Abstract
Background:
Exposure to particulate matter, particularly with aerodynamic diameter <2.5 μm (PM2.5), may increase inflammation and oxidative stress in pregnant women and affect fetal growth. We examined trimester specific PM2.5 exposure levels and small for gestational age (SGA) using the statewide birth registry of Ohio from 2007 to 2010.
Methods:
Exposure to PM2.5 in each trimester and for each gestational week was determined using data from 57 Environmental Protection Agency network monitoring stations across the state of Ohio. We restricted the data to 224,921 singleton live births, with a gestational age of 20-42 weeks, no genetic disorders or congenital abnormalities, and who had home addresses within a 10 km radius of any PM2.5 monitoring station. We estimated odds ratios of SGA using Generalized Linear Models (GLMs) and Distributed Lag Models (DLMs), and adjustment for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of the baby.
Results:
Mean PM2.5 levels during the entire pregnancy were 13.03 μg/m3 with a standard deviation of 1.57 μg/m3. Covariates adjusted odds ratios and 95% confidence intervals of a 10 μg/m3 increase in PM2.5 levels with a 10 km buffer radius for SGA and trimesters modeled separately were 0.94 (0.88, 1.00) for the first trimester, 0.93 (0.86, 1.00) for the second trimester, 1.07 (1.00, 1.15) for the third trimester, and 0.92 (0.81, 1.06) for the entire pregnancy. When a 5 km buffer radius was used, adjusted odds ratios and 95% confidence intervals for SGA were 0.97 (0.89, 1.05) for the first trimester, 0.96 (0.88, 1.05) for the second trimester, 1.09 (1.02, 1.17) for the third trimester, and 0.99 (0.85, 1.14) for the overall pregnancy, indicating sensitivity to buffer choice. DLMs showed gestational weeks 30-35 to be a particular window of vulnerability.
Conclusion:
Increasing exposure to PM2.5 during the third trimester of pregnancy was associated with a small increase in risk of SGA in this population-based study. Selection of a buffer radius significantly impacted our results in the first trimester, but not in the third trimester.
Keywords: PM2.5, particulate matter, air pollution, fetal growth, SGA
Graphical Abstract

1. Introduction
Air pollution is a widely accepted environmental and human health hazard that is composed of many different constituent solids, liquids, and gases. The particulate matter fraction of this mixture that measures <2.5 μm in aerodynamic diameter (PM2.5) is usually generated from fossil fuel combustion from motor vehicles (Zhu et al., 2002). Once PM2.5 is inhaled and reaches the lungs, it induces local inflammatory changes that result in a systemic cytokine response causing oxidative stress and inflammation throughout the body (Brucker et al., 2013; Patel et al., 2013).
Recently, efforts have been made to reduce air pollution in the United States and other countries, resulting in decreasing levels of PM2.5 and other pollutants in the air (United States Environmenal Protection Agency, 2017). Although levels are decreasing, they remain high enough that we continue to discover associations between relatively low exposure and human health. Exposure to air pollution has been linked to a host of health conditions including adverse pregnancy outcomes such as pre-eclampsia (Olsson et al., 2013; Wu et al., 2009), gestational hypertension (Van Den Hooven et al., 2011; Vinikoor-Imler et al., 2012), infant death (Defranco et al., 2015; Glinianaia et al., 2004), low birth weight (Dadvand et al., 2013), infant congenital malformations (Ren et al., 2017), preterm birth (Defranco et al., 2016), and infants born small-for-gestational-age (SGA) (Hyder et al., 2014; Parker et al., 2005). A joint consensus statement by the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society discusses the negative outcomes which babies born SGA face over their lifespans including decreased growth, cognitive impairment, endocrine abnormalities, insulin resistance, and cardiovascular disease (Clayton et al., 2007).
Although several studies have shown associations between prenatal exposure to PM2.5 and increased risk for SGA, a recent meta-analysis (Zhu et al., 2015) highlights the need for more studies that specifically examine trimester specific effects of this association, as several only report results for the whole pregnancy. We used a geo-spatially defined birth cohort study located in Ohio to examine the relationship between prenatal PM2.5 exposure and SGA births with particular attention to trimester specific exposures at concentrations experienced in many other parts of the country as well. We also utilized distributed lag models (DLMs) to determine specific windows of vulnerability over the course of the pregnancy.
2. Materials and Methods
In this geo-spatial, population based birth cohort study, we utilized a dataset consisting of live births in the state of Ohio from January 1, 2007 through December 31, 2010 provided by the Ohio Department of Health. This study was approved by the Ohio Department of Health and Human Subjects Institutional Review Board and was exempt from review by the University of Cincinnati Institutional Review Board. The exposure of interest was PM2.5, measured daily throughout pregnancy, and the outcome of interest was SGA births. We defined SGA as <10th percentile of United States 2009-2010 sex- and gestational-age-specific fetal growth curves (Talge et al., 2014). Gestational age was gathered from the birth record, and we used best obstetric estimate based on last menstrual period and ultrasound parameters.
2.1. Study Population
We used state birth record data to evaluate numbers of SGA and non-SGA babies born in Ohio during the time period of interest. Subjects were included in our analysis if they were 1) singleton live births, 2) gestational age 20-42 weeks, 3) without genetic disorders or congenital abnormalities, 4) resided within a 10 kilometer radius from a monitoring station, and 5) had PM2.5 level data for all three trimesters. Subjects were excluded if they had missing data for exposure or outcome variables.
