Abstract
Objectives:
Transcranial photobiomodulation (t-PBM) consists of the delivery of near-infrared (NIR) or red light to the scalp designed to penetrate to subjacent cortical areas of the brain. NIR t-PBM has recently emerged as a potential therapy for brain disorders. This study assessed the efficacy of repeated sessions of NIR t-PBM on sexual dysfunction.
Methods:
We performed a secondary analysis of a double-blind clinical trial on t-PBM for major depressive disorder (MDD). Twenty individuals received NIR t-PBM (n = 9) or sham therapy (n = 11) twice a week for 8 weeks. Sexual desire, arousal, and orgasm were assessed using the Systematic Assessment for Treatment-Emergent Effects-Specific Inquiry (SAFTEE-SI).
Results:
The mean improvement in sexual function (decrease in SAFTEE sex total score) in subjects receiving t-PBM in NIR-mode was significantly greater than in subjects receiving sham-mode in the whole sample (NIR [n = 9] −2.55 ± 1.88 vs. sham [n = 11] −0.45 ± 1.21; z = 2.548, P = 0.011]) and in the completers (NIR [n = 5] −3.4 ± 1.95 vs. sham [n = 7] −0.14 ± 1.21; z = 2.576, P = 0.010])
Conclusion:
This exploratory study with a small sample size indicates that repeated sessions of NIR t-PBM may be associated with therapeutic effects on sexual dysfunction. The latter appeared unrelated to the antidepressant effect of t-PBM in our cohort. Lasers Surg. Med.
Keywords: photobiomodulation, transcranial, sexual dysfunction
INTRODUCTION
Sexual dysfunction is common and it affects 43% of women and 31% of men in the Unites States [1]. Sexual dysfunction might include decreased sexual desire (libido), insufficient sexual arousal, such as incomplete engorgement of the penis or of the clitoris or lack of vaginal lubrication, and delayed or absent sexual orgasm (anorgasmia), or a combination of the above.
The causes for sexual dysfunction are disparate and include psychiatric, neurological, endocrine, cardiovascular and pelvic conditions, as well as side effects of commonly prescribed medications [2]. While any of the listed causes is sufficient to produce sexual dysfunction, a multifactorial causation is rather the norm than the exception. If we consider major depressive disorder (MDD)—a common cause of sexual dysfunction with up to 50% of untreated depressed patients suffering from sexual dysfunction [3,4]—MDD is frequently comorbid with other physical illnesses [5], and its prevalence is twice as high in patients who also suffers from at least two other chronic medical conditions [6]. The same patients are also likely to receive medications for MDD or for other medical conditions, and to take supplements and medications for wellness, such as oral contraceptives. It is then apparent that multiple causes of sexual dysfunction can coexist, such as psychiatric disorders, other medical conditions, and pharmacotherapy [7], whether medications are prescribed for the treatment of the same medical disorders or taken for wellness. Our sample of patients with sexual dysfunction—which we hereby report on—exemplifies the multifactorial nature of causality of sexual dysfunction.
Multiple treatments exist for women and men with sexual dysfunction [8]; however, as patients increasingly turn to alternative medicine and to non-pharmacological interventions to treat their ailments [9,10], we need scientifically sound alternatives to the current medications for the treatment of sexual dysfunction.
Transcranial photobiomodulation (t-PBM) with near-infrared light (NIR) is a novel modality of neuromodulation, based on the penetration of invisible light through the skull and absorption of the same light by the neuronal mitochondria [11]. The NIR absorbed by mitochondria results in increased cellular energy metabolism. Beneficial effects of t-PBM have been documented such as pro-cognitive [12–15], antidepressant [16–18], and anxiolytic effects [17]; we report on the effects of t-PBM on sexual dysfunction in a cohort of depressed patients with medical and psychiatric comorbidity and concomitant pharmacological therapies.
