Abstract
Background:
Animal studies suggest that prenatal exposure to diethylstilbestrol (DES) causes epigenetic alterations in primordial germ cells that affect the next generation, but human studies are sparse.
Methods:
We assessed hormonally mediated outcomes in third generation women whose mothers were prenatally DES-exposed and unexposed.
Results:
Compared to the unexposed, DES-exposed third generation women had an increased risk of irregular menses and amenorrhea; the respective prevalence ratios and 95% confidence intervals (CI) in follow-up data were 1.32 (95% CI: 1.10, 1.60) and 1.26 (95% CI: 1.06, 1.49); associations were more apparent in third generation women whose prenatally DES-exposed mothers were affected by vaginal epithelial changes. The follow-up data also indicated an association with preterm delivery (relative risk (RR): 1.54; 95% CI: 1.35, 1.75).
Conclusion:
DES third generation women may have an increased risk of irregular menstrual cycles, amenorrhea, and preterm delivery, consistent with intergenerational effects of endocrine disrupting chemical exposure in humans.
Keywords: Diethylstilbestrol, DES, third generation women, granddaughters, intergenerational, epigenetic
Introduction
Diethylstilbestrol (DES), belongs to the family of endocrine disrupting chemicals (EDC) and is an established transplacental teratogen and carcinogen in humans (reviewed in1). A synthetic, nonsteroidal estrogen, DES was administered to pregnant women under the mistaken belief it would reduce pregnancy complications and losses. From the late 1930s through the early 1970s, DES was given to nearly two million pregnant women in the US alone2. Use of DES in pregnancy was discontinued after a seminal report showed a strong association with vaginal clear cell adenocarcinoma in prenatally exposed women3. A recent analysis of the US National Cancer Institute (NCI) DES Combined Cohort Follow-up Study showed elevated relative risks of twelve adverse health outcomes, including reproductive tract anomalies, infertility, pregnancy complications, cervical intraepithelial neoplasia, and clear cell adenocarcinoma in women with prenatal DES exposure4.
Laboratory studies of mice show alterations in gene expression (reviewed in5,6) as well as an increased occurrence of reproductive tract structural anomalies and tumors in females born to animals exposed to DES in utero 7,8,9. The mouse model has accurately predicted or replicated outcomes observed in women given DES during pregnancy and women exposed in utero 10 Thus, evidence of epigenetic alterations and intergenerational effects in mice has raised concerns that gestational exposure to DES alters gene expression in fetal primordial germ cells, with health consequences for the next generation5,6,10. Studies in humans have provided some support for an intergenerational transmission of DES effects. A few studies of third generation men; i.e., sons born to prenatally DES-exposed mothers, showed an increased risk of hypospadias11–13, although the association was equivocal in our previous report, in which DES exposure status was verified14. Our previous studies of third generation women; i.e., daughters born to prenatally DES-exposed mothers, showed an increased frequency of menstrual irregularity, and suggested possibly increased risks of infertility15, and ovarian cancer 16. An earlier study17, as well as our previous report15, found no association between DES and menarcheal age, and a small clinical study found no physical evidence of reproductive tract anomalies in third generation women18.
In this report, we assessed outcomes in third generation women – those whose mothers were and were not prenatally exposed to DES. Due to the endocrine disrupting potential of DES, we focused our investigation on reproductive and hormonally-mediated outcomes. Our analyses were based on combined data from two baseline data collection phases, and on follow-up data collected from a subset of third generation women.
Methods
NCI DES Combined Cohort Follow-up Study.
In the early 1990s, the NCI established the DES Combined Cohort Follow-up Study to assess health outcomes in women exposed to DES during pregnancy (first generation), and in women and men who were prenatally DES-exposed (second generation). As part of that study, previously followed cohorts were reassembled, and new cohorts were assembled for the first time. The previously followed cohorts of DES-exposed and unexposed first generation women were those who participated in the Women’s Health Study (WHS) (New Hampshire and Maine), or in a clinical trial of DES conducted at the University of Chicago (the Dieckmann trial). Newly identified second generation participants comprised the prenatally DES-exposed and unexposed daughters and sons of women who participated in the WHS or the Dieckmann trial. Previously followed second generation cohorts included prenatally DES-exposed and unexposed men who had participated in a study at the Mayo Clinic; prenatally DES-exposed and unexposed women and men identified through a private infertility clinic in Boston; and prenatally DES-exposed women who participated in the National Cooperative Diethylstilbestrol Adenosis Project (DESAD) project, along with their unexposed sisters or unexposed women identified through the same record sources.
For all first and second generation study participants, exposure to DES, or the absence of exposure, was verified by the medical record or a physician’s note. Approximately half of the prenatally DES-exposed second generation women, those who participated in DESAD or the Dieckmann clinical trial, had been classified with regard to the presence or absence of vaginal epithelial changes (VEC), a marker of early and high cumulative DES exposure19, when participating in these studies.
In 1994, the first NCI DES Combined Cohort Follow-up Study questionnaires were mailed to 6,551 second generation women, including 4,459 exposed to DES in utero, and 2,092 unexposed. Completed questionnaires were returned by 5,707 women (88% of the exposed and 84% of the unexposed). In subsequent years, responses to questionnaire mailings were 94% and 94% (exposed, unexposed) in 1997, 90% and 90% (exposed, unexposed) in 2001, and 82% and 85% (exposed, unexposed) in 2006. A detailed description of the second generation women enrolled in the NCI DES Combined Cohort Follow-up Study has been published previously20.
