Abstract
Stress granule (SG)-like antiviral granules (AVG) had been found in some vaccinia virus infection conditions and shown to repress translation. Similar RNA granules are also associated with translational inhibition and poxvirus restriction mediated by the host restriction factor SAMD9, but their function is less clear. We studied the composition of these RNA granules by immunofluorescence and found them enriched with SG component TIA1 and viral dsRNA binding protein E3. However, TIA1 was not required for granule formation or SAMD9-mediated poxvirus restriction, in contrast to its critical role in SG formation and AVG function. The granule formation was abolished by blocking viral DNA replication or intermediate viral gene transcription, suggesting that post-replicative viral mRNA was important for granule formation. Our data show that TIA1 is not universally antiviral against poxviruses and support a model that the RNA granules are formed as the result of untranslated mRNA accumulation in viral DNA factories.
Keywords: poxvirus, vaccinia virus, stress granule, host range gene, host restriction factor, TIA1
Introduction
A variety of environmental stresses, including viral infection, can trigger translational arrest in eukaryotic cells, leading to the formation of discrete cytoplasmic foci known as stress granules (SGs) (Anderson and Kedersha, 2008). SGs contain stalled messenger ribonucleoproteins (mRNPs), playing an important role in translational control (Ivanov et al., 2018). The condensation of mRNPs into SGs is mediated by specific RNA binding proteins, including TIA1 (cytotoxic granule associated RNA binding protein) and G3BP1 (G3BP stress granule assembly factor 1). TIA1 (Gilks et al., 2004) and G3BP1 (Tourriere et al., 2003) are crucial components of SG and serve as markers for SG detection by immunofluorescence microscopy. TIA1 can also function as a translational silencer (Piecyk et al., 2000). SGs have been linked to innate immunity against viruses, and some RNA viruses are known to target specific SG components (McCormick and Khaperskyy, 2017; Tsai and Lloyd, 2014). TIA1 was also suggested to have direct antiviral activity, as the replication of several RNA and DNA viruses enhanced in MEFs (mouse embryo fibroblasts) lacking TIA1 (Li et al., 2002; Simpson-Holley et al., 2011).
Poxviruses, a family of DNA viruses that replicate in the cytoplasm, were found to induce the formation of SG-like RNA granules in infected cells, especially when their replications were crippled by the removal of viral inhibitors of innate immunity (Simpson-Holley et al., 2011). In cells infected with a vaccinia virus (VACV) mutant with deletion in the double-stranded RNA (dsRNA) binding protein E3 (vΔE3), stable RNA granules enriched with TIA1 and G3BP1 accumulated around viral DNA factories. These RNA granules demonstrated antiviral effect and required TIA1 for their function, and the replication of vΔE3 was enhanced in MEFs lacking TIA1 (Simpson-Holley et al., 2011). These RNA granules also formed in wild-type (WT) VACV-infected cells with lower frequency and shown to repress viral protein synthesis (Rozelle et al., 2014). Similar RNA granules had also been found to be associated with host restriction of poxvirus replication mediated by SAMD9 (Liu and McFadden, 2015). SAMD9 (Liu et al., 2011) and its paralog, SAMD9L (Meng et al., 2018), are host restriction factors against poxviruses and could be antagonized by two distinct classes of poxvirus inhibitors, represented by K1 and C7 of VACV. K1 is encoded only by orthopoxviruses, whereas functional C7 homologs are encoded by nearly all mammalian poxviruses (Meng et al., 2008), including myxoma virus (MYXV). VACV mutant with deletion in both K1 and C7 (vK1−C7−) or MYXV mutant with deletion in a C7 homolog, M062, replicated abortively in many mammalian cells and were highly attenuated in animal models (Liu et al., 2011; Meng et al., 2018). SAMD9 restricted vK1−C7− replication through inhibition of viral protein synthesis, albeit the mechanism of translational inhibition is unknown (Sivan et al., 2018). In cells infected by either vK1−C7− or M062-deletion MYXV, RNA granules enriched with G3BP1 accumulated in and around viral DNA factories (Liu and McFadden, 2015). However, G3BP1 and G3BP2 proteins are not required for either granule formation or host restriction of vK1−C7− (Sivan et al., 2018). To better understand the role of these RNA granules in poxvirus restriction, we further characterized the composition of the RNA granules associated with SAMD9-mediated poxvirus restriction and studied factors that could be critical for the granule formation.