2.2. PM2.5 Exposure
To assess PM2.5 exposure in the state of Ohio, we analyzed United States Environmental Protection Agency (EPA) data from 57 air quality monitoring stations in Ohio to develop a map of PM2.5 exposure throughout the state (United States Environmenal Protection Agency, 2018). Using ArcGIS 10.1 (ESRI, Redlands CA, USA), we geocoded maternal address at the time of birth and calculated the mother’s residential distance to the nearest EPA monitoring station with methods previously described (Hall et al., 2014). In this analysis we included births at gestational ages 20 through 42 weeks with a maternal address within 10 km of a monitoring station (n=224,921) (Defranco et al., 2016).
2.3. Statistical Analysis
Subject exposure to PM2.5 was calculated as a mean by gestational week, by trimester, and for the entire pregnancy based on daily monitoring data from the EPA station closest to the subject’s residential address. We also rounded PM2.5 exposure means to the nearest whole number and calculated the proportion of SGA births at each exposure level.
To examine the relationship between PM2.5 exposure and SGA with consideration of clustering within a monitoring station, we used Generalized Estimating Equations (GEE) in the Generalized Linear Model with logit link, and adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use during pregnancy, prenatal care initiation, birth year, season of birth, and sex of the baby (Fiaz et al., 2012). We chose covariates based on significant differences in bivariate analysis, biologic plausibility, and parsimony in the statistical model.
Four different statistical models were used to examine the relationship between PM2.5 exposure and SGA. First, we determined the adjusted odds ratios (aORs) and 95% confidence intervals (CIs) at each exposure level (integer, μg/m3) by trimester and for the entire pregnancy in relation to SGA proportions. Only PM2.5 levels with at least 100 total births were included in the analysis to minimize the influence of unstable SGA percentage at both ends of the exposure range. Second, we modeled continuous PM2.5 exposure levels for each trimester and for the entire pregnancy to calculate a linear trend of dose-response. We used separate models for each trimester as well as a joint model for all three trimesters to take into account potential bias from separate models (Wilson et al., 2017). Third, we used binary PM2.5 exposure levels to calculate the association of high (≥15 μg/m3) versus low (<15 μg/m3) exposure. This cut-off was chosen because 15 μg/m3 was the EPA annual standard during the period of study. Additionally, we dichotomized the PM2.5 exposure levels to high (≥12 μg/m3) versus low (<12 μg/m3) exposure to consider the current EPA annual standard. Fourth, we used DLMs to examine weekly mean exposure to PM2.5 levels from gestational weeks 0-35 to identify potential windows of susceptibility that may be finer than trimester, because the latter is arbitrary for starting point and length of windows. The DLM constrains the association of weekly specific exposure with outcome to be smooth across gestational weeks, and can consider either linear or non-linear associations (Gasparrini, 2014). We considered three dose-response patterns between PM2.5 exposure and SGA proportion: linear, B-spline, and natural cubic spline, and modeled the gestational week and response relation as B-spline (Hsu et al., 2015; Sheffield et al., 2018; Wu et al., 2018). The degrees of freedom of the splines and the dose-response patterns were determined using smaller Akaike Information Criterion (Gasparrini, 2011; Sheffield et al., 2018). The DLMs were adjusted for the above-mentioned covariates. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA), and the DLMs were implemented using the dlnm package Version 2.3.6 in R 3.5.1 (Vienna, Australia). Tests with a probability value of <0.05 were considered statistically significant.
3. Results
There were 597,000 live births in Ohio from 2007-2010 captured for analysis. Of those, we excluded births in which: the gestational age was <20 weeks, >42 weeks, or missing; genetic disorders or congenital anomalies were present; the birth weight was <350 grams; there was a multifetal gestation; the mother lived ≥10 kilometers from a monitor station; or exposure data was not available for all trimesters, leaving a total of 224,921 births for the final analysis. Of these births, 24,299 were SGA (10.8%) (Figure 1).
Figure 1:
Flow diagram of the study population.
Demographic, social, and socioeconomic characteristics of the cohort are presented in Table 1. Briefly, 67.4% of mothers were less than 30 years old at the time of birth, 61.8% were white, 54.5% had completed some post-secondary education, 44.5% had private health insurance, and 19.1% used tobacco during their pregnancy. SGA percentages were higher in mothers with a younger age, who are black, with less than high school education, having prepregnancy body mass index (BMI) <18.5 kg/m2, receiving Medicaid, who smoked during pregnancy, and who had late or no prenatal care initiation. An analysis on mothers excluded because they lived >10 km from a monitoring station (mostly rural) showed that this group had slightly lower PM2.5 exposure, less SGA proportion, less teen pregnancy, higher white percentage, higher education, less Medicaid, and more prenatal care in the first trimester (data not shown).
Table 1:
Study Population Characteristics and SGA percentages.
