1. INTRODUCTION
Styrene is a monocyclic aromatic hydrocarbon mainly used in polymer industries where plastics, synthetic rubbers, and resins are manufactured.1 In 2008, over 12 billion pounds of styrene were produced in the United States.2 Ethylbenzene is found naturally in coal tar and petroleum and is used in the manufacturing process of styrene. Both styrene and ethylbenzene are classified by the International Agency for Research on Cancer (IARC) as group 2B agents, possibly carcinogenic to humans.3, 4 Ethylbenzene and styrene (EB/S) released from polymers and resins may lead to indoor air contamination and inhalation exposures.5, 6 Inhalation is thought to be the primary non-occupational route of EB/S exposure for the general population.3, 6 The median level of ethylbenzene in outdoor suburban air was reported to be 2.7 µg/m3 (0.62 ppb); however, indoor EB/S air levels are often higher due to environmental tobacco smoke and emissions from other consumer products.7 Styrene intakes from ambient air for the Canadian general population have been estimated to be 0.004 – 0.17 μg/kg body weight.8 Ethylbenzene content in tobacco smoke ranges from 83–142 µg/cigarette while styrene content in mainstream tobacco smoke is typically 3.5 – 76.2 µg/cigarette.6, 9 The general population is also exposed to EB/S through ingestion. Styrene can be found in low levels in wine (up to 8 ppb) and as high as 30 ppb in wheat beer.5, 10 In addition, styrene can migrate into food packed in polystyrene materials. Relative to the total EB/S exposures for the general population (~125 µg/person/day), the intakes attributable to ingestion are estimated to be low (0.2 – 1.2 µg/person/day); up to 99% of EB/S exposure is thought to come from inhalation sources and only 1–2% from food consumption.5
Once absorbed, styrene is metabolized predominantly by cytochrome P450 isozymes to styrene-7,8 oxide (7,8-SO).2 Epoxide hydrolase subsequently converts 7,8-SO to styrene glycol (phenylethylene glycol) followed by its oxidization to mandelic acid (MA) and then phenylglyoxylic acid (PGA).3 7,8-SO also forms glutathione conjugates that are further converted into phenyl hydroxyethyl mercapturic acids (PHEMs; N-acetyl-S-(1-phenyl-2-hydroxyethyl)-L-cysteine + N-acetyl-S-(2-phenyl-2-hydroxyethyl)-L-cysteine); however, this pathway is minor in humans. PHEM excretion represents less than one percent of total urinary styrene metabolites.11 Human In vivo studies have shown that MA and PGA are also the major urinary metabolites of ethylbenzene exposure.12–14 Ethylbenzene is initially hydroxylated at the side chain and further oxidized to phenylethylene glycol, the same downstream metabolite of styrene. Subsequent oxidation converts phenylethylene glycol to MA and then PGA, as with styrene metabolism.15, 16 Previous studies show concentrations of MA and PGA in urine correlate with concentrations of styrene in air and blood.17–20 The American Conference of Governmental Industrial Hygienists (ACGIH) has defined styrene occupation exposure guidelines based on the sum of MA + PGA.21
Occupational styrene exposure via inhalation has been linked to ischemic heart disease22 and effects such as changes in color vision, hearing impairment, and symptoms of neurotoxicity.2 Some cohort studies report that elevated styrene exposure is associated with increased cancer prevalence and mortality3 while other studies find no relation of styrene exposure to cancer.23 Occupational workers exposed to a mixture of solvents including ethylbenzene were reported to express increased lymphocyte counts, decreased hemoglobin concentrations, and hearing loss24, 25; however, these studies did not isolate the effect source as ethylbenzene. Although many occupational exposure studies have been performed, few studies examine EB/S exposure in the general population, inclusive of all occupations and lifestyle habits. One report shows MA and PGA data for NHANES 2011 – 2012 alone, but does not identify EB/S exposure sources or include applicable data from NHANES 2005 – 2006.26 Due to availability of NHANES data and particular interest in urinary metabolites, this research focuses solely on concentrations of urinary EB/S metabolites (MA, PGA, PHEM) from the NHANES 2005 – 2006 and 2011 – 2012 survey cycles in order to evaluate exposure in the U.S. population. Additionally, we examine whether smoking status, food, alcohol, and other demographic variables influence EB/S exposure. We present results of both MA and PGA independently as well as the molar sum of MA + PGA to assess exposure to ethylbenzene and/or styrene.
2. MATERIALS AND METHODS
2.1. Study Design
NHANES is a population-based survey conducted to assess the health and nutritional status of the United States population based on data from regular cross-sectional multistage probability samples representative of the non-institutionalized United States civilian population. The National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) conducts the NHANES survey.27 Specimens were collected during physical examinations in mobile examination centers (MEC). Informed written consent was obtained from all subjects before they took part in the study, and the CDC/NCHS Research Ethics Review Board reviewed and approved the study (NCHS ERB Approval Protocols #2005–06, 2011–17).
Spot urine samples from an environmental subsample (N = 5815) of two NHANES cycles (NHANES 2005 – 2006 and 2011 – 2012; participants ≥ 6-years old) were analyzed for MA, PGA, and PHEM. Results reported here, however, are from a smaller dataset (N = 4690) comprising records remaining after applying eligibility criteria and discarding records with incomplete data for analytic variables (this attrition is detailed in Statistical Analysis below).
2.2. Chemical Analysis
NHANES 2005 – 2006 and 2011 – 2012 urine specimens were kept at −70°C before analysis. Samples were assayed for MA, PGA, and PHEM by running a 50 µL aliquot of each specimen through an ultra-high performance liquid chromatography system (Waters Inc., Milford, MA) coupled with electrospray ionization tandem mass spectrometry (Sciex API 5500 Triple Quad, Applied Biosystems, Foster City, CA) (UPLC-ESI-MS/MS) using Analyst software (Applied Biosystems, Foster City, CA).28 The mass spectrometer was operated using negative ion ESI and in scheduled multiple reaction monitoring (SMRM) mode. The ion source temperature was held at 650°C. The electrospray ion voltage was −4000 V. Urine specimens were assayed with a 1:10 dilution (50 µl urine + 25 µl mixed internal standard + 425 µl 15 mM ammonium acetate, pH 6.8). Chromatographic separation was achieved using an Acquity UPLC® HSS T3, 100 Å, 1.8 µm, 2.1 mm x 150 mm column (Waters Inc., Milford, MA). The mobile phase consisted of 15 mM ammonium acetate, pH 6.8 (Solvent A) and acetonitrile (Solvent B). Unknown concentrations were determined using the peak area ratio of a known standard to the stable isotope-labeled internal standard. For MA, m/z 151→107 and m/z 151→77 were monitored as the quantitation and confirmation ion transitions, respectively. For PGA, the quantitation and confirmation ion transitions monitored were m/z 149→77 and m/z 149→105, correspondingly. For PHEM, we monitored the m/z 282→153 quantitation ion transition and m/z 282→123 confirmation ion transitions. Regarding labeled internal standards, we monitored ion transitions m/z 156→112 for MA-d52H5, m/z 154→82 for PGA-d5 2H5, and m/z 288→159 for PHEM-13C6. The limits of detection (LODs) were 12 µg/L for MA and PGA and 0.7 µg/L for PHEM.
Two quality control (QC) pools (QL and QH) were prepared by spiking desired levels of 35 urinary metabolites in a nonsmoker human urine lot pooled and screened by O2si Smart Solutions (Charleston, SC). Each QC pool was characterized (N = 20 independent determinations) for target analytes over a three month period. Aliquots from the QL and QH pools were analyzed together with each batch of unknowns to confirm acceptable assay precision for all analytes. Absolute assay accuracy was verified by blinded analysis of these 35 analytes prepared in water. Four proficiency testing (PT) samples containing urinary metabolites were prepared by Absolute Standards, Inc. (Hamden, CT) to cover the calibration range for each metabolite. PT samples were blind-coded by an independent QC officer. PT samples were run biannually and following major instrument maintenance.