Material and Methods
Cells, viruses and antibodies.
VERO (ATCC CCL-81), HeLa 229 (ATCC CCL-2.1), RK13-A8/23 cells (Warren et al., 2012) (kindly provided by Dr. Bernard Moss) were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum (FBS). The culture medium for RK13-A8/23 cells was supplemented with 300 μg/ml Zeocin. WT WR strain of VACV and vK1−C7− (Meng et al., 2008) were propagated in VERO cells. A8 and A23 deletion VACV (vA8Δ and vA23Δ) (Warren et al., 2012), kindly provided by Dr. Bernard Moss, were propagated in RK13-A8/23 cells. The antibodies used for immunofluorescence and Western blot analysis were: goat anti- TIA1 (Santa Cruz, sc-1751), J2 mouse anti-dsRNA (Scicons), mouse anti-G3BP1 (BD Transduction Laboratory, 611126), rabbit anti-SAMD9 (Sigma-Aldrich, HPA021319), rabbit polyclonal anti-E3 (kindly provided by Dr. Jingxin Cao) (Dueck et al., 2015), and TW2.3 mouse monoclonal anti-E3 (kindly provided by Dr. Bernard Moss) (Yuwen et al., 1993).
Fluorescent microscopy.
HeLa cells grown on coverslips were infected at 0.5 PFU/cell. In some infection, cytosine arabinoside (AraC) was added at 40 μg/ml to inhibit DNA replication. At different time post infection, the cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.2% Triton X-100 for 5 min, blocked with 10% FBS for 60 min, and stained with various antibodies for 1 h and an appropriate secondary antibody for an additional hour. The DNA was stained with DAPI (Invitrogen). Coverslips were imaged with an Olympus IX-81 fluorescence microscope.
Generation of TIA1 knockout cells.
The plasmid used for TIA1 knockout was constructed from lentiCRISPRv2 (a gift from Feng Zhang, Addgene plasmid #52961) according to the published protocol (Sanjana et al., 2014). In brief, lentiCRISPRv2 was digested with BsmBI and ligated with a pair of oligonucleotides with the specific guide sequence for TIA1 (5’-ATGAAACTCCACAAAACAAT-3’). The lentiCRISPRv2-derived plasmid was used in making lentiviruses in 293 FT cells for transduction of HeLa cells. 24 hr after transduction, the cells were subjected to puromycin selection for 7 days. Clones of the knockout cells were isolated and validated by Western blotting against TIA1 and genotyping for TIA1, similarly as described (Meng et al., 2018).
Virus yield determination.
HeLa cells in 12-well tissue culture plate were infected at a MOI of 1 PFU per cell. After 1 h of adsorption at room temperature, the inoculum was removed and the cells were washed with DMEM plus 1% FBS. At various times after infection, cells were harvested and viral titers in the cell lysates were determined by plaque assay in VERO cells.
Western blot analysis
The Western blot analysis was performed as previously described (Meng and Xiang, 2006). Briefly, the samples were solubilized in sodium dodecyl sulfate (SDS) sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes and blocked with Tris-buffered saline supplemented with 5% nonfat dried milk and 0.05% Tween-20 for 1 h at room temperature. Subsequently, membranes were incubated with the antibodies and analyzed with chemiluminescence reagent (Pierce).
Results
RNA granules associated with SAMD9-mediated poxvirus restriction are similar to antiviral granules in composition.