MATERIALS AND METHODS
This single-site study (ELATED-2) was approved by the Massachusetts General Hospital (MGH) institutional review board (IRB). Recruitment began in February 2014, and the study was completed in August 2015. The ELATED-2 clinical trial has already been described in detail [19].
Inclusion and Exclusion Criteria
Adult subjects (age 18–65 years) meeting the DSM-IV SCID criteria for MDD, with the degree of depression severity rated at least moderate (Hamilton Depression Rating Scale, HAM-D17 total score ranging 14–24), were included in the study after providing written informed consent. During the current episode, subjects could have failed no more than one FDA-approved antidepressant medication (for at least 6 weeks) and no more than one course of structured psychotherapy for depression (for at least 8 weeks). Other exclusionary conditions included active substance use disorders (prior 6 months), lifetime psychotic episodes, bipolar disorder, active suicidal ideation, and homicidal ideation, in addition to unstable medical illness and recent stroke (prior 3 months). Women of child-bearing potential were required to use a birth-control method if sexually active; pregnancy and lactation were exclusionary. To allow maximum light penetration and to minimize potential risks of local tissue damage from the use of NIR, the following conditions were also exclusionary: (i) having a forehead skin condition; (ii) taking a light-activated medication (prior 14 days); and (iii) having a head-implant.
Study Design and Treatment
Eligible subjects were randomized to an 8-week study with twice weekly double-blind NIR t-PBM versus sham therapy (16 sessions total). At each session, NIR or sham were administered to the forehead bilaterally, directed to the F3 and F4 sites—per the EEG placement map—for the dorsolateral prefrontal cortex (dlPFC). The study clinician had the option to adjust the duration of light exposure after reaching week 4 and week 6 (after 6 and 10 sessions respectively) from 20 to 25 and 30 minutes, respectively, based on tolerability and efficacy. The study devices employed an LED source (Omnilux New U, light emitting diode, manufactured by Photo-medex Inc.) emitting NIR at a wavelength of 823 nm, with an average irradiance up to 36.2 mW/cm2 and fluence over 30 minutes up to 65.2 J/cm2, with a treatment window of 28.7 cm2 per each of the two devices. All but three subjects remained on stable antidepressant treatment during the trial; their data were censored after change in concomitant antidepressant therapies.
Randomization and Blinding
Two t-PBM device types were available for each modality (NIR and sham). The apparent behavior (i.e., all visible and audible indicators) of the devices was identical for both modalities. However, only NIR-mode t-PBM device produced the therapeutic NIR energy. NIR light is invisible and undetectable to patients and physicians. The study research assistant used permuted block randomization with varying block sizes to randomize subjects in 1:1 fashion to each pair of instruments as “A” and “B.” Only the research assistant was able to identify each pair of instruments as “A” and “B.” The investigators and the subjects remained blind to the subject assignment, since the label on each device was covered prior to treatment administration. Photomedex, Inc. provided the blinding codes of NIR and sham, which were kept in a sealed envelope at the study site, for each labeled pair of devices. Prior to this secondary paper, the blinding in ELATED-2 had already been tested, and there were no significant correlations between treatment assignment and the guess made by the subjects as to which treatment they were receiving [19].
Clinical Outcome Measures
The outcome measure for this secondary analysis of the ELATED-2 study was the overall change in sexual dysfunction, assessed by weekly and systematic self-reporting by the Systematic Assessment for Treatment-Emergent Effects-Specific Inquiry (SAFTEE-SI) scale [20]: a checklist of 55 adverse symptoms commonly or possibly experienced during treatment and categorized by severity as: 0-none, 1-mild, 2-moderate, 3-severe. From the SAFTEE scale, three items were identified as pertinent to the sexual dysfunction: item 36, loss of sexual interest; item 37, problems with sexual arousal (erection or lubrication); and item 38, delayed or absent orgasm. A composite score was generated by adding the rating for each of the three items: the SAFTEE sex total score (ranging 0–9), indicating the overall sexual dysfunction, the higher the score the worse the dysfunction. The use of a composite score was justified by the high correlation between all three measures of sexual dysfunction, ranging from 0.73 to 0.89. No attributions were made as to whether the sexual dysfunction was influenced by the underling major depressive disorder, by concomitant medications, by comorbid illnesses or disorders or by other factors, including study participation and t-PBM. The instructions for self-rating were in fact generic: “below is a list of symptoms people sometimes have; please read each item; indicate how bothersome each symptom has been for you by circling the appropriate number to the right of the item.”
