal neurologic symptoms consistent with a vascular territory and without an alternative explanation at any postoperative exam; 2) Silent Infarct: acute cerebral lesion on DWI-MRI and negative postoperative neurologic exam; 3) No Ischemic Event: no acute cerebral lesion on DWI-MRI and negative postoperative neurologic exam.
Statistical Analyses
Stata/MP 14.2 (26) was used for analyses. Student’s T-tests, Mann-Whitney U tests, and chi square analyses examined group differences in demographics. Generalized estimating equation models assuming an exchangeable correlation structure and robust variance estimates examined group differences in cognition with adjustment for demographic variables. Comparisons between the groups at each time point and within groups (i.e., baseline vs. 4–6 weeks and 1-year) were computed from the marginal predictions. p values were computed using Sidak adjustments for multiple comparisons. Sensitivity to potentially non-random dropout was examined using shared parameter models (27).
RCI analyses provided incidence and type of POCD at each post-surgical time (i.e., change >1.67 SD than change in the non-surgical group). Differences between participants with vs. without POCD were examined using Student’s T-tests, Mann-Whitney U tests, chi-square analyses, Poisson distribution tests (number of acute cerebral lesions), or two-part tests (NIH Stroke Scale, acute cerebral lesion volume). Two-part tests combine the proportion of participants with stroke or acute lesion(s) with the distribution of the scores/lesion size conditional on having a stroke/lesion to generate a single test that is more representative of the outcome than looking at either part independently (28). Statistical significance was set at p < 0.05.
Results
Characteristics of Surgery and Non-Surgery Participants
Groups differed significantly in age, education, race, and medical comorbidities (Table 1). Surgery participants were one year older, more often Caucasian, and had two fewer years of education, more medical comorbidities, and more depression symptoms, although mood was well within normal limits. Demographic and mood variables were included as covariates in group analyses.
Table 1.
Group Characteristics
| Surgery (n = 190) |
Non-Surgery (n = 198) |
p Value | |||
|---|---|---|---|---|---|
| M/% | SD | M/% | SD | ||
| Age | 75.7 | 6.0 | 74.3 | 6.3 | 0.02 |
| Male | 63% | 67% | 0.36 | ||
| Education, years | 13.8 | 3.0 | 15.0 | 3.2 | <.01 |
| Caucasian | 95% | 84% | <.01 | ||
| Past stroke | 4% | 5% | 0.36 | ||
| Past transient ischemic attack | 11% | 14% | 0.32 | ||
| Charlson Comorbidity Index | 1.4 | 1.3 | 1.1 | 1.2 | <.01 |
| Geriatric Depression Scalea | 4.1 | 4.1 | 2.6 | 3.4 | <.01 |
| Mini Mental Status Examb | 27.5 | 1.9 | 27.8 | 1.9 | 0.07 |
max = 30 (worst)
max = 30 (best).