2.3. Statistical Analysis
Participants were identified as exclusive users of combusted tobacco products (named “exclusive combusted tobacco users” or “exclusive smokers” in this report) if they responded “yes” to SMQ680 and “yes” to at least one of SMQ690A – SMQ690C (cigarettes, pipes, cigars). Participants were identified as non-users if they answered “no” to SMQ680 or were both missing a response to SMQ680 and had serum cotinine ≤10 ng/mL. The serum cotinine threshold of >10 ng/mL has been identified as consistent with active use of combusted tobacco products29, and was used to stratify self-identified exclusive smokers and non-users in statistical analyses reported herein. Participants were excluded from analysis for use of smokeless tobacco and nicotine replacement therapy (N=230), for missing serum cotinine data (N=284), or for missing data for other variables used in regression models (N=609). In addition, two participants were excluded because preliminary regression analysis indicated they imposed excessive leverage on regression parameter estimates (see Results). This attrition left 4,690 study participants eligible for statistical analysis.
Because NHANES participants are recruited through a multistage sampling design, it is necessary to estimate variances properly and to produce unbiased, nationally representative statistics. Rather than simple random sampling, NHANES utilizes stratum and cluster in order to properly represent any underrepresented groups of people. Robust estimation may be accomplished by applying survey sample weights to each participant’s data and using Taylor series linearization to produce variance estimates. We used this estimation approach as it was implemented in the DESCRIPT subroutine of the statistical software package SUDAAN®, Version 11.0.0 (Research Triangle Institute 2012), called from the SAS® statistical software application, Version 9.4, as well as the SURVEYREG subroutine of SAS® 9.4 (SAS® Institute 2010). Sample-weighted linear regression models stratified by tobacco use status (exclusive smokers vs. non-users) were fit to NHANES data from the 2005 – 2006 and 2011 – 2012 survey cycles (NHANES), where the dependent variables were the urinary concentrations of MA and PGA (µg/L). Because the distribution of measurements was strongly right-skewed, which would have adversely affected hypothesis testing, the concentration data was transformed with the natural log for regression analysis. We report slopes from these models along with their 95 percent confidence intervals and p-values. In addition, to facilitate interpretability, we report the slopes transformed to represent the absolute change in biomarker concentration ∆Y associated with a unit-increase in the predictor , as adapted from Rodríguez-Barranco, et al., 201730:
where is the sample-weighted geometric mean of biomarker concentration. The tabulated regression results assume , so that represents the absolute change associated with a unit-increase in the predictor and resembles a conventional linear regression slope where the dependent variable is untransformed. The 95 percent confidence interval is
where is the sample-weighted standard error of the slope. Both and its 95%CI are calculated at , which is reported in the caption accompanying the tabulated regression results. Since this geometric mean is treated as a fixed quantity, the width of the 95%CI may be slightly underestimated. In addition, at values different from the geometric mean, the value of and the width of its 95%CI will vary, owing to the transformation of the dependent variable with the natural log. Statistical significance was set to α ≤ 0.05.
The analytic dataset for the CPD models comprised exclusive combusted tobacco users and non-users, excluded participants who used smokeless tobacco or nicotine replacement therapy (N = 230), who could not be assigned to a CPD category (N = 499), or who were missing data for other variables used in the regression model (N = 584), or because they imposed excessive leverage on regression parameter estimates (N = 2), leaving 4500 participants eligible for statistical analysis. Exposure in the CPD models was classified in ranges of ≤0.05 ng/mL serum cotinine (unexposed to tobacco smoke); >0.05 – ≤10 ng/mL (presumptively exposed to second-hand tobacco smoke); 1 – 10 CPD (0.5 pack), 11 – 20 (1 pack), and >20 (>1 pack), where the reference category was unexposed participants. The unexposed category was defined at ≤0.05 ng/mL serum cotinine, which was its LOD in the 1999 – 2000 NHANES cycle, and although this improved in 2001 to 0.015 ng/mL, we use 0.05 ng/mL to permit historical comparison of serum cotinine results.
Ethylbenzene and styrene whole blood NHANES 2005 – 2006 data was excluded from analysis due to insufficient data available for comparison against the urinary 2005 – 2006 and 2011 – 2012 NHANES cycles. Additionally, urinary EB/S biomarkers have a longer half-life than EB/S in blood, and thus average exposure over a longer period of time, and are less influenced by varied and recent exposures.31 Based on reliability of sampling as well as cross-referencing with BEI standards, urinary biomarker data was preferred for this analysis.
Reported results met the accuracy and precision specifications of the quality control/quality assurance program of the CDC National Center for Environmental Health, Division of Laboratory Sciences.32 Measurements below the LOD were substituted with the quotient of the LOD divided by the square root of two.33
3. RESULTS
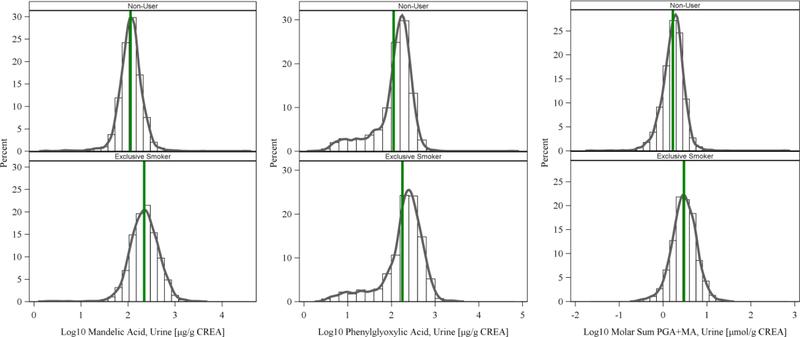
The detect rate of MA and PGA was 98.9 and 90.6 percent, respectively, but was only 19.6 percent for PHEM, so PHEM was excluded from further analysis. Table 1 presents demographic distributions for the 4690 participants reported here. Table 2 presents sample-weighted summary statistics for urinary MA and PGA levels. Tables S2 – S5 present sample-weighted geometric means and percentiles for MA and PGA concentrations adjusted and non-adjusted for creatinine. Sample-weighted median MA among exclusive smokers (246 µg/g creatinine) was approximately twice as high as non-users (121 µg/g creatinine), and similarly, median PGA among exclusive smokers (258 µg/g creatinine) was considerably higher than non-users (164 µg/g creatinine). The molar sum (MA + PGA) median concentrations were 3.40 µmol/g among exclusive smokers and 1.89 µmol/g for non-users. These differences appear in the histograms of urinary MA and PGA among exclusive smokers and non-users (Figure 1).
Table 1.
Demographic distribution of NHANES 2005 – 2006 and 2011 – 2012 participants ≥ 6 years-old (N = 4690).
| Variable | Exclusive Smokera | Non-userb | ||
|---|---|---|---|---|
| Nc | % (SEd) | Nc | % (SEd) | |
| NHANES Cycle | ||||
| 2005 – 2006 | 569 | 11.22 (0.86) | 2261 | 36.85 (2.15) |
| 2011 – 2012 | 298 | 9.06 (0.64) | 1562 | 42.86 (2.25) |
| Age (yr) | ||||
| 6–11 | 0 | 0.00 (0.00) | 276 | 4.04 (0.37) |
| 12–19 | 133 | 1.33 (0.15) | 1002 | 11.13 (0.73) |
| 20–39 | 306 | 7.82 (0.68) | 968 | 23.47 (1.17) |
| 40–59 | 279 | 8.41 (0.59) | 756 | 24.44 (1.12) |
| ≥60 | 149 | 2.73 (0.36) | 821 | 16.63 (1.11) |
| Race/ethnicity | ||||
| Mexican-American | 97 | 1.10 (0.22) | 828 | 7.53 (0.99) |
| Non-Hispanic Black | 275 | 2.77 (0.41) | 956 | 8.67 (1.24) |
| Non-Hispanic White | 403 | 14.45 (1.03) | 1448 | 54.11 (2.15) |
| Other Hispanic or Other/Multi Race | 92 | 1.97 (0.29) | 591 | 9.40 (0.81) |
| Sex | ||||
| Female | 354 | 8.97 (0.62) | 2065 | 42.57 (0.88) |
| Male | 513 | 11.32 (0.72) | 1758 | 37.15 (0.75) |
| Poverty Statuse | ||||
| No | 621 | 16.26 (0.83) | 2971 | 69.41 (1.52) |
| Yes | 246 | 4.03 (0.47) | 852 | 10.30 (1.10) |
| BMIf | ||||
| Healthy weight | 293 | 6.79 (0.61) | 1467 | 28.22 (1.51) |
| Overweight/Obese | 549 | 12.91 (0.87) | 2289 | 50.34 (1.25) |
| Underweight | 25 | 0.59 (0.17) | 67 | 1.15 (0.22) |
Participants reporting using cigarettes, cigars, or pipes during 5 days prior to physical examination and with serum cotinine measurement >10 ng/mL
Participants reporting not using tobacco or nicotine products during 5 days prior to physical examination or with serum cotinine measurement ≤10 ng/mL
Sample size, not sample-weighted
Standard error, sample-weighted
No: Poverty Income Ratio (PIR) ≥ 1.00; Yes: PIR < 1.00
For children and teens 6 – 19 yr of age:
Healthy weight: BMI 5th to 85th percentile
Overweight/Obese: BMI ≥ 85th percentile
Underweight: BMI < 5th percentile
For adults ≥ 20 yr of age:
Healthy weight: BMI 18.5 to < 25.0 kg/M2.