| Non-SGA, n (row %) |
SGA, n (row %) |
Total, n (column %) |
|
|---|---|---|---|
| Births | 200,622 (89.20) | 24,299 (10.80) | 224,921 (100) |
| Demographic Factors | |||
| Maternal Age*, years | |||
| <20 | 23,383 (84.36) | 4,335 (15.64) | 27,718 (12.32) |
| 20-24 | 52,118 (87.22) | 7,640 (12.78) | 59,758 (26.57) |
| 25-29 | 57,756 (90.23) | 6,256 (9.77) | 64,012 (28.46) |
| 30-34 | 43,884 (91.82) | 3,912 (8.18) | 47,796 (21.25) |
| 35-39 | 19,480 (91.87) | 1,723 (8.13) | 21,203 (9.43) |
| ≥40 | 4,001 (90.23) | 433 (9.77) | 4,434 (1.97) |
| Maternal Race* | |||
| Non-Hispanic White | 126,109 (91.66) | 11,467 (8.34) | 137,576 (61.75) |
| Non-Hispanic Black | 56,502 (84.16) | 10,635 (15.84) | 67,137 (30.13) |
| Other Non-Hispanic | 5,681 (86.43) | 892 (13.57) | 6,573 (2.95) |
| Hispanic | 10,425 (90.49) | 1,095 (9.51) | 11,520 (5.17) |
| Maternal Education* | |||
| Less than high school | 38,319 (84.34) | 7,117 (15.66) | 45,436 (20.4) |
| High school graduate | 48,946 (87.64) | 6,902 (12.36) | 55,848 (25.07) |
| Some post-secondary education | 111,484 (91.78) | 9,981 (8.22) | 121,465 (54.53) |
| Child Sex* | |||
| Male | 101,934 (89.04) | 12,544 (10.96) | 114,478 (50.9) |
| Female | 98,688 (89.36) | 11,755 (10.64) | 110,443 (49.1) |
| Social Behaviors and Socioeconomic Factors | |||
| Parity* | |||
| 0 | 79,822 (87.18) | 11,733 (12.82) | 91,555 (41.51) |
| 1 | 61,371 (91.10) | 5,999 (8.90) | 67,370 (30.54) |
| ≥2 | 55,584 (90.18) | 6,053 (9.82) | 61,637 (27.95) |
| Pre-pregnancy Body Mass Index* | |||
| <18.5 | 7,914 (81.39) | 1,810 (18.61) | 9,724 (4.59) |
| 18.5-24.9 | 90,613 (88.59) | 11,668 (11.41) | 102,281 (48.24) |
| 25-29.9 | 45,695 (90.33) | 4,894 (9.67) | 50,589 (23.86) |
| ≥30 | 44,968 (91.00) | 4,448 (9.00) | 49,416 (23.31) |
| Health Insurance Type* | |||
| Medicaid | 83,786 (86.11) | 13,517 (13.89) | 97,303 (43.26) |
| Private Insurance | 92,416 (92.44) | 7,553 (7.56) | 99,969 (44.45) |
| Other | 24,420 (88.32) | 3,229 (11.68) | 27,649 (12.29) |
| Maternal Tobacco Use During Pregnancy* | |||
| Yes | 35,670 (83.22) | 7,190 (16.78) | 42,860 (19.06) |
| No | 164,952 (90.60) | 17,109 (9.40) | 182,061 (80.94) |
| Prenatal Care Initiation* | |||
| First Trimester | 100,691 (90.48) | 10,592 (9.52) | 111,283 (67.24) |
| Second Trimester | 35,102 (87.53) | 4,999 (12.47) | 40,101 (24.23) |
| Third Trimester | 7,449 (86.57) | 1,156 (13.43) | 8,605 (5.2) |
| Never | 4,703 (85.29) | 811 (14.71) | 5,514 (3.33) |
| Season of Birth* | |||
| Spring | 50,716 (89.58) | 5,898 (10.42) | 56,614 (25.17) |
| Summer | 51,770 (89.25) | 6,238 (10.75) | 58,008 (25.79) |
| Fall | 49,115 (88.75) | 6,224 (11.25) | 55,339 (24.6) |
| winter | 49,021 (89.19) | 5,939 (10.81) | 54,960 (24.44) |
Significant difference in SGA percentage (p<0.05)
Table 2 shows that the range of exposure to PM2.5 was 8.28-18.57 μg/m3 for the whole pregnancy, the average PM2.5 concentration was 13.03±1.57 (mean ± SD), and the median PM2.5 concentration was 13.04 μg/m3. The trimester specific exposure range was wider than that for the whole pregnancy, at 6.22-24.15 μg/m3, and the third trimester had the largest range of exposures for the cohort. The SGA percentages by each integer of PM2.5 concentration with a given trimester or entire pregnancy were shown in Supplemental Table S1. The correlation coefficient of exposures was 0.21 between the first and second trimesters, 0.31 between the first and the third trimesters, and 0.25 between the second and the third trimesters.
Table 2:
Mean PM2.5 (μg/m3) concentrations during the entire pregnancy in each of the study years, Ohio 2007-2010.
| Year | n | Mean±SD | Median | Range |
|---|---|---|---|---|
| 2007 | 60,559 | 13.72±1.30 | 13.79 | 9.65-18.57 |
| 2008 | 57,677 | 13.87±1.48 | 13.95 | 9.80-18.20 |
| 2009 | 54,964 | 11.95±1.22 | 11.81 | 8.33-15.65 |
| 2010 | 51,721 | 12.42±1.33 | 12.36 | 8.28-16.92 |
| Total | 224,921 | 13.03±1.57 | 13.04 | 8.28-18.57 |
Figure 2 shows the aORs and 95% CIs of rounded mean PM2.5 concentrations during each trimester and for the whole pregnancy with reference set at 10 μg/m3. At high exposure levels there were negative associations in the first and second trimesters, but positive associations in the third trimester. The dose response curve for the whole pregnancy was generally flat.
Figure 2:
Adjusted ORs and 95% CIs of mean PM2.5 concentrations during each trimester and during the entire pregnancy for SGA infants, with reference at 10 μg/m3. Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
When PM2.5 exposure was modeled continuously, there was a reduced risk in the first trimester in separate and joint models, and a higher risk in the third trimester in both model types (Table 3). We noted the same pattern when PM2.5 exposure was categorized as a binary using 15 μg/m3 as a cut-point, but there were no associations when 12 μg/m3 was used as the cut-point (Table 4).
