Abstract
Background:
Autism spectrum disorder (ASD) is suspected to have environmental and genetic contributions. Polychlorinated biphenyls (PCBs) are environmental risk factors of interest due to their potential as neurodevelopmental toxicants and environmental persistence despite a US production ban in the 1970s.
Methods:
Participants were mother-child pairs from MARBLES, a high-risk pregnancy cohort that enrolls families who have one child diagnosed with ASD and are planning to have another child. PCB concentrations were measured in maternal blood at each trimester of pregnancy using gas chromatography coupled with triple quadruple mass spectrometry. Concentrations were summed into total PCB and two categories based on function/mechanisms of action: dioxin-like (DL), and ryanodine receptor (RyR)-activating PCBs. Multinomial logistic regression assessed risk of clinical diagnosis of ASD and non-typical development (Non-TD) compared to typically developing (TD) in the children at 3 years old.
Results:
A total of 104 mother-child pairs were included. There were no significant associations for total PCB; however, there were borderline significant associations between DL-PCBs and decreased risk for Non-TD diagnosis (adjusted OR: 0.41 (95% CI 0.15 to 1.14)) and between RyR-activating PCBs and increased risk for ASD diagnosis (adjusted OR: 2.63 (95% CI 0.87 to 7.97)).
Conclusion:
This study does not provide strong supporting evidence that PCBs are risk factors for ASD or Non-TD. However, these analyses suggest the need to explore more deeply into subsets of PCBs as risk factors based on their function and structure in larger cohort studies where non-monotonic dose-response patterns can be better evaluated.
Keywords: Polychlorinated Biphenyls, Autism, Pregnancy, Ryanodine Receptor, Prospective Study
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder (NDD) affecting approximately 1.5% of children in high-income countries (Lyall et al., 2017a; Newschaffer et al., 2007). ASD encompasses a range of symptoms and severities, with key areas of disability including difficulty communicating, interacting, learning, and behaving (Centers for Disease Control and Prevention, 2017). ASD incidence has risen since the 1970’s in the United States (Nevison, 2014) and continues to increase, with a current Center for Disease Control and Prevention (CDC) estimate that 1 in 59 children has ASD (Centers for Disease Control and Prevention, 2018). This rise is only partially explained by changing diagnostic criteria, inclusion of milder cases and earlier age of diagnosis (Hertz-Picciotto and Delwiche, 2009). Despite a strong genetic component in a small percentage of ASD cases, genetic factors alone cannot account for the steady increase (Hallmayer et al., 2011; Herbert, 2010). Thus, further etiologic research is needed to identify ASD risk factors with scientific evidence pointing to environmental contaminants (Kalkbrenner et al., 2014; Kim et al., 2010).
Polychlorinated biphenyls (PCB) are a group of synthesized man-made chemicals that are generally accepted as human neurodevelopmental toxicants, with early life exposure linked to neurological deficits in children (Berghuis et al., 2015; Boucher et al., 2009; Schantz et al., 2003). There are 209 PCB congeners differing in degree and placement of chlorine atoms on a biphenyl structure. Despite the late 1970s ban of PCB production in the United States, PCBs persist in the environment due to their long half-life and are frequently detected in environmental media (Marek et al., 2017) and human tissues (Marek et al., 2014; Needham et al., 2011). PCBs leach into the environment from breakdown of old buildings and waste facilities (Herrick et al., 2007; Klosterhaus et al., 2014) and continue to be produced as unintentional byproducts of paint pigment synthesis (Guo et al., 2014). PCB exposure is widespread amongst women of child bearing age (Hopf et al., 2009; Koh et al., 2015) and levels in mothers correlate to levels in their children (Koh et al., 2015). Although the exact PCB placental transfer rate is still under investigation, studies suggest that PCBs are able to cross the placental barrier and enter the in utero environment (Eguchi et al., 2018; Lancz et al., 2015).
Though some studies have reported a null association between PCBs and ASD risk (Braun et al., 2014; Brown et al., 2018), other evidence suggests that PCBs may be risk factors for the development of ASD (Eubig et al., 2010) with studies showing increased risk for NDDs with perinatal PCB exposure (Cheslack-Postava et al., 2013; Lyall et al., 2017b; Sagiv et al., 2010). One study found that PCB exposure in utero was associated with poorer cognitive ability at 42 months of age (Patandin et al., 1999), and several animal model studies have also identified an association between prenatal PCB exposure and poorer spatial learning ability and motor coordination (Boix et al., 2010; Widholm et al., 2004).
The Markers of Autism Risk in Babies – Learning Early Signs (MARBLES) study (Hertz-Picciotto et al., In Press) includes mother-child pairs in families where at least one older sibling of the child had a diagnosis of ASD. Having one older sibling with ASD has been associated with up to an 18.7% increased risk of ASD in the younger sibling (Ozonoff et al., 2011). This study expands current literature focused on the general population by examining the unique risk in Northern California families with increased genetic susceptibility for ASD, while also analyzing a broad range of structurally/mechanistically diverse PCBs. PCBs have previously been divided into two categories, dioxin-like (DL) or non-dioxin-like (NDL), based on activity at the aryl hydrocarbon receptor (White and Birnbaum, 2009), which is an integral receptor for normal neurodevelopment (Petersen et al., 2006). Early research focused mainly on DL-PCBs, but NDL-PCBs are extremely potent sensitizers of the ryanodine receptor (RyR), a calcium channel critical for typical neurodevelopment (Pessah et al., 2010; Wong et al., 1997). To capture two main sensitive targets of PCBs, a subset of the range of congeners selected in this study are considered to be DL-PCBs and some are potent activators of the RyR. Therefore, our study assesses a uniquely susceptible population and expands on the number of PCBs analyzed by assessing the effects of developmental exposure to 17 PCB congeners in utero, while also addressing DL-PCBs and RyR-active PCBs.