Study Completion
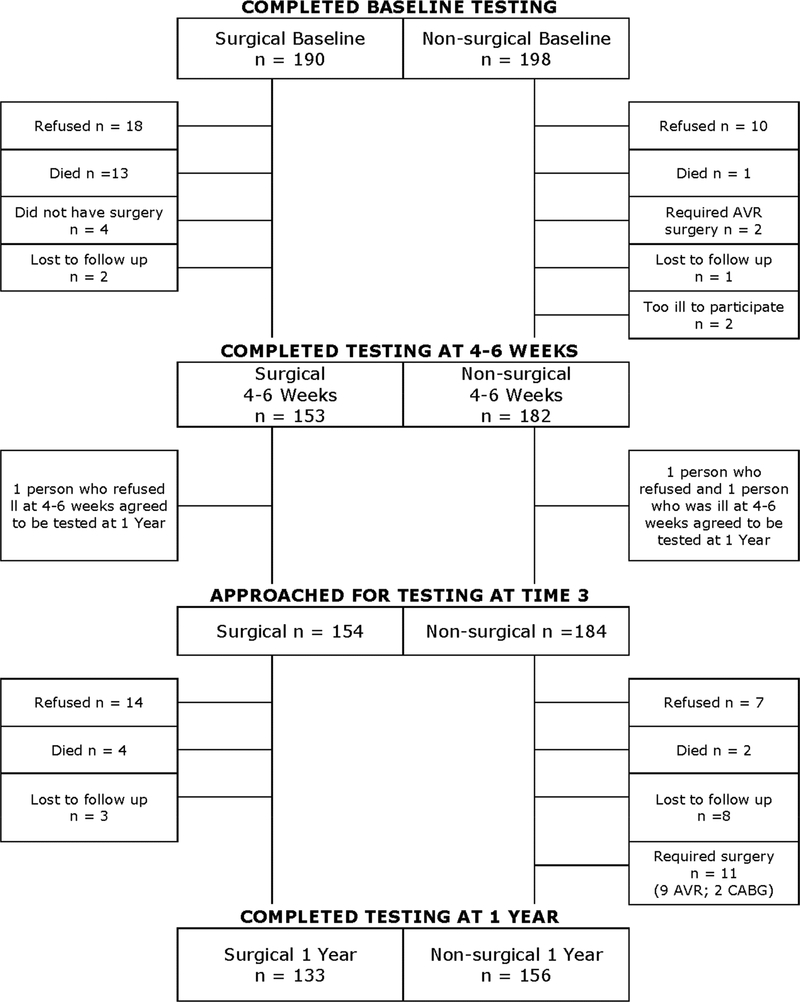
Eighty-one percent of surgery and 92% of non-surgery participants completed the 4–6 week evaluation (χ2 = 9.7, p < 0.01; Figure 1). Among surgical drop-outs, 49% refused and 35% died. Among completers at 4–6 weeks, 86% of surgery and 85% non-surgery participants completed the 1-year evaluation (χ2 = 0.07, p = 0.79). Relative to the non-surgery group, surgical group dropouts were less educated, more often non-Caucasian, had lower baseline cognitive function, and more depression symptoms and medical comorbidities (Charlson Comorbidity Index), including TIA. Consequently, there were fewer demographic and clinical differences between the surgical and non-surgical participants who completed the study (see Supplemental Table 2). Surgical dropouts also were more likely to have had a postoperative clinical stroke compared to surgical study completers (χ2 = 11.09, p < 0.01).
Fig 1.
Flow diagram of cognitive test completion at each study time point.
Postoperative Cognitive Outcomes
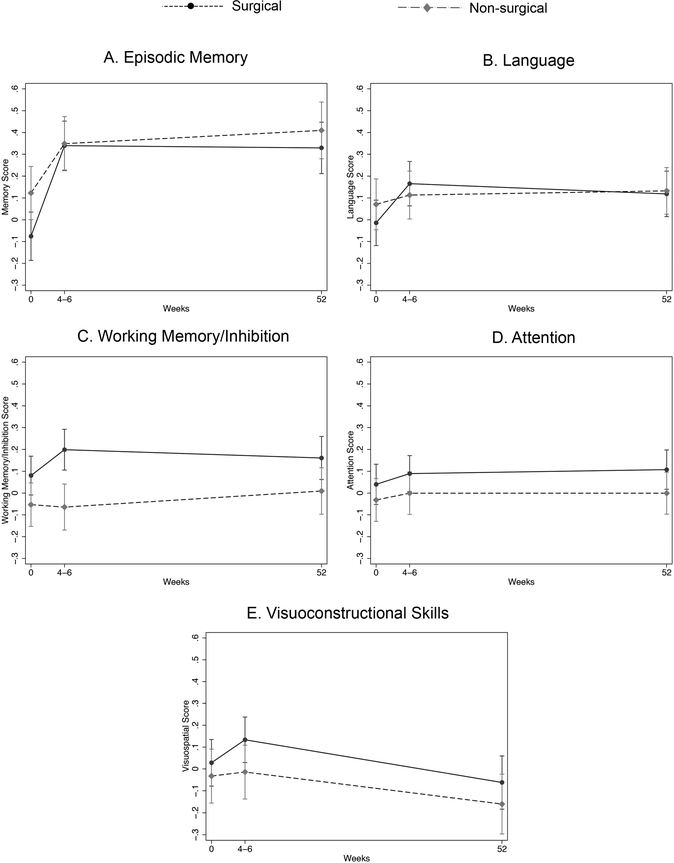
Group marginal predicted scores over time, with 95% confidence intervals, were generated from the generalized estimating equation models after adjustment for demographic differences (Figure 2). After controlling for demographic differences and multiple comparisons, the surgical and non-surgical groups differed only on the working memory/inhibition score at 4–6 weeks post-surgery [mean difference(standard error, SE) = 0.26(0.07), 95% Confidence Interval (CI) = 0.09 to 0.44, p = 0.001; all other p > 0.060].