Study Sample and Data Cleaning
A total of 21 randomized subjects who received at least one t-PBM session were included in the original ELATED-2 sample. However, for this secondary analysis, on repeated measures of self-rated scales, the sample is limited to 20 subjects, since one subject in the original pool consistently skipped several answers on the scales for the duration of the study. This subject had received a diagnosis of attention-deficit disorder and reported significant difficulties concentrating while off stimulants. As briefly mentioned, three subjects were censored as part of the a priori data cleaning process due to their starting new psychoactive treatments during the course of the study. All other available data were included, regardless of drop-outs.
Analyses
Descriptive statistics were used for the prevalence of conditions and treatments commonly associated with sexual dysfunction. The prevalence of clinically significant sexual dysfunction at baseline was also reported (SAFTEE sex total score ≥2). Baseline characteristics for the NIR and sham groups were compared by unpaired Mann-Whitney (two-tailed) and Pearson’s Chi-square test, respectively, for continuous and nominal variables. A Mann–Whitney U-test (two-tailed) was also chosen to compare the change in the total severity score for sexual dysfunction (SAFTEE sex total score) from baseline to endpoint—last evaluation carried forward—across the two study groups. The same analysis was repeated in the following subsamples: in completers, in all women, and in all men. For specific types of sexual dysfunction such as impaired libido, arousal, and orgasm, the change in severity score for each of the three SAFTEE items (36, 37, 38) was compared, in the whole sample and in completers (Mann–Whitney U-test, two-tailed). Because of the small sample size, we used non-parametric tests and we refrained from covaring for the change in depression severity. As post hoc analyses—to better discern whether the antidepressant effect was driving the effect on sexual functioning—we (i) calculated Cohen’s d effect size of t-PBM for the change of severity of sexual dysfunction and for the change in severity of depression (HAM-D17 total score) and (ii) assessed the timing of significant changes in sexual dysfunction and in depression severity, since plotting of means suggested an early improvement of sexual functioning (last evaluation carried forward up to the specific time point and Mann–Whitney U-test, two-tailed for the change from baseline). The analyses on our primary outcome—the overall change in sexual dysfunction—were repeated after removing two subjects with no assessments after baseline (n = 18; Mann–Whitney U-test, two-tailed). For all analyses, significance was set at P ≤ 0.05.
RESULTS
Etiology of Sexual Dysfunction, Baseline Characteristics, and Retention
Per inclusion criteria, all subjects (n = 20) had major depressive disorder, a common cause of sexual dysfunction. Of note, in the same group 45% and 25% of subjects were diagnosed with at least one or two additional psychiatric disorders, respectively; the most common being generalized anxiety disorder, post-traumatic stress disorder, and social anxiety disorder. Nearly all subject (85%) were also affected by non-psychiatric, medical conditions; many of which are potentially implicated in sexual dysfunction. Endocrine conditions were among the most common (30%) and included thyroid and parathyroid diseases and thyroid hormone supplementation. Neurological conditions were equally common (30%) and included history of concussion, spinal cord injury, and chronic pain syndromes. Cardiovascular diseases and metabolic syndrome (25%) included hypertension, hyperlipidemia, and obesity. One case of pelvic disorder was due to hysterectomy. Nearly all subjects (80%) received pharmacological therapies. Prescribed medications with sexual dysfunction described as a potential side-effect were antidepressants or other psychiatric medications taken by 45% of the subjects; antiepileptic medications by 15%; α2-agonists, diuretics, statins, or calcium channel blockers also by 15%; one subject took narcotics and NSAIDs; and last, oral contraceptive (or other means of contraception) were required for study entry of women of child-bearing age. See Table 1 for an overview on these medical conditions and treatments commonly affecting sexual functioning, and for their prevalence rates in our study sample. In sum, 90% of study subjects had at least one additional explanation—other than depression or other psychiatric diagnoses—for their sexual dysfunction.