Table 3:
Adjusted ORs and 95% CIs of continuous PM2.5 concentrations for SGA infants.
| Model | Trimester | aOR per 10 μg/m3 PM2.5 | 95% CI | p |
|---|---|---|---|---|
| Separate | First | 0.94 | 0.88-1.00 | 0.051 |
| Second | 0.93 | 0.86-1.00 | 0.130 | |
| Third | 1.07 | 1.00-1.15 | 0.046 | |
| Entire pregnancy | 0.92 | 0.81-1.06 | 0.243 | |
| Joint | First | 0.92 | 0.86-0.99 | 0.027 |
| Second | 0.93 | 0.85-1.02 | 0.111 | |
| Third | 1.08 | 1.01-1.16 | 0.021 |
Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
Table 4:
Adjusted ORs and 95% CIs of dichotomous PM2.5 concentrations for SGA infants.
| Cut-off of PM2.5 (μg/m3) |
Trimester | Low exposure | High exposure | Modeled separately | Modeled jointly | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % SGA | n | % SGA | aOR | 95% CI | aOR | 95% CI | ||
| ≥15 | First | 175,649 | 10.82 | 49,272 | 10.73 | 0.96 | 0.92-0.99 | 0.96 | 0.92-0.99 |
| Second | 185,883 | 10.78 | 39,038 | 10.93 | 0.97 | 0.94-1.01 | 0.97 | 0.93-1.01 | |
| Third | 181,665 | 10.65 | 43,256 | 11.44 | 1.04 | 1.00-1.09 | 1.04 | 1.00-1.09 | |
| Entire pregnancy | 200,259 | 10.76 | 24,662 | 11.16 | 1.00 | 0.96-1.04 | |||
| ≥12 | First | 74,283 | 10.71 | 150,638 | 10.85 | 0.99 | 0.97-1.02 | 0.99 | 0.96-1.01 |
| Second | 76,331 | 10.70 | 148,590 | 10.85 | 0.99 | 0.96-1.03 | 0.99 | 0.96-1.03 | |
| Third | 82,293 | 10.54 | 142,628 | 10.95 | 1.02 | 0.99-1.05 | 1.02 | 0.99-1.05 | |
| Entire pregnancy | 63,232 | 10.61 | 161,689 | 10.88 | 0.97 | 0.93-1.00 | |||
Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
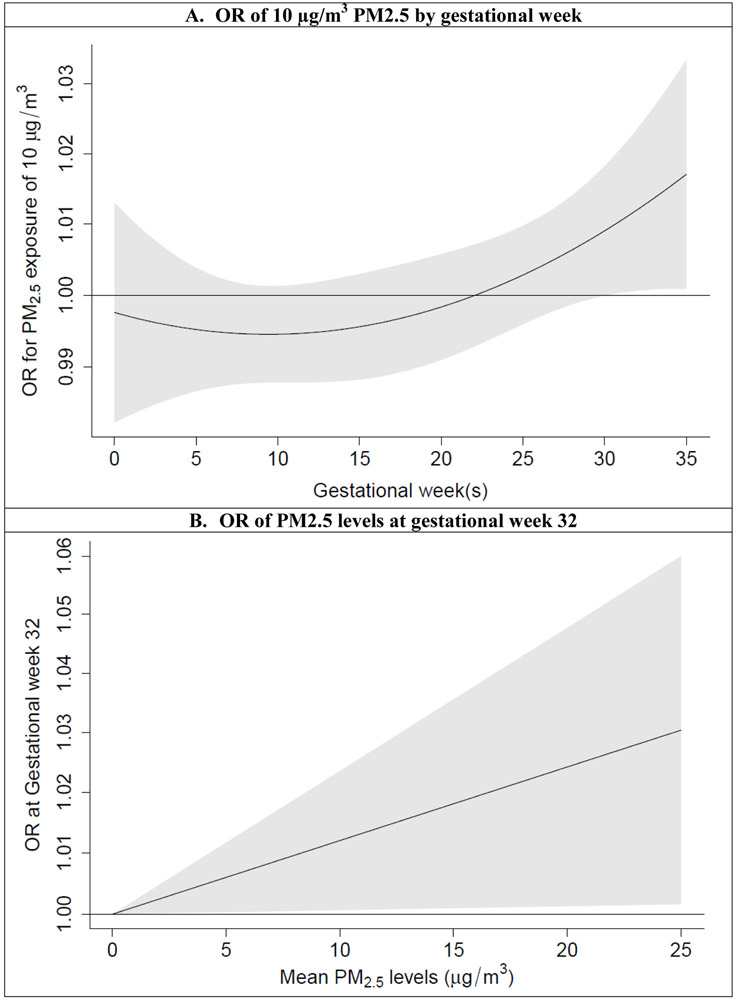
In the DLMs, the linear pattern between PM2.5 exposure and the SGA proportion had the lowest Akaike Information Criterion and was used to describe the association. Figure 3 shows the ORs for an increase of 10 μg/m3 PM2.5, with a “J” shape across gestational weeks. The results indicate decreased risk of SGA for exposure between gestational weeks 5-15, and increased risk of SGA for exposure after week 30.
Figure 3.