In 2000, the NCI assembled a cohort of third generation women, also known as the granddaughters generation. Potential third generation study participants were the granddaughters of first generation women who were known to be DES-exposed and unexposed during pregnancy, and the daughters of second generation women (prenatally DES-exposed and unexposed) participating in the NCI Combined Cohort Follow-up Study. We identified potential third generation study participants by reviewing the parity records of their prenatally DES-exposed and unexposed (second generation) mothers. The parity review identified 1781 (966 exposed and 815 unexposed) age-eligible (≥ 18) potential third generation study participants. We asked the second generation women to provide their daughters’ contact information. Contact information was obtained for 898 (515 exposed (53.3%), and 383 unexposed (47.0%) potential third generation study participants. Of these, 793 (463 exposed (89.9%), 330 unexposed (86.2%) third generation women completed a baseline questionnaire.
From 2009 – 2012, using similar methods, we enrolled a new group of age-eligible third generation women who had turned 18 since the previous baseline enrollment phase. Of the 1195 (857 exposed, 338 unexposed) newly identified third generation women, contact information from the mother was obtained for 577, including 416 (48.5%) exposed and 161 (47.6%) unexposed. Of these, 472, including 333 (80.0%) exposed, and 139 (86.3%) unexposed third generation women completed the baseline questionnaire.
Over the same time period, we sent a follow-up questionnaire to 789 (462 exposed and 327 unexposed) third generation women who were previously enrolled in the 2001 baseline study (4 previous participants could not be re-approached due to Institutional Review Board regulations at one of the study centers). Of these, 381 (82.5%) exposed and 280 (85.6%) unexposed participated in follow-up. On average, the interval between completing the baseline and follow-up questionnaires was 8.8 years in both the exposed and unexposed third generation women. The average age at follow-up was 32.6 in the exposed and 34.3 in the unexposed third generation women. The baseline and follow-up questionnaires were completed by mail, telephone, or online using a web-based instrument.
The current study was approved by the institutional review boards at each participating center (Dartmouth College, Boston University, New England Medical Center, the University of Chicago, and Texas Methodist Health Center), and at the NCI.
Outcome variables
On both the baseline and follow-up questionnaires, we asked about the regularity of menstrual periods, defined as usually predictable within 5 days. The follow-up questionnaire asked whether periods were usually predictable within 5 days when not pregnant or taking hormones. On both questionnaires, amenorrhea was defined as six or more weeks without a menstrual period in the past 12 months while not pregnant, breast-feeding, or using oral contraceptives. The follow-up questionnaire asked women whether they had experienced menopause, defined as 12 or more months without a menstrual period.
On the baseline questionnaire, infertility was defined as ever attempting to become pregnant for at least one year without success; on the follow-up questionnaire, the time-frame for the same question was since the date of the most recent (i.e., baseline) questionnaire response, which was printed on the questionnaire page. Pregnancy and pregnancy outcomes ascertained on both questionnaires included ever having been pregnant, ever having had a live birth and the following adverse events: ectopic pregnancy, miscarriage (first or second trimester loss), third trimester pregnancy loss/stillbirth, preterm delivery (a live birth occurring more than 3 weeks before the due date), and neonatal death (in the first month of life). We also ascertained first pregnancy outcomes in the baseline and follow-up data. In all analyses, pregnancy outcomes were assessed in women who had ever been pregnant.
The baseline questionnaire asked third generation study participants whether they usually wrote with their right hand, left hand, or either (analyzed as left/either vs. right in the analysis). Four questions were asked only on the follow-up questionnaire, including whether a health care provider ever told them they had endometriosis or a thyroid condition. The follow-up questionnaire also asked about usual sexual partners in adulthood; the response options of only men, mostly men, mostly women, only women, no sexual contact were analyzed as only men versus all other responses combined. Finally, the follow-up questionnaire asked the participant’s own birth weight, which was converted to ounces in the analysis.
Statistical approach
The primary analyses assessed DES in relation to outcomes reported in the third generation women’s combined baseline data (796 exposed; 469 unexposed) from the two baseline enrollment periods. We used log binomial regression models with robust variance estimates to generate prevalence ratios (PR) and 95% confidence intervals (CI) to estimate the association between DES and prevalent outcomes of interest21. The same modeling approach was used to explore the role of the prenatally DES-exposed mothers’ VEC status in relation to outcomes in their daughters, the DES-exposed third generation women.
We performed additional analyses of incident outcomes occurring during follow-up in the subset of third generation women who had participated in the earlier baseline and in the follow-up study (381 exposed; 280 unexposed). For these analyses, we used log binomial regression models with robust variance estimates to calculate risk ratios (RR) and 95% CI. Stratified analyses were used to assess infertility as an outcome within age groups (<30; ≥30 years). We used linear regression to assess mean differences (MD) and 95% CI between the exposed and unexposed third generation women for continuous outcomes. All analyses were minimally adjusted for age (continuous), and robust cluster variance estimates were used to account for correlation within original cohort22. The subsets of third generation women involved in the combined baseline and follow-up analyses are shown in the Diagram.