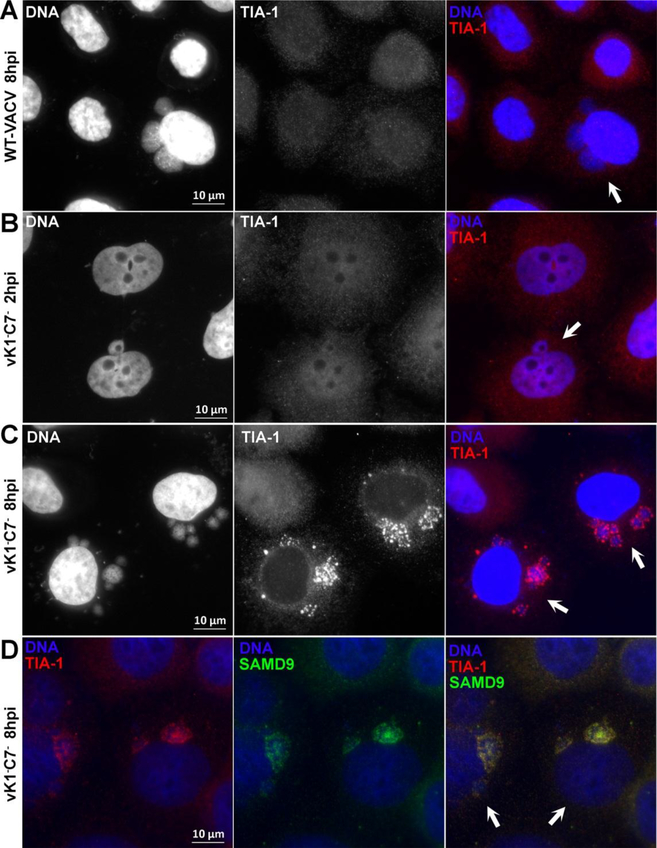
Previous studies had identified some components of the RNA granules associated with SAMD9-mediated poxvirus restriction (Liu and McFadden, 2015; Sivan et al., 2018). We performed a similar study of the RNA granules with immunofluorescence analysis and confirmed that the granules contained SAMD9, G3BP1 and dsRNA (data not shown). Since TIA1 has been shown to be crucial for SG formation and the function of the antiviral granules against VACV, we further studied whether TIA1 is also part of the RNA granules that are associated with SAMD9-mediated poxvirus restriction. We infected HeLa cells with WT VACV or vK1−C7− and examined the cellular localization of TIA1 at different time post infection. A low MOI was used, so both uninfected cells and infected cells with distinctive cytoplasmic DNA factories could be analyzed together in the same field of view. In uninfected or WT VACV-infected cells, TIA1 distributed diffusely throughout the cells (Fig. 1A). In vK1−C7−-infected cells, TIA1 had a similarly diffusive distribution at 2 h post infection (Fig. 1B), but it had aggregated into large granules within or surrounding the viral DNA factories by 8 h post infection (Fig. 1C). Staining with antibodies against both TIA1 and SAMD9 showed that TIA1 colocalized with SAMD9 in the granules (Fig. 1D), indicating that TIA1 is a component of the RNA granules associated with SAMD9-mediated poxvirus restriction.
Figure 1. TIA1 is enriched in RNA granules that are associated with SAMD9-mediated poxvirus restriction.
HeLa cells were infected with vK1−C7− or WT VACV at a MOI of 0.5 PFU/cell. At the indicated time (2 or 8 h post infection), the infected cells were fixed, permeabilized, and stained with primary antibodies (goat anti-TIA1 or/and rabbit anti-SAMD9), followed by DAPI and secondary antibodies (donkey anti-goat IgG coupled to Alexa Fluor 594 or/and goat anti-rabbit IgG coupled to Alexa Fluor 488). The fluorescence signals from DAPI and TIA1 are shown in white in the first two column from A-C. The arrows point to infected cells. Note uninfected cells were included in the same field of view.
E3 co-localizes with dsRNA in granules associated with SAMD9-mediated poxvirus restriction.