Adjusted ORs and 95% CIs of PM2.5 levels for SGA infants in distributed lag models. A) by gestational week; B) at gestational week 32. Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
Additionally, we performed sensitivity analyses restricting to 1) pregnant women living within a 5km radius to the closest PM2.5 monitoring station, and 2) nulliparous women to remove the impact of previous pregnancy history. The association was modeled with continuous exposure, binary exposure, and DLMs. The analysis restricting to nulliparous women was similar to the results in Tables 3 and 4 (Supplemental Tables S2 and S3, and Supplemental Figure S1), but the analysis with buffer radius set to <5 km did not find any associations between PM2.5 and SGA during the first trimester (Tables 5 and 6, and Figure 4).
Table 5:
Adjusted ORs and 95% CIs of continuous PM2.5 concentrations for SGA infants in women living <5 km from the nearest EPA monitoring station (N=112,627, SGA=13,963, 12.40%).
| Model | Trimester | aOR per 10μg/m3 PM2.5 | 95% CI | p |
|---|---|---|---|---|
| Separate | First | 0.97 | 0.89-1.05 | 0.459 |
| Second | 0.96 | 0.88-1.05 | 0.327 | |
| Third | 1.09 | 1.02-1.17 | 0.013 | |
| Entire pregnancy | 0.99 | 0.85-1.14 | 0.849 | |
| Joint | First | 0.95 | 0.87-1.04 | 0.271 |
| Second | 0.95 | 0.87-1.04 | 0.291 | |
| Third | 1.10 | 1.02-1.18 | 0.009 |
Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
Table 6:
Adjusted ORs and 95% CIs of dichotomous PM2.5 concentrations for SGA infants in women living <5 km from the nearest EPA monitoring station.
| Cut-off of PM2.5 (μg/m3) |
Trimester | Low exposure | High exposure | Modeled separately | Modeled jointly | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % SGA | n | % SGA | aOR | 95% CI | aOR | 95% CI | ||
| ≥15 | First | 87,021 | 12.40 | 25,606 | 12.39 | 0.98 | 0.94-1.03 | 0.98 | 0.94-1.03 |
| Second | 92,048 | 12.39 | 20,579 | 12.42 | 0.97 | 0.93-1.02 | 0.97 | 0.92-1.02 | |
| Third | 89,917 | 12.25 | 22,710 | 12.99 | 1.05 | 1.00-1.09 | 1.05 | 1.01-1.09 | |
| Entire pregnancy | 99,332 | 12.34 | 13,295 | 12.85 | 1.04 | 0.98-1.10 | |||
| ≥12 | First | 36,153 | 12.36 | 76,474 | 12.41 | 1.00 | 0.97-1.04 | 0.99 | 0.96-1.03 |
| Second | 36,825 | 12.46 | 75,802 | 12.37 | 0.99 | 0.95-1.02 | 0.99 | 0.95-1.03 | |
| Third | 40,032 | 12.15 | 72,595 | 12.53 | 1.04 | 0.99-1.08 | 1.04 | 0.99-1.08 | |
| Entire pregnancy | 29,926 | 12.43 | 82,701 | 12.39 | 0.97 | 0.94-1.01 | |||
Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
Figure 4.
Adjusted ORs and 95% CIs of PM2.5 levels for SGA infants in women living <5 km from the nearest EPA monitoring station in distributed lag models. A) by gestational week; B) at gestational week 32. Adjusted for maternal age, race, education, parity, body mass index, insurance type, tobacco use, prenatal care initiation, birth year, season of birth, and sex of baby.
4. Discussion
4.1. General Findings
In this study of PM2.5 concentration and Ohio births between 2007 and 2010, we found that first trimester exposure to increasing levels of PM2.5 was associated with a slightly decreased risk of SGA in infants, and third trimester exposure was associated with a slightly increased risk. DLMs indicate that gestational weeks 30-35 in particular are a potential window of vulnerability to PM2.5 exposure for SGA. Both the first and third trimester associations held when exposure was modeled continuously, dichotomized, and with DLMs. However, an analysis restricting the buffer radius to <5km from the nearest EPA monitoring station showed that only the third trimester positive association remained significant.
4.2. Strengths and Limitations
An obvious limitation of our study is the use of EPA monitoring station data to assess individual exposure to PM2.5 during pregnancy. We restricted our primary analysis to mothers that lived within 10 km of a monitoring station, but this does not account for dynamic exposure during time spent away from the home or from indoor air pollution. Additionally, the 10 km buffer radius around stations is an imprecise estimate of daily exposure and actual personal exposure during the study period may have varied to an unknown degree. We also were unable to assess whether women moved during their pregnancies, however a 2014 review showed that pregnant women in the United States have approximately 24% residential mobility, but the moves tend to be <10km apart, which would reduce exposure misclassification (Bell and Belanger, 2012). Ideally, we would have obtained personal monitoring data to more accurately assess exposure, but that would not be feasible for a study of this size. Also, EPA monitoring stations are not distributed evenly throughout the state, creating inherent sample bias in our methodology because we excluded births in areas without nearby monitoring stations, most of which are in rural areas. Similar to the findings of others, the population that we were able to include in this analysis was significantly different from those excluded because of distance to the nearest monitoring station (Bell and Ebisu, 2012). These conditions with regard to the monitoring data may bias our study toward the null.
The exposure range measured in this study did not include PM2.5 levels <5 or >21 μg/m3, and therefore these results cannot be extrapolated to very pristine or very urban environments. However, according to the EPA regional PM2.5 data trends, average yearly levels of PM2.5 in the majority of the United States do fall within the range described in this study (United States Environmenal Protection Agency, 2017). Although it is difficult to generalize our results to other regions and states because of population differences, this study describes pollution levels more similar to what the average US resident may experience as opposed to other studies that describe only very high levels of exposure (Coker et al., 2015; Sram et al., 2013; Vinikoor-Imler et al., 2012).