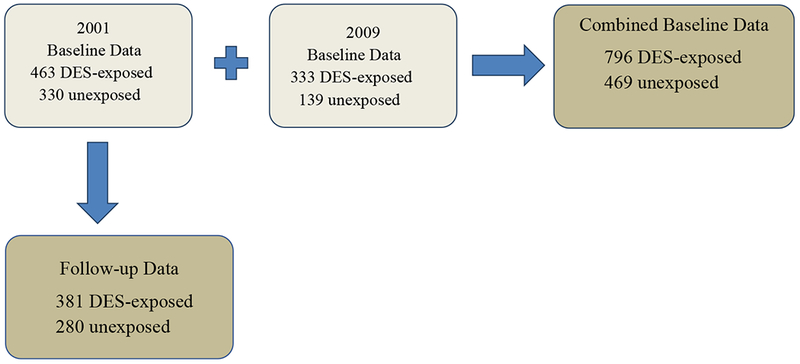
Diagram showing Third Generation Study women in the present analysis (dark gray).
Results
The frequency distribution of age and age-adjusted covariates among third generation women at baseline is shown in Table 1. Most study participants were white and less than thirty years of age. Compared with the unexposed, DES-exposed participants were generally younger, were more likely to have completed college, and to have been breast-fed as infants. The DES-exposed were slightly less likely to be under- or over-weight, to have ever married, smoked cigarettes, or used oral contraceptives.
Table 1.
Third generation women’s characteristicsa at baseline, according to the mothers’ prenatal DES exposure status
| Mother’s prenatal DES exposure status | ||
|---|---|---|
| Characteristic | Exposed (n = 796) |
Unexposed (n = 469) |
| No. (%) | No. (%) | |
| Age | ||
| ≤20 | 121 (15.2) | 71 (15.1) |
| 20-24 | 383 (48.1) | 190 (40.5) |
| 25-29 | 208 (26.1) | 123 (26.2) |
| ≥30 | 84 (10.6) | 85 (18.1) |
| Average age | 24.20 (4.13) | 25.09 (5.00) |
| Race/Ethnicity | ||
| White | 777 (98.0) | 455 (96.9) |
| Non-white | 16 (2.0) | 13 (3.1) |
| Education | ||
| HS graduate or less | 321 (39.8) | 212 (44.0) |
| Post HS | 119 (14.8) | 86 (18.6) |
| College graduate | 352 (45.4) | 171 (37.4) |
| Breast-fed as infant | ||
| No | 196 (23.4) | 143 (27.2) |
| Yes | 522 (68.5) | 274 (63.3) |
| Unknown | 68 (8.2) | 44 (9.4) |
| BMI | ||
| ≤18.5 | 30 (3.8) | 28 (6.0) |
| 18.5-<25 | 548 (69.8) | 284 (60.9) |
| 25-<30 | 134 (17.1) | 94 (20.2) |
| ≥30 | 73 (9.3) | 60 (12.9) |
| Marital status | ||
| Never married | 615 (81.0) | 333 (73.4) |
| Ever married | 178 (19.0) | 136 (26.6) |
| Ever used oral contraceptives | ||
| No | 169 (21.8) | 88 (18.6) |
| Yes | 624 (78.2) | 379 (81.4) |
| Ever smoked cigarettes | ||
| No | 585 (74.2) | 327 (71.3) |
| Yes | 209 (25.8) | 141 (28.7) |
Abbreviations: DES, diethylstilbestrol; HS, high school; BMI, body mass index.
Characteristics are age-adjusted based on the age distribution in the unexposed.
At the time of baseline enrollment, nearly all third generation women had reached menarche (791/796 exposed; 466/469 unexposed). The average age at first menses was 12.5 in the exposed and 12.7 in the unexposed (MD: −0.20; 95% CI: −0.57, 0.16). We found no indication that DES exposure was associated with menarche before 11 years of age (PR: 0.93; 95% CI: 0.78,1.18). Among those with regular periods, regularization of menstrual periods occurred on average at age 14.3 in the exposed and 14.0 in the unexposed (MD: −0.25; 95% CI: −0.81, 0.31). Comparing the DES-exposed to the unexposed, the PR was 1.04 (95% CI: 0.86,1.25) for attaining menstrual regularization at age 15 or later. Analyses using other dichotomous outcome groupings gave no indication that menarche or menstrual regularization occurred at an earlier or later age in the exposed compared to the unexposed (data not shown).
The age and cohort adjusted PR for usual menstrual regularity and amenorrhea during the past year, as reported at baseline and at follow-up, are shown in Table 2. In the baseline data, comparing DES-exposed to unexposed third generation women, the PR was 1.22 (95% CI: 1.12,1.33) for irregular menstrual cycles, and 1.22 (95% CI: 0.85, 1.75) for amenorrhea. In the follow-up data, the PR was 1.32 (95% CI: 1.10, 1.60) for irregular menstrual cycles, and 1.26 (95% CI: 1.06, 1.49) for amenorrhea. None of the third generation women had experienced menopause.
Table 2.
Menstrual outcomes in third generation women, according to their mother’s prenatal DES exposure status..
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Mother’s prenatal DES exposure status | Mother’s prenatal DES exposure status | |||||
| Outcome | Exposed (n = 796) No. (%) |
Unexposed (n = 469) No. (%) |
PRa (95% CI) | Exposed (n = 381) No. (%) |
Unexposed (n = 280) No. (%) |
PRa (95% CI) |
| Menstrual cycles usually regular | ||||||
| Yes | 623 (78.9) | 385 (83.0) | 287 (75.3) | 226 (80.7) | ||
| No | 167 (21.1) | 79 (17.0) | 1.22 (1.12,1.33) | 94 (24.7) | 54 (19.3) | 1.32 (1.10, 1.60) |
| Amenorrhea in last 12 months | ||||||
| No | 661 (83.8) | 405 (86.9) | 314 (82.4) | 238 (85.0) | ||
| Yes | 128 (16.2) | 61 (13.1) | 1.22 (0.85,1.75) | 67 (17.6) | 42 (15.0) | 1.26 (1.06, 1.49) |
Abbreviations: DES, diethylstilbestrol; PR, prevalence ratios; CI, confidence intervals.
