Abstract
Context:
Symptoms affect quality of life (QOL), functional status, and cognitive function in cancer survivors, but older survivors are understudied.
Objectives:
To identify prototypical pre-systemic therapy psychoneurological symptom clusters among older breast cancer survivors, and determine whether these symptom clusters predicted cognition and QOL over time.
Methods:
Women with newly diagnosed non-metastatic breast cancer (n=319) and matched non-cancer controls (n=347) aged 60+ completed questionnaires and neuropsychological tests before systemic therapy and 12- and 24-months later. Latent class analysis identified clusters of survivors based upon their pre-therapy depression, anxiety, fatigue, sleep disturbance, and pain. Linear mixed-effects models examined changes in objective cognition, perceived cognition, and functional status (instrumental activities of daily living (IADL) disability, functional well-being, and breast cancer-specific QOL) by group, controlling for covariates.
Results:
Nearly one-fifth of older survivors were classified as having a high pre-therapy symptoms (n=51; 16%); the remainder had a low symptoms (n=268; 84%); both groups improved over time on all outcomes. However, compared to the low symptom group and controls, survivors with high symptoms had lower baseline objective cognition and lower perceived cognition at baseline and 24-months, lower functional well-being at baseline and 12-months, greater IADL disability at baseline, and lower breast cancer-specific QOL at all time points (all p<0.05).
Conclusion
Nearly one-fifth of older breast cancer survivors had high psychoneurological symptoms at diagnosis, which, predict clinically meaningful decrements in perceived cognition and function in the first 24 months post-diagnosis. Pre-treatment psychoneurological symptom clusters could identify survivors for monitoring or intervention.
Keywords: symptoms, symptom cluster, geriatric assessment, cognition, quality of life
Introduction
Anxiety, depression, fatigue, sleep disturbance, and pain are prevalent and distressing psychoneurological symptoms1–3 that have individually been associated with cognitive problems such as difficulties with concentration, decision-making, and memory in cancer survivors.6,7 Older survivors may be at particularly high risk for these symptoms, as well as adverse cognitive function and quality of life (QOL) outcomes due to concurrent forces of aging, comorbid conditions, and risk for neurodegeneration.7,8 Psychoneurological symptoms often co-occur, or cluster, and their combined effects on QOL can be greater than the sum of their individual effects.9–17 Psychoneurological symptom clusters and cognitive problems appear to share some common underlying mechanisms such as proinflammatory cytokines, hormone dysregulation, and genetic vulnerabilities.10–12,14,18–20 These observations lead some to suggest that cognitive problems are part of the psychoneurological symptom cluster;10,14 however, psychoneurological symptoms prior to systemic treatment may be a risk factor for subsequent cognitive dysfunction as well as poor QOL.6,7 Cancer survivors, especially older cancer survivors (ages 60+), are particularly concerned about cognitive problems as an outcome of their health conditions.2,21,22
Despite the fact that 74% of the 15.5 million US cancer survivors are aged 60 and older,23 there is a paucity of symptom research focused on older survivors. An early study in this area found that older breast cancer survivors have significantly greater anxiety, depression, and fatigue prior to systemic cancer treatment than matched cancer-free controls at the same time point.24 Although these symptoms were not associated with baseline objective cognitive function,24 the longitudinal relationships between pre-treatment psychoneurological symptom clusters and subsequent cognitive functioning, functional status, and QOL have yet to be examined. This evidence would be important to help identify subgroups of older breast cancer survivors who may be at risk for poor functional outcomes, in order to inform potential intervention targets and survivorship care planning for older survivors as they age.
We used data from the longitudinal Thinking and Living with Cancer (TLC) study of older breast cancer survivors and matched cancer-free controls to address these knowledge gaps.24 In this report we evaluate whether pre-systemic treatment symptom clusters are associated with cognition and QOL outcomes over time. We hypothesized that older survivors with high pre-treatment symptoms would have worse objective and perceived cognitive function, greater functional disability, and poorer breast cancer-specific QOL relative to those with low symptoms and matched non-cancer controls.
Methods
This is a secondary analysis of data from the longitudinal multisite TLC study, which was established to examine cognitive function in older breast cancer survivors.24 Participants included in the current analyses were recruited from five sites from August 2010 to December 2015; recruitment and follow-up are ongoing. The research protocol was registered at ClinicalTrials.gov (#NCT03451383), met Health Insurance Portability and Accountability (HIPAA) standards, and was approved by all institutional review boards (IRBs).
Participants
Participants were aged ≥60 years and fluent in English. Survivors were newly diagnosed with primary non-metastatic breast cancer (stages 0-III). Controls were frequency-matched to survivors based on age (in 5-year groups), race (i.e., White, Black/African American, Hispanic, and Asian American/Pacific Islander), education (i.e., ≤high school, some college+), and site. Participants were excluded if they had a stroke, head injury, major psychiatric disorder or neurodegenerative disorder, prior chemotherapy or hormonal therapy, or treatment for another cancer within the past five years. Those taking psychoactive medications were eligible if the dose was stable for at least two months prior to enrollment.
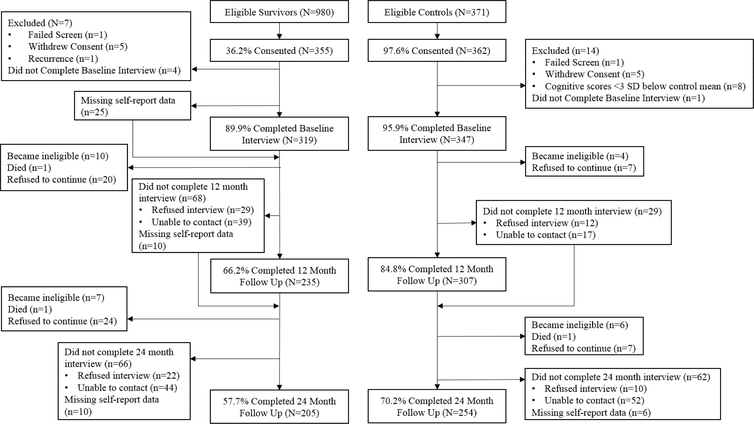
Among those eligible, 36.2% of survivors and 97.6% of controls consented (Figure 1). Survivors’ consent rates varied across sites from 17.2–72.7% (median 62.5%), with the lowest consent rate at a large urban cancer center that had many competing research studies. Participants were screened after informed consent to ensure ability to complete the study and were ineligible if they scored <24 on the Mini-Mental State Examination (MMSE) or <3rd grade on the Wide Range Achievement Test-4th Edition (WRAT-4); one survivor and one control were excluded for these reasons. The analytic sample included 319 breast cancer survivors and 347 controls.