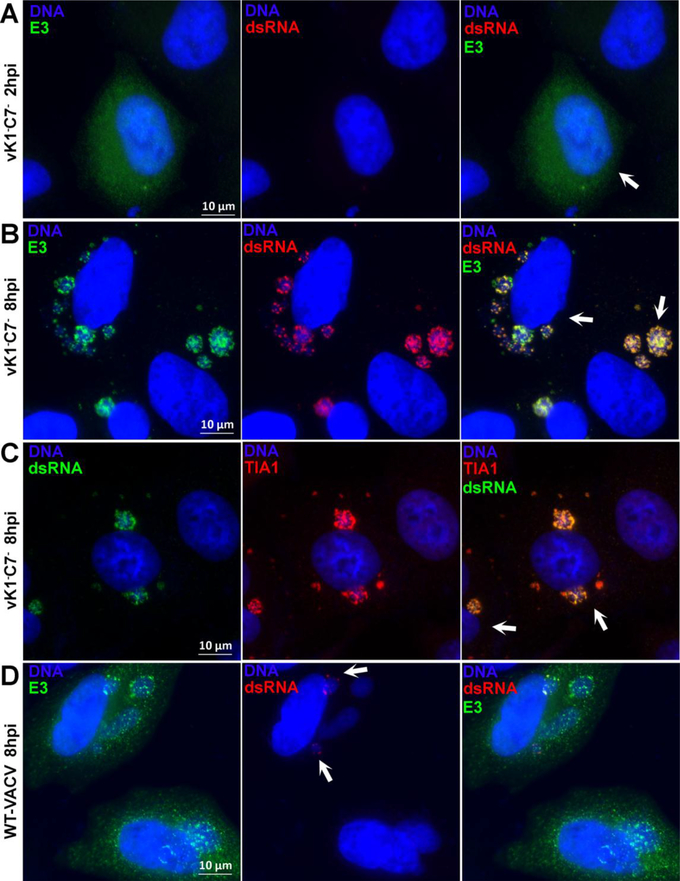
Antiviral granules against VACV were most effectively induced by vΔE3, and their induction can be attributed to cellular stress responses to dsRNA, which were usually sequestered by E3 during WT VACV infection. Since RNA granules associated with SAMD9-mediated poxvirus restriction were induced by a virus that encodes E3, we studied whether there was any defect in dsRNA sequestration by E3. At 2 h post infection with either vK1−C7− or WT VACV, E3 distributed evenly throughout the cells and no dsRNA can be detected with anti-dsRNA antibody (Fig. 2A). At 8 h post infection, E3 staining became more granular, and dsRNA granules can be detected in a small fraction of viral DNA factories in WT VACV-infected cells (Fig. 2D, arrows), along with E3 granules. In contrast, dsRNA granules could be detected in all viral DNA factories in vK1−C7−-infected cells, and E3 was found to strictly colocalize with dsRNA (Fig. 2B), suggesting that dsRNA was sequestered by E3. In addition, TIA1 also colocalized with dsRNA in DNA factories in vK1−C7−-infected cells (Fig. 2C).
Figure 2. VACV E3 and TIA1 colocalize with dsRNA in RNA granules that are associated with SAMD9-mediated poxvirus restriction.
HeLa cells were infected and processed for immunofluorescence analysis as in Fig. 1. The primary antibodies used were J2 mouse monoclonal anti-dsRNA, rabbit polyclonal anti-E3 and goat anti-TIA1.Arrows in A-C point to infected cells. Arrows in D point to factories with dsRNA.
TIA1 is not required for the formation of RNA granules or host restriction of vK1−C7−.