2. Materials and methods
2.1. Participants
The University of California (UC) Davis MIND Institute in Sacramento, CA facilitates research in developmental disorders and is where clinical assessments are conducted for the MARBLES cohort. This cohort was established in 2006 as a prospective, high-risk cohort, and still enrolls families. The MARBLES cohort is comprised of the younger siblings in families with at least one older child diagnosed with ASD. Mothers are recruited either before conception or during the pregnancy of the younger child. Families must reside within 2.5 hours driving distance from the MIND Institute. Questionnaire data and bio-specimens are collected throughout pregnancy and after childbirth, including blood samples. Data includes many potential confounders and effect modifiers, which were included in the assessment of PCB risk. The outcomes of ASD, non-typical development (non-TD), or typically developing (TD) in the younger child of interest, are assessed at 3 years of age at the MIND Institute by trained personnel that formally attain research reliability, and using an algorithm including clinical diagnosis and scores from two research-grade measures, Autism Diagnostic Observation Schedule (ADOS) and Mullen Scales of Early Learning (MSEL) (Ozonoff et al., 2014). ASD was diagnosed if children had scores over ADOS cutoff and were classified as ASD using the DSM-4. Children were diagnosed as Non-TD if they did not meet criteria for ASD, but had low MSEL scores, more than one score 1.5 SD below the mean or at least one score 2 SD below the mean, or high ADOS scores. If children scored at least three points below ADOS cutoff and had all four MSEL scores within 2.0 SD of the normative mean, or had only one MSEL subscale score 1.5 SD below the mean, they were considered to be typically developing.
Inclusion criteria included confirmed diagnosis of ASD in an older sibling of the child and active status in the MARBLES cohort, meaning participants had no loss to follow-up and had not declined further participation in the study. Participant observations were excluded if the child of interest was a twin or had no final diagnosis. In the case of multiple siblings only the older sibling was included. A total of 115 mother-child pairs met these criteria and had a maternal sample analyzed for PCB concentration, out of the 143 mother-child pairs enrolled during the same period. Though some mothers had a sample analyzed from all three trimesters, many only had concentrations available from one or two trimesters. Of the 115 participants, there were 29 samples from the 1st trimester, 44 samples from the 2nd trimester, and 104 samples from the 3rd trimester. This analysis focused on exposure during the third trimester due to sparse data in the first and second trimester. The UC Davis Institutional Review Board has approved this study, and written and informed consent was obtained before collecting data and biospecimens.
2.2. Exposure data
Maternal samples were collected during prenatal visits at the participant’s home. Plasma was isolated after centrifugation at 1200xg for 15 minutes at 4°C, after which samples were stored at −80°C. PCB analysis was performed at UC Davis using a validated standard operating procedure to extract, separate, and detect PCBs by gas chromatography coupled with triple quadruple mass spectrometry (GC/MS/MS, Scion TQ triple quadruple mass spectrometer, Bruker, Fremont, CA, USA). Sample preparation details and GC-MS/MS parameters were reported elsewhere (Lin et al., 2013). In brief, 500μl of plasma were extracted by solid phase extraction (SPE), further purified by silica cartridges and analyzed for PCB congeners 11, 28, 52, 66, 77, 84, 91, 95, 101, 118, 131, 132, 135, 136, 138, 149, 153, 174, 175, 176, 180, and 196. 13C12 labeled PCB 97 was used as surrogate internal standard throughout the procedures (Cambridge Isotope Laboratories, Inc, Tewksbury, MA, USA), and Mirex, was added after extraction to monitor any shifts in instrument performance. Standard reference material (SRM1957) was purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). Human control serum (DDC Mass Spect Gold®, MSG 3000, Golden West Biologicals, InC. Temecula, CA, USA) was fortified with all PCB standard solutions (Accustandard, Inc., New Haven, CT, USA) at concentrations of 0.1, 0.8 and 4 ng/ml for quality control purposes. The analytical laboratory participates in the Artic Monitoring and Assessment Program (also known as AMAP Ring Test of Persistent Organic Pollutants in Human Serum) (The Centre de toxicology du Québec, 2018) for PCB analyses and consistently showed excellent performance for PCB congener analysis with a −2< Z’-score < 2.
Congeners were selected based on prior knowledge of PCB use in manufacturing and potential for neurotoxicity (Eckhardt et al., 2007; Erickson and Kaley, 2010; Herrick et al., 2016). For statistical analysis, a congener could have no more than 30% of the observations be below the level of detection (LOD) (Dorgan et al., 1999; Needham et al., 2005). PCB congeners 28, 66, 131, 135, and 149 were excluded due to the aforementioned criteria. The remaining congeners’ values below the LOD were imputed with (Nichols et al., 2007; Sonneborn et al., 2008). Because PCB analysis was performed in three separate batches and LODs differed between batches for all congeners except PCB 196, values below the LOD were imputed using batch- and PCB-specific LODs.
PCB values were lipid-standardized using plasma total lipid (TL) values collected for each mother at the same time point as the PCB. Plasma TL content was calculated from total cholesterol (TC) and total triglycerides (TG) using the formula: TL = 2.27 × TC + TG + 0.623 in units of g/l (Bernert et al., 2007; Phillips et al., 1989). TC and TG were determined using standard clinical chemistry enzymatic methods in the UC Davis Health System Department of Pathology and Laboratory Medicine (Davis, CA, USA) (Allain et al., 1974; Bucolo and David, 1973; Pinter et al., 1967; Trinder, 1969). We converted raw serum concentrations to lipid-standardized concentrations using PCB Lipid-Standardized = (PCB Raw Serum Concentration × 1000) / TL.
To reduce multiple hypothesis testing, we analyzed 3 groupings of PCBs, by calculating congener sums: total PCB sum (PCB congeners 11, 52, 77, 84, 91, 95, 101, 118, 132, 136, 138, 153, 174, 175, 176, 180, and 196), DL-PCB congeners (PCB congeners 77 and 118), and RyR activating congeners (PCB congeners 52, 84, 95, 136, and 176) (Fig. 1). DL-PCBs were chosen based on their agonistic activity at the aryl hydrocarbon receptor, and RyR activating congeners by having an EC2x, a 200% activation at the receptor, greater than 1 μM (Pessah et al., 2010). In light of other possible PCB groupings (Ritchie et al., 2005; Wolff et al., 1982), these functionally specific groupings were chosen due to the emerging interest in calcium channel signaling (Ebert and Greenberg, 2013), as regulated by RyR-activation (Wayman et al., 2012a), and its association with ASD, and research in DL-toxins and their association with neurodevelopment and ASD (Guo et al., 2018; White and Birnbaum, 2009).