For outcomes prevalent at baseline and at follow-up, adjusted for age and cohort.
We explored the role of VEC among third generation women whose mothers had been members of the DESAD and Dieckmann cohorts, in which DES-exposed participants were examined and classified (positive/negative) with regard to VEC status. In the combined baseline data, we identified 678 exposed and 222 unexposed third generation women whose mothers had been members of the DESAD and Dieckmann cohorts. Of the 678 DES-exposed third generation women, 322 (47.5%) had mothers who were positive for VEC. Compared to the unexposed, the PR for menstrual irregularity was 1.30 (95% CI: 1.17, 1.46) for DES-exposed participants whose mothers had VEC, and 1.04 (95% CI: 0.74, 1.46) for DES-exposed participants whose mothers did not have VEC. The PR for amenorrhea at baseline were similar regardless of the mothers’ VEC status (PR: 0.99; 95% CI: 0.71, 1.37 with VEC, and PR: 0.96; 95% CI: 0.69, 1.34 without VEC).
In the follow-up data, we identified 298 DES-exposed and 118 unexposed third generation women whose mothers’ VEC status was known. Of the 298 DES-exposed third generation women, 130 (43.6%) had mothers who were positive for VEC. Compared to the unexposed, the PR for menstrual irregularity was 1.33 (95% CI: 1.04, 1.70) for DES-exposed participants whose mothers had VEC, and 1.16 (95% CI: 0.82, 1.63) for DES-exposed participants whose mothers did not. For amenorrhea, the PR at follow-up was 1.48 (95% CI: 0.86, 2.57) for DES-exposed third generation women whose mothers had VEC and 1.16 (95% CI: 0.68, 1.96) for DES-exposed women whose mothers did not.
Table 3 shows the age and cohort adjusted PR and RR associated with pregnancy, pregnancy outcomes, and infertility in the baseline and follow-up data, respectively. At baseline, DES-exposed third generation women, compared to the unexposed, were less likely to have ever been pregnant (PR: 0.91; 95% CI: 0.86, 0.97), but fewer DES exposed (19.0%) than unexposed (26.6%) were married at baseline. Among baseline participants who had ever been pregnant, the average age at first pregnancy was similar for the exposed (22.3) and unexposed (21.5); MD: 0.75 (95% CI: − 0.56,2.06). Also among baseline participants who had ever been pregnant, we found no association between DES and having had a live birth (PR: 1.04; 95% CI: 0.87, 1.25). In the follow-up data, the RR was 0.99 (95% CI: 0.92, 1.06) for DES in relation to pregnancy during follow-up, and 1.08 (95% CI: 1.00, 1.16) for a live birth during follow-up.
Table 3.
Reproductive outcomes in third generation women, according to their mother’s prenatal DES exposure status.
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Mother’s prenatal DES exposure status | Mother’s prenatal DES exposure status | |||||
| Outcome | Exposed (n =796) No. (%) |
Unexposed (n = 469) No. (%) |
PRa (95% CI) | Exposed (n = 381) No. (%) |
Unexposed (n = 280) No. (%) |
RRb (95% CI) |
| Ever been pregnant | ||||||
| No | 588 (74.0) | 304 (64.8) | 144 (44.2) | 87 (38.7) | ||
| Yes | 207 (26.0) | 165 (35.2) | 0.91 (0.86,0.97) | 182 (55.8) | 138 (61.3) | 0.99 (0.92,1.06) |
| Ever had a live birthc | ||||||
| No | 64 (31.2) | 48 (29.3) | 18 (9.9) | 22 (15.9) | ||
| Yes | 141 (68.8) | 116 (70.7) | 1.04 (0.87,1.25) | 164 (90.1) | 116 (84.1) | 1.08 (1.00, 1.16) |
| Ever have an adverse pregnancy outcomed | ||||||
| No | 142 (68.6) | 118 (71.5) | 129 (70.9) | 98 (71.0) | ||
| Yes | 65 (31.4) | 47 (28.5) | 1.12 (0.87,1.44) | 53 (29.1) | 40 (29.0) | 1.01 (0.72,1.41) |
| Miscarriaged | 48 (23.2) | 36 (21.8) | 1.06 (0.75,1.50) | 31 (17.0) | 29 (21.0) | 0.80 (0.40,1.63) |
| Preterm deliveryd | 21 (10.1) | 14 (8.5) | 1.31 (0.81,2.10) | 22 (12.1) | 11 (8.0) | 1.54 (1.35,1.75) |
| Ectopicd | 6 (2.9) | 3 (1.8) | 1.57 (0.41,5.95) | 7 (3.9) | 1 (0.7) | 6.00 (0.45,78.3) |
| Infertility | ||||||
| No | 757 (95.3) | 448 (95.5) | 345 (91.0) | 246 (88.2) | ||
| Yes | 37 (4.7) | 21 (4.5) | 1.53 (0.92,2.53) | 34 (9.0) | 33 (11.8) | 0.85 (0.56, 1.29) |
| Sought medical help for infertility | ||||||
| No | 764 (96.2) | 449 (95.7) | 336 (88.4) | 253 (90.7) | ||
| Yes | 30 (3.8) | 20 (4.3) | 1.38 (0.83,2.28) | 44 (11.6) | 26 (9.3) | 1.49 (0.89,2.49) |
Abbreviations: DES, diethylstilbestrol; PR, prevalence ratios; RR, risk ratios; CI, confidence intervals.