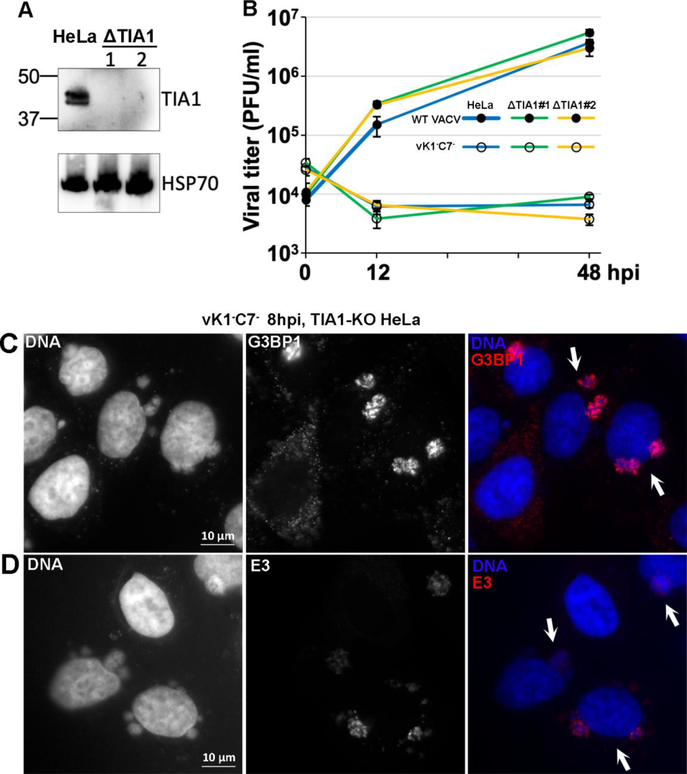
TIA1 was previously shown to be critical for the function of the antiviral granules against vΔE3 (Simpson-Holley et al., 2011). To determine whether TIA1 also contributes to SAMD9-mediated poxvirus restriction, we knocked out TIA1 from HeLa cells with Crispr-cas9 technology. Two cell clones that had no detectable level of TIA1 proteins were identified by Western blot (Fig. 3A), and indels were found in their TIA1 gene transcripts (not shown). We found no significant difference in VACV replication in the parental and TIA1 knockout (ΔTIA1) HeLa cells. In both cell types, WT VACV replicated productively with similar viral yields, while vK1−C7− had similar growth defect (Fig. 3B). When infected by vK1−C7−, both cells showed similar G3BP1 (Fig. 3C) and E3 (Fig. 3D) granules in viral DNA factories. Altogether, our data show that TIA1 is not required for granule formation or SAMD9-mediated poxvirus restriction.
Figure 3. TIA1 is not required for the formation of RNA granules or host restriction of vK1−C7−.
(A). The validation of HeLa cell lines with knockout in TIA1 (ΔTIA1) by Western blot. The clonally selected knockout cells were established with CRISPR-Cas9 technology. The levels of TIA1 and HSP70 (loading control) proteins in the cell lysates of two ΔTIA1 clones (#1 and #2) were determined by Western blotting. The parental HeLa cells were used as controls. (B). TIA1 is not required for host restriction of vK1−C7−. Parental or ΔTIA1 (#1 and #2) HeLa cells were infected with WT-VACV (filled circle) or vK1−C7− (open circle) at a MOI of 1 PFU/cell. Viral titers at 0, 12, and 48 hpi were determined by a plaque assay in VERO cells. (C, D). TIA1 is not required for the formation of RNA granules. ΔTIA1 HeLa cells were infected with vK1−C7− at a MOI of 0.5 PFU/cell for 8 h and then processed for immunofluorescence analysis as in Fig. 1. Primary antibodies used were mouse anti-G3BP1 or mouse anti-E3. The arrows point to infected cells.
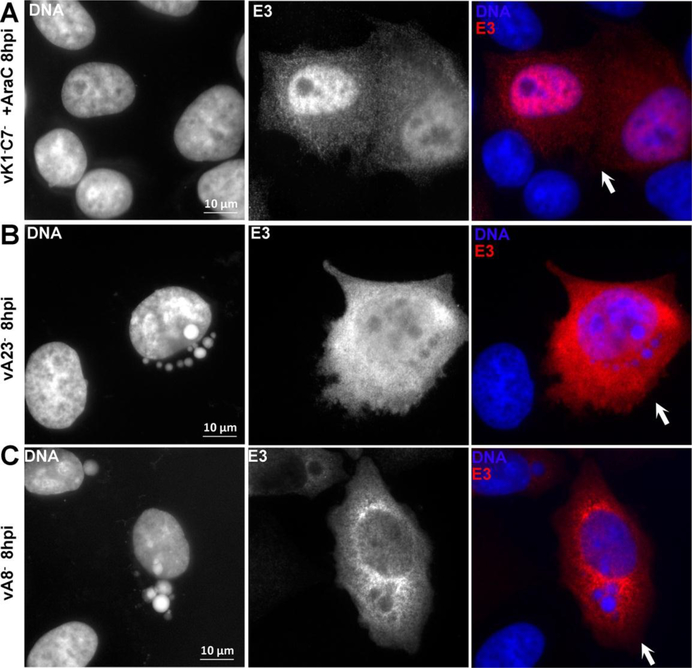
The formation of RNA granules requires viral DNA replication and intermediate gene transcription.