TABLE 1.
Prevalence of Conditions and Treatments Commonly Affecting Sexual Functioning (Sample: n = 20)
| Neurological |
| Concussion, spinal cord injury, and chronic pain (n = 6) |
| Antiepileptic drugs (n = 3) |
| Narcotics and NSAIDs (n = 1) |
| Endocrine |
| Thyroid disease and thyroid hormone supplementation and parathyroid disease (n = 6) |
| Cardiovascular & metabolic syndrome |
| Obesity, hypertension, and hyperlipidemia (n = 5) |
| Alpha2-agonist, diuretics, statins, or calcium channel blockers (n = 3) |
| Psychiatric |
| Major depressive disorder (n = 20) |
| Generalized anxiety disorder (n = 5) |
| Post-traumatic stress disorder (n = 5) |
| Social anxiety disorder (n = 3) |
| Antidepressants or other psychiatric medications (n = 9) |
| Pelvic disorders |
| Hysterectomy (n = 1) |
There were no significant differences among the two groups at baseline in terms of demographic and clinical characteristics as well as concurrent antidepressant treatment (Table 2). Clinically significant sexual dys-function was prevalent (85% of our sample), both in the t-PBM NIR group (8 out of 9 subjects) and in the t-PBM sham group (9 out of 11). During the study, all subjects continued their baseline antidepressant treatment, if any, except one subject in the t-PBM NIR group, who discontinued her psychotherapy at baseline. Eight subject did not complete their 8-week trial for the following reasons: four subjects failed to return to follow-up, three subjects started a concomitant intervention to treat their mood and anxiety symptoms, and one subject performed the last assessment outside of the time window.
TABLE 2.
Demographic and Clinical Characteristics of the Sample
| Active (n = 9) |
Sham (n = 11) |
P value |
|
|---|---|---|---|
| Age | 47.3 ± 11.1 | 50.7 ± 13.3 | 0.361 |
| Gender (female) | 5 (56%) | 6 (55%) | 0.964 |
| Race | 0.360 | ||
| White | 8 (89%) | 10 (91%) | |
| Asian | 1 (11%) | 0 | |
| African American | 0 | 1 (9%) | |
| Ethnicity | |||
| Not Hispanic or Latino | 8 (100%) | 11 (100%) | - |
| Using AD medication | 3 (33%) | 3 (27%) | 0.769 |
| SAFTEE sex total score | 3.3 ± 1.9 | 4.2 ± 3.5 | 0.783 |
| HAM-D | 19.7 ± 1.9 | 20.2 ± 4.3 | 0.847 |
| CGI severity of depression | 4.4 ± 0.5 | 4.4 ± 0.5 | 0.916 |
AD, antidepressant; HAM-D17, Hamilton depression rating scale (17 items); CGI-S, clinical global impression severity scale.