Overweight/Obese: BMI ≥ 25.0 kg/M2.
Underweight: BMI < 18.5 kg/M2.
Table 2.
Sample-weighted median urinary MA and PGA concentrations (creatinine adjusted) among participants ≥ 6 years-old in NHANES 2005 – 2006 and NHANES 2011 – 2012 (N = 4690).
| Variable | Sample Size | MA (µg/g) creatinine Median [25th, 75th percentile] | PGA (µg/g) creatinine Median [25th, 75th percentile] | Molar Sum MA + PGA (µmol/g) creatinine Median [25th, 75th percentile] | ||||
|---|---|---|---|---|---|---|---|---|
| Non-usera | Exclusive Smokerb | Non-usera | Exclusive Smokerb | Non-usera | Exclusive Smokerb | Non-usera | Exclusive Smokerb | |
| All | 3823 | 867 | 121 [88.4, 161] | 246 [159, 382] | 164 [101, 231] | 258 [156, 416] | 1.89 [1.34, 2.49] | 3.40 [2.25, 5.11] |
| NHANES Cycle | ||||||||
| 2005 – 2006 | 2261 | 569 | 120 [88.1, 161] | 239 [160, 389] | 120 [42.4, 190] | 216 [98.3, 331] | 1.59 [1.09, 2.21] | 3.00 [2.04, 4.66] |
| 2011 – 2012 | 1562 | 298 | 122 [88.9, 164] | 255 [158, 370] | 193 [140, 254] | 306 [188, 451] | 2.11 [1.61, 2.72] | 3.69 [2.62, 5.61] |
| Age (yr) | ||||||||
| 6–11 | 276 | 0 | 129 [94.6, 176] | N/Ad | 227 [178, 305] | N/Ad | 2.41 [1.87, 3.10] | N/Ad |
| 12–19 | 1002 | 133 | 102 [77.3, 137] | 165 [119, 266] | 155 [93.2, 208] | 240 [136, 338] | 1.69 [1.15, 2.20] | 2.78 [1.67, 3.73] |
| 20–39 | 968 | 306 | 117 [88.1, 160] | 212 [137, 323] | 146 [102, 215] | 230 [141, 371] | 1.76 [1.30, 2.38] | 2.99 [2.04, 4.24] |
| 40–59 | 756 | 279 | 129 [97.1, 168] | 272 [179, 472] | 165 [91.0, 231] | 286 [170, 436] | 1.94 [1.40, 2.52] | 3.69 [2.48, 5.64] |
| ≥60 | 821 | 149 | 125 [86.8, 169] | 274 [199, 399] | 176 [99.2, 246] | 292 [174, 500] | 2.00 [1.38, 2.58] | 3.82 [2.71, 5.64] |
| Race/ethnicity | ||||||||
| Mexican-American | 828 | 97 | 121 [93.5, 159] | 168 [111, 276] | 157 [98.4, 222] | 181 [87.9, 268] | 1.84 [1.34, 2.41] | 2.13 [1.50, 3.32] |
| Non-Hispanic Black | 956 | 275 | 102 [74.0, 137] | 196 [136, 287] | 133 [90.2, 186] | 205 [146, 307] | 1.56 [1.14, 2.11] | 2.73 [1.99, 3.89] |
| Non-Hispanic White | 1448 | 403 | 124 [89.9, 167] | 260 [174, 411] | 173 [102, 239] | 280 [167, 434] | 1.95 [1.38, 2.56] | 3.60 [2.49, 5.48] |
| Other Hispanic or Multi Race | 591 | 92 | 123 [94.3, 162] | 239 [156, 362] | 163 [109, 231] | 291 [166, 409] | 1.85 [1.44, 2.51] | 3.64 [1.93, 4.83] |
| Sex | ||||||||
| Female | 2065 | 354 | 128 [93.7, 171] | 268 [179, 448] | 176 [104, 247] | 290 [173, 439] | 2.00 [1.42, 2.65] | 3.76 [2.72, 5.57] |
| Male | 1758 | 513 | 113 [84.4, 151] | 222 [141, 354] | 152 [92.3, 213] | 218 [140, 377] | 1.77 [1.25, 2.31] | 3.00 [2.03, 4.59] |
| Poverty Statusc | ||||||||
| No | 2971 | 621 | 122 [89.4, 163] | 248 [162, 371] | 164 [98.4, 231] | 249 [145, 406] | 1.90 [1.34, 2.49] | 3.35 [2.24, 5.05] |
| Yes | 852 | 246 | 115 [80.7, 153] | 234 [155, 406] | 164 [114, 230] | 284 [169, 433] | 1.83 [1.32, 2.44] | 3.47 [2.47, 5.36] |
| BMIe | ||||||||
| Healthy weight | 1467 | 293 | 121 [89.8, 166] | 256 [158, 409] | 168 [102, 242] | 269 [133, 438] | 1.92 [1.35, 2.57] | 3.54 [2.03, 5.49] |
| Overweight/Obese | 2289 | 549 | 121 [87.1, 160] | 240 [159, 371] | 161 [100, 226] | 255 [166, 388] | 1.87 [1.33, 2.43] | 3.29 [2.31, 5.03] |
| Underweight | 67 | 25 | 130 [103, 159] | 185 [177, 315] | 188 [144, 243] | 262 [170, 420] | 2.05 [1.89, 2.58] | 3.52 [2.41, 4.09] |
Participants with serum cotinine measurement ≤10 ng/mL
Participants with serum cotinine measurement >10 ng/mL
No: Poverty Income Ratio (PIR) ≥ 1.00; Yes: PIR < 1.00
N/A: not applicable
For children and teens 6 – 19 yr of age:
Healthy weight: BMI 5th to 85th percentile
Overweight/Obese: BMI ≥ 85th percentile
Underweight: BMI < 5th percentile
For adults ≥ 20 yr of age:
Healthy weight: BMI 18.5 to < 25.0 kg/M2.
Overweight/Obese: BMI ≥ 25.0 kg/M2.
Underweight: BMI < 18.5 kg/M2.
Figure 1.
Histograms (not sample weighted) of log10 creatinine-adjusted urinary concentrations of MA and PGA (µg/g CREA) among exclusive smokers (N = 867) and non-users (N = 3823). Vertical reference line at average concentration.
Multivariable regression analyses evaluated the association between urinary MA and PGA with tobacco smoke exposure and food intake, controlling for potential confounders, stratified by exclusive smokers (Table 3) and non-users (Table 4). The following demographic variables were included in the regression models: age, sex, race/ethnicity, body mass index (BMI), poverty income ratio (PIR; ratio of self-reported family income to the U.S. Census poverty threshold), and fasting time (time between specimen collection and when participant last ate or drank anything other than water). Urinary biomarker concentrations can be influenced by urine dilution, which can vary markedly from void to void and may confound statistical inference.34 Urine dilution can be accounted for by scaling urinary analyte concentration to the urinary concentration of creatinine, a compound formed endogenously by lean body mass and excreted at a fairly constant rate. The summary statistics of urinary concentrations are reported as the ratio of MA and PGA to creatinine [µg/g creatinine]. For the regression models, however, we accounted for urinary dilution by including urinary creatinine [g/L] as a predictor variable. Tables S6 and S7 present similar multivariable regression analyses on molar sum (MA + PGA) concentrations for smokers and non-users, respectively. Preliminary regression analysis discovered two participants with large sample-weighted Studentized residuals (89.9, 38.9) corresponding to absolute Gaussian quantiles < 1E–28, which is indicative of excessive influence, or leverage, on the regression parameter estimates. Leverage was also evaluated with sample-weighted Cook’s Distance (1.2, 0.8), which were also beyond a desirable range. Consequently, these two observations were dropped from all analyses reported here.