Another limitation of our study inherent to using birth records is the limited data available for covariate adjustment. We may have unmeasured confounding left in the model that could be biasing our estimates either toward or away from the null. We also were unable to account for other aspects of air pollution in our model such as CO, NO2, or SO2 due to the differing placement and smaller numbers of those EPA monitoring stations. However, the PM2.5 exposure data that we did have access to is more complete, and both weight and gestational age are expected to have been recorded with high validity.
The geo-spatial cohort design of our study is a significant strength as it allows us to examine a large sample size with temporal data in a single population. To our knowledge, this cohort also has the most recent birth records dates of any other large studies of PM2.5 and SGA with the exception of Smith et al., who studied a cohort from 2006-2010 (Smith et al., 2017). It is important to continue to study more recent cohorts of individuals with regard to air pollution exposure because both the composition and quantity of pollution changes over time (United States Environmenal Protection Agency, 2017), potentially leading to differing health consequences.
4.3. Interpretation
Our sensitivity analysis examining associations between PM2.5 exposure and SGA infants born <5 km from the nearest EPA monitoring station differed significantly from our primary analysis, which used a 10 km buffer radius, in that it did not find a lower risk from exposure in the first trimester. Generally, individual exposure estimates from stationary monitors are thought to be more accurate when smaller buffer radii are drawn, and larger radii are more prone to exposure misclassification. Even though the 5 km buffer radius analysis had a smaller sample size (n=112,627), we do not believe that inadequate power is the reason we do not see an association during the first trimester. Our other sensitivity analysis that used a 10 km buffer and restricted to nulliparous women had an even smaller sample size (n=91,555) and still found the spurious association in the first trimester. Another potential explanation for the finding of lower risk in the first trimester is due to our inclusion of only live births. It is possible that the fetuses most predisposed to adverse effects from PM2.5 were lost in early pregnancy or stillborn, leaving the remaining cohort biased. Additionally, the third trimester is a more biologically plausible window of vulnerability because that is when the most rapid fetal growth occurs (Alexander et al., 1996; Grantz et al., 2018).
There is some inconsistency in the literature around PM2.5 exposure and SGA, particularly with regard to whole pregnancy and trimester specific effects. A recent paper by Stieb et al. utilized birth record data from the large Canadian national registry and found an increased risk for SGA (OR= 1.04, 95% CI= 1.01-1.07) with a 10 μg/m3 increase in PM2.5 over the whole pregnancy, but no significant results by trimester (Stieb et al., 2015). Mannes et al. only found second trimester exposure to be significantly associated with SGA (OR= 1.34, 95% CI= 1.10-1.63) (Mannes et al., 2005). However, Rich et al. found PM2.5 exposure during both the first (OR=1.11, 95% CI=1.01-1.23) and third (OR=1.11, 95% CI= 1.01-1.21) trimesters to be significantly associated with SGA (Rich et al., 2009). A recent study by DeFranco et al. also examined a similar cohort of Ohio births and found that exposure to high levels of PM2.5 in the third trimester was associated with a 42% increased risk for stillbirth (Defranco et al., 2015). This could indicate that the exposure levels found in this study are sufficient to disrupt multiple fetal processes in the third trimester.
The discrepancy of trimester specific estimates of risk related to PM2.5 concentrations could be from the exposure variation within trimesters, the composition and sources of PM2.5 exposure, and population characteristics. Our use of DLMs allowed us to model PM2.5 exposure on a weekly basis during pregnancy and discover that gestational weeks 30-35 are a particular window of vulnerability. Additionally, we showed that dichotomizing exposure at a cut-off of 15 μg/m3, but not 12 μg/m3 revealed trimester specific differences in SGA odds.
The present study contributes to the body of knowledge in this area with weekly and trimester specific assessments of births in Ohio, a population not previously studied in this way. Our study population consisted of 30.13% black women, which is much higher than the US average of 13.4% (United States Census Bureau, 2017), and could account for some of the differences between our findings and other authors’. This difference in population characteristics may also explain why we had slightly more SGA babies in our cohort when we would expect based on the definition of SGA as babies in <10th percentile of US 2009-2010 sex- and gestational-age-specific fetal growth curves. National data shows black women are almost twice as likely to deliver a SGA baby than white women (United States Census Bureau, 2017).
The mechanisms underlying exposure to PM2.5 and impaired fetal growth are still unclear. Previously hypothesized mechanisms include coagulability changes, inflammation and oxidative stress, and direct effects on the autonomic nervous system (Glinianaia et al., 2004). Another compelling hypothesis is that PM2.5 promotes placental dysfunction via vascular changes that results in decreased fetal growth (Liu et al., 2016; Veras et al., 2008). Since PM2.5 has also been associated with an increased risk of preterm birth (Defranco et al., 2016), additional impact with impaired fetal growth put highly exposed babies at an even greater risk for early delivery and small size, which have short- and long-term risks on child physical, pulmonary, cardiovascular, and neuropsychological development.
Although we only found a 5-10% increased risk of SGA from increased PM2.5 exposure in the third trimester, particulate air pollution is a ubiquitous exposure in US women, and continuing to improve air quality is still a worthwhile public health measure. While we studied SGA as the outcome of this investigation, the adverse impact of particulate matter exposure goes beyond fetal growth. Since the association we found was for the third trimester of pregnancy, when fetal weight is steadily increasing, there is a potential to intervene and reduce exposure to PM2.5 in the pregnant women.