Overall Sexual Dysfunction
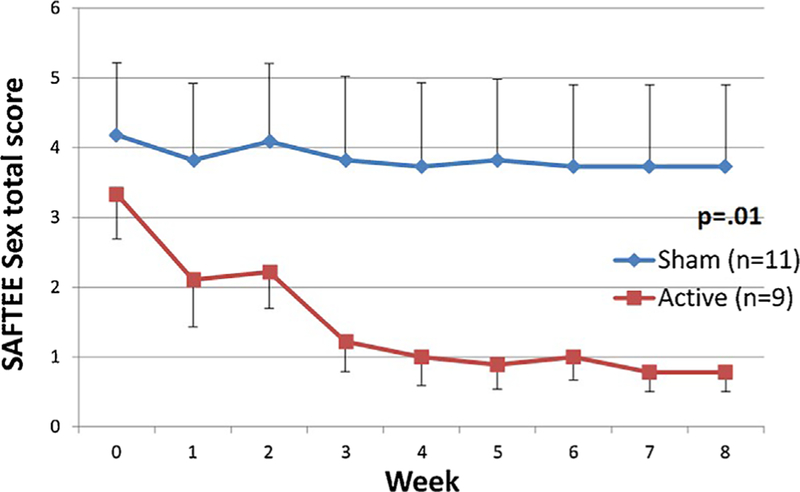
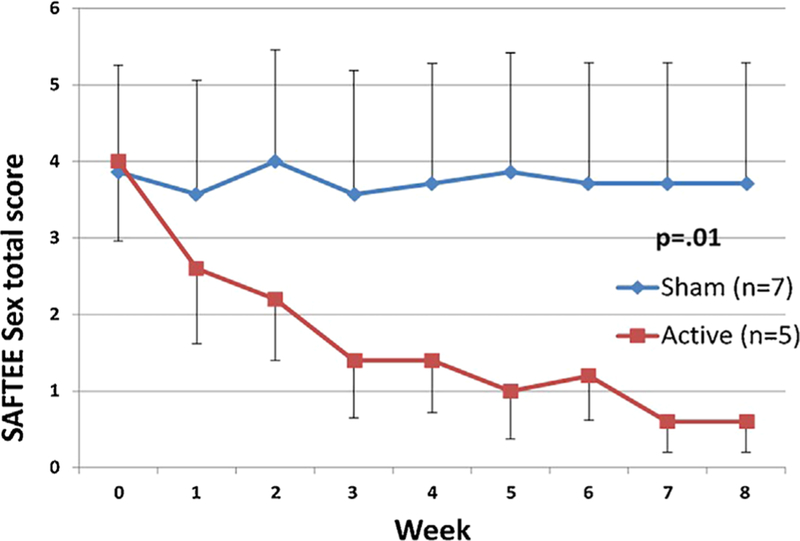
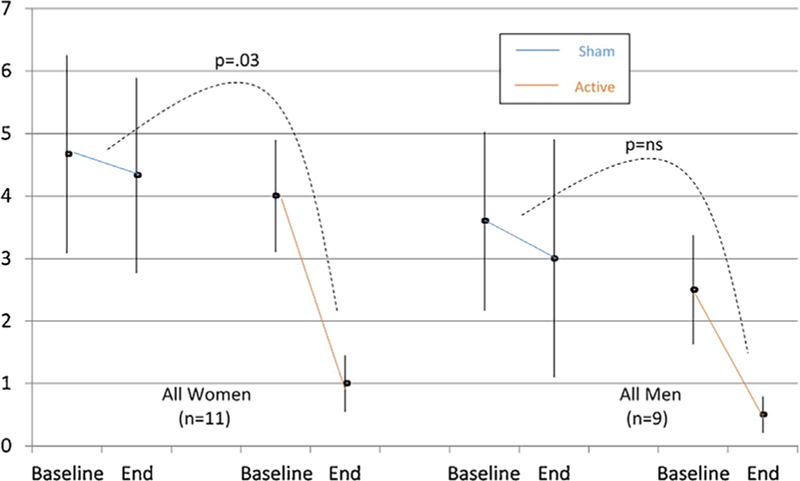
The mean change in SAFTEE sex total score in subjects receiving t-PBM in NIR-mode was significantly greater than in subjects receiving sham-mode in the whole sample (NIR [n = 9] −2.55 ± 1.88 vs. sham [n = 11] −0.45 ± 1.21; z = 2.548, P = 0.011]) and in the completers (NIR [n = 5] −3.4 ± 1.95 vs. sham [n = 7] −0.14 ± 1.21; z = 2.576, P = 0.010]) Figures 1 and 2 illustrate the mean SAFTEE sex total scores over the course of the study for the two t-PBM groups (NIR and sham), in the whole sample and in completers. After removing two subjects from the analyses—due to lack of post-treatment assessments—findings were confirmed (NIR [n = 9] −2.55 ± 1.88 vs. sham [n = 9] −0.55 ± 1.33; z = 2.215, P = 0.027]). When splitting the overall sample based on gender, significance for the same comparison was reached in women (NIR [n = 5] −3.0 ± 2.24 vs. sham [n = 6] −0.3 ± 0.82; z = 2.196, P = 0.028]) but not in the smaller sample of men (NIR [n = 4] −2.0 ± 1.41 vs. sham [n = 5] −0.6 ± 1.014, P= 0.310]). See Figure 3.