For outcomes prevalent at baseline, adjusted for age and cohort.
For new events occurring during follow-up, adjusted for age and cohort.
All pregnancy outcomes assessed among women who were ever pregnant.
The denominator for calculating percents is the number of women ever pregnant.
In the baseline data, the PR was 1.12 (95% CI: 0.87, 1.44) for DES in relation to ever having had at least one adverse pregnancy outcome overall, 1.06 (95% CI: 0.75,1.50) for miscarriage, and 1.31 (95% CI: 0.81,2.10) for preterm delivery (Table 3). The latter finding was attenuated when further adjusted for body mass index (BMI) and infertility (PR: 1.16; 95% CI: 0.75, 1.82). In the follow-up data, the RR was 1.01 (95% CI: 0.72, 1.41) for DES in relation to an adverse pregnancy outcome overall, 0.80 (95% CI: 0.40, 1.63) for miscarriage, and 1.54 (95% CI: 1.35, 1.75) for a preterm delivery. The RR for a preterm delivery was 1.67 (95% CI: 1.31,2.12) when additionally adjusted for BMI and infertility. We were unable, due to the small numbers of cases, to assess whether VEC influenced the risk of preterm birth or other adverse pregnancy outcomes in the DES-exposed third generation women.
Based on 6 exposed and 3 unexposed cases, the PR for ectopic pregnancy was 1.57 (95% CI: 0.41, 5.95) in the baseline data. Seven new exposed ectopic pregnancies and one unexposed ectopic pregnancy were reported at follow-up; the RR was 6.00 (95% CI: 0.45,78.30). Combining the baseline findings with new cases reported during follow-up, there were 13 ectopic pregnancies in the exposed and 4 in the unexposed.
Although statistical power was limited, we explored DES in relation to miscarriage and preterm delivery as first pregnancy outcomes (not in table). For DES in relation to preterm delivery as a first pregnancy outcome in the baseline data, the age and cohort adjusted PR was 3.08 (95% CI: 0.97, 9.80), and 2.72 (95% CI: 1.02, 7.23) when additionally adjusted for BMI and infertility. In the follow-up data, the age and cohort adjusted RR for DES in relation to preterm delivery as a first pregnancy outcome was 1.53 (95% CI: 0.50, 4.66), and 2.11 (95% CI: 0.95, 4.67) after further adjustment for BMI and infertility. There was no indication DES was associated with overall risk of an adverse pregnancy outcome or of miscarriage at first pregnancy in either the baseline or follow-up data.
In the baseline data, the PR for DES exposure in relation to infertility was 1.53 (95% CI: 0.92, 2.53) (Table 3). When further adjusted for BMI, the PR was 1.75 (95% CI: 1.06, 2.88). The PR for DES exposure in relation to infertility was 1.65 (95% CI: 0.67, 4.05) among women who were age 30 or older at baseline. Also at baseline, the PR was 1.38 (95% CI: 0.83, 2.28) for DES in relation to seeking medical help for infertility; this finding was unchanged after additional adjustment for education (PR: 1.38; 95% CI: 0.83, 2.31). In analyses confined to follow-up events, the RR was 0.85 (95% CI: 0.56, 1.29) for the association between DES and infertility. The RR was 1.49 (0.89, 2.49) for seeking medical treatment for infertility, and similar when additionally adjusted for education (RR: 1.41; 95% CI: 0.88, 2.28) (not in table). Among women who sought medical attention, the baseline PR was 0.77 (95% CI: 0.59, 0.99) and the follow-up RR was 1.01 (95% CI: 0.87,1.18) for the relationship between DES and infertility attributed to the woman or both partners, rather than the male partner or an unknown cause (not in table).
The PR for DES in relation to left-handedness, available from baseline data, was 0.82 (95% CI: 0.42, 1.61) (not in table). Questions regarding endometriosis, thyroid conditions, sexual orientation, and birth weight were asked for the first time at follow-up (not in table). The PR was 1.06 (95% CI: 0.48, 2.36) for DES in relation to having had a diagnosis of endometriosis. Among those affected, mean age at diagnosis of endometriosis was comparable for the exposed (25.2) and unexposed (25.0) women (MD: −0.49; 95% CI: − 5.21, 4.23). The PR was 1.63 (95% CI: 0.94, 2.82) for DES in relation to ever having had a thyroid condition. The PR was 0.76 (95% CI: 0.54, 1.07) for the relation between DES and not having exclusively male partners in adulthood, compared to only male partners. The mean birth weight in ounces was significantly lower in the exposed (109.0 ounces) compared to the unexposed (116.6 ounces); MD: −8.54 (95% CI: − 13.24, − 3.84).
Discussion
Our data suggest an increased risk of menstrual irregularity, amenorrhea, preterm birth, and possibly ectopic pregnancy in the DES-exposed third generation women; i.e., women whose mothers were exposed prenatally to DES, and whose grandmothers were given DES during pregnancy. These findings are consistent with the notion that prenatal exposure to DES can influence outcomes in the next generation of offspring.