Fig. 1.
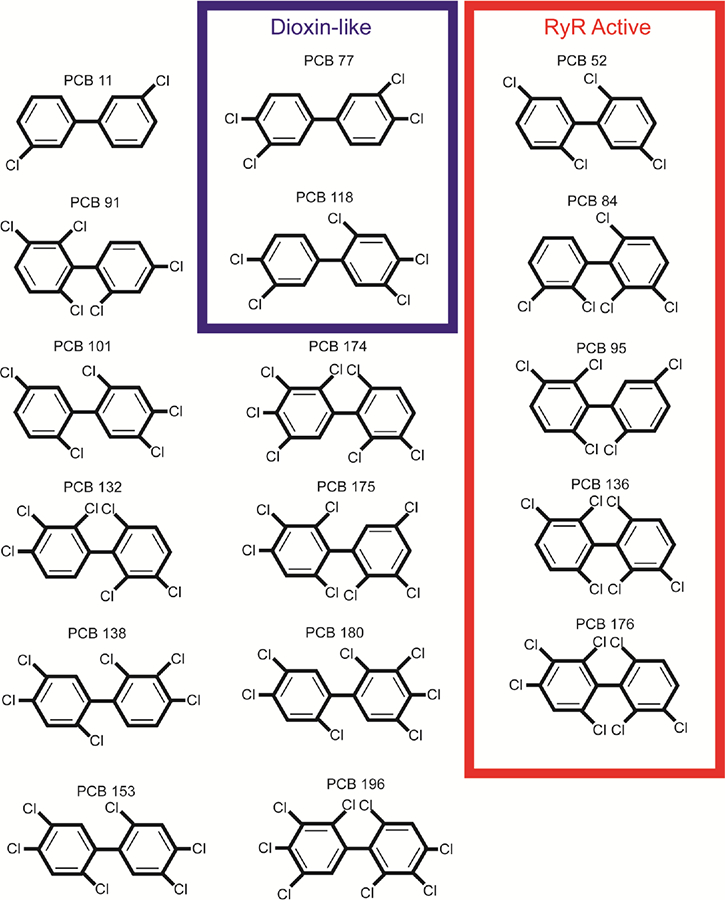
The unique configurations of polychlorinated biphenyls (PCB) included in the analysis shown by functional/structural groupings, dioxin-like congeners and ryanodine receptor (RyR) active congeners. All congeners shown were included in the total PCB sum.
2.3. Statistical analyses
In exploratory analysis, we examined PCB concentrations for linearity using empirical logit plots and smoothing splines. The analyses suggested a non-linear relationship between PCB concentrations and diagnostic outcome. We then categorized PCBs using the quartiles of concentration to examine dose-trends. The first quartile, Q1, contained the values from the 26 mothers with the lowest concentrations of PCBs, and was used as the reference group. Quartiles were made using the full cohort, regardless of child outcome, with 26 mothers in each quartile.
Given evidence of a non-linear dose response and because of limited sample sizes for quartiles, for the main analysis PCB concentration levels were dichotomized using a median split that placed mothers with the 50% lowest concentration of PCBs into the lower median/reference group (n = 52), and mothers with the 50% highest concentration of PCBs into the higher median group (n = 52). This was done separately for each grouping for both non-standardized and lipid-standardized sums. Descriptive analysis was also conducted on individual congeners using the median split.
We used multinomial logistic regression to assess the risk of ASD or Non-TD compared to TD. P-values ≤ 0.05 were considered statistically significant.
Each PCB grouping was examined separately for confounding and effect modification. A directed acyclic graph (DAG) was developed during the preliminary analysis plan to identify possible confounders (Hernán et al., 2004). The following covariates were evaluated as potential confounders: child gender, child ethnicity (Hispanic, non-Hispanic), maternal age, maternal pre-pregnancy BMI (obese with BMI ≥ 30, not obese with BMI <30), prenatal vitamin use in month 1 of pregnancy, pre-pregnancy smoking (smoking prior to pregnancy at any time point, no history of smoking), maternal birth place (US, non-US), insurance delivery payer (private, public), home ownership, and maternal education (less than bachelor degree, bachelor degree or higher). Child gender, child ethnicity, maternal age, maternal pre-pregnancy BMI, prenatal vitamin use, and pre-pregnancy smoking were also evaluated as potential effect modifiers (Casas et al., 2015; Hertz-Picciotto et al., 2005; Schmidt et al., 2011; Verhulst et al., 2009; Vreugdenhil et al., 2002; Ward et al., 2009).
We considered as confounder candidates, covariates broadly associated (p-value ≤ 0.3) with both outcome and exposure. Covariates were then added to the univariate multinomial logistic regression model individually. If the addition of the covariate changed the exposure of interest’s beta coefficient or odds ratio (OR) value by 10% it was considered for model building. For effect modification, a p-value ≤ 0.2 was needed for the product term between exposure and outcome. If a covariate met this criterion a stratified model was completed. Due to the unequal male to female ratio of ASD prevalence (Centers for Disease Control and Prevention, 2017), and biologic plausibility for sex effects in ASD and Non-TD etiology, stratification for child gender was examined.
Model building was completed using step-wise selection based on changes in the effect estimate for the PCB grouping of interest. When observations were missing a covariate, specifically prenatal vitamin use, the adjusted model was compared to a crude model including only participants with the covariate. Separate models were created for non-standardized and lipid-standardized PCB sums.