5. Conclusions
The findings reported in this study indicate that pregnant women exposed to increasing PM2.5 levels in their third trimester are at a modestly increased risk for delivering SGA babies, with a particular window of vulnerability between 30 and 35 weeks gestation. We also found that the selection of buffer radius significantly impacted our results in the first trimester, but not the third trimester of pregnancy. Although this study was conducted exclusively on women in Ohio, the exposure ranges we describe are experienced by many urban areas across the country, and this work contributes to a growing literature describing the deleterious impact of PM2.5 on human health. Additional research including personal monitoring data on a more diverse group of women would strengthen the science in this field, however we should also focus on continuing to decrease individuals’ exposure to particulate air pollution.
Supplementary Material
Highlights:
Exposure to PM2.5 during pregnancy has been associated with impaired fetal growth, but trimester-specific effects are still unclear.
This study examines the association between PM2.5 and SGA in a Ohio geo-spatial cohort from 2007-2010 with n= 224,921.
Exposure to increasing levels of PM2.5 during the third trimester of pregnancy, particularly gestational weeks 30-35, was associated with a 5-10% increased risk for SGA.
This study was the first of its kind to examine Ohio births and also uses the more recent birth record data to examine PM2.5 exposure and SGA.
Acknowledgements:
This work was supported by the Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; March of Dimes Grant 22-FY13-543 for the March of Dimes Prematurity Research Center Ohio Collaborative; and the University of Cincinnati Medical Scientist Training Program Grant 2T32GM063483-1.
Abbreviations:
- PM2.5
particulate matter <2.5 μm in aerodynamic diameter
- SGA
small-for-gestational-age
- EPA
United States Environmental Protection Agency
- aOR
adjusted odds ratio
- CI
confidence interval
- GEE
generalized estimating equation
- DLM
distributed lag model
References:
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M, 1996. A United States National Reference for Fetal Growth. Obstet. Gynecol 87, 163–168. [DOI] [PubMed] [Google Scholar]
- Bell ML, Belanger K, 2012. Review of research on residential mobility during pregnancy : consequences for assessment of prenatal environmental exposures. J. Expo. Sci. Environ. Epidemiol 22, 429–438. 10.1038/jes.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, 2012. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect 120, 1699–704. 10.1289/ehp.1205201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker N, Moro AM, Charão MF, Durgante J, Freitas F, Baierle M, Nascimento S, Gauer B, Bulcão RP, Bubols GB, Ferrari PD, Thiesen FV, Gioda A, Duarte MMMF, de Castro I, Saldiva PH, Garcia SC, 2013. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci. Total Environ 463–464, 884–893. https://doi.Org/10.1016/j.scitotenv.2013.06.098 [DOI] [PubMed] [Google Scholar]
- Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol AD, 2007. Consensus statement: Management of the child born small for gestational age through to adulthood: A consensus statement of the international societies of pediatric endocrinology and the growth hormone research society. J. Clin. Endocrinol. Metab 92, 804–810. 10.1210/jc.2006-2017 [DOI] [PubMed] [Google Scholar]
- Coker E, Ghosh J, Jerrett M, Gomez-Rubio V, Beckerman B, Cockburn M, Liverani S, Su J, Li A, Kile ML, Ritz B, Molitor J, 2015. Modeling spatial effects of PM2.5 on term low birth weight in Los Angeles County. Environ. Res 10.1016/j.envres.2015.06.044 [DOI] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, Gehring U, Glinianaia SV, Gouveia N, Ha EH, Leem JH, van den Hooven EH, Jalaludin B, Jesdale BM, Lepeule J, Morello-Frosch R, Morgan GG, Pesatori AC, Pierik FH, Pless-Mulloli T, Rich DQ, Sathyanarayana S, Seo J, Slama R, Strickland M, Tamburic L, Wartenberg D, Nieuwenhuijsen MJ, Woodruff TJ, 2013. Maternal exposure to particulate air pollution and term birth weight: A multi-country evaluation of effect and heterogeneity. Environ. Health Perspect 121, 367–373. 10.1289/ehp.1205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranco E, Hall E, Hossain M, Chen A, Haynes EN, Jones D, Ren S, Lu L, Muglia L, 2015. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS One 10, 1–12. 10.1371/journal.pone.0120594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranco E, Moravec W, Xu F, Hall E, Hossain M, Haynes EN, Muglia L, Chen A, 2016. Exposure to airborne particulate matter during pregnancy is associated with preterm birth : a population-based cohort study. Environ. Heal 15, 1–8. 10.1186/s12940-016-0094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaz AS, Rhoads GG, Demissie K, Kruse L, Lin Y, Rich DQ, 2012. Ambient Air Pollution and the Risk of Stillbirth. Am. J. Epidemiol 176, 308–316. 10.1093/aje/kws029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2014. Modeling exposure-lag-response associations with distributed lag nonlinear models. Stat. Med 33, 881–899. 10.1002/sim.5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2011. Distributed Lag Linear and Non-Linear Models in R : The Package dlnm. J. Stat. Softw 43, 1–20. [PMC free article] [PubMed] [Google Scholar]
- Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D, 2004. Does particulate air pollution contribute to infant death? A systematic review. Environ. Health Perspect 112, 1365–1370. 10.1289/ehp.6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz KL, Hediger ML, Liu D, Louis GMB, 2018. Fetal growth standards : the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health organization Multicentre Growth Reference Study. Am. J. Obstet. Gynecol 218, S641–S655.e28. 10.1016/j.ajog.2017.11.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ES, Connolly N, Jones DE, DeFranco EA, 2014. Integrating public data sets for analysis of maternal airborne environmental exposures and stillbirth. AMIA Annu Symp Proc 2014, 599–605. [PMC free article] [PubMed] [Google Scholar]