Fig 2.
Marginal predicted composite scores at each time point for surgical (dashed line) and non-surgical (solid line) participants. (A) Episodic memory, (B) Language, (C) Working Memory/inhibition, (D) Attention, and (E) Visuoconstructional skills. Error bars reflect 95% confidence intervals. An asterisk indicates significantly different scores between surgery and non-surgery groups. Significant differences from baseline to 4–6 weeks/1-year are not indicated in the figure (see text).
Over time, both groups showed a significant improvement in episodic memory scores from baseline to 4–6 weeks [p < 0.001; non-surgical: mean difference(SE) = 0.42(0.03), 95% CI = 0.33 to 0.50; surgical: mean difference (SE) = 0.23 (0.04), 95% CI = 0.12 to 0.34] and from baseline to 1-year [p < 0.001; non-surgical: mean difference (SE) = 0.41 (0.04), 95% CI = 0.30 to 0.51; surgical: mean difference (SE) = 0.29 (0.04), 95% CI = 0.18 to 0.40]. The non-surgical group also showed significant improvement on working memory/inhibition scores from baseline to 4–6 weeks [p < 0.001; mean difference(SE) = 0.12 (0.02), 95% CI = 0.06 to 0.18,) and 1-year (p = 0.019; mean difference(SE) = 0.08(0.03), 95% CI = 0.01 to 0.15] and on language scores from baseline to 4–6 weeks [p < 0.001, mean difference(SE) = 0.18(0.03), 95% CI = 0.10 to 0.26,] and 1-year (p < 0.001). No other differences in scores over time were significant (p > 0.236 for all).
Shared parameter models for the cognition scores were examined to evaluate the effect of participant attrition on the results. These models produced results similar to the generalized estimating equation models.
Cognitive RCI Defining POCD Frequency
Nineteen surgery participants (12.4%) met our POCD criteria at 4–6 weeks (RCI showed >1.67 SD decline compared to non-surgical group in one or more cognitive domain). Of the 19 participants with POCD at 4–6 weeks, five did not complete the 1-year evaluation (3 died; 1 lost to follow-up; 1 refused). Only 3 of the remaining POCD participants continued to meet POCD criteria at 1-year (3/14; 2%). At 1-year, 7 additional participants met criteria, with a grand total of 10 (7.5%) surgery participants with POCD at 1-year. See Supplemental Table 3.
Stroke, Acute Cerebral Infarcts, and POCD
Patients with POCD at 4–6 weeks were significantly older and showed a trend for more clinical strokes (p = 0.05) than participants without POCD (Table 2). Of those with postoperative cerebral infarcts, participants with POCD had significantly more and larger acute cerebral infarcts, with approximately twice the number in the POCD versus non-POCD group (incidence rate ratio = 2.13, 95% CI = 1.52 to 2.98).
Table 2.