3. Results
We included 104 mothers in our analysis, 60 of whose child was TD at 3 years, 21 with ASD, and 23 with Non-TD. Three mothers were missing prenatal vitamin use data, 1 with a child with TD, and 2 with Non-TD. Table 1 displays descriptive statistics of the population by child diagnosis. There was a significant difference in child gender across child diagnosis. As reflected in Table 1, maternal age, prenatal vitamin use, and maternal education displayed different averages and proportions across diagnostic groups, although differences were not statistically significant.
Table 1.
Demographic and clinical characteristics of children included in the study, stratified by child diagnosis
| Child 36 Month Diagnosis | ||||
|---|---|---|---|---|
| ASD (n = 21) | Non-TD (n = 23) | TD (n = 60) | P-value | |
| Child male gender, n (%) | 18 (85.7) | 13 (56.5) | 32 (53.3) | 0.03 |
| Child race white, n (%) | 7 (33.3) | 8 (34.8) | 44 (73.3) | 0.71 |
| Child Hispanic ethnicity | 8 (38.1) | 7 (30.4) | 18 (30.0) | 0.78 |
| Mother age (years), mean (SD) | 35.2 (5.2) | 34.8 (4.6) | 33.4 (4.8) | 0.13 |
| Father age (years), mean (SD) | 38.5 (5.9) | 37.2 (6.1) | 36.4 (6.2) | 0.38 |
| Mother Pre-pregnancy BMI, n (%) | 0.28 | |||
| Normal | 6 (28.6) | 12 (52.2) | 33 (55.0) | |
| Overweight | 10 (47.6) | 7 (30.4) | 15 (25.0) | |
| Obese | 5 (23.8) | 4 (17.4) | 12 (20.0) | |
| Mother born outside US, n (%) | 5 (23.8) | 3 (13.0) | 10 (16.7) | 0.63 |
| Prenatal Vitamin Use in Month 1, n (%)1 | 7 (33.3) | 9 (42.9) | 33 (55.9) | 0.17 |
| Maternal cigarette smoking before pregnancy, n (%)2 | 2 (10.0) | 3 (14.3) | 3 (5.1) | 0.38 |
| Insurance delivery type, n (%)3 | 0.41 | |||
| Private | 16 (76.2) | 16 (69.6) | 48 (82.8) | |
| Public | 5 (24.8) | 7 (30.4) | 10 (17.2) | |
| Own Home, n (%)1 | 13 (61.9) | 14 (66.7) | 36 (61.0) | 0.90 |
| Maternal Education, n (%) | 0.08 | |||
| Less than a bachelor degree | 13 (61.9) | 15 (65.2) | 25 (41.7) | |
| Bachelor, master’s, professional, or doctorate degree | 8 (38.1) | 8 (34.8) | 35 (58.3) | |
Note: Only MARBLES children whose mothers had PCB measurements during 3rd Trimester were included in the current study.
ASD = Autism Spectrum Disorder; Non-TD = Non-Typical Development; TD = Typical Development;
P-values obtained from chi-square test;
Frequency missing = 1 in TD group, 2 in Non-TD group;
Frequency missing = 1 in TD group, 1 in ASD group, 2 in Non-TD group;
Frequency missing = 2 in TD group.
Table 2 provides the distribution of PCB concentration in the third trimester by individual congener. Concentrations had a skewed distribution and unequal mean and median values. The most abundant congener was PCB 11, followed by PCB 118.
Table 2.
| Congener | Minimum (ng/g) | Mean (ng/g) | SD (ng/g) | Geometric Mean (ng/g) | Median (ng/g) | Maximum (ng/g) |
|---|---|---|---|---|---|---|
| PCB 11 | 1.24 | 53.74 | 45.43 | 32.01 | 42.68 | 157.78 |
| PCB 52 | 1.33 | 9.82 | 6.36 | 7.53 | 8.87 | 28.88 |
| PCB 77 | 0.36 | 3.06 | 1.40 | 2.76 | 2.90 | 9.79 |
| PCB 84 | 0.55 | 3.40 | 3.85 | 2.55 | 2.41 | 27.23 |
| PCB 91 | 0.57 | 2.04 | 2.06 | 1.64 | 1.73 | 18.10 |
| PCB 95 | 0.54 | 3.29 | 2.31 | 2.89 | 3.00 | 20.56 |
| PCB 101 | 0.95 | 9.98 | 15.31 | 6.78 | 6.85 | 145.75 |
| PCB 118 | 0.51 | 11.53 | 6.78 | 8.78 | 11.87 | 43.85 |
| PCB 132 | 0.65 | 3.27 | 2.24 | 2.67 | 2.71 | 14.68 |
| PCB 136 | 0.14 | 2.48 | 2.52 | 1.79 | 1.95 | 20.70 |
| PCB 138 | 0.77 | 4.84 | 3.90 | 3.96 | 4.24 | 32.71 |
| PCB 153 | 0.81 | 9.94 | 7.72 | 6.80 | 8.54 | 45.67 |
| PCB 174 | 0.54 | 3.04 | 5.92 | 1.79 | 1.72 | 46.79 |
| PCB 175 | 0.38 | 3.39 | 2.98 | 2.68 | 2.78 | 25.70 |
| PCB 176 | 0.12 | 3.43 | 12.72 | 1.46 | 1.58 | 126.12 |
| PCB 180 | 0.57 | 9.53 | 14.84 | 6.44 | 5.92 | 131.69 |
| PCB 196 | 0.12 | 2.09 | 2.53 | 1.31 | 1.56 | 17.71 |
PCB = Polychlorinated Biphenyl; SD = Standard Deviation
Distribution only includes mothers that met all eligibility for inclusion in the analysis,
Distribution includes imputed values using to replace missing observations
The first PCB grouping examined was the summed total of all PCBs detected (Table 3). There were no significant findings in either crude or adjusted models.
Table 3.