To assess the stage of viral replication that is required for the formation of RNA granules, we first determined whether the granule formation can be abolished by blocking viral DNA synthesis. HeLa cells were infected with vK1−C7− or WT VACV for 8 h in the presence of a DNA replication inhibitor, and granule formation was examined by immunofluorescence. No TIA1, G3BP1 or E3 granules could be found in infected cells (Fig. 4A and data not shown), indicating that viral DNA replication is required for the granule formation. The transcription of VACV intermediate genes requires a viral transcription factor complex consisted of VACV A8 and A23, and the deletion of either A8 or A23 blocks intermediate gene transcription (Warren et al., 2012). While granular E3 proteins were frequently observed around DNA factories in WT VACV-infected cells at 8 h post infection (Fig. 2D), no E3 granules were observed around DNA factories in cells infected with either A8-deletion or A23-deletion VACV mutant (Fig. 4B&C). In addition, while G3BP1 and TIA1 granules were observed in a small fraction of WT VACV-infected cells, no granules were observed in cells infected with either A8-deletion or A23-deletion (not shown). Altogether the data suggest that post-replicative mRNA is important for granule formation.
Figure 4. The formation of RNA granules requires viral DNA replication and intermediate gene transcription.
(A). HeLa cells were infected with vK1−C7− at a MOI of 0.5 PFU/cell for 8 h in the presence of AraC. HeLa cells were infected with vA23Δ (B) or vA8Δ (C) for 8 h. The cells were processed for immunofluorescence analysis as in Fig. 1. The primary antibodies were TW2.3 mouse monoclonal anti-E3. The fluorescence signals from DAPI and anti-E3 are shown in white in the first two columns. The arrows point to infected cells.
Discussion
SG-like RNA granules are associated with abortive replication of many viruses (McCormick and Khaperskyy, 2017; Tsai and Lloyd, 2014), but whether they are directly antiviral are unclear in many cases. In the case of vaccinia virus infection, SG-like granules were found to be associated with nonproductive replication of a VACV mutant with deletion in E3 (vΔE3) and with a minority of WT VACV replication (Simpson-Holley et al., 2011) (Fig. 5). These granules are similar to SG in containing TIA1 and G3BP1, but they are more stable than SGs and cannot be dispersed with translation initiation inhibitors (Simpson-Holley et al., 2011). They were described as antiviral granules and shown to repress translation (Rozelle et al., 2014). Interestingly, RNA granules similar to the antiviral granules were also found in cells that were infected by poxvirus mutants that lack inhibitors against the host restriction factor SAMD9 (Liu and McFadden, 2015). Previous studies showed that these RNA granules were similar to the antiviral granules in containing G3BP1 and nascent viral mRNA (Liu and McFadden, 2015; Sivan et al., 2018). We showed here that these RNA granules also contain TIA1, which is a crucial component of both SG and the antiviral granule (Simpson-Holley et al., 2011). These RNA granules are thus similar to antiviral granules in their composition and their close association with viral DNA factories. The mechanism by which TIA1 contributes to antiviral activity of the antiviral granules is unclear, but it was suggested that TIA1 may either directly inhibit viral gene expression by binding viral mRNA or alter splicing of TIA1 target genes (Simpson-Holley et al., 2011). However, we found knockout of TIA1 from HeLa cells had no negative effect on either granule formation or SAMD9-mediated host restriction, indicating that TIA1 in RNA granules is not universally antiviral against vaccinia virus.