Figure 1.
Study flow chart. Those that did not complete an assessment or have missing self-report data remain eligible to complete the next assessment unless they refuse to continue study participation.
Data Collection
Data collection procedures have been described previously.24 Survivors completed baseline assessment before radiation or systemic therapy and after surgery (except for seven treated with neo-adjuvant therapy). Survivors’ medical records were reviewed for clinical data, including subsequent recurrences. Biospecimens were collected for APOE genotyping.25,26 Follow-up assessments were conducted at 12- and 24-months post-enrollment. Controls were assessed contemporaneously. Assessments included in-person neuropsychological testing and an in-person or telephone interview.
Measures
Outcomes
The battery of neuropsychological assessments used in this study has been previously described.27,28 Two cognitive domains were assessed: attention/processing speed/executive function (APE; six tests: Neuropsychological Assessment Battery [NAB] digits forward, NAB digits backward, Controlled Oral Word Association Test, Trail making A, Trail making B, and Digit Symbol Coding; baseline, 12- and 24-month Cronbach’s α=0.66–0.72)29,30 and learning/memory (LM; five tests: Logical memory I, Logical memory II, NAB list learning immediate recall, NAB list learning short delay, NAB list learning long delay; α=0.85–0.90).27,31 These domains were selected because they are affected in cancer,32 can change over time,33 and are relevant to aging.34 Raw scores from neuropsychological tests were standardized to the means and standard deviations for age and education strata-matched control scores at baseline.28 Domain scores were created from the standardized z-scores for each test.
Self-reported outcomes included: 1) Perceived cognitive function assessed with the Functional Assessment of Cancer Therapy-Cognitive Function35 (FACT-Cog; α=0.95–0.96; scores range from 0–148; minimal clinically important difference (MCID)=7–10 points36); 2) Instrumental Activities of Daily Living (IADL) Disability assessed using the Older Americans Resource Scale37 (OARS; scores range from 1–7; MCID=3 points38); 3) The functional well-being (FWB) subscale of the FACT-B (or FACT-G for controls)39 assessed ability to function, do work, and enjoy activities (α=0.79–0.87; scores range from 6–24; MCID=2–3 points;40 one item addressing sleep was excluded to limit overlap with sleep measure); 4) The Breast Cancer Subscale (BCS) of the FACT-B assessed quality of life (QOL) attributable to breast cancer concerns (α=0.42–0.75; scores range from 9–45; MCID=2–3 points;40 one item addressing pain was excluded to limit overlap with symptom measures).
Predictors
Symptoms of anxiety, depression, fatigue, sleep disturbance, and pain were selected a priori based on prior research.9 Anxiety was assessed with the State-Trait Anxiety Inventory (STAI; α=0.86). Depression was assessed with the Center for Epidemiological Studies-Depression (CES-D) scale, excluding the item on sleep problems (α=0.86). Fatigue was assessed with the FACIT-F (α=0.90). Assessments of sleep problems and pain were adapted from the FACT-B and CES-D. Sleep problems were assessed by participants’ 5-point Likert-scale ratings on two questions: “I have been sleeping well in the past week” and “My sleep was restless in the past week” (α=0.78). Pain was assessed on a 5-point Likert-scale with two questions: “I have had pain in the past week” and “I have had certain parts of my body where I experience pain” (α=0.77). Higher scores on each symptom measure indicated higher symptom levels, and scores were scaled to T-scores based on control means and standard deviations.
Covariates
Covariates were determined a priori. Cancer stage and treatments were collected from medical records. Cancer stage was coded stage 0–1 vs. 2–3. There was little variability in treatment type, dosage, or duration; therefore, treatment was coded as chemotherapy with or without hormone therapy and vs. hormone therapy alone. Demographics included age, education, and race. Self-reported comorbidities were assessed using the OARS comorbidity scale.24 APOE genotype26 was determined by standard single nucleotide polymorphism (SNP) genotyping. APOE genotype status was categorized as carrier (presence of any ε4 allele) vs. non-carrier (no ε4 alleles). The WRAT-4 was used to measure cognitive reserve.41 Study site was also included as a covariate due to the variability in survivors’ consent rates across sites.
Statistical Analyses
Demographic and clinical characteristics of survivors and controls were compared with t and χ2 tests. Latent class analysis was performed in Mplus42 and used to identify groups of survivors with high versus low symptoms. Models were run iteratively and the Vuong-Lo-Mendell-Rubin Likelihood (VLMR) Likelihood ratio test43 was used to determine the number of symptom cluster groups among survivors. Model entropy is also reported, with values above 0.80 indicating a good fitting solution.
Linear mixed-effects models predicting objective cognition, perceived cognition and functional scores over time (APE, LM, FACT-Cog, FACT-FWB, and IADLs) examined differences between symptom and control groups on outcomes and the group-by-time interaction, adjusting for age, race, cognitive reserve, site, comorbidity, and APOE status (any + vs. no ε4). Among survivors, linear mixed-effects models also examined concurrent and longitudinal associations of symptom group with FACT-BCS score and group-by-time interactions, adjusting for covariates plus stage and treatment. Maximum likelihood estimation was employed to use all available data.