Table 3.
Sample-weighted multiple regression slopes for urinary MA and PGA concentrations (µg/L) among NHANES 2005 – 2006 and NHANES 2011 – 2012 exclusive smokersa (N = 867).
| Variable | MA (µg/L) | PGA (µg/L) | ||||
|---|---|---|---|---|---|---|
| Slope [95% CI]d | p-valuee | 𝚫Y [95%CI]f | Slope [95% CI]d | p-valuee | 𝚫Y [95%CI]f | |
| Intercept | 4.47 [4.20, 4.75] | <.0001 | 2.08E+04 [1.59E+04, 2.72E+04] | 3.45 [2.99, 3.90] | <.0001 | 6.24E+03 [3.95E+03, 9.80E+03] |
| Creatinine, urine [g/L]b | 0.622 [0.534, 0.709] | <.0001 | 207 [171, 247] | 0.913 [0.784, 1.04] | <.0001 | 307 [247, 375] |
| Fasting time [HH.00]c | 0.007 [−0.003, 0.017] | 0.1554 | 1.74 [−0.602, 4.11] | 0.016 [−0.004, 0.037] | 0.1155 | 3.40 [−0.712, 7.60] |
| Cotinine, serum [ng/mL] | 0.001 [0.0005, 0.002] | 0.0006 | 0.267 [0.129, 0.405] | 0.0008 [0.0004, 0.001] | 0.0003 | 0.174 [0.0908, 0.258] |
| NHANES Cycle | ||||||
| 2005 – 2006 | Ref. | . | Ref. | Ref. | . | Ref. |
| 2011 – 2012 | −0.155 [−0.334, 0.025] | 0.0881 | −34.5 [−67.1, 4.29] | 0.447 [0.293, 0.600] | <.0001 | 116 [71.6, 167] |
| Age (yr) | ||||||
| 6–11 | — | . | — | — | . | — |
| 12–19 | −0.114 [−0.280, 0.0514] | 0.1695 | −26.0 [−57.6, 11.1] | −0.022 [−0.399, 0.354] | 0.9042 | −4.56 [−65.7, 83.3] |
| 20–39 | Ref. | . | Ref. | Ref. | . | Ref. |
| 40–59 | 0.189 [0.059, 0.320] | 0.0058 | 50.1 [15.8, 88.9] | 0.102 [−0.149, 0.353] | 0.4155 | 22.0 [−26.8, 84.2] |
| ≥60 | 0.185 [0.005, 0.364] | 0.0443 | 48.8 [2.85, 103] | 0.276 [−0.019, 0.570] | 0.0659 | 65.2 [−1.64, 154] |
| Race/ethnicity | ||||||
| Mexican-American | −0.219 [−0.432, −0.006] | 0.0439 | −47.3 [−83.1, −3.43] | −0.337 [−0.637, −0.037] | 0.0289 | −58.8 [−95.6, −9.68] |
| Non-Hispanic Black | −0.105 [−0.238, 0.028] | 0.1166 | −24.0 [−49.9, 5.49] | −0.227 [−0.369, −0.085] | 0.0027 | −41.8 [−62.7, −17.7] |
| Non-Hispanic White | Ref. | . | Ref. | Ref. | . | Ref. |
| Other Hispanic or Multi Race | −0.057 [−0.245, 0.131] | 0.5411 | −13.3 [−50.8, 31.6] | 0.072 [−0.214, 0.359] | 0.6101 | 15.4 [−37.8, 85.6] |
| Sex | ||||||
| Female | 0.135 [0.005, 0.264] | 0.0416 | 34.6 [2.49, 71.0] | 0.226 [0.037, 0.416] | 0.0206 | 52.2 [9.31, 104] |
| Male | Ref. | . | Ref. | Ref. | . | Ref. |
| Poverty Status | ||||||
| No | Ref. | . | Ref. | . | Ref. | |
| Yes | 0.038 [−0.116, 0.193] | 0.6164 | 9.40 [−25.1, 49.5] | 0.149 [−0.034, 0.331] | 0.1066 | 32.9 [−5.44, 78.6] |
| BMI | ||||||
| Healthy weight | Ref. | . | Ref. | Ref. | . | Ref. |
| Overweight/Obese | −0.002 [−0.141, 0.138] | 0.9826 | −0.361 [−30.4, 34.0] | 0.092 [−0.135, 0.319] | 0.4169 | 19.7 [−24.5, 74.8] |
| Underweight | 0.103 [−0.169, 0.374] | 0.4466 | 26.0 [−35.2, 105] | 0.229 [−0.161, 0.620] | 0.2399 | 53.0 [−27.9, 171] |
| Food Group | ||||||
| Milk Products [kg/d] | −0.067 [−0.226, 0.091] | 0.3936 | −15.6 [−47.4, 21.4] | −0.047 [−0.387, 0.293] | 0.7806 | −9.41 [−64.1, 66.4] |
| Meat, Poultry [kg/d] | −0.124 [−0.385, 0.137] | 0.3408 | −28.0 [−75.2, 32.6] | −0.006 [−0.494, 0.482] | 0.9798 | −1.25 [−77.8, 121] |
| Eggs [kg/d] | −0.249 [−0.920, 0.422] | 0.4547 | −53.0 [−142, 117] | 0.979 [−0.382, 2.34] | 0.1525 | 342 [−57.8, 1.82E+03] |
| Legumes, Nuts, Seeds [kg/d] | 0.024 [−0.959, 1.01] | 0.9615 | 5.71 [−145, 393] | 0.636 [−0.107, 1.38] | 0.0906 | 183 [−15.5, 588] |
| Grain Products [kg/d] | 0.172 [0.010, 0.334] | 0.0382 | 45.0 [3.89, 93.1] | 0.248 [−0.104, 0.600] | 0.1604 | 57.9 [−17.8, 164] |
| Fruits [kg/d] | −0.315 [−0.589, −0.041] | 0.0255 | −65.0 [−106, −12.1] | −0.850 [−1.32, −0.386] | 0.0007 | −118 [−149, −68.2] |
| Vegetables [kg/d] | −0.328 [−0.597, −0.059] | 0.0184 | −67.3 [−107, −16.1] | −0.176 [−0.617, 0.264] | 0.4208 | −33.3 [−92.8, 57.8] |
| Fats, Oils, Salad Dressings [kg/d] | −0.459 [−2.72, 1.80] | 0.6817 | −88.4 [−223, 1.09E+03] | −2.44 [−6.40, 1.52] | 0.2185 | −188 [−205, 604] |
| Sugars, Sweets, Beverages [kg/d] | −0.029 [−0.071, 0.013] | 0.1714 | −6.81 [−16.0, 2.79] | −0.003 [−0.061, 0.055] | 0.9197 | −0.594 [−11.7, 11.2] |
| Beer [kg/d] | 0.042 [−0.101, 0.185] | 0.5559 | 10.2 [−21.9, 47.1] | 0.065 [−0.093, 0.224] | 0.4076 | 13.9 [−17.1, 50.0] |
| Wine [kg/d] | −0.163 [−1.10, 0.773] | 0.7258 | −36.0 [−157, 262] | −0.143 [−0.700, 0.415] | 0.6054 | −27.4 [−101, 99.2] |
Participants with serum cotinine measurement >10 ng/mL
Although urinary creatinine concentration is usually reported in mg/dL, here this concentration is in g/L so that its slope simplifies to the more readily interpretable scale of μg/g creatinine.
The time between when the participant last ate or drank anything other than water and the time of the venipuncture.