Vaginal epithelial changes (VEC) are a non-obligate precursor of clear cell adenocarcinoma (CCA) of the vagina, the disease that signaled the adverse effects of prenatal DES exposure3. VEC, which are associated with high and early gestational doses of DES19, affect 34-91% of prenatally DES-exposed women, and rarely are seen in unexposed women (reviewed in 23). Associations with established DES-related outcomes are generally stronger in prenatally DES-exposed women who are also affected by VEC4. Thus, in addition to being a marker of early and high dose exposure, VEC may also serve as a marker of DES effects on the developing fetus. In our baseline and follow-up data, associations with menstrual irregularity were more apparent in exposed third generation women whose prenatally exposed mothers were affected by VEC. Additionally, although not statistically significant, the association with amenorrhea in the follow-up data was more evident in third generation women whose prenatally DES-exposed mothers had VEC. These findings, although preliminary, lend additional support to the possibility that prenatal exposure to DES has intergenerational effects in humans.
Reports of adult disease in prenatally exposed populations are based largely on individuals with prenatal exposure to DES4 or prenatal exposure to severe undernutrition (e.g., the Dutch Hunger Winter) 24. Less is known, however, about the effects of prenatal exposures on adult disease in the next generation of offspring; i.e., the third generation. Overviews of studies of prenatal under-nutrition note equivocal evidence of health effects in the offspring of prenatally exposed individuals24, or effects limited to descendants of the grandpaternal line25. In the setting of DES, findings in the third generation are suggestive, but can be inconsistent (e.g. with regard to elevated risk of hypospadias11–14), or preliminary (e.g., with regard to increased risk of ovarian cancer16).
An increasing body of evidence, however, suggests that prenatal exposures may induce epigenetic alterations of primordial germ cells in the developing fetus, affecting health outcomes in the next generation26. Numerous studies of animal species have shown diverse prenatal exposures (reviewed in 26), including the EDC bisphenol A27, DDT28, methoxychlor29, and vinclozolin30 in relation to outcomes in multiple descendant generations. Although studies in laboratory mice have shown epigenetic changes in prenatally DES-exposed animals as well as an increased frequency of reproductive tract tumors in the next generation of offspring 9, only one study has shown tumorigenic effects extending to the fourth generation of females; i.e., the granddaughters of animals prenatally exposed to DES31. Because the fourth generation lacks direct DES exposure to germ cells, this finding suggests true transgenerational transmission of epigenetic alterations, and raises concerns about multi-generational DES effects in humans31. To our knowledge, fourth generation effects of prenatal exposures in humans have not been reported.
Consistent with a previous study of third generation women17, as well as our earlier report arising from a subset of the women studied here15, the current data do not support an association between DES exposure and age at menarche. Studies of menstrual irregularity in prenatally exposed women have produced mixed results; at least one study32, but not others33,34 have shown an association with DES. As in our previous report of third generation women15, the present analysis indicated modestly elevated risks of irregular menstrual periods and amenorrhea, although the latter finding was statistically significant only in the follow-up data. Associations seen in the follow-up data are unlikely to reflect baseline enrollment bias. Although self-selection among the affected exposed may have occurred at both baseline and follow-up, menstrual irregularity and amenorrhea are not established outcomes in prenatally DES-exposed women, so greater participation among exposed third generation women with these conditions seems unlikely. We did not assess patterns of oral contraceptive use, so cannot address the possibility that DES-exposed third generation women experienced amenorrhea more often than the unexposed due to more frequent interruptions in their use of oral contraceptives. However, the proportion of women using OCs was similar in the exposed and unexposed, and there is no a priori reason to suspect patterns of use were different in the two groups.
Studies of laboratory mice have shown infertility in prenatally DES-exposed dams35, but not in their female offspring7. In the present study, an association between DES and infertility was suggested by the baseline data, but the confidence intervals were wide, and the follow-up data, which comprised older women, most of whom had started their families, did not indicate an association. Infertility is an established consequence of prenatal DES exposure in women4,32,34. To the extent that awareness of this association carried over to the next generation, our baseline findings may reflect greater initial enrollment among exposed women who had experienced infertility, rather than a true association. While the follow-up data may reflect a true absence of association, we cannot entirely rule-out the possibility that participation in follow-up was elevated among unexposed women who experienced infertility. Although we found no evidence, at baseline or follow-up, that infertility was due to a problem solely with the woman, DES-exposed women were more likely to seek medical treatment for infertility, perhaps reflecting heightened concern. A more complete assessment of infertility will be possible when the women are older.
The baseline data suggested that DES-exposed third generation women, compared to the unexposed, were less likely to have been pregnant. However, fewer exposed women had been married, and the apparent delay in pregnancy is likely an artifact of delayed marriage. The women were young at baseline, and most had never been married or pregnant. As with infertility outcomes, a more meaningful assessment of pregnancy outcomes will become possible as the women age. The follow-up data, involving older women, did not indicate a difference in the likelihood of pregnancy for the exposed and unexposed.
Increased risk of several adverse pregnancy outcomes, including miscarriage, preterm delivery, stillbirth, ectopic pregnancy, and neonatal death are known to affect women who were prenatally exposed to DES4. In the present study, we did not find an association between DES and overall risk of at least one adverse pregnancy outcome, nor between DES and risk of miscarriage. An increased risk of preterm delivery was evident in the follow-up data, and suggested as a possible outcome of first pregnancy in both the baseline and follow-up data. Although the numbers were small, and confidence intervals were wide, the baseline and follow-up data also suggested a possible association between DES and ectopic pregnancy. Ectopic pregnancy usually arises from tubal irregularities and is strongly associated with prenatal DES exposure, which is known to cause reproductive tract anomalies4. We do not have sufficient data concerning the indication for DES in the grandmother to determine whether adverse pregnancy outcomes in the third generation might resemble those of their grandmothers.