Fig. 1.
SAFTEE sex total score (mean ± SE) over time in all subjects (n = 20). Participants in the active group showed a significant decrease (P = 0.01) in symptoms of sexual dysfunction, as measured by the SAFTEE sex assessment, compared to the sham group. Intent to treat (ITT), evaluation carried forward (ECF) graphs, and P-value for comparison of delta pre- minus post-treatment between groups.
Fig. 2.
SAFTEE sex total score (mean ± SE) over time in the completers sample (n = 12). Participants in the active group showed a significant decrease (P = 0.01) in symptoms of sexual dysfunction, as measured by the SAFTEE sex assessment, compared to the sham group. Intent to treat (ITT), evaluation carried forward (ECF) graphs, and P-value for comparison of delta pre- minus post-treatment between groups.
Fig. 3.
SAFTEE sex total score (mean ± SE) pre- and post-treatment in all women (n = 11) and all men (n = 9). Women in the active group showed a significant decrease (P =0.03) in the sexual dysfunction symptoms, as measured by the SAFTEE sex assessment, compared to the sham group. These between-group differences were not found in the smaller sample of men. Intent to treat (ITT), evaluation carried forward (ECF) bars, and P-value for comparison of delta pre- minus post-treatment between groups.
The effect sizes for the improvement in overall sexual functioning were d = 1.33 (n = 20) and d = 1.23 (n = 18) (d = 2.01 [n = 12] for completers and d = 0.41 [n = 8] in noncompleters) (Cohen’s d). These effects were 55% and 73% greater than the ones observed for the decrease in severity of depression (d = 0.86 [n = 20] and d = 0.71 [n = 18]). Also, a significant decrease in the overall sexual dysfunction, but not in the depression severity, was documented as early as at completion of week 2 in the overall sample (NIR [n = 9] −1.11 ± 1.17 vs. sham [n = 11] −0.09 ± 1.05; z = 1.972, P =.049]) and after completion of week 3, when removing two subjects with lack of post-treatment assessments (NIR [n = 9] −2.11 ± 1.45 vs. sham [n = 9] −0.44 ± 1.24; z = 2.313, P = 0.021)]. The decrease in severity of depression was never significant in both samples, at any timepoint (n = 20 and n = 18; of note both samples were less than the original ELATED-2 sample of n = 21, which our group previously reported on).