Results
Participants ranged in age from 60–98 (Table 1). Survivors and controls were similar in baseline demographics, cognitive reserve, comorbidities, APOE genotype, and objective and perceived cognitive function.
Table 1.
Participant Characteristics
| Characteristic | All Survivors (N=319) | High Symptom (N=51) | Low Symptom (N=268) | Controls (N=347) |
|---|---|---|---|---|
| Completed 12-month follow-up, n (%) | 235 (73.7) | 33 (64.7) | 202 (75.4) | 307 (88.5) |
| Completed 24-month follow-up, n (%) | 205 (64.3) | 29 (56.9) | 176 (65.7) | 254 (73.2) |
| Age, Mean (SD) Race, n (%) | 68.2 (6.1) | 67.5 (5.1) | 68.4 (6.3) | 67.8 (7.0) |
| Othera | 66 (20.7) | 13 (25.5) | 53 (19.8) | 73 (21.0) |
| Non-Hispanic White | 253 (79.3) | 38 (74.5) | 215 (80.2) | 273 (78.7) |
| Years of Education, Mean (SD) | 15.2 (2.2) | 14.9 (2.1) | 15.2 (2.2) | 15.4 (2.3) |
| WRAT-4 Standardized Score, Mean (SD) | 111.2 (15.4) | *107.0 (15.1) | 112.0 (15.4) | 111.8 (16.1) |
| Total Number of Comorbidities, Mean (SD) | 2.6 (1.9) | *3.2 (2.4) | 2.4 (1.8) | 2.4 (1.8) |
| APOE ε4 Carrier, n (%) | 57 (17.9) | 9 (17.6) | 48 (17.9) | 84 (24.2) |
| AJCC Stage, n (%) | ||||
| 0–1 | 213 (66.8) | *26 (51.0) | 187 (69.8) | n/a |
| 2–3 | 105 (32.9) | *24 (47.1) | 81 (30.2) | |
| Surgery, n (%) | ||||
| Lumpectomy | 177 (55.5) | 28 (54.9) | 149 (55.6) | |
| Mastectomy | 140 (43.9) | 23 (45.1) | 117 (43.7) | |
| Cancer Treatment Received, n (%) | ||||
| Hormonal only | 90 (28.2) | *21 (41.2) | 69 (25.7) | |
| Chemotherapy+/-Hormonal | 217 (68.0) | *30 (58.8) | 187 (69.8) | |
| Radiation Treatment, n (%) | 171 (53.6) | 27 (52.9) | 144 (53.7) | |
| Pre-treatment Symptom T-score, Mean (SD) | ||||
| Anxiety | 51.9 (11.6) | *70.4 (14.9) | 48.5 (6.5) | 48.2 (7.8) |
| Depression | 51.8 (11.6) | *73.7 (10.7) | 47.9 (6.0) | 48.3 (7.9) |
| Fatigue | 52.3 (12.2) | *70.7 (18.2) | 48.8 (6.1) | 47.8 (6.6) |
| Sleep Disturbance | 51.6 (10.5) | *61.8 (9.5) | 49.7 (9.5) | 48.5 (9.3) |
| Pain | 50.7 (10.4) | *60.4 (12.2) | 48.9 (8.9) | 49.2 (9.6) |
Significantly different from low symptom group at p<0.05. WRAT-4=Wide Range Achievement Test, 4th edition.
Other race includes Black, Hispanic, and American Indian/Alaska Native. Numbers may not add to 100% due to missing data.
Symptom Clusters in Survivors
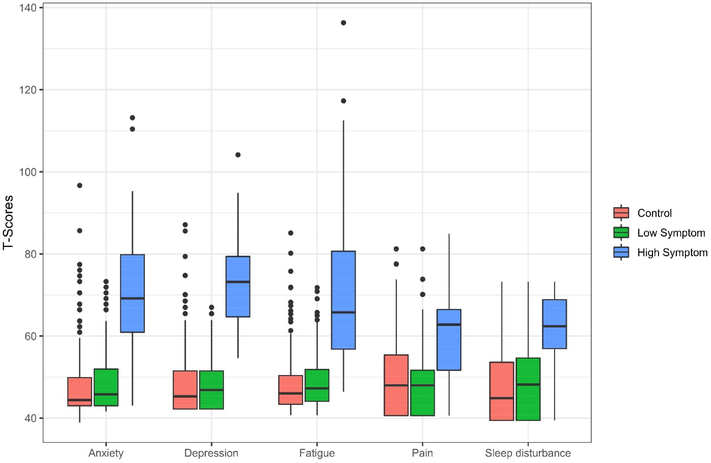
The results of the latent class analysis indicated that a two-group solution fit the data better than a one-group model (VLMR p<0.001, Entropy=0.946). Although the entropy was good for the three-group solution (0.911), the VLMR indicated that it did not provide a statistically better fit to the data (p=0.504). Therefore, the two-group solution was considered as final. The high symptom group included 16% (n=51) and the low symptom group 84% (n=268) of survivors. The symptom groups among survivors were significantly different on all five individual symptoms (p<0.05); however, the low symptom group had similar symptom T-scores to controls (Figure 2). Compared to the low symptom group, the high symptom group had lower average WRAT-4 scores, greater total comorbidities, was more likely to have later stage disease, and was less likely to receive chemotherapy with or without hormone therapy (Table 1) (all p<0.05).
Figure 2.
Distributions of T-scores for symptoms by symptom group or control.