95% confidence interval
p-Values estimated from identical models where the dependent variable was natural log-transformed
𝚫Y is the expected change in biomarker concentration in µmol/L associated with a unit-increase in the predictor, controlling for other predictors in the model. 𝚫Y [95%CI] computed with GM[Y] = 240 µg/L for MA and GM[Y] = 206 µg/L for PGA.
Table 4.
Sample-weighted multiple regression slopes for urinary MA and PGA concentrations (µg/L) among NHANES 2005 – 2006 and NHANES 2011 – 2012 non-usersa (N = 3823).
| Variable | MA (µg/L) | PGA (µg/L) | ||||
|---|---|---|---|---|---|---|
| Slope [95% CI]d | p-valuee | 𝚫Y [95%CI]f | Slope [95% CI]d | p-valuee | 𝚫Y [95%CI]f | |
| Intercept | 3.75 [3.51, 3.99] | <.0001 | 4.54E+03 [3.57E+03, 5.76E+03] | 3.08 [2.77, 3.39] | <.0001 | 2.42E+03 [1.77E+03, 3.30E+03] |
| Creatinine, urine [g/L]b | 0.734 [0.667, 0.800] | <.0001 | 118 [104, 133] | 0.93 [0.855, 1.01] | <.0001 | 180 [158, 204] |
| Fasting time [HH.00]c | −0.002 [−0.009, 0.005] | 0.5566 | −0.218 [−0.935, 0.503] | 0.016 [0.007, 0.024] | 0.0005 | 1.82 [0.897, 2.75] |
| Cotinine, serum [ng/mL] | 0.021 [0.009, 0.033] | 0.0014 | 2.33 [1.02, 3.66] | 0.039 [0.010, 0.068] | 0.0097 | 4.63 [1.31, 8.04] |
| NHANES Cycle | ||||||
| 2005 – 2006 | Ref. | . | Ref. | Ref. | . | Ref. |
| 2011 – 2012 | 0.017 [−0.068, 0.103] | 0.6833 | 1.91 [−6.89, 11.5] | 0.734 [0.621, 0.847] | <.0001 | 126 [101, 154] |
| Age (yr) | ||||||
| 6–11 | 0.032 [−0.085, 0.150] | 0.5816 | 3.56 [−8.49, 17.1] | 0.310 [0.159, 0.461] | 0.0002 | 42.3 [20.9, 67.1] |
| 12–19 | −0.078 [−0.164, 0.008] | 0.0729 | −8.21 [−16.2, 0.488] | −0.063 [−0.177, 0.051] | 0.2693 | −7.12 [−18.5, 5.59] |
| 20–39 | Ref. | . | Ref. | Ref. | . | Ref. |
| 40–59 | 0.095 [−0.003, 0.193] | 0.0564 | 10.9 [0.103, 22.7] | 0.090 [−0.039, 0.219] | 0.1627 | 11.0 [−3.85, 27.8] |
| ≥60 | 0.085 [−0.014, 0.184] | 0.0893 | 9.71 [−1.10, 21.6] | 0.160 [0.031, 0.288] | 0.0164 | 20.2 [4.29, 38.2] |
| Race/ethnicity | ||||||
| Mexican-American | 0.082 [−0.003, 0.167] | 0.0593 | 9.33 [−0.0197, 19.5] | −0.039 [−0.173, 0.095] | 0.5559 | −4.46 [−18.0, 10.9] |
| Non-Hispanic Black | −0.108 [−0.200, −0.017] | 0.0219 | −11.2 [−19.5, −2.19] | −0.271 [−0.395, −0.147] | <.0001 | −27.6 [−37.6, −16.4] |
| Non-Hispanic White | Ref. | . | Ref. | Ref. | . | Ref. |
| Other Hispanic or Multi Race | 0.012 [−0.077, 0.102] | 0.7811 | 1.35 [−7.75, 11.3] | −0.050 [−0.165, 0.066] | 0.3903 | −5.62 [−17.3, 7.44] |
| Sex | ||||||
| Female | 0.019 [−0.066, 0.105] | 0.6475 | 2.13 [−6.65, 11.7] | 0.168 [0.049, 0.286] | 0.0071 | 21.3 [6.40, 37.9] |
| Male | Ref. | . | Ref. | Ref. | . | Ref. |
| Poverty Status | ||||||
| No | Ref. | . | Ref. | Ref. | . | Ref. |
| Yes | −0.029 [−0.125, 0.068] | 0.5499 | −3.07 [−12.5, 7.22] | 0.051 [−0.073, 0.175] | 0.4056 | 6.12 [−7.64, 21.6] |
| BMI | ||||||
| Healthy weight | Ref. | . | Ref. | Ref. | . | Ref. |
| Overweight/Obese | 0.023 [−0.045, 0.092] | 0.4918 | 2.58 [−4.54, 10.2] | 0.004 [−0.081, 0.090] | 0.9222 | 0.482 [−8.76, 10.5] |
| Underweight | 0.071 [−0.113, 0.256] | 0.4363 | 8.09 [−11.0, 30.9] | 0.265 [−0.001, 0.530] | 0.0505 | 35.3 [1.09, 79.4] |
| Food Group | ||||||
| Milk Products [kg/d] | 0.037 [−0.104, 0.178] | 0.5969 | 4.13 [−10.3, 20.6] | 0.111 [−0.015, 0.236] | 0.0817 | 13.6 [−1.16, 30.3] |
| Meat, Poultry [kg/d] | 0.076 [−0.083, 0.235] | 0.3363 | 8.65 [−8.08, 28.2] | 0.016 [−0.189, 0.221] | 0.8742 | 1.88 [−19.3, 27.7] |
| Eggs [kg/d] | 0.692 [0.201, 1.18] | 0.0072 | 109 [26.8, 241] | 0.267 [−0.733, 1.27] | 0.5904 | 35.6 [−58.3, 281] |
| Legumes, Nuts, Seeds [kg/d] | −0.041 [−0.388, 0.305] | 0.8090 | −4.43 [−34.1, 37.0] | −0.450 [−0.994, 0.094] | 0.1019 | −42.2 [−72.5, 8.90] |
| Grain Products [kg/d] | 0.048 [−0.078, 0.174] | 0.4448 | 5.36 [−7.73, 20.1] | 0.077 [−0.087, 0.241] | 0.3462 | 9.29 [−9.02, 30.7] |
| Fruits [kg/d] | 0.004 [−0.144, 0.152] | 0.9529 | 0.473 [−14.1, 17.2] | −0.381 [−0.499, −0.261] | <.0001 | −36.8 [−45.5, −27.2] |
| Vegetables [kg/d] | −0.149 [−0.354, 0.055] | 0.1467 | −15.2 [−32.0, 5.31] | −0.056 [−0.298, 0.185] | 0.6374 | −6.39 [−29.2, 22.4] |
| Fats, Oils, Salad Dressings [kg/d] | 0.666 [−0.455, 1.79] | 0.2352 | 103 [−37.0, 516] | −1.28 [−3.09, 0.540] | 0.1620 | −84.0 [−111, 70.1] |
| Sugars, Sweets, Beverages [kg/d] | −0.014 [−0.046, 0.019] | 0.3890 | −1.51 [−4.82, 1.91] | −0.011 [−0.050, 0.028] | 0.5775 | −1.25 [−5.50, 3.16] |
| Beer [kg/d] | 0.141 [0.023, 0.260] | 0.0210 | 16.6 [3.01, 31.7] | 0.135 [0.029, 0.240] | 0.0138 | 16.8 [3.95, 31.0] |
| Wine [kg/d] | 0.223 [−0.233, 0.680] | 0.3263 | 27.3 [−21.2, 103] | −0.472 [−1.10, 0.152] | 0.1332 | −43.8 [−76.6, 16.0] |
Participants with serum cotinine measurement >10 ng/mL
Although urinary creatinine concentration is usually reported in mg/dL, here this concentration is in g/L so that its slope simplifies to the more readily interpretable scale of μg/g creatinine.
The time between when the participant last ate or drank anything other than water and the time of the venipuncture.