- Hsu HL, Chiu YM, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ, 2015. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children Identifying Sensitive Windows and Sex Differences. Am. J. Respir. Critial Care Med 192, 1052–1059. 10.1164/rccm.201504-0658OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML, 2014. PM2.5 Exposure and Birth Outcomes: Use of satellite- and monitor-based data. Epidemiology 25, 58–67. 10.1097/EDE.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Wang F, Li C, 2016. Effect of Fine Particulate Matter (PM2.5) on Rat Placenta Pathology and Perinatal Outcomes. Med. Sci. Monit 22, 3274–3280. 10.12659/MSM.897808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S, 2005. Impact of ambient air pollution on birth weight in Sydney, Australia. Occup. Environ. Med 10.1136/oem.2004.014282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson D, Mogren I, Forsberg B, 2013. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: A register-based cohort study. BMJ Open 3, 1–8. 10.1136/bmjopen-2012-001955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Schoendorf KC, 2005. Air Pollution and Birth Weight Among Term Infants in California. Pediatrics 115, 121–128. 10.1542/peds.2004-0889 [DOI] [PubMed] [Google Scholar]
- Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL, 2013. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ. Res 121, 71–78. 10.1016/j.envres.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Haynes E, Hall E, Hossain M, Chen A, Muglia L, Lu L, DeFranco E, 2017. Periconception Exposure to Air Pollution and Risk of Congenital Malformations. J. Pediatr 193, 76–84.e6. 10.1016/j.jpeds.2017.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Demissie K, Lu SE, Kamat L, Wartenberg D, Rhoads GG, 2009. Ambient air pollutant concentrations during pregnancy and the risk of fetal growth restriction. J. Epidemiol. Community Health 63, 488–496. 10.1136/jech.2008.082792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Speranza R, Chiu YM, Hsu L, Curtin PC, Renzetti S, Pajak A, Coull B, Schwartz J, Kloog I, Wright RJ, 2018. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, Ghosh RE, Hansell AL, Kelly FJ, Ross Anderson H, Toledano MB, 2017. Impact of London’s road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ 359, 1–13. 10.1136/bmj.j5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Dostal M, Merkerova-Dostalova M, Libalova H, Milcova A, Rossner P, Rossnerova A, Schmuczerova J, Svecova V, Topinka J, Votavova H, 2013. Health impact of air pollution to children. Int. J. Hyg. Environ. Health 10.1016/j.ijheh.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Beckerman BS, Jerrett M, Crouse DL, Omariba DWR, Peters PA, van Donkelaar A, Martin RV, Burnett RT, Gilbert NL, Tjepkema M, Liu S, Dugandzic RM, 2015. Associations of Pregnancy Outcomes and PM2.5 in a National Canadian Study. Environ. Health Perspect 10.1289/ehp.1408995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Mudd LM, Sikorskii A, Basso O, 2014. United States Birth Weight Reference Corrected For Implausible Gestational Age Estimates. Pediatrics 133, 844–853. 10.1542/peds.2013-3285 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau, 2017. QuickFacts [WWW Document]. census.gov. URL https://www.census.gov/quickfacts/fact/table/US/PST045217 (accessed 7.27.18).
- United States Environmenal Protection Agency, 2018. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US [WWW Document]. epa.gov. URL https://www.epa.gov/outdoor-air-quality-data (accessed 7.18.18).
- United States Environmenal Protection Agency, 2017. Particulate Matter (PM 2.5) Trends [WWW Document]. epa.gov. URL https://www.epa.gov/air-trends/particulate-matter-pm25-trends#pmreg (accessed 7.13.18).
- Van Den Hooven EH, De Kluizenaar Y, Pierik FH, Hofman A, Van Ratingen SW, Zandveld PYJ, MacKenbach JP, Steegers EAP, Miedema HME, Jaddoe VWV, 2011. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: The generation r study. Hypertension 57, 406–412. 10.1161/HYPERTENSIONAHA.110.164087 [DOI] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Caldini EG, Ribeiro AACM, Mayhew TM, Saldiva PHN, Dolhnikoff M, 2008. Particulate Urban Air Pollution Affects the Functional Morphology of Mouse Placental. Biol. Reprod 79, 578–584. 10.1095/biolreprod.108.069591 [DOI] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, Gray SC, Edwards SE, Miranda ML, 2012. The effects of exposure to particulate matter and neighbourhood deprivation on gestational hypertension. Paediatr. Perinat. Epidemiol 26, 91–100. 10.1111/j.1365-3016.2011.01245.x [DOI] [PubMed] [Google Scholar]
- Wilson A, Chiu YHM, Hsu HHL, Wright RO, Wright RJ, Coull BA, 2017. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am. J. Epidemiol 186, 1281–1289. 10.1093/aje/kwx184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Jiang B, Zhu P, Geng X, Liu Z, Cui L, Yang L, 2018. Associations between maternal weekly air pollutant exposures and low birth weight: A distributed lag non-linear model. Environ. Res. Lett 13 10.1088/1748-9326/aaa346 [DOI] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B, 2009. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the South Coast Air Basin of California. Environ. Health Perspect 117, 1773–1779. 10.1289/ehp.0800334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J, 2015. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ. Sci. Pollut. Res 22, 3383–3396. 10.1007/s11356-014-3458-7 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C, 2002. Concentration and size distribution of ultrafine particles near a major highway. J. Air Waste Manag. Assoc 52, 1032–1042. 10.1080/10473289.2002.10470842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.