Association between Total PCB concentration sum1 (dichotomized using a median split) and ASD and Non-TD
| ASD vs. TD | Non-TD vs. TD | ||||||
|---|---|---|---|---|---|---|---|
| Exposure | TD (n = 60) | ASD (n = 21) | Non-TD (n = 23) | OR (95% CI) | P-Value | OR (95% CI) | P-Value |
| Frequency (%) | |||||||
| Total PCB Lipid-Standardized Crude Model | |||||||
| M1 (33.63 – 117.97 ng/g of lipid) | 33 (55) | 9 (43) | 10(43) | Reference | Reference | ||
| M2 (119.35 – 662.34 ng/g of lipid) | 27 (45) | 12 (57) | 13 (57) | 1.63 (0.60 to 4.44) | 0.34 | 1.59 (0.60 to 4.19) | 0.35 |
| Total PCB Lipid-Standardized Adjusted Model2,3 | |||||||
| M1 (33.63 – 117.97 ng/g of lipid) | 33 (56) | 9 (43) | 8 (38) | Reference | Reference | ||
| M2 (119.35 – 662.34 ng/g of lipid) | 26 (44) | 12 (57) | 13 (62) | 1.50 (0.53 to 4.23) | 0.44 | 1.87 (0.65 to 5.37) | 0.25 |
PCB = Polychlorinated Biphenyl, TD = Typical Development, ASD = Autism Spectrum Disorder, Non-TD = Non-Typical Development, M1 = Lowest 50% PCB concentration, M2 = Highest 50% PCB concentration
Sum included concentration from: PCB 11, PCB 52, PCB 77, PCB 84, PCB 91, PCB 95, PCB 101, PCB 118, PCB 132, PCB 136, PCB 138, PCB 153, PCB 174, PCB 175, PCB 176, PCB 180 and PCB 196 collected during the 3rd trimester of pregnancy,
Loss of 3 observations due to missing prenatal vitamin use,
Adjusted for maternal education and prenatal vitamin use in month 1 of pregnancy
No significant results were found for DL-PCBs; however, the direction of association between DL-PCBs and ASD differed from the association between DL-PCBs and Non-TD (Table 4). There were elevated ORs for ASD in the mothers with the highest amount of lipid-standardized DL-PCB concentrations (adjusted OR: 1.87 (95% CI 0.66 to 5.29)), but decreased ORs for Non-TD diagnosis in the children of mothers within the 50% highest amount of DL-PCB category (adjusted OR: 0.41 (95% CI 0.15 to 1.14)).
Table 4.
Association between Dioxin-Like PCB concentration sum1 (dichotomized using a median split) and ASD and Non-TD
| ASD vs. TD | Non-TD vs. TD | ||||||
|---|---|---|---|---|---|---|---|
| Exposure | TD (n = 60) | ASD (n = 21) | Non-TD (n = 23) | OR (95% CI) | P-Value | OR (95% CI) | P-Value |
| Frequency (%) | |||||||
| Dioxin-Like PCB Lipid-Standardized Crude/Final Model2 | |||||||
| M1 (2.47 – 14.78 ng/g of lipid) | 29 (48) | 7 (33) | 16 (70) | Reference | Reference | ||
| M2 (14.83 – 46.18 ng/g of lipid) | 31 (52) | 14 (67) | 7 (30) | 1.87 (0.66 to 5.29) | 0.24 | 0.41 (0.15 to 1.14) | 0.09 |
PCB = Polychlorinated Biphenyl, TD = Typical Development, ASD = Autism Spectrum Disorder, Non-TD = Non-Typical Development, M1 = Lowest 50% PCB concentration, M2 = Highest 50% PCB concentration
Sum included concentration from: PCB 77 and PCB 118 collected during the 3rd trimester of pregnancy,
No covariates met criteria as confounders
RyR-active PCBs models showed the same differences in direction of effect estimates between ASD and Non-TD with lipid-standardized concentrations, but were not statistically significant (Table 5). There was an elevated OR (adjusted OR: 2.63 (95% CI 0.87 to 7.97)) for ASD diagnosis in children of mothers with the highest amount of RyR-active PCBs and decreased OR (adjusted OR: 0.77 (95% CI 0.27 to 2.25)) for Non-TD diagnosis.
Table 5.
Association between RyR Active PCB concentration sum1 (dichotomized using a median split) and ASD and Non-TD
| ASD vs. TD | Non-TD vs. TD | ||||||
|---|---|---|---|---|---|---|---|
| Exposure | TD (n = 60) | ASD (n = 21) | Non-TD (n = 23) | OR (95% CI) | P-Value | OR (95% CI) | P-Value |
| Frequency (%) | |||||||
| RyR Active PCB Lipid-Standardized Crude Model | |||||||
| M1 (4.68 – 16.31 ng/g of lipid) | 33 (55) | 6 (29) | 13 (57) | Reference | Reference | ||
| M2 (16.74 – 170.03 ng/g of lipid) | 27 (45) | 15 (71) | 10 (43) | 3.06 (1.04 to 8.95) | 0.04 | 0.94 (0.36 to 2.48) | 0.90 |
| RyR Active PCB Lipid-Standardized Adjusted Model2,3 | |||||||
| M1 (4.68 – 16.31 ng/g of lipid) | 33 (56) | 6 (29) | 12 (57) | Reference | Reference | ||
| M2 (16.74 – 170.03 ng/g of lipid) | 26 (44) | 15 (71) | 9 (43) | 2.63 (0.87 to 7.97) | 0.09 | 0.77 (0.27 to 2.25) | 0.64 |
PCB = Polychlorinated Biphenyl, TD = Typical Development, ASD = Autism Spectrum Disorder, Non-TD = Non-Typical Development, RyR = Ryanodine receptor, M1 = Lowest 50% PCB concentration, M2 = Highest 50% PCB concentration
Sum included concentration from: PCB 52, PCB 84, PCB 95, PCB 136, and PCB 176 collected during the 3rd trimester of pregnancy
Loss of 3 observations due to missing prenatal vitamin use,
Adjusted for maternal education and prenatal vitamin use in month 1 of pregnancy
Associations between individual congeners and ASD and Non-TD risk were examined in exploratory analysis, and corrected for multiple hypotheses testing using false discovery rate adjustment (Supplement Table 1).