95% confidence interval
p-Values estimated from identical models where the dependent variable was natural log-transformed
𝚫Y is the expected change in biomarker concentration in µmol/L associated with a unit-increase in the predictor, controlling for other predictors in the model. 𝚫Y [95%CI] computed with GM[Y] = 109 µg/L for MA and GM[Y] = 116 µg/L for PGA.
Urinary MA was significantly and positively associated with serum cotinine among exclusive smokers (ΔY=0.267 MA µg/L per cotinine ng/mL) and non-users (ΔY=2.33 MA µg/L per cotinine ng/mL), controlling for potential confounders. Dietary exposure was explored by assessing the mass NHANES participants consumed within each USDA (US Department of Agriculture) food group for the 24 hour period (midnight to midnight) preceding the dietary recall interview conducted in person as part of the physical examination. Standardized hierarchical food groups can be identified from the USDA code, where the first digit represents one of nine major food groups, and each subsequent digit represents subgroups of increasing specificity.35 Each participant’s dietary intake was first apportioned over nine food groups: milk products; meat, poultry; eggs; legumes, nuts, seeds; grain products; fruits; vegetables; fats, oils, salad dressings; and sugars, sweets, beverages. In addition, we distinguished beer and wine as food subgroups. The USDA food codes and logic for apportioning dietary intake are detailed in Table S1. Among exclusive smokers, MA was positively associated with eating grain products (ΔY=45.0 MA µg/L per kg consumed), while negatively associated with eating fruits (ΔY=–65.0 MA µg/L per kg consumed) and vegetables (ΔY=–67.3 MA µg/L per kg consumed). Among non-users, MA was positively associated with eating eggs (ΔY=109 MA µg/L per kg consumed) and drinking beer (ΔY=16.6 MA µg/L per kg consumed). Median values for the consumption of each food group, cigarettes smoked per day, and serum cotinine were calculated in order to quantify MA and PGA exposure effects when multiplied by their respective regression slopes (Table 5). Among exclusive smokers, MA levels was significantly higher in females compared to males, and highest among older adults (40 – 59 years and ≥ 60 years) compared to the 20 – 39 year olds. Among non-users, MA was higher among 40 – 59 years and ≥ 60 years and among Mexican Americans compared to non-Hispanic whites.
Table 5.
Sample-weighted medians and selected percentiles of serum cotinine, cigarettes smoked per day, and consumption of each food group among NHANES 2005 – 2006 and NHANES 2011 – 2012.
| Variable | N | Percentile | |||
|---|---|---|---|---|---|
| 50th | 75th | 90th | 95th | ||
| Serum Cotinine [ng/mL], nonsmokers | 3823 | 0.02 | 0.0769 | 0.407 | 1.31 |
| Serum Cotinine [ng/mL], smokers | 867 | 197.6 | 302 | 392.2 | 462 |
| Cigarettes Per Day | 678 | 10.91 | 19.5 | 24.5 | 30.4 |
| Milk Products [kg/d] | 4690 | 0.128 | 0.342 | 0.594 | 0.804 |
| Meat, Poultry [kg/d] | 4690 | 0.159 | 0.299 | 0.456 | 0.605 |
| Eggs [kg/d] | 4690 | . | . | 0.101 | 0.144 |
| Legumes, Nuts, Seeds [kg/d] | 4690 | . | 0.0160 | 0.0863 | 0.171 |
| Grain Products [kg/d] | 4690 | 0.264 | 0.440 | 0.668 | 0.860 |
| Fruits [kg/d] | 4690 | 0.0456 | 0.248 | 0.458 | 0.617 |
| Vegetables [kg/d] | 4690 | 0.120 | 0.246 | 0.392 | 0.509 |
| Fats, Oils, Salad Dressings [kg/d] | 4690 | . | 0.0138 | 0.0367 | 0.0609 |
| Sugars, Sweets, Beverages [kg/d] | 4690 | 1.84 | 2.75 | 3.89 | 4.80 |
| Beer [kg/d] | 4690 | . | . | 0.358 | 1.07 |
| Wine [kg/d] | 4690 | . | . | . | 0.147 |
Urinary PGA was positively associated with serum cotinine among exclusive smokers (ΔY=0.174 PGA µg/L per cotinine ng/mL) and non-users (ΔY=4.63 PGA µg/L per cotinine ng/mL). Among exclusive smokers, PGA was negatively associated with eating fruits (ΔY=–118 µg/L PGA per kg consumed). Like MA, PGA was positively associated with eating eggs (ΔY=342 µg/L PGA per kg consumed), but only at a borderline significant level. Among non-users, PGA was positively associated with consuming milk products (ΔY=13.6 µg/L PGA per kg consumed) and drinking beer (ΔY=16.8 µg/L PGA per kg consumed), while negatively associated with eating fruits (ΔY=–36.8 µg/L PGA per kg consumed). Among exclusive smokers, PGA was significantly higher in females. Among non-users, PGA was higher in females and among 6 – 11 year olds, followed by ≥60 year olds.
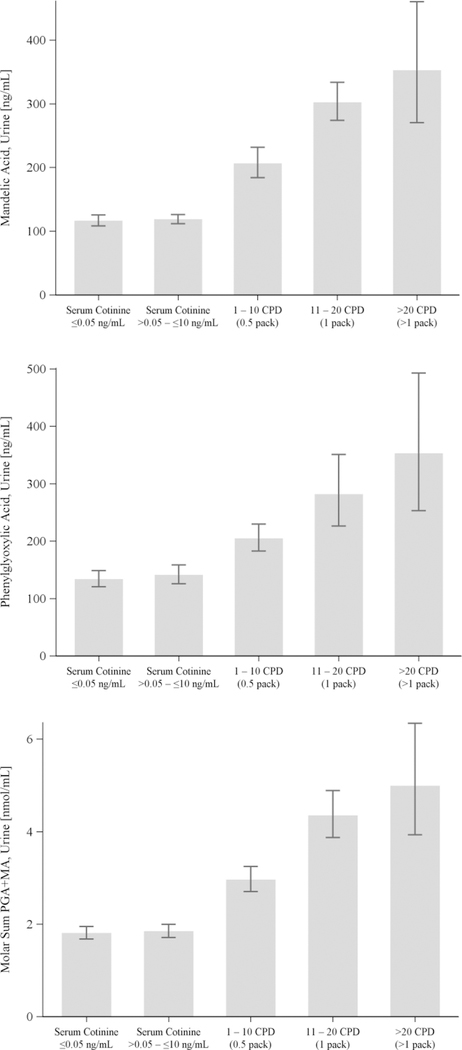
Among the 4500 participants in the CPD model, 8.50 percent smoked 1 – 10 CPD, 5.11 percent smoked 11 – 20 CPD, and 1.41 percent smoked >20 CPD. Beginning with non-users (with and without SHS exposure), both MA and PGA among exclusive smokers increased in direct proportion to CPD rate. The relationships of urinary MA and PGA to CPD among exclusive smokers compared to non-users with serum cotinine levels of ≤0.05 ng/mL and 0.05 – 10 ng/mL were also examined in sample-weighted regression models. For the CPD regression models (Tables S8 and S9), serum cotinine was used as a continuous predictor and was replaced with CPD in the same models as summarized in Tables 3 and 4. The CPD regression model of molar sums (MA + PGA) is presented in Table S9. When adjusted for urinary creatinine, age, race/ethnicity, sex, poverty status, BMI and food intake, the CPD models show that all CPD groups had significantly higher urinary MA and PGA levels (p <.0001) compared to those whose serum cotinine levels were ≤0.05 ng/mL. Smoking 0.5 pack cigarettes per day predicted increased MA (97.9 µg/L) and PGA (69.3 µg/L). Consistent with the exclusive smoker and non-user regression models (Tables 3 and 4), the CPD regression model for PGA identified that the median level of fruit consumption was a significant decrease in PGA (−2.43 µg/L). Figure 2 displays the sample-weighted least-square means of urinary MA and PGA by CPD category, adjusted for sex, age, race/ethnicity, BMI, fasting time, urinary creatinine, impoverishment, and diet. Figure 2 also shows the dose-dependent response of MA and PGA concentrations with respect to CPD. In addition, participants with 0.05 – 10 ng/mL serum cotinine (presumptively exposed to secondhand tobacco smoke) did not have significantly increased urinary MA or PGA compared to participants with ≤0.05 ng/mL serum cotinine.