Surgery Participants with POCD vs without POCD at 4–6 Weeks and 1-year
| No POCD (n = 80) |
POCD 4–6 Weeks (n = 12) |
p Value | POCD 1-year (n = 10) |
p Value | ||||
|---|---|---|---|---|---|---|---|---|
| M/% | SD | M/% | SD | M/% | SD | |||
| Demographics | ||||||||
| Age | 74.1 | 5.5 | 78.2 | 4.9 | 0.02 | 77.5 | 5.3 | 0.09 |
| Male | 68 | 58 | 0.37 | 78 | 0.42 | |||
| Education, Years | 14.2 | 3 | 15.2 | 3 | 0.36 | 15.6 | 2.5 | 0.19 |
| Caucasian | 96 | 92 | 0.43 | 100 | 0.72 | |||
| Past transient ischemic attack | 6 | 0 | 0.49 | 0 | 0.60 | |||
| Past Stroke | 1 | 8 | 0.25 | 0 | 0.90 | |||
| Charlson Comorbidity Index | 1.2 | 1.2 | 1 | 0.9 | 0.82 | 1 | 1.1 | 0.69 |
| Preoperative AFIB | 23 | 42 | 0.15 | 22 | 0.68 | |||
| Geriatric Depression Scale | 3.3 | 3.3 | 3.2 | 2.6 | 0.82 | 3.11 | 2.7 | 0.91 |
| Mini Mental-Status Exam | 27.9 | 1.6 | 27.5 | 1.9 | 0.47 | 27.7 | 1.4 | 0.42 |
| Postoperative Stroke | ||||||||
| Percent silent infarct | 55 | 17 | 0.01 | 22 | 0.08 | |||
| Percent stroke | 10 | 33 | 0.05 | 33 | 0.08 | |||
| Median postoperative NIH Stroke Scalea | 1 | 2 | 0.28 | 8 | 0.08 | |||
| Percent with lesion(s) on MRI | 63 | 42 | 0.17 | 56 | 0.68 | |||
| Median number acute cerebral infarctb, c | 2 | 4 | 0.01 | 4 | 0.05 | |||
| Median total acute cerebral infarct volume (mm3)b, d | 305 | 1597 | 0.01 | 585 | 0.09 | |||
| Median maximum single acute cerebral infarct volume (mm3)b, d | 198 | 723 | 0.03 | 222 | 0.41 | |||
Medians are reported only for participants in the “stroke” group
Medians are reported only for participants with acute cerebral lesions
Poisson test was performed
Two-part test was performed.
p values in bold <0.05.
A similar pattern of results was observed at 1-year although several differences were reduced to trends, likely due to sample size. Trends suggest that POCD participants at 1-year (n = 10) were slightly older and had more postoperative clinical strokes that were rated as more clinically severe (NIH Stroke Scale). The 1-year POCD group had 2.6 times more acute cerebral infarcts than the non-POCD group (incidence rate ratio = 2.65, 95% CI = 1.88 to 3.74; p = 0.05), and acute infarct volumes tended to be larger.
Comment
To our knowledge, this is the largest study of cognitive outcomes in older adults 1-year after AVR. Patients undergoing AVR had lower scores on tests of working memory/inhibition at 4–6 weeks compared to older adults with similar cardiovascular disease who did not have surgery. However, the two groups did not differ in cognitive performance at 1-year. At 4–6 weeks, only 12.4% of the surgery group met RCI criteria for POCD; only 7.5% met the criteria after 1-year. POCD at 4–6 weeks was associated with more and larger acute cerebral infarcts.
The rate of POCD in our cohort was generally comparable (10) or lower (11, 29) than the rate reported in younger patients undergoing CABG. There are currently no universally accepted criteria for POCD, and methodological differences likely influence prevalence rates. However, even overall group analyses of cognitive outcomes showed only minimal short-term cognitive disruption in our high-risk, elderly sample. Thus, advanced age alone should not contraindicate surgical AVR due to concern for cognitive decline.
One to two small acute cerebral infarcts were observed in most surgical participants, but those with POCD generally suffered more and larger acute cerebral infarcts. The relation between acute cerebral infarcts and POCD remains unclear; studies showing no relation report relatively small total lesion volumes (<1,000 mm3; (30, 31)). Our data suggest a threshold effect, with poor cognitive outcomes observed after the burden of multiple infarcts and/or a single large infarct reaches a tipping-point. In fact, larger lesion volumes independently predict worse outcomes in first-time stroke patients (32), and a threshold effect has been proposed for the relation between chronic leukoarariosis on MRI and cognitive impairment in pathological aging (33). Future research should aim to identify a cerebral infarct threshold for POCD.