Cognition Outcomes
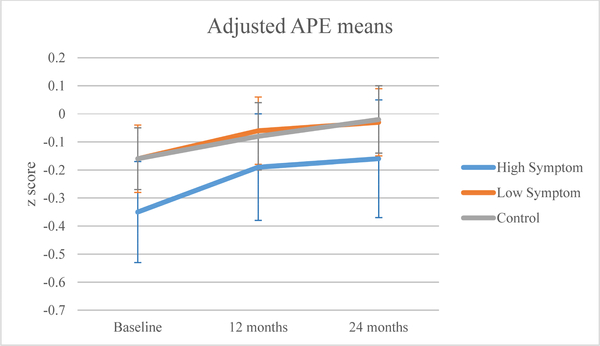
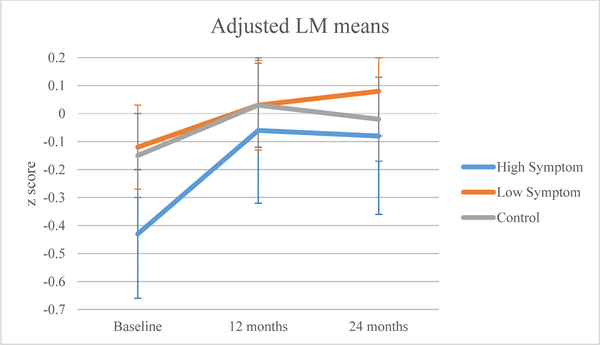
The high symptom group had statistically lower baseline APE (p=0.025) and LM (p=0.018) scores than the control group (Table 2). All groups showed improvement in neuropsychological scores over time (APE p<0.0001; LM p<0.0001; Figures 3a, 3b). There were no significant symptom cluster group-by-time interactions.
Table 2.
Associations with Cognitive and Functional Outcomes in Models with Survivors and Controls
| APE | LM | FACT-Cog | FACT-FWB | OARS-IADLs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | β | SE | β | SE | β | SE | β | SE | β | SE |
| Intercept | 0.14 | 0.27 | 0.11 | 0.35 | 126.62*** | 7.93 | 15.71*** | 1.48 | −1.34*** | 0.35 |
| Group - High Symptom | −0.20* | 0.09 | −0.27* | 0.11 | −15.28*** | 2.69 | −6.66*** | 0.55 | 0.95*** | 0.14 |
| Group - Low Symptom | 0.00 | 0.05 | 0.03 | 0.06 | 1.01 | 1.42 | −0.82** | 0.29 | 0.11 | 0.07 |
| Time - 12 month | 0.07*** | 0.02 | 0.18*** | 0.03 | −0.05 | 0.88 | −0.21 | 0.23 | 0.04 | 0.04 |
| Time - 24 month | 0.13*** | 0.02 | 0.14*** | 0.04 | −0.36 | 0.91 | −0.18 | 0.25 | 0.10 | 0.05 |
| GroupXTime - High | ||||||||||
| Symptom, 12 month | 0.09 | 0.07 | 0.19 | 0.10 | 7.75** | 2.84 | 4.13*** | 0.74 | −0.57*** | 0.14 |
| GroupXTime - High | ||||||||||
| Symptom, 24 month | 0.06 | 0.08 | 0.21 | 0.11 | 3.11 | 2.87 | 5.25*** | 0.79 | −0.79*** | 0.16 |
| GroupXTime - Low | ||||||||||
| Symptom, 12 month | 0.03 | 0.03 | −0.03 | 0.05 | −2.74* | 1.39 | 0.92* | 0.36 | −0.10 | 0.07 |
| GroupXTime - Low | ||||||||||
| Symptom, 24 month | 0.00 | 0.04 | 0.07 | 0.06 | −2.13 | 1.43 | 0.98* | 0.39 | −0.13 | 0.08 |
| Age | −0.02*** | 0.00 | −0.03*** | 0.00 | −0.03 | 0.10 | 0.01 | 0.02 | 0.02*** | 0.00 |
| Race - other race | −0.35*** | 0.06 | −0.25*** | 0.07 | 0.79 | 1.63 | 0.35 | 0.30 | 0.02 | 0.07 |
| Cognitive Reserve | 0.01*** | 0.00 | 0.02*** | 0.00 | 0.08 | 0.04 | 0.01 | 0.01 | 0.00 | 0.00 |
| Comorbidity | −0.03** | 0.01 | 0.01 | 0.02 | −1.57*** | 0.35 | −0.36*** | 0.06 | 0.10*** | 0.01 |
p<0.05
p<0.01
p<0.001
Reference groups: control for group, baseline for time, White for race, ε4 negative for APOE. The models also controlled for study site. Estimates for each group by time interaction represent the average difference in rate of change for the respective symptom groups relative to the control group after adjusting for covariates.
Figure 3a.
Mean attention, processing speed, and executive function (APE) domain z-scores by symptom or control group, after adjusting for covariates.
Figure 3b.
Adjusted mean learning and memory (LM) domain z-scores by symptom or control group.
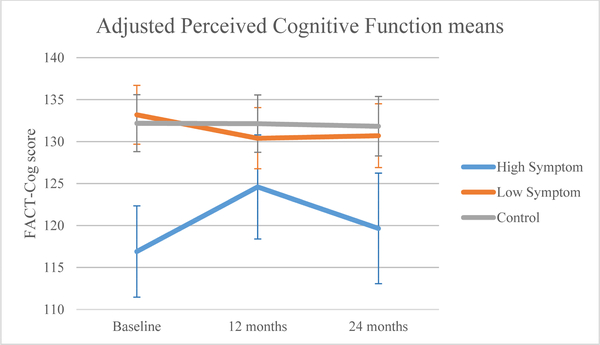
Perceived cognition (FACT-Cog) was similar at baseline between the low symptom and control groups, but the high symptom group had statistically and clinically meaningful worse baseline perceived cognitive scores than controls (high: 116.91, controls: 132.19, p<0.0001; Figure 3c). There was also a significant group-by-time interaction (p=0.007), with perceived cognition scores worsening from baseline to 12 months for the low symptom group (p=0.049), and improving in the high symptom group (p=0.007). Despite improvement for the high symptom group, 95% confidence intervals (CI) of adjusted means (Table 3) showed that the high symptom group reported statistically and clinically worse perceived cognition than the control group at 12 months (high: 124.60, controls: 132.14, p<0.05) and both groups at 24 months (high: 119.66, low: 130.70, controls: 131.83, p<0.05).