Although small studies of prenatally DES-exposed women have suggested an association with bisexual or same-sex orientation36,37, the findings of one36 were not confirmed in a later replication effort38, nor were they confirmed in the large NCI study of women with prenatal DES exposure39. The present study of third generation women provides no indication that DES exposure is associated with having nonheterosexual partners in adulthood. The data were limited, however, for examining separate variables representing exclusively same sex partners, bisexuality or lack of sexual contact. Also, we did not query women for gender identity.
A few studies have suggested a relation between prenatal DES exposure and left-handedness, a marker of brain lateralization and masculinization40,41, but this was not seen in the NCI study of prenatally exposed women39, and we found no evidence, in the present study, of an association between DES and left-handedness in third generation women.
Our data suggested a possible increase of thyroid conditions in exposed women who responded to the follow-up questionnaire. Emerging evidence suggests that prenatal exposure to EDC may disrupt thyroid function42,43, but data are lacking for EDC effects in the offspring generation. Finally, compared with the unexposed, DES-exposed third generation women were of lower birth weight, a finding that was expected due to pregnancy complications, particularly preterm delivery, experienced by their mothers.
Conclusions.
Our data suggest an increased risk of menstrual irregularity, amenorrhea, preterm birth, as well as a possibly increased risk of ectopic pregnancy in the daughters of women who were exposed prenatally to DES. In addition, irregular menstrual periods were more evident in DES-exposed third generation women whose mothers had VEC, a marker of DES exposure and effects. These findings are consistent with an intergenerational transmission of epigenetic alterations affecting the primordial germ cells of the DES-exposed fetus. The intergenerational transmission of DES effects has implications for the influence of other pharmaceutical and environmental EDC on human health. Additional follow-up of this relatively young cohort is needed to determine whether DES is related to infertility and specific adverse pregnancy outcomes, in particular, ectopic pregnancy.
Highlights.
Studies of mice indicate intergenerational effects of DES exposure; i.e., effects in the offspring of prenatally DES-exposed animals.
Using data from the US National Cancer Institute Third Generation DES Study, we assessed DES exposure in relation to outcomes in a cohort of third generation women whose mothers were prenatally DES-exposed and unexposed.
The results suggested that third generation DES-exposed women have increased risks of irregular menstrual periods and amenorrhea. These risks generally were more apparent in women whose prenatally DES-exposed mothers had vaginal epithelial changes.
The data also suggested an elevated risk of preterm birth and possibly of ectopic pregnancy in the third generation DES-exposed women.
The data did not indicate an increase in same-sex orientation in DES-exposed third generation women.
DES-exposed third generation women have an increased risk of menstrual aberrations and possibly of specific adverse reproductive outcomes. These findings may have implications for intergenerational effects of endocrine disrupting chemicals in humans.
Acknowledgments:
We are grateful for the technical support provided by Judith Harjes, Suzanne Lenz, Helen Bond, Diane Anderson, and Mary Ziegler. Special thanks are due to the women who participated in this study, and to their mothers. We dedicate this paper to the memory of our colleague Raymond Kaufman, MD, an early pioneer in DES research who had a special interest in the Third Generation Study.
This study was supported by the US National Cancer Institute, Contracts HHSN261201500027C and HHSN261201000120C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report.
References
- 1.Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects C Embryo Today, 2013; 99: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noller KL. In utero exposure to diethylstilbestrol In: Jones HW III, Wentz AC, Burnett LS, eds. Novak’s Textbook of Gynecology (11th Edition). Baltimore: Williams and Wilkins, 1988; p. 623–42. [Google Scholar]
- 3.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 1971;284:878–81. [DOI] [PubMed] [Google Scholar]
- 4.Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. New Engl J med. 2011. ;365(14):1304–14. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan JA, Burow M, Chiang T-C, Li SF. Gene imprinting in developmental toxicology: a possible interface between physiology and pathology. Toxicol Ltrs 2001; 120: 161–4. [DOI] [PubMed] [Google Scholar]
- 6.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 2006;147:S11–7. [DOI] [PubMed] [Google Scholar]
- 7.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 1998; 19: 1655–63. [DOI] [PubMed] [Google Scholar]
- 8.Turusov VS, Trukhanova LS, Parfenov YD, Tomatis L. Occurrence of tumors in descendants of CBA male mice prenatally treated with diethylstilbestrol. Int J Cancer 1992;50:131–5. [DOI] [PubMed] [Google Scholar]
- 9.Walker BE. Tumors in female offspring of mice exposed prenatally to diethylstilbestrol. J Natl Cancer Inst 1984;73:133–40. [PubMed] [Google Scholar]
- 10.Newbold R Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect 1995; 103 Suppl 7: 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE; OMEGA Project Group. Lancet 2002; 359: 1102–7. [DOI] [PubMed] [Google Scholar]