As a whole, the surgical group did not show evidence for cognitive decline. The group difference in working memory/inhibition at 4–6 weeks emerged because the surgical group did not improve as much as the non-surgical group (i.e., diminished practice effect). Muted practice effects have been identified as an early marker of Alzheimer’s disease (34). Although findings are mixed regarding cardiac surgery and increased risk for dementia/mild cognitive impairment (8, 9)], longer-term studies focused on practice effects and elderly AVR patients are lacking.
The fact that group differences were noted only on tests of working memory/inhibition is potentially important. Past studies have shown that post-operative changes in working memory/inhibition are strongly associated with functional limitations (23). Thus, characterization of POCD subtypes may elucidate mechanisms and long-term functional consequences in future research.
We acknowledge several study limitations. First, only 6% of the eligible recruitment pool at UPHS was non-white (16). Our sample reflects the University of Pennsylvania’s patient population but limits generalizability. Second, participants were recruited from an established heart valve disease program; outcomes may have differed in a multi-site study that included hospitals with less experience in AVR and fewer complicated cases. Third, the non-surgical group had less severe aortic stenosis and differed on several demographic variables. These differences were statistically controlled for in between-group analyses but were not controlled in the RCI analyses; nevertheless, results were generally consistent across analytic approaches, and the group differences make the low rate of POCD in the RCI analysis even more striking, as demographic/clinical differences would suggest better cognitive function in the non-surgical group (e.g., younger age, etc.). Finally, 19% and 30% of the surgery group did not complete testing at 4–6 weeks and 1-year, respectively, which is comparable to or lower than attrition in other large, longitudinal studies of POCD following CABG (6, 7, 29). In sample size planning, we anticipated a 20% loss to follow-up. Thus, this degree of attrition was not unexpected, as sample was quite elderly with multiple comorbidities. Shared parameter analyses adjusting for participant drop out did not raise concern, and the results of study completers are quite clear — the large majority of this high-risk older adult sample survived AVR, completed the study, and demonstrated cognitive outcomes comparable to non-surgical participants at 1-year. These findings are generally consistent with prior large studies of CABG surgery that included nonsurgical comparisons (6, 7) but our findings are even more remarkable given the advanced age, extent of cardiovascular and valvular disease, and associated comorbidities of our sample.
In conclusion, despite the high risk for POCD in elderly AVR patients and the high rate of clinical stroke and acute cerebral infarcts in our cohort (16), the overwhelming majority of participants who survived the surgery and completed the study demonstrated intact postoperative cognitive abilities. POCD in the minority of surgery participants was associated with more and larger acute cerebral infarcts.