Figure 3c.
Adjusted mean perceived cognitive function by symptom or control group.
Table 3.
Adjusted Least Squares Means for Cognitive and Quality of Life Outcomes
| High Symptom Group | Low Symptom Group | Controls | |
|---|---|---|---|
| Outcome | |||
| Time point | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| APE scorea | |||
| Baseline | −0.35 (-0.53,-0.18) | −0.16 (-0.28,-0.04) | −0.16 (-0.27,-0.04) |
| 12 months | −0.19 (-0.38,0.00) | −0.06 (-0.18,0.07) | −0.08 (-0.20,0.03) |
| 24 months | −0.16 (-0.37,0.05) | −0.03 (-0.15,0.10) | −0.02 (-0.14,0.10) |
| LM scorea | |||
| Baseline | −0.43 (-0.66,-0.19) | −0.12 (-0.27,0.03) | −0.15 (-0.30,-0.01) |
| 12 months | −0.06 (-0.32,0.21) | 0.03 (-0.13,0.19) | 0.03 (-0.12,0.18) |
| 24 months | −0.08 (-0.36,0.20) | 0.08 (-0.08,0.24) | −0.02 (-0.17,0.13) |
| Perceived cognitive functionb | |||
| Baseline | 116.91 (111.47,122.34) | 133.19 (129.69,136.70) | 132.19 (128.80,135.58) |
| 12 months | 124.60 (118.40,130.80) | 130.40 (126.76,134.04) | 132.14 (128.72,135.55) |
| 24 months | 119.66 (113.08,126.24) | 130.70 (126.90,134.50) | 131.83 (128.29,135.36) |
| Functional well-beingc | |||
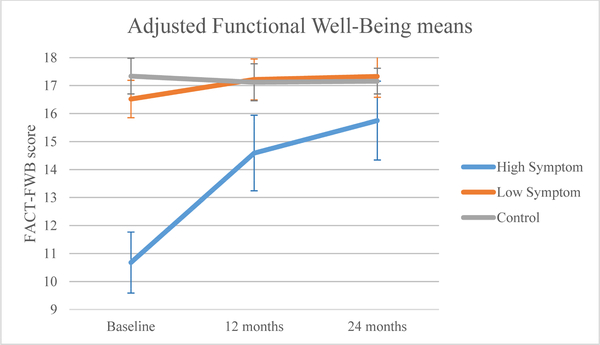
| Baseline | 10.68 (9.59,11.76) | 16.52 (15.85,17.19) | 17.34 (16.70,17.98) |
| 12 months | 14.59 (13.23,15.95) | 17.22 (16.49,17.95) | 17.12 (16.46,17.79) |
| 24 months | 15.75 (14.34,17.16) | 17.33 (16.58,18.08) | 17.16 (16.48,17.85) |
| IADLsd | |||
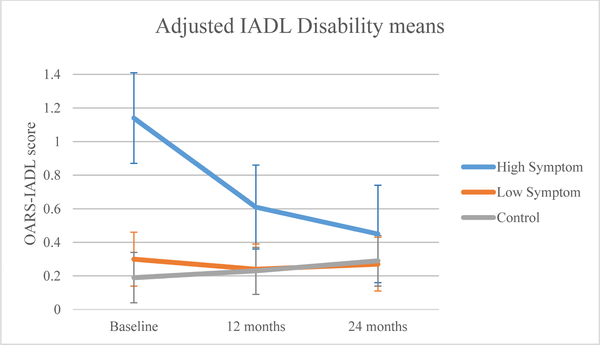
| Baseline | 1.14 (0.87,1.41) | 0.30 (0.14,0.46) | 0.19 (0.04,0.34) |
| 12 months | 0.61 (0.36,0.86) | 0.24 (0.09,0.39) | 0.23 (0.09,0.37) |
| 24 months | 0.45 (0.16,0.75) | 0.27 (0.11,0.43) | 0.29 (0.14,0.45) |
| QOL - breast cancer concernse | |||
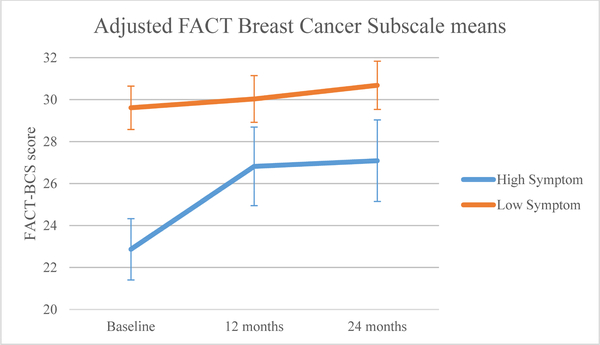
| Baseline | 22.87 (21.41,24.33) | 29.61 (28.58,30.64) | |
| 12 months | 26.82 (24.95,28.69) | 30.03 (28.92,31.14) | n/a |
| 24 months | 27.09 (25.15,29.02) | 30.68 (29.53,31.83) | |
APE=Attention, processing speed, and executive function; LM=Learning and memory; z-scores standardized to the baseline scores of age- and education-matched controls.
Based on FACT-Cog; scores range from 0–148; higher scores indicate better cognition; MCID=7–10 points.
Based on FACT Functional Well-Being subscale without the question on sleep; scores range from 6–24; higher scores indicate better QOL; MCID=2–3 points.
Based on OARS-IADLs score; scores range from 1–7; higher scores indicate greater IADL disability; MCID=3 points.
Based on FACT-Breast Cancer Subscale without the question on pain; scores range from 9–45; higher scores indicate better QOL; MCID=2–3 points.
All results are adjusted for age, race, WRAT score, recruitment site, comorbidity, and APOE status. Results for FACT-Breast Cancer Subscale are also adjusted for stage of disease and receipt of chemotherapy.