Figure 2.
Sample-weighted least-square means [95% confidence intervals] of urinary MA and PGA concentrations (µg/L) for each CPD category (N = 4500).
4. DISCUSSION
We assessed EB/S exposure in a representative sampling of the US population by measuring urinary EB/S biomarkers MA, PGA, and PHEM. Our analysis showed that PHEM was detected in 19.6 percent of urine specimens. This relatively lower detection rate compared to MA and PGA likely results from the fact that less than one percent of the absorbed dose of EB/S is excreted in the form of urinary PHEM.11 Conversely, both MA and PGA were widely detected (>90 percent) and account for a larger fraction of the absorbed dose. Thus MA and PGA may be more suitable biomarkers than PHEM for assessing non-occupational EB/S exposure.36 The high detection rate of MA and PGA in this study is also consistent with an Italian population study where MA and PGA was detected in all subjects not occupationally exposed to styrene.37 High detection rates of EB/S metabolites MA and PGA among presumably unexposed populations might indicate the presence of uncharacterized exposure sources besides tobacco smoke.
Both MA and PGA urinary levels were higher in exclusive smokers than non-users in NHANES 2005 –2006 and NHANES 2011 – 2012. Our results show that median MA levels in the U.S. population were higher in exclusive smokers compared to levels of non-users (246 vs. 121 µg/g creatinine), and median PGA levels of exclusive smokers (258 µg/g creatinine) were significantly higher than that of non-users (164 µg/g creatinine). These findings are consistent with the amounts of EB/S in cigarette smoke; smoking one pack of cigarettes a day would likely lead to inhalation of milligram quantities of EB/S.9 The MA and PGA histograms shown in Figure 1 both illustrate higher EB/S biomarker levels in exclusive smokers compared with non-users. Our regression model revealed that both MA and PGA correlate with serum cotinine in exclusive smokers (Table 3) and follow a dose response pattern with CPD (Figure 2, Tables S8 – S9). Furthermore, tobacco smoke is known to contain significant amounts of ethylbenzene and styrene.9 Combined, these results support tobacco smoke as a significant source of EB/S exposure in the U.S. population. The least-square means presented in Figure 2 show that both urinary MA and PGA were higher in participants with presumptive SHS exposure (0.05 – 10 ng/mL serum cotinine) compared with those with no SHS exposure (<0.05 ng/mL serum cotinine), but this increase was not statistically significant after controlling for confounders (Tables S8 – S9).
Higher urinary concentrations of MA and PGA were observed with increasing age, where young adults (12 – 19 years) had the lowest concentrations and the 40 – 59 year and ≥60 year age groups had higher MA and PGA concentrations (Tables 3 and 4). This age-related difference cannot be explained by differences in tobacco smoke exposure because serum cotinine was included in the model. Age-related differences in urinary excretion of MA and PGA could be attributed to the fact that persons ≥60 years tend to have lower lean body mass and thus lower urinary creatinine.34 Statistically significant differences in creatinine levels between females and males may also explain why creatinine-adjusted concentrations of MA and PGA were higher among females compared to males (Table 3) yet lower in females compared to males before creatinine adjustments (Tables S2 & S4).
The gender and racial biases of urinary excretion of EB/S metabolites could also be the result of combined metabolic differences. One study found that the heterozygous genotypes CYP2E1*5b and CYP2E1*6 result in significantly reduced excretion of MA and PGA.38 This report was supported by another study which found that individuals with the CYP2E1*5b heterozygote allele had decreased excretion of EB/S metabolites in comparison to the wild type homozygote.39 Between genders, women have higher CYP2B6 expression in the liver40; CYP2B6 has been shown to be the most effective Cytochrome P450 form, which initiates the conversion of styrene to styrene-7,8-oxide.41
We investigated the consumption of nine different food groups as well as wine and beer in order to characterize potential dietary sources of exposure to ethylbenzene and styrene. Our models indicate that fruit consumption is a statistically significant negative predictor of urinary PGA concentrations in the U.S. population (Tables 3 and 4). While we cannot fully interpret the effects of fruit consumption on EB/S exposure, grapefruit has been reported to create an inhibitory effect on cytochrome P450, thus possibly slowing metabolism of ethylbenzene and styrene.42, 43 Consuming food groups such as eggs and grains was linked to increased MA levels in smokers; however, the magnitudes of the exposure effect of these food groups were much smaller than the exposure effect in the CPD model. Based on the sample-weighted regression slope for grain consumption and the median dietary intake of grain in the U.S. (estimated from NHANES diet data), median grain consumption is associated with 11.1 µg/L increased MA. This contrasts with smoking 0.5 pack cigarettes per day, which is associated with a 97.9 µg/L increase in MA.
We explored the effects of wine and beer consumption on EB/S exposure. Beer was a significant predictor of increased EB/S exposure in non-users of tobacco, but wine and beer were not significant predictors of EB/S exposure in exclusive smokers. Neither wine nor beer were shown to impact the molar sum (MA + PGA) of EB/S exposure in the population (Table S9). Published literature claims that wheat beer can contain up to about 30 ppb styrene10, 44 and wine contains a range of styrene from 1–3 ppb, with a maximum of 8 ppb.5 Wine was not a significant predictor in our models. Cigarette smoke contains 3.5 – 76.2 µg/cigarette, so the EB/S exposure from beer or wine would be negligible compared to smoking a pack of cigarettes.9 Published literature also reports that alcohol consumption can inhibit the rate of urinary MA and PGA excretion.45 While the kinetics of excretion are affected by ethanol consumption, total concentrations excreted are not significantly altered.46 Due to these kinetic effects on EB/S metabolite excretion, we cannot provide conclusive information regarding the overall effects of alcohol on the measured urinary MA and PGA levels.
The NHANES 2005 – 2006 and 2011 – 2012 MA and PGA data provide the first reference levels of these EB/S exposure biomarkers in a representative sample of the U.S. population. Our study also establishes tobacco smoke as a significant source of ethylbenzene and styrene exposure. Further monitoring of these EB/S exposure biomarkers as part of subsequent cycles of NHANES will provide useful information about changes in ethylbenzene and styrene exposure in the U.S. population over time.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the National Center for Health Statistics and Westat for planning and directing the National Health and Nutrition Examination Survey (NHANES). We thank the urinary metabolites team for the analysis of thousands of NHANES urine specimens to generate the published dataset. We thank Connie Sosnoff (CDC) and her team for the serum cotinine data as well as Denise Tevis, Christopher Reese, and John Ruhl for quality assessment. The findings and conclusions in this report are solely those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding: NHANES biomarker measurements were funded by the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the Centers for Disease Control and Prevention or U. S. Food and Drug Administration. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the U.S. Food and Drug Administration.
The authors declare that they have no actual or potential competing financial interests.
Declarations of interest: none.