None of the models using non-standardized PCB concentrations had significant or borderline significant results. For comparison to other studies, these tables have been placed in the supplemental material (Supplement Tables 2–4).
Stratification by sex produced cell sizes <5 and unstable effect estimates for females (Supplement Tables 5–7). With a borderline significance of the interaction term, the stratified analyses by child gender are provided in the supplemental material.
We examined risk of ASD and Non-TD using quartiles to evaluate dose-response. Due to small frequencies these were treated as exploratory analysis. The models displayed non-monotonic trends in risk estimates as quartiles increased (Supplement Table 8–10). Lipid-standardized DL-PCBs had the highest risk of ASD in children of mothers in the 4th quartile (adjusted OR: 4.50 (95% CI 0.83 to 24.38)) (Supplement Table 9), though highest risk of ASD was found in the 3rd quartile for both lipid-standardized total PCBs (adjusted OR: 4.93 (95% CI 0.81 30.21)) (Supplement Table 8) and lipid-standardized RyR-active PCBs (adjusted OR: 4.64 (95% CI 1.01 to 21.41)) (Supplement Table 10).
4. Discussion
The present study does not provide substantial evidence of an association between PCB concentration and ASD, or PCB concentration and Non-TD in high-risk siblings; however, there were interesting patterns that emerged to add to the weight of evidence suggesting PCBs as risk factors for ASD.
Previous literature has shown developmental exposure to PCBs generally has detrimental effects on neurodevelopment (Berghuis et al., 2015; Boucher et al., 2009; Schantz et al., 2003). Some studies that found no effect of PCBs may have been limited by how the PCBs were grouped, small sample sizes, or outcome measurements focused on symptomology rather than clinically-confirmed diagnosis (Boucher et al., 2009; Braun et al., 2014; Ribas-Fitó et al., 2001). Additionally, there are 209 distinct PCB congeners that possess a wide range of toxicological activities, and previous studies included different selections of congeners from this study. It should be noted that when the congeners that were measures matched, the PCB congener concentrations in the MARBLES participants were comparable to those previously reported, particularly when compared to studies conducted in the US (Braun et al., 2014; Lyall et al., 2017b). Studies vary in how they group PCB congener sums, which makes practical comparison difficult across studies. As mechanistic and functional groupings increase, it would be advantageous to develop a standardized approach to function-specific groupings to increase comparability.
When examining the lipid-standardized models, we observed differences between directions of associations between ASD and Non-TD in relation to the DL-PCB and RyR-active PCB sum exposures above the median. Although results were not statistically significant, this highlights the potential for elevated risk specific to ASD, and the usefulness of focusing on PCB groupings based on function/structure. This was comparable to other studies that also found differences in associations for ASD compared to associations for a separate non-ASD NDDs group (Lyall et al., 2017b). However, non-elevated ORs for Non-TD could be an effect of all other non-ASD developmental disorders being combined into a single Non-TD group, which could attenuate individual associations between PCBs and specific disorders. The increased risk for both ASD and Non-TD in the child for the total PCB sum may indicate the possibility of potentially unexamined PCB groupings that may increase risk for NDDs other than ASD. Focusing on ASD risk, Cheslack-Postava et al. also found increased risk of ASD among those above the 90th percentile of PCB concentration. Brown et al. found decreased ASD risk among those above the 75th percentile of PCB concentration, though neither study reported statistically significant results.
This study’s limitations include a small sample size, restriction to one trimester during pregnancy, the potential for missing important congeners and interactions with other exposures, and residual confounding. Though the analysis focused on one time point, the third trimester, PCB concentrations were relatively stable throughout the pregnancy for the participants with multiple observations. There may be additional mechanisms through which PCBs have the potential to increase risk for NDDs, but in order to minimized multiple hypothesis testing, this study remained focused on three priority PCB groupings. Though observational studies have opportunities for residual confounding, the multitude of potential confounders that the MARBLES study collected allowed control for multiple confounding factors.
This study was novel in the high-risk population examined, and adds to the literature regarding functional groupings of PCBs. A unique aspect in this study is the statistical analysis of non-lipid-standardized concentrations. Many previous studies have solely included results for lipid-standardized values due to the lipophilic property of PCBs. Over the course of a pregnancy lipids are highly variable based on a number of factors, including pre-pregnancy BMI and potential metabolic dysregulation before and during pregnancy (Lain and Catalano, 2007; Vahratian et al., 2010). PCB exposure may remain relatively steady throughout the pregnancy period and normalizing PCB levels to total lipids could alter the final values used in statistical analyses from actual levels present in the serum. Here we examined the third trimester, when lipids tend to increase significantly, therefore including both allows a more comprehensive interpretation due to lipid normalization. Additionally, animal models are not consistently able to standardize by lipids (Boix et al., 2010; Widholm et al., 2004), and providing both sets of results can start to bridge gaps between animal and human studies.
The non-monotonic dose response in the exploratory quartile analysis supports results from developmental PCB exposures in animal models, which highlight possible threshold effects. It should be noted that due to cell sizes <5 in the quartile and stratified analyses, caution should be used when interpreting the results. Rats developmentally exposed to an industrial mixture of PCBs showed learning and memory deficits at the lower PCB dose and the high PCB dose group performed similarly to the vehicle controls in the Morris water maze task (Yang et al., 2009). Similar non-monotonic effects were also seen both in vivo and in vitro with RyR-active PCBs (Wayman et al., 2012b; Yang et al., 2014; Yang et al., 2009). A study following women in Southern California also saw a slightly non-monotonic response, although non-significant, in their total PCB and ASD outcome measurement (Lyall et al., 2017b).
Further research in PCBs and ASD risk would also benefit from gene-environment interaction analysis. Although the MARBLES cohort is comprised of families with increased genetic susceptibility, the sample size in this study subset did not allow sufficient power for interaction analysis. A call has been placed to examine ASD risk from exposure to environmental toxins by varying genotypes (Stamou et al., 2013) based on evidence from animal studies (Lesiak et al., 2014; Wayman et al., 2012b). Additional studies, with adequate power, could provide insight to the level of risk that functionally grouped PCBs have in combination with different genetic variants.