Supplementary Material
List of Abbreviations
- AVR
aortic valve replacement
- CABG
coronary artery bypass graft
- CI
confidence interval
- DWI
diffusion-weighted imaging
- NIH
National Institute of Health
- POCD
post-operative cognitive dysfunction
- RCI
reliable change index
- SE
standard error
- SD
standard deviation
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hogue CW Jr, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999;100:642–7. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez RA, Rubens FD, Wozny D, Nathan HJ. Cerebral emboli detected by transcranial Doppler during cardiopulmonary bypass are not correlated with postoperative cognitive deficits. Stroke 2010;41:2229–35. [DOI] [PubMed] [Google Scholar]
- 3.Westaby S, Saatvedt K, White S, Katsumata T, van Oeveren W, Halligan PW. Is there a relationship between cognitive dysfunction and systemic inflammatory response after cardiopulmonary bypass? Ann Thorac Surg 2001;71:667–72. [DOI] [PubMed] [Google Scholar]
- 4.Jensen BO, Hughes P, Rasmussen LS, Pedersen PU, Steinbruchel DA. Cognitive outcomes in elderly high-risk patients after off-pump versus conventional coronary artery bypass grafting: a randomized trial. Circulation 2006;113:2790–5. [DOI] [PubMed] [Google Scholar]
- 5.Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1–2 years after noncardiac surgery in the elderly. Ispocd group. International study of post-operative cognitive dysfunction. Acta Anaesthesiol Scand 2000;44:1246–51. [DOI] [PubMed] [Google Scholar]
- 6.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol 2008;63:581–90. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk D, Moons KG, Nathoe HM, et al. Cognitive outcomes five years after not undergoing coronary artery bypass graft surgery. Ann Thorac Surg 2008;85:60–4. [DOI] [PubMed] [Google Scholar]
- 8.Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology 2005;65:986–90. [DOI] [PubMed] [Google Scholar]
- 9.Evered LA, Silbert BS, Scott DA, Maruff P, Ames D. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology 2016;125:62–71. [DOI] [PubMed] [Google Scholar]
- 10.Selnes OA, Grega MA, Borowicz LM, Royall RM Jr, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg 2003;75:1377–84; discussion 1384–6. [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA 2002;287:1405–12. [DOI] [PubMed] [Google Scholar]
- 12.Uekermann J, Suchan B, Daum I, Kseibi S, Perthel M, Laas J. Neuropsychological deficits after mechanical aortic valve replacement. J Heart Valve Dis 2005;14:338–43. [PubMed] [Google Scholar]
- 13.Ebert AD, Walzer TA, Huth C, Herrmann M. Early neurobehavioral disorders after cardiac surgery: a comparative analysis of coronary artery bypass graft surgery and valve replacement. J Cardiothorac Vasc Anesth 2001;15:15–9. [DOI] [PubMed] [Google Scholar]
- 14.Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010;121:870–8. [DOI] [PubMed] [Google Scholar]
- 15.Zimpfer D, Czerny M, Kilo J, et al. Cognitive deficit after aortic valve replacement. Ann Thorac Surg 2002;74:407–12; discussion 412. [DOI] [PubMed] [Google Scholar]
- 16.Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation 2014;129:2253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massaro A, Messe SR, Acker MA, et al. Pathogenesis and risk factors for cerebral infarct after surgical aortic valve replacement. Stroke 2016;47:2130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 20.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 1995;59:1289–95. [DOI] [PubMed] [Google Scholar]
- 21.Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia 2002;40:435–45. [DOI] [PubMed] [Google Scholar]
- 22.Brandt J The Hopkins verbal learning test: Development of a new verbal memory test with six equivalent forms. Clin Neuropsychol 1991;5:125–42. [Google Scholar]
- 23.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology 2008;108:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price CC, Tanner JJ, Schmalfuss I, et al. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology 2014;120:601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–31. [DOI] [PubMed] [Google Scholar]
- 26.StataCorp. Stata statistical software: release 14. College Station, TX, StataCorp LP, 2015. [Google Scholar]
- 27.Schluchter MD. Methods for the analysis of informatively censored longitudinal data. Stat Med 1992;11:1861–70. [DOI] [PubMed] [Google Scholar]
- 28.Lachenbruch PA. Comparisons of two-part models with competitors. Stat Med 2001;20:1215–34. [DOI] [PubMed] [Google Scholar]
- 29.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 30.Cook DJ, Huston J 3rd, Trenerry MR, Brown RD, Jr, Zehr KJ, Sundt TM 3rd. Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg 2007;83:1389–95. [DOI] [PubMed] [Google Scholar]
- 31.Knipp SC, Matatko N, Wilhelm H, et al. Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg 2008;85:872–9. [DOI] [PubMed] [Google Scholar]
- 32.Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke 2000;31:2597–602. [DOI] [PubMed] [Google Scholar]
- 33.Price CC, Mitchell SM, Brumback B, et al. MRI-leukoaraiosis thresholds and the phenotypic expression of dementia. Neurology 2012;79:734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zehnder AE, Blasi S, Berres M, Spiegel R, Monsch AU. Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer disease? Am J Alzheimers Dis Other Demen 2007;22:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.