Functional Outcomes
For functional well-being QOL (FACT-FWB; Figure 4a), controls exhibited significantly better scores at baseline than the low (p=0.005) and high symptom groups (p<0.0001). All groups showed improvement over time (p<0.0001). The group-by-time interaction was statistically significant (p<0.0001); controls maintained functional well-being QOL over time, and there was improvement from baseline to 12 and 24 months for the low (12 months: p=0.012; 24 months: p=0.012) and high (12 months: p<0.0001; 24 months: p<0.0001) symptom groups. Despite this improvement, scores remained statistically and clinically worse for the high compared to the low symptom group and controls at 12 months (high: 14.59, low: 17.22, controls: 17.12, p<0.05), but the statistically significant difference was not clinically significant at 24 months (high: 15.75, low: 17.33, controls: 17.16, p<0.05).
Figure 4a.
Adjusted mean functional well-being by symptom or control group.
At baseline, controls and the low symptom group had similar IADL disability scores to each other, but the high symptom group had greater IADL disability (p<0.0001; Figure 4b). All groups showed improvement over time (p<0.001). The group-by-time interaction was statistically significant (p<0.0001), with the high symptom group showing greater improvement in IADL disability from baseline at 12 (p<0.0001) and 24 months (p<0.0001) than the low symptom group and controls. Despite this improvement, the high symptom group did not differ from the low symptom group and controls at 12 (high: 0.61, low: 0.24, controls: 0.23, p>0.05) or 24 months (high: 0.45, low: 0.27, controls: 0.29, p>0.05).
Figure 4b.
Adjusted mean instrumental activities of daily living (IADL) disability by symptom or control group.
Breast Cancer-Specific QOL
The high symptom group exhibited statistically and clinically meaningful worse baseline breast cancer QOL than the low symptom group (Table 4; Figure 4c). Both groups significantly improved over time (p<0.0001). The group-by-time interaction was significant (p<0.0001). Relative to the low symptom group, the high symptom group showed significantly improved scores from baseline to 12 months (p=0.001) and 24 months (p=0.002). However, the high symptom group remained statistically and clinically lower than the low symptom group at baseline (high: 22.87, low: 29.61, p<0.05), 12 (high: 26.82, low: 30.03, p<0.05), and 24 months (high: 27.09, low: 30.68, p<0.05).
Table 4.
Associations of Symptom Cluster with Survivors’ Breast Cancer-Specific QOL
| FACT-BCS |
||
|---|---|---|
| Effect | β | SE |
| Intercept | 23.90*** | 3.02 |
| Group - High Symptom (vs. low) | −6.74*** | 0.72 |
| Time - 12 month | 0.43 | 0.36 |
| Time - 24 month | 1.07** | 0.39 |
| GroupXTime - High Symptom, 12 month | 3.53*** | 0.97 |
| GroupXTime - High Symptom, 24 month | 3.15** | 1.05 |
| Age | 0.10* | 0.04 |
| Race - other race | −0.25 | 0.59 |
| Cognitive Reserve (WRAT score) | 0.00 | 0.02 |
| Comorbidity | −0.58*** | 0.13 |
| APOE - ε4 positive | 0.75 | 0.57 |
| Stage - Stage 2–3 | −0.52 | 0.51 |
| Treatment - Hormone only | 0.07 | 0.55 |
p<0.05
p<0.01
p<0.001
Reference groups: low symptom for group, baseline for time, White for race, ε4 negative for APOE, stage 0–1 for stage, chemotherapy with or without hormone therapy for treatment. The model also controlled for study site. Estimates for each group by time interaction represent the average difference in rate of change for the high symptom group relative to the low symptom group after adjusting for covariates.
Figure 4c.
Adjusted mean breast cancer quality of life by symptom group.
Discussion
This study is among the first to examine the relationship between pre-treatment symptom clusters and longitudinal cognitive and functional outcomes over two years in a large prospective study of older breast cancer survivors and matched cancer-free controls. Nearly one-fifth of older breast cancer survivors reported high symptoms before systemic therapy. Despite some improvement over time, high pre-treatment symptoms were significantly associated with persistently worse perceived cognition and breast cancer specific QOL scores. Additionally, the high symptom group had lower objective cognition at baseline than non-cancer controls. In contrast, the low symptom group appeared similar to matched non-cancer controls on all outcomes across time.
Our findings for older breast cancer survivors converge with prior research showing that a subgroup of survivors report initial high levels of anxiety, depression, fatigue, sleep disturbance, and pain.9 Prior data also support a relationship between high (vs. low) symptoms and worse QOL and functional status, with similar effect sizes as seen in our sample.14–17 The number of survivors in the high symptom group is also similar to prior research (14–28%) in survivors from diverse age groups.16,17,44,45 Although we expected older survivors to experience greater pre-treatment symptoms due to aging, prior findings indicate that younger survivors are more likely than older survivors to report high symptoms.16,45 Potentially, older survivors may generally report low levels of symptoms due to a shift in their pattern of responding as they become accustomed to age-related physical ailments.46
Specific symptoms of anxiety, depression, fatigue, and sleep disturbance have each been independently associated with worse perceived cognition in cancer samples. 7,47,48 Research on symptom clusters in cancer occasionally includes cognitive complaints in the cluster because they often co-occur.10,14 However, poor performance on objective tests of cognitive function is inconsistently related to these symptoms.49,50 Our findings confirm that these symptoms and self-reported cognition are associated. The lack of overall effect for objective cognitive function may reflect the very subtle changes in these measures over time. Perceived cognitive function, QOL, and functional status remain clinically important and should be assessed early in care processes51 because, beyond QOL and cognition, they predict treatment toxicity and mortality.52
Our findings have clinical implications for geriatric assessment and survivorship interventions. Assessment of pre-treatment symptomatology as part of a comprehensive geriatric assessment may help clinicians identify older cancer survivors at risk for functional impairment and worse QOL. Although our results indicate that those in the high symptom group gradually improved over time, lower perceived cognition and QOL persisted over two years in the high symptom group. In contrast, survivors in the low symptom group appear similar to women without cancer on all outcomes. The ability to distinguish older survivors who are at greatest risk prior to treatment from those similar to cancer-free older adults should help providers determine which patients are most in need of support. Symptoms at diagnosis may be a promising method to help providers identify this potentially higher-risk older survivor group, and make early referrals for interventions.