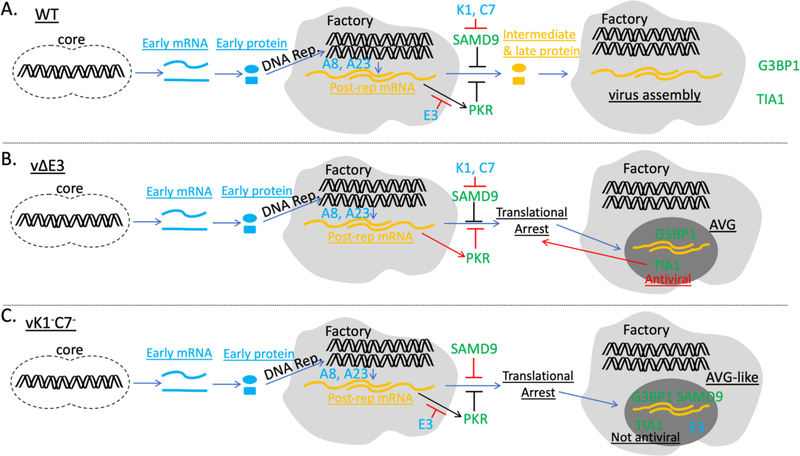
Figure 5. Model of granule formation in cells that undergo productive or nonproductive VACV infection.
(A). In WT VACV infected cells, E3 sequesters dsRNA formed by post-replicative mRNA, preventing PKR activation, while K1 and C7 inhibit SAMD9-mediated translational arrest. G3BP1 and TIA1-containg granules are only present in a minority of infected cells. (B). In vΔE3-infected cells, dsRNA activates PKR, resulting in translational arrest and the formation of AVG containing G3BP1 and TIA1. TIA1 was found to be crucial for AVG function. (C). In vK1−C7−-infected cells, SAMD9 inhibits the translation of post-replicative mRNA in a PKR-independent manner. Untranslated viral mRNA accumulates in DNA factories and aggregates with TIA1, G3BP1 and E3, forming RNA granules that are similar to AVG in composition but does not require TIA1 for SAMD9-mediated translational arrest. Viral DNA replication and A8- and A23-depedent intermediate gene transcription are required for granule formation.
Antiviral granules against VACV were induced after dsRNA activated PKR and triggered the phosphorylation of eIF2α, and the granule formation could be abolished by preventing PKR activation (Simpson-Holley et al., 2011). dsRNA was also found to be a prominent component of the RNA granules associated with SAMD9-mediated poxvirus restriction, but eIF2α phosphorylation was not connected with the granule formation (Liu and McFadden, 2015) and PKR knockdown or knockout had no effect on SAMD9-mediated poxvirus restriction (Meng et al., 2009; Sivan et al., 2018). In this study, we found dsRNA strictly colocalized with E3 in the granules, indicating that they were sequestered by E3 and thus unlikely to drive granule formation through PKR activation. The granule formation was abolished when viral DNA replication or intermediate gene transcription was blocked, indicating that post-replicative mRNA is required for granule formation. Previous studies showed that the majority of dsRNA produced during VACV replication was from overlapping transcription of post-replicative mRNA (Liu and Moss, 2016; Willis et al., 2011). Although these dsRNA may not drive granule formation through PKR activation, they may be essential for granule formation by bringing together various RNA binding proteins including TIA1, G3BP1 and E3. However, we showed here that TIA1 is not required for granule formation. A recent report had showed that G3BP1 and G3BP2 are not required for the granule formation and SAMD9-mediated host restriction (Sivan et al., 2018). Therefore, it appears that the formation of RNA granules associated with SAMD9-mediated poxvirus restriction requires no specific SG components. It is thus likely that the granules formation could be driven by an array of RNA protein interactions, none of which was individually required for the granule formation. Our data suggest a model that the RNA granules are formed as a consequence of SAMD9-mediated translation inhibition, which resulted in the accumulation of untranslated mRNA in viral DNA factories, leading to the aggregation of RNA protein complex into cytoplasmic granules (Fig. 5).