5. Conclusions
Altogether, these findings build on the hypothesis that PCBs are plausible risk factors for ASD if specific function and mechanism of PCB congeners are considered, which aligns with previous literature also reporting elevated ORs for developmental PCB exposure and ASD outcome (Cheslack-Postava et al., 2013; Lyall et al., 2017b). In particular, this study implicates that these environmental contaminants could influence recurrence risk for ASD in younger siblings of children with ASD who are genetically pre-disposed. Further research using larger sample sizes and general or normal-risk populations are needed to add confidence to the findings presented here and their generalizability; however, by examining PCBs that are detectable in this Northern California high-risk population we are adding to current knowledge of this environmentally persistent toxin in genetically susceptible families which has the potential for informing public health intervention and education strategies.
Supplementary Material
Highlights:
PCBs may disrupt neurodevelopment in children if mothers are exposed prenatally
Grouping PCBs by function/structure may provide more specific risk insight
No evidence was found for associations between total PCBs and either Non-TD or ASD
Evidence for associations with RyR and dioxin-like PCBs needs further investigation
Acknowledgments
The authors thank Dr. Irva Hertz-Picciotto, original PI of the MARBLES study, for access to study data, and the MARBLES study participants.
Declaration of interest
Dr. Schmidt has received lodging for The Baby Siblings Research Consortium Meeting; travel and lodging for invited talks at the University of Sherbrooke, Sherbrooke, Québec, Canada; the University of California Santa Cruz. Santa Cruz, California (Lodging); Epigenomics 2016, Puerto Rico (Lodging); Neurotoxicity Society & International Neurotoxicology Association, Florianópolis, Brazil; RISE 2017 Second International Meeting on Environmental Health in Strasbourg. Strasbourg, France. Dr. Schmidt also received Autism Speaks grant funding to develop an online autism environmental questionnaire.
Dr. Iosif is a Department of Defense Peer Reviewer (honoraria), NIH Ad Hoc Study Section Member/Service (honoraria), and Lancet Psychiatry Reviewer (honorarium).
Dr. Ozonoff has received research grant funding from the National Institutes of Health and Autism Speaks, travel reimbursement and honoraria for editorial activities from Autism Speaks, Autism Science Foundation, and Wiley, and book royalties from Guilford Press and American Psychiatric Press, Inc.
Funding
The MARBLES Study has been supported by NIH grants from the National Institute of Environmental Health Sciences [grant numbers P01-ES011269, R01-ES020392, R01-ES025574, P30-ES023513, and R01-ES028089], the Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Network [grant number U54-HD079125], for the UC Davis Clinical and Translational Sciences Center [grant numbers NIHUL1-TR000002 and K12-HD051958], by U.S. Environmental Protection Agency [grants numbers R-829388 & R-833292], by Department of Defence [grant number AR110194], and by the UC Davis MIND Institute. Furthermore, work has been supported by EPA STAR [grant number RD-83329201] and NIH [grant number R24-ES028533 and P50-MH106438]. This study was also supported by [grant number T32-ES007059], the Floyd and Mary Schwall Medical Research Fellowship Program predoctoral fellowship, and [grant number F32-HD088016].
The University of California, Davis Institutional Review Board has approved this study, and informed consent was obtained prior to data and sample collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allain CC, et al. , 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20, 470–475. [PubMed] [Google Scholar]
- Berghuis SA, et al. , 2015. Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol. 89, 687–709. [DOI] [PubMed] [Google Scholar]
- Bernert JT, et al. , 2007. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 68, 824–31. [DOI] [PubMed] [Google Scholar]
- Boix J, et al. , 2010. Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved. Neuroscience. 167, 994–1003. [DOI] [PubMed] [Google Scholar]
- Boucher O, et al. , 2009. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect. 117, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect. 122, 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, et al. , 2018. Association of maternal insecticide levels with autism in offspring from a national birth cohort. Am J Psychiatry (Advanced Online Publication). doi: 10.1176/appi.ajp.2018.17101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo G, David H, 1973. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 19, 476–482. [PubMed] [Google Scholar]
- Casas M, et al. , 2015. Prenatal exposure to PCB-153, p,p′-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int. 74, 23–31. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2017. Facts about ASD. Available: <https://www.cdc.gov/ncbddd/autism/facts.html>(Accessed October 2017).
- Centers for Disease Control and Prevention, 2018. Data & statistics Available: <https://www.cdc.gov/ncbddd/autism/data.html>(Accessed April 2018).