Initial evidence suggests that cognitive-behavioral therapy (CBT) can reduce severity of symptoms such as depression, fatigue, and insomnia; CBT and pharmacological treatment both may be effective for treating mood, adjustment, and sleep disorders, and in turn, improve cancer survivors’ QOL and functional status.53–55 Other behavioral interventions may also be effective in lowering symptoms, including yoga, meditation, and journaling.56,57 Therefore, providers may also consider behavioral interventions to mitigate symptomatology.
Limitations of the current study should be considered. Although the sample of survivors was ethnically and racially representative of older cancer survivors in the United States, the sample was well-educated and recruited mainly from academic cancer centers and their affiliated community sites. Survivors’ consent rates also differed across study sites. Therefore, results may not generalize to broadly representative survivor groups. Although the sample was large, the small subgroup of survivors with high symptoms may have limited power to detect some relationships with outcomes. Attrition may also have limited power. Additionally, assessment of symptoms, perceived cognition, IADL disability, and quality of life was self-reported, and any systematic error or bias in responses could have affected the magnitude and directions of the observed relationships. However, the consistency of associations between symptoms and several diverse types of outcomes suggests that results are robust. Measures of sleep disturbance and pain were not validated measures, although they showed good internal consistency; and the other symptoms were from well validated, reliable scales. Finally, differences in IADLs at baseline did not appear to be clinically meaningful according to current benchmarks for older adults; however, little is known regarding clinically meaningful differences in subtle IADL difficulties for older breast cancer survivors.
Overall, nearly one-fifth of older breast cancer survivors in this study experienced high symptoms prior to systemic cancer treatment, and being in the high symptom group predicted poor outcomes. Identifying older breast cancer survivors who are at risk for cognitive complaints and poor QOL has implications for geriatric assessment and referral for interventions to support symptom management and mental health.
Acknowledgments
Disclosures: This work was supported by the National Institutes of Health [R01CA129769, R35CA197289, P30CA51008, T32CA117865, F31CA220964, P30AG10133, R01AG19771, and R01LM011360]; American Cancer Society [IRG-92–152]. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: A.D.: Cardinal Health (Advisory board). M.E.: GTx (research support). A.H.: Seattle Genetics, Amgen Pharmaceuticals, and Genentech (consultation); Glaxo Smith Kline, Abraxis Bioscience, and Celgene (research support). C.I.: Genentech, Pfizer, and AstraZeneca (consulting and speakers bureau); Novartis and Nanostring (consulting). HJ: RedHill Biopharma, Janssen Scientific Affairs (consulting). All remaining authors have declared no conflicts of interest.
ClinicalTrials.gov Identifier: NCT03451383
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst . 2014;106:1–8. doi: 10.1093/jnci/dju129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer . 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroenke K, Johns SA, Theobald D, Wu J, Tu W. Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Support Care Cancer . 2013;21:765–773. doi: 10.1007/s00520-012-1578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piper BF, Cella D. Cancer-related fatigue: Definitions and clinical subtypes. J Natl Compr Canc Netw . 2010;8:958–966. doi: 10.6004/jnccn.2010.0070 [DOI] [PubMed] [Google Scholar]
- 5. Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol . 2018. doi: 10.1200/jco.2018.78.6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon DE, Cohen R, Chen H, et al. The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: A 2-year longitudinal study. J Cancer Res Clin Oncol . 2016;142:1461–1474. doi: 10.1007/s00432-016-2163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol . 2017;35:506–514. doi: 10.1200/JCO.2016.68.5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Extermann M, Leeuwenburgh C, Samiian L, et al. Impact of chemotherapy on medium-term physical function and activity of older breast cancer survivors, and associated biomarkers. J Geriatr Oncol . 2017;8:69–75. doi: 10.1016/j.jgo.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H-J, Barsevick AM, Beck SL, Dudley W. Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: A secondary analysis. Oncol Nurs Forum . 2012;39:E20-E30. doi: 10.1188/12.ONF.E20-E30 [DOI] [PubMed] [Google Scholar]
- 10.Kim H-J, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs . 2012;35:E1-E20. doi: 10.1097/NCC.0b013e318233a811 [DOI] [PubMed] [Google Scholar]
- 11.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun . 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doong S-H, Dhruva A, Dunn LB, et al. Associations Between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs . 2015;17:237–247. doi: 10.1177/1099800414550394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland CS, Mayer M, Dreyer NA, et al. Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: Results from a substudy of the VIRGO observational cohort study. Breast . 2014;23:763–769. doi: 10.1016/j.breast.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Starkweather AR, Lyon DE, Elswick RJ, Montpetit AJ, Conley Y, McCain NL. A conceptual model of psychoneurological symptom cluster variation in women with breast cancer: Bringing nursing research to personalized medicine. Curr Pharmacogenomics Person Med . 2013;11:224–230. doi: 10.2174/18756921113119990004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs . 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas BC, Waller A, Malhi RL, et al. A longitudinal analysis of symptom clusters in cancer patients and their sociodemographic predictors. J Pain Symptom Manage . 2014;47:566–578. doi: 10.1016/j.jpainsymman.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Trudel-Fitzgerald C, Savard J, Ivers H. Longitudinal changes in clusters of cancer patients over an 18-month period. Health Psychol . 2014;33:1012–1022. doi: 10.1037/a0033497 [DOI] [PubMed] [Google Scholar]
- 18.Myers JS, Koleck TA, Sereika SM, Conley YP, Bender CM. Perceived cognitive function for breast cancer survivors: Association of genetic and behaviorally related variables for inflammation. Support Care Cancer . 2017;25:2475–2484. doi: 10.1007/s00520-017-3654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers JS. Proinflammatory cytokines and sickness behavior: Implications for depression and cancer-related symptoms. Oncol Nurs Forum . 2008;35:802–807. doi: 10.1188/08.Onf.802-807 [DOI] [PubMed] [Google Scholar]