Acknowledgements
This work was supported by a grant from NIAID to Y. Xiang (AI079217). We thank Dr. Bernard Moss, NIAID, for providing the A23 and A8 deletion VACV mutants and the complementary cell line. We thank Dr. Jingxin Cao, Public Health Agency of Canada, for providing antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson P, Kedersha N, 2008. Stress granules: the Tao of RNA triage. Trends Biochem Sci 33, 141–150. [DOI] [PubMed] [Google Scholar]
- Dueck KJ, Hu YS, Chen P, Deschambault Y, Lee J, Varga J, Cao J, 2015. Mutational analysis of vaccinia virus E3 protein: the biological functions do not correlate with its biochemical capacity to bind double-stranded RNA. J Virol 89, 5382–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P, 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15, 5383–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Kedersha N, Anderson P, 2018. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb Perspect Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, Moreno GT, Brinton MA, 2002. Cell proteins TIA-1 and TIAR interact with the 3’ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol 76, 11989–12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McFadden G, 2015. SAMD9 is an innate antiviral host factor with stress response properties that can be antagonized by poxviruses. J Virol 89, 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wennier S, Zhang L, McFadden G, 2011. M062 is a host range factor essential for myxoma virus pathogenesis and functions as an antagonist of host SAMD9 in human cells. J Virol 85, 3270–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Moss B, 2016. Opposing Roles of Double-Stranded RNA Effector Pathways and Viral Defense Proteins Revealed with CRISPR-Cas9 Knockout Cell Lines and Vaccinia Virus Mutants. J Virol 90, 7864–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Khaperskyy DA, 2017. Translation inhibition and stress granules in the antiviral immune response. Nat Rev Immunol 17, 647–660. [DOI] [PubMed] [Google Scholar]
- Meng X, Chao J, Xiang Y, 2008. Identification from diverse mammalian poxviruses of host-range regulatory genes functioning equivalently to vaccinia virus C7L. Virology 372, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Jiang C, Arsenio J, Dick K, Cao J, Xiang Y, 2009. Vaccinia virus K1L and C7L inhibit antiviral activities induced by type I interferons. J Virol 83, 10627–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Xiang Y, 2006. Vaccinia virus K1L protein supports viral replication in human and rabbit cells through a cell-type-specific set of its ankyrin repeat residues that are distinct from its binding site for ACAP2. Virology 353, 220–233. [DOI] [PubMed] [Google Scholar]
- Meng X, Zhang F, Yan B, Si C, Honda H, Nagamachi A, Sun LZ, Xiang Y, 2018. A paralogous pair of mammalian host restriction factors form a critical host barrier against poxvirus infection. PLoS Pathog 14, e1006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P, 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J 19, 4154–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozelle DK, Filone CM, Kedersha N, Connor JH, 2014. Activation of stress response pathways promotes formation of antiviral granules and restricts virus replication. Mol Cell Biol 34, 2003–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F, 2014. Improved vectors and genome-wide libraries for CRISPR screening. Nature methods 11, 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holley M, Kedersha N, Dower K, Rubins KH, Anderson P, Hensley LE, Connor JH, 2011. Formation of antiviral cytoplasmic granules during orthopoxvirus infection. J Virol 85, 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan G, Glushakow-Smith SG, Katsafanas GC, Americo JL, Moss B, 2018. Human host-range restriction of the vaccinia virus C7/K1 double deletion mutant is mediated by an atypical mode of translation inhibition. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J, 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol 160, 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tsai WC, Lloyd RE, 2014. Cytoplasmic RNA Granules and Viral Infection. Annu Rev Virol 1, 147–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RD, Cotter CA, Moss B, 2012. Reverse genetics analysis of poxvirus intermediate transcription factors. Journal of virology 86, 9514–9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis KL, Langland JO, Shisler JL, 2011. Viral double-stranded RNAs from vaccinia virus early or intermediate gene transcripts possess PKR activating function, resulting in NF-kappaB activation, when the K1 protein is absent or mutated. J Biol Chem 286, 7765–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwen H, Cox JH, Yewdell JW, Bennink JR, Moss B, 1993. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology 195, 732–744. [DOI] [PubMed] [Google Scholar]