- 12.Brouwers MM, Feltz WF, Roelofs LA, Klemeny LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod 2006: 21:666–9. [DOI] [PubMed] [Google Scholar]
- 13.Pons JC, Papiernik E, Billon A, Hessabi M, Duyme M. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Prenat Diagn 2005; 25: 418–9. [DOI] [PubMed] [Google Scholar]
- 14.Palmer JR, Wise LA, Robboy SJ, Titus-Ernstoff L, Noller KL, Herbst AL, Troisi R, Hoover RN. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology 2005; 16: 583–6. [DOI] [PubMed] [Google Scholar]
- 15.Titus-Ernstoff L, Troisi R, Hatch EE, et al. Menstrual and Reproductive Characteristics of Women whose Mothers were Exposed to DES in utero. Int J Epidemiol 2006;35(4):862–8. [DOI] [PubMed] [Google Scholar]
- 16.Titus-Ernstoff L, Troisi R, Hatch EE, et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology 2008;19(2):251–7. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox AJ, Umbach DM, Hornsby PP, Herbst AL. Age at menarche among diethylstilbestrol granddaughters. Am J Obstet Gynecol 1995; 173: 835–836, [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RH, Adam E. Findings in female offspring of women exposed in utero to diethylstilbestrol.Obstet Gynecol. 2002;99:197–200. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien PC, Noller KL, Robby SJ, Barnes AB, Kaufman RH, Tilley BC, Townsend DE. Vaginal epithelial changes in young women enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstet Gynecol 1979; 53: 300–8. [PubMed] [Google Scholar]
- 20.Hatch EE, Palmer JR, Titus-Ernstoff L, Kaufman RH, Noller KL, Mittendorf R, Robboy SJ, Hyer M, Cowan DM, Adam E, Colton T, Hartge P, Hoover RN. Cancer risk after long term follow-up of women exposed to diethylstilbestrol (DES) in utero. J Am Med Assoc 1998;280:630–634. [DOI] [PubMed] [Google Scholar]
- 21.Zou G A modified poisson regression approach to prospective studies with binarydata. Am J Epidemiol. 2004; 159: 702–706 [DOI] [PubMed] [Google Scholar]
- 22.Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. J Finan Quantitative Anal. 1989;24:333–355 [Google Scholar]
- 23.Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation 2013; 84: 252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health 2011; 32: 10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Guckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. Brit Jour Obs Gyn 2013; 120: 548–53. [DOI] [PubMed] [Google Scholar]
- 26.Skinner MK, Manikka M, Guerroro-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol 2011; 31: 337–43, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolstenholme JT, Savera ME, Shetty RJ, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol A produces transgenerational changes in behavior and gene expression. Endocrinol 2012; 153: 3828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Medicine 2013; 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manikkam M, Hague MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One 2014; 9(7) e102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinol 2006; 147: 5515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker BE, Haven MI. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis 1997; 18: 791–3. [DOI] [PubMed] [Google Scholar]
- 32.Herbst AL, Hubby MM, Azizi F, Makii MM. Reproductive and gynecologic surgical experience in diethylstilbestrol-exposed daughters. Am J Obstet Gynecol. 1981;141:1019–28. [DOI] [PubMed] [Google Scholar]
- 33.Barnes AB. Menstrual history and fecundity of women exposed and unexposed in utero to diethylstilbestrol. J Reprod Med. 1984;29:651–5. [PubMed] [Google Scholar]
- 34.Senekjian EK, Potkul RK, Frey K, Herbst AL. Infertility among daughters either exposed or not exposed to diethylstilbestrol. Am J Obstet Gynecol 1988; 158: 493–8. [DOI] [PubMed] [Google Scholar]
- 35.McLachlan JA, Newbold RR, Shah HC, Hogan MD, Dixon RL. Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES) Fertil Steril. 1982;38:364–71. [DOI] [PubMed] [Google Scholar]
- 36.Ehrhardt AA, Meyer-Bahlburg HF, Rosen LR, Feldman JF, Veridiano NP, Zimmerman I, McEnewn BS. Sexual orientation after prenatal exposure to exogenous estrogen. Arch Sex Behav 1985; 14: 57–77. [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Bahlburg HFL, Erhardt AA, Rosen LR, Gruen RS, Veridiano NP, Fann FH, Neuwalder HF. Prenatal estrogens and the development of homosexual orientation. Develop Psychol 1995; 31: 12–21. [Google Scholar]
- 38.Lish JD, Erhardt AA, Meyer-Bahlburg HF, Rosen LR, Gruen RS, Veridiano NP. Gender-related behavior development in females exposed to diethylstilbestrol (DES): An attempted replication. J Am Acad Child Adolesc Psychiatry 1991; 30: 29–37. [DOI] [PubMed] [Google Scholar]
- 39.Titus-Ernstoff L, Perez K, Hatch EE, Troisi R, Palmer JR, Hartge P, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K, Pickett KE, Hoover R. Psychosexual characteristics of men and women exposed prenatally to diethylstilbestrol. Epidemiology 2003; 14: 155; 876504960. [DOI] [PubMed] [Google Scholar]
- 40.Smith LL, Hines M. Language lateralization and handedness in women prenatally exposed to DES. Psychoneuroendocrinol 2000; 25: 497–512. [DOI] [PubMed] [Google Scholar]
- 41.Scheirs JG, Vingerhoets AJ. Handedness and other laterality indices in women prenatally exposed to DES. J Clin Exp Neuropsychol 1995; 17: 725–30. [DOI] [PubMed] [Google Scholar]
- 42.Boas M Feldt-Rasmussen U, Main KM. Thryoid effects of endocrine disruptors. Molec Cell Biol 2012; 355: 240–8. [DOI] [PubMed] [Google Scholar]
- 43.Molehin D, Nikert MD, Richard K. Prenatal exposures to multiple thyroid hormone disruptors: Effects on glucose and lipid metabolism. J Thyroid Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