- 20.Numakawa T, Richards M, Nakajima S, et al. The role of brain-derived neurotrophic factor in comorbid depression: Possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry . 2014;5:1–12. doi: 10.3389/fpsyt.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tometich DB, Mosher CE, Hirsh AT, et al. Metastatic breast cancer patients’ expectations and priorities for symptom improvement. Support Care Cancer . 2018;26:3781–3788. doi: 10.1007/s00520-018-4244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champion VL, Wagner LI, Monahan PO, et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer . 2014;120:2237–2246. doi: doi: 10.1002/cncr.28737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017 . American Cancer Society; Atlanta: 2016. [Google Scholar]
- 24.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol . 2014;32:1909–1918. doi: 10.1200/JCO.2013.54.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koleck TA, Bender CM, Sereika SM, et al. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum . 2014;41:313–325. doi: 10.1188/14.onf.e313-e325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology . 2003;12:612–619. doi: 10.1002/pon.742 [DOI] [PubMed] [Google Scholar]
- 27.Stern RA, White T. NAB, Neuropsychological Assessment Battery: Administration, scoring, and interpretation manual . Lutz, FL: Psychological Assessment Resources (PAR); 2003. [Google Scholar]
- 28.Clapp JD, Luta G, Small BJ, et al. The impact of using different reference populations on measurement of breast cancer-related cognitive impairment rates. Arch Clin Neuropsychol . 2018. doi: 10.1093/arclin/acx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaton R, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults . Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 30.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo's older Americans normative studies: Age-and IQ-adjusted norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clin Neuropsychol . 2005;19:329–377. doi: 10.1080/13854040590945210 [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D Wechsler Memory Scale - Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 32.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry . 2014;26:102–113. doi: 10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: New evidence from neuroimaging of the aging brain. Curr Opin Neurol . 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48 [DOI] [PubMed] [Google Scholar]
- 34.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat . 2010;123:819–828. doi: 10.1007/s10549-010-1088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner LI, Sweet J, Butt Z, Lai J-s, Cella D. Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol . 2009;7:W32-W39. [Google Scholar]
- 36.Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol . 2014;67:811–820. doi: 10.1016/j.jclinepi.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 37.George LK, Fillenbaum GG. OARS Methodology. J Am Geriatr Soc . 1985;33:607–615. doi: 10.1111/j.1532-5415.1985.tb06317.x [DOI] [PubMed] [Google Scholar]
- 38.Abdulaziz KE, Brehaut J, Taljaard M, et al. National survey of family physicians to define functional decline in elderly patients with minor trauma. BMC Fam Pract . 2016;17:1–7. doi: 10.1186/s12875-016-0520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol . 1997;15:974–986. doi: 0732-183X/97/1503-0050$3.00/0 [DOI] [PubMed] [Google Scholar]
- 40.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes . 2003;1:79–79. doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manly JJ, Schupf N, Tang M- X, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol . 2005;18:213–217. doi: 10.1177/0891988705281868 [DOI] [PubMed] [Google Scholar]
- 42.Muthén BO, Muthén LK. Mplus Users Guide (v. 7) . Los Angeles, CA: Muthén & Muthén; 2014. [Google Scholar]
- 43.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika . 2001;88:767–778. doi: 10.1093/biomet/88.3.767 [DOI] [Google Scholar]
- 44.Astrup GL, Hofsø K, Bjordal K, et al. Patient factors and quality of life outcomes differ among four subgroups of oncology patients based on symptom occurrence. Acta Oncol . 2017;56:462–470. doi: 10.1080/0284186X.2016.1273546 [DOI] [PubMed] [Google Scholar]
- 45.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer . 2014;120:2371–2378. doi: 10.1002/cncr.28699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprangers MAG, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol . 1999;10:747–749. doi: 10.1023/A:1008305523548 [DOI] [PubMed] [Google Scholar]
- 47.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: Examining the relationship with neuropsychological test performance. J Natl Cancer Inst . 2013;105:791–801. doi: 10.1093/jnci/djt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower JE. Behavioral symptoms in breast cancer patients and survivors: Fatigue, insomnia, depression, and cognitive disturbance. J Clin Oncol . 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ono M, Ogilvie JM, Wilson JS, et al. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol . 2015;5:1–19. doi: 10.3389/fonc.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debess J, Riis JØ, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Res Treat . 2010;121:91–100. doi: 10.1007/s10549-010-0756-8 [DOI] [PubMed] [Google Scholar]
- 51.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol . 2016;7:242–248. doi: 10.1016/j.jgo.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol . 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jassim GA, Whitford DL, Hickey A, Carter B. Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst Rev . 2015(5):Cd008729. doi: 10.1002/14651858.CD008729.pub2 [DOI] [PubMed] [Google Scholar]
- 54.Carvalho AF, Hyphantis T, Sales PM, et al. Major depressive disorder in breast cancer: A critical systematic review of pharmacological and psychotherapeutic clinical trials. Cancer Treat Rev . 2014;40:349–355. doi: 10.1016/j.ctrv.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 55.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev . 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 56.Duncan M, Moschopoulou E, Herrington E, et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open . 2017;7:e015860. doi: 10.1136/bmjopen-2017-015860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garland SN, Rouleau CR, Campbell T, Samuels C, Carlson LE. The comparative impact of Mindfulness-Based Cancer Recovery (MBCR) and Cognitive Behavior Therapy for Insomnia (CBT-I) on sleep and mindfulness in cancer patients. Explore (NY) . 2015;11:445–454. doi: 10.1016/j.explore.2015.08.004 [DOI] [PubMed] [Google Scholar]