- Cheslack-Postava K, et al. , 2013. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol Teratol. 38, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorgan JF, et al. , 1999. Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control. 10, 1–11. [DOI] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME, 2013. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 493, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt S, et al. , 2007. Record high peaks in PCB concentrations in the Arctic atmosphere due to long-range transport of biomass burning emissions. Atmos Chem Phys. 7, 4527–4536. [Google Scholar]
- Eguchi A, et al. , 2018. Maternal–fetal transfer rates of PCBs, OCPs, PBDEs, and dioxin-like compounds predicted through quantitative structure–activity relationship modeling. Environ Sci Pollut Res Int. 25, 7212–7222. [DOI] [PubMed] [Google Scholar]
- Erickson MD, Kaley RG, 2010. Applications of polychlorinated biphenyls. Environ Sci Pollut Res Int. 18, 135–151. [DOI] [PubMed] [Google Scholar]
- Eubig PA, et al. , 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect. 118, 1654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, et al. , 2014. Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments. Environ Sci Technol. 48, 8573–80. [DOI] [PubMed] [Google Scholar]
- Guo Z, et al. , 2018. Dioxins as potential risk factors for autism spectrum disorder. Environment International. 121, 906–915. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, et al. , 2011. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 68, 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, 2010. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 23, 103–10. [DOI] [PubMed] [Google Scholar]
- Hernán MA, et al. , 2004. A Structural Approach to Selection Bias. Epidemiology. 15, 615–625. [DOI] [PubMed] [Google Scholar]
- Herrick RF, et al. , 2007. Soil contamination from PCB-containing buildings. Environ Health Perspect. 115, 173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, et al. , 2016. Review of PCBs in US schools: a brief history, an estimate of the number of impacted schools, and an approach for evaluating indoor air samples. Environ Sci Pollut Res Int. 23, 1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, et al. , 2005. In Utero Polychlorinated Biphenyl Exposures in Relation to Fetal and Early Childhood Growth. Epidemiology. 16, 648–656. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Delwiche L, 2009. The rise in autism and the role of age at diagnosis. Epidemiology. 20, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, et al. , In Press. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: Design, protocols and preliminary data from the MARBLES study. Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf NB, et al. , 2009. Background levels of polychlorinated biphenyls in the U.S. population. Sci Total Environ. 407, 6109–19. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, et al. , 2014. Environmental Chemical Exposures and Autism Spectrum Disorders: A Review of the Epidemiological Evidence. Current Problems in Pediatric and Adolescent Health Care. 44, 277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, et al. , 2010. Exposure to environmental toxins in mothers of children with autism spectrum disorder. Psychiatry Investig. 7, 122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterhaus S, et al. , 2014. Polychlorinated biphenyls in the exterior caulk of San Francisco Bay Area buildings, California, USA. Environ Int. 66, 38–43. [DOI] [PubMed] [Google Scholar]
- Koh WX, et al. , 2015. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ Sci Technol. 49, 8105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lain KY, Catalano PM, 2007. Metabolic changes in pregnancy. Clin Obstet Gynecol. 50, 938–948. [DOI] [PubMed] [Google Scholar]
- Lancz K, et al. , 2015. Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int J Hyg Environ Health. 218, 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak A, et al. , 2014. The Environmental Neurotoxicant PCB 95 Promotes Synaptogenesis via Ryanodine Receptor-Dependent miR132 Upregulation. The Journal of Neuroscience. 34, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, et al. , 2013. Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography-tandem mass spectrometry in human serum and plasma. Talanta. 113, 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, et al. , 2017a. The changing epidemiology of Autism Spectrum Disorder. Annu Rev Public Health. 38, 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, et al. , 2017b. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ Health Perspect. 125, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, et al. , 2014. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ Sci Technol. 48, 13459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, et al. , 2017. Airborne PCBs and OH-PCBs Inside and Outside Urban and Rural U.S. Schools. Environ Sci Technol. 51, 7853–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, et al. , 2005. Concentrations of environmental chemicals associated with neurodevelopmental effects in U.S. population. Neurotoxicology. 26, 531–45. [DOI] [PubMed] [Google Scholar]
- Needham LL, et al. , 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 45, 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison CD, 2014. A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, et al. , 2007. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 28, 235–58. [DOI] [PubMed] [Google Scholar]
- Nichols BR, et al. , 2007. Age-specific reference ranges for polychlorinated biphenyls (PCB) based on the NHANES 2001–2002 survey. J Toxicol Environ Health A. 70, 1873–7. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, et al. , 2014. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry. 53, 398–407 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, et al. , 2011. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 128, e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, et al. , 1999. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr. 134, 33–41. [DOI] [PubMed] [Google Scholar]
- Pessah IN, et al. , 2010. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 125, 260–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, et al. , 2006. The aryl hydrocarbon receptor pathway and sexual differentiation of neuroendocrine functions. Endocrinology. 147, S33–42. [DOI] [PubMed] [Google Scholar]
- Phillips DL, et al. , 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Tox. 18, 495–500. [DOI] [PubMed] [Google Scholar]
- Pinter JK, et al. , 1967. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 121, 404–414. [DOI] [PubMed] [Google Scholar]
- Ribas-Fitó N, et al. , 2001. Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J. Epidemiol. Community Health. 55, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, et al. , 2005. Comparison of proposed frameworks for grouping polychlorinated biphenyl congener data applied to a case-control pilot study of prostate cancer. Environ Res. 98, 104–13. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, et al. , 2010. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 171, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, et al. , 2003. Effects of PCB Exposure on Neuropsychological Function in Children. Environmental Health Perspectives. 111, 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, et al. , 2011. Prenatal Vitamins, One-carbon Metabolism Gene Variants, and Risk for Autism. Epidemiology. 22, 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn D, et al. , 2008. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birthweight. Paediatr Perinat Epidemiol. 22, 202–13. [DOI] [PubMed] [Google Scholar]
- Stamou M, et al. , 2013. Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol. 36, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Centre de toxicology du Québec, 2018. AMAP: AMAP ring test for persistent organic pollutants in human serum. Available: <https://www.inspq.qc.ca/en/ctq/eqas/amap/description>(Accessed April 2018).
- Trinder P, 1969. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 6, 24–27. [Google Scholar]
- Vahratian A, et al. , 2010. Prepregnancy body mass index and gestational age-dependent changes in lipid levels during pregnancy. Obstet Gynecol. 116, 107–113. [DOI] [PubMed] [Google Scholar]
- Verhulst SL, et al. , 2009. Intrauterine Exposure to Environmental Pollutants and Body Mass Index during the First 3 Years of Life. Environmental Health Perspectives. 117, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil HJI, et al. , 2002. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. The Journal of Pediatrics. 140, 48–56. [DOI] [PubMed] [Google Scholar]
- Ward MH, et al. , 2009. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 117, 1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, et al. , 2012a. PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect. 120, 1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, et al. , 2012b. PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect. 120, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS, 2009. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 27, 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, et al. , 2004. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 82, 577–89. [DOI] [PubMed] [Google Scholar]
- Wolff MS, et al. , 1982. Disposition of polychlorinated biphenyl congeners in occupationally exposed persons. Toxicol Appl Pharmacol. 62, 294–306. [DOI] [PubMed] [Google Scholar]
- Wong PW, et al. , 1997. Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J Biol Chem. 272, 15145–15153. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. , 2014. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci. 138, 379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, et al. , 2009. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 117, 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