DECLARATIONS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Kim KW Effects of Styrene-metabolizing Enzyme Polymorphisms and Lifestyle Behaviors on Blood Styrene and Urinary Metabolite Levels in Workers Chronically Exposed to Styrene. Toxicol Res 2015; 31: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ATSDR. Toxicological profile for Styrene U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, 2010. [Google Scholar]
- 3.IARC. Styrene: Lyon, France, 2002. [Google Scholar]
- 4.IARC. Ethylbenzene International Agency for Research on Cancer: Lyon, France, 2000. [Google Scholar]
- 5.Tang W, Hemm I, Eisenbrand G Estimation of human exposure to styrene and ethylbenzene. Toxicology 2000; 144: 39–50. [DOI] [PubMed] [Google Scholar]
- 6.ATSDR. Toxicological profile for Ethylbenzene U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, 2010. [Google Scholar]
- 7.Wallace L, Pellizzari E, Hartwell TD, Perritt R, Ziegenfus R Exposures to benzene and other volatile compounds from active and passive smoking. Arch Environ Health 1987; 42: 272–279. [DOI] [PubMed] [Google Scholar]
- 8.Newhook R, Caldwell I Exposure to styrene in the general Canadian population. IARC Sci Publ 1993; e-pub ahead of print 1993/01/01 27–33. [PubMed]
- 9.Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ et al. Mainstream Smoke Levels of Volatile Organic Compounds in 50 U.S. Domestic Cigarette Brands Smoked With the ISO and Canadian Intense Protocols. Nicotine Tob Res 2016; 18: 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langos D, Granvogl M Studies on the Simultaneous Formation of Aroma-Active and Toxicologically Relevant Vinyl Aromatics from Free Phenolic Acids during Wheat Beer Brewing. J Agric Food Chem 2016; 64: 2325–2332. [DOI] [PubMed] [Google Scholar]
- 11.Manini P, Andreoli R, Poli D, De Palma G, Mutti A, Niessen WM Liquid chromatography/electrospray tandem mass spectrometry characterization of styrene metabolism in man and in rat. Rapid Commun Mass Spectrom 2002; 16: 2239–2248. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein L An overview of environmental and toxicological aspects of aromatic hydrocarbons. IV. Ethylbenzene. Sci Total Environ 1985; 44: 269–287. [DOI] [PubMed] [Google Scholar]
- 13.Sams C, Loizou GD, Cocker J, Lennard MS Metabolism of ethylbenzene by human liver microsomes and recombinant human cytochrome P450s (CYP). Toxicol Lett 2004; 147: 253–260. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom K, Riihimaki V, Laine A Urinary disposition of ethylbenzene and m-xylene in man following separate and combined exposure. Int Arch Occup Environ Health 1984; 54: 355–363. [DOI] [PubMed] [Google Scholar]
- 15.Drummond L, Caldwell J, Wilson HK The metabolism of ethylbenzene and styrene to mandelic acid: stereochemical considerations. Xenobiotica 1989; 19: 199–207. [DOI] [PubMed] [Google Scholar]
- 16.Korn M, Gfrorer W, Herz R, Wodarz I, Wodarz R Stereometabolism of ethylbenzene in man: gas chromatographic determination of urinary excreted mandelic acid enantiomers and phenylglyoxylic acid and their relation to the height of occupational exposure. Int Arch Occup Environ Health 1992; 64: 75–78. [DOI] [PubMed] [Google Scholar]
- 17.Guillemin MP, Berode M Biological monitoring of styrene: a review. Am Ind Hyg Assoc J 1988; 49: 497–505. [DOI] [PubMed] [Google Scholar]
- 18.Lauwerys RR, Hoet P. Industrial chemical exposure : guidelines for biological monitoring Lewis Publishers: Boca Raton, FL, 2001. [Google Scholar]
- 19.Pekari K, Nylander-French L, Pfäffli P, Sorsa M, Aitio A Biological monitoring of exposure to styrene-assessment of different approaches. J Occup Med Toxicol 1993; 2: 115–126. [Google Scholar]
- 20.Relationship Between Exposure to Xylenes and Ethylbenzene Expressed Either in Concentration in Air and Amount of Their Metabolites Excreted in the Urine. Cham Springer International Publishing, 2016. [Google Scholar]
- 21.ACGIH. Threshold limit values for chemical substances and physical agents and biological exposure indices Cincinatti, OH, 2015. [Google Scholar]
- 22.Matanoski GM, Tao XG Styrene exposure and ischemic heart disease: a case-cohort study. Am J Epidemiol 2003; 158: 988–995. [PubMed] [Google Scholar]
- 23.Boffetta P, Adami HO, Cole P, Trichopoulos D, Mandel JS Epidemiologic studies of styrene and cancer: a review of the literature. J Occup Environ Med 2009; 51: 1275–1287. [DOI] [PubMed] [Google Scholar]
- 24.Angerer J, Wulf H Occupational chronic exposure to organic solvents. XI. Alkylbenzene exposure of varnish workers: effects on hematopoetic system. Int Arch Occup Environ Health 1985; 56: 307–321. [DOI] [PubMed] [Google Scholar]
- 25.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, Kotylo P, Fiszer M, Dudarewicz A et al. Hearing loss among workers exposed to moderate concentrations of solvents. Scand J Work Environ Health 2001; 27: 335–342. [DOI] [PubMed] [Google Scholar]
- 26.Jain RB Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of U.S. adults. Environ Toxicol Pharmacol 2015; 40: 471–479. [DOI] [PubMed] [Google Scholar]
- 27.NHANES. National Healthy and Nutrition Examination Survey (https://www.cdc.gov/nchs/nhanes/index.htm). In, 2017.
- 28.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta 2012; 750: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 1996; 275: 1233–1240. [PubMed] [Google Scholar]
- 30.Rodriguez-Barranco M, Tobias A, Redondo D, Molina-Portillo E, Sanchez MJ Standardizing effect size from linear regression models with log-transformed variables for meta-analysis. BMC Med Res Methodol 2017; 17: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wigaeus E, Lof A, Bjurstrom R, Nordqvist MB Exposure to styrene. Uptake, distribution, metabolism and elimination in man. Scand J Work Environ Health 1983; 9: 479–488. [DOI] [PubMed] [Google Scholar]
- 32.Caudill SP, Schleicher RL, Pirkle JL Multi-rule quality control for the age-related eye disease study. Stat Med 2008; 27: 4094–4106. [DOI] [PubMed] [Google Scholar]
- 33.Hornung RW, Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990; 5: 46–51. [Google Scholar]
- 34.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005; 113: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahuja JK, Moshfegh AJ, Holden JM, Harris E USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr 2013; 143: 241S–249S. [DOI] [PubMed] [Google Scholar]
- 36.De Palma G1 MP, Mozzoni P, Andreoli R, Bergamaschi E, Cavazzini S, Franchini I, Mutti A. Polymorphism of xenobiotic-metabolizing enzymes and excretion of styrene-specific mercapturic acids. Chem Res Toxicol 2001; 14: 1393–1400. [DOI] [PubMed] [Google Scholar]
- 37.Manini P, De Palma G, Andreoli R, Goldoni M, Mutti A Determination of urinary styrene metabolites in the general Italian population by liquid chromatography-tandem mass spectrometry. Int Arch Occup Environ Health 2004; 77: 433–436. [DOI] [PubMed] [Google Scholar]
- 38.Carbonari D, Mansi A, Proietto AR, Paci E, Bonanni RC, Gherardi M et al. Influence of genetic polymorphisms of styrene-metabolizing enzymes on the levels of urinary biomarkers of styrene exposure. Toxicol Lett 2015; 233: 156–162. [DOI] [PubMed] [Google Scholar]
- 39.Prieto-Castello MJ, Cardona A, Marhuenda D, Roel JM, Corno A Use of the CYP2E1 genotype and phenotype for the biological monitoring of occupational exposure to styrene. Toxicol Lett 2010; 192: 34–39. [DOI] [PubMed] [Google Scholar]
- 40.Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML et al. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther 2003; 307: 906–922. [DOI] [PubMed] [Google Scholar]
- 41.Sumner SJ, Fennell TR Review of the metabolic fate of styrene. Crit Rev Toxicol 1994; 24 Suppl: S11–33. [DOI] [PubMed] [Google Scholar]
- 42.Bailey DG, Kreeft JH, Munoz C, Freeman DJ, Bend JR Grapefruit juice-felodipine interaction: effect of naringin and 6’,7’-dihydroxybergamottin in humans. Clin Pharmacol Ther 1998; 64: 248–256. [DOI] [PubMed] [Google Scholar]
- 43.Hukkanen J, Jacob P 3rd, Benowitz NL Effect of grapefruit juice on cytochrome P450 2A6 and nicotine renal clearance. Clin Pharmacol Ther 2006; 80: 522–530. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz KJ, Stubner R, Methner FJ Formation of styrene dependent on fermentation management during wheat beer production. Food Chem 2012; 134: 2121–2125. [DOI] [PubMed] [Google Scholar]
- 45.Cerny S, Mraz J, Flek J, Tichy M Effect of ethanol on the urinary excretion of mandelic and phenylglyoxylic acids after human exposure to styrene. Int Arch Occup Environ Health 1990; 62: 243–247. [DOI] [PubMed] [Google Scholar]
- 46.Wilson HK, Robertson SM, Waldron HA, Gompertz D Effect of alcohol on the kinetics of mandelic acid excretion in volunteers exposed to styrene vapour. Br J Ind Med 1983; 40: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.