Impaired Libido
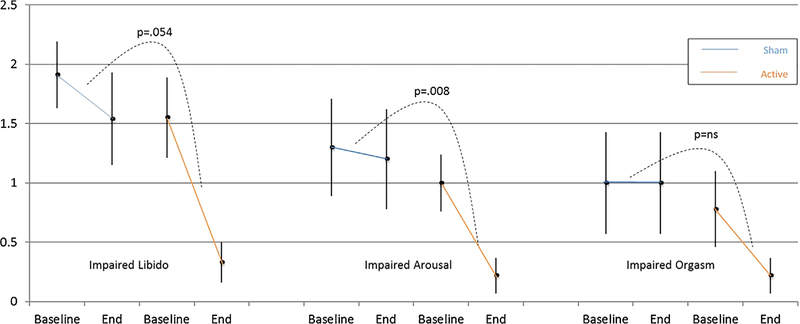
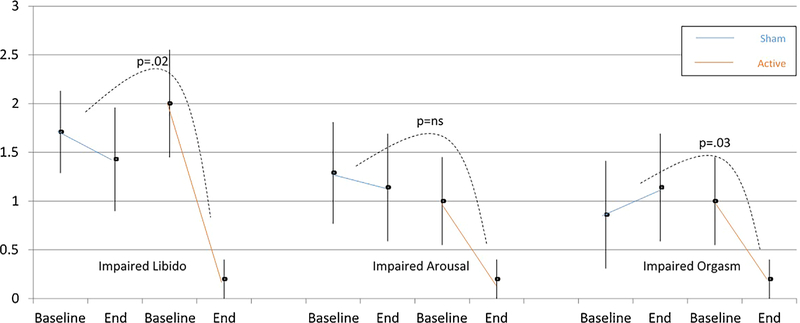
The comparison of the mean change in SAFTEE item 36 score “loss of sexual interest” approached significance in the whole sample (NIR [n = 9] −1.2 ± 1.09 vs. sham [n = 11] −0.4 ± 0.67; z = 1.930, P = 0.054]) and was significant in the completers (NIR [n = 5] −1.8 ± 1.09 vs. sham [n = 7] −0.3 ± 0.49; z = 2.276, P = 0.023]). The percent decrease in severity of “loss of sexual interest” in the t-PBM NIR group was −79% and −90%, respectively, in the whole sample and in completers. See Figures 4 and 5.
Fig. 4.
SAFTEE sex item score (mean ± SE) pre- and post-treatment in the whole sample (n = 20). The active group showed a significant improvement (P = 0.008) in arousal compared to the sham group, as well as a trend toward greater improvement in libido (P = 0.054). There were no significant differences between groups in orgasm. Intent to treat (ITT), evaluation carried forward (ECF) bars, and P-value for the comparison of delta pre- minus post-treatment between groups.
Fig. 5.
SAFTEE sex item score (mean ± SE) pre- and post-treatment in the completers sample (n = 12). The active group showed a significant improvement in libido (P = 0.02) and in orgasm (P = 0.03) compared to the sham group. There were no significant differences between groups in arousal. Intent to treat (ITT), evaluation carried forward (ECF) bars, and P-value for comparison of delta pre- minus post-treatment between groups.
Impaired Sexual Arousal
The comparison of the mean change in SAFTEE item 37 “problems with sexual arousal (erection or lubrication)” reached significance in the whole sample (NIR [n = 9] −0.8 ± 0.67 vs. sham [n = 11] −0.1 ± 0.30; z = 2.633, P < .001]) but failed to in the completers (NIR=[n = 5] −0.8 ± 0.84 vs. sham [n = 7] −0.1 ± 0.38; z = 1.659, P = 0.097]). The percent decrease in severity of “problems with sexual arousal” in the t-PBM NIR group was −78% and −80%, respectively, in the whole sample and completers.
Delayed or Absent Orgasm
The comparison of the mean change in SAFTEE item 38 “delayed or absent orgasm” was only significant in completers (NIR [n = 09] −0.6 ± 0.73 vs. sham [n = 11] 0.0 ± 0.89; z = 1.738, P = 0.08]; NIR [n = 5] −0.8 ± 0.84 vs. sham [n = 7] −0.3 ± 0.76; z = 2.228, P = 0.026]). The percent decrease in severity of “delayed or absent orgasm” in the t-PBM NIR group was −72% and −80%, respectively, in the whole sample and in completers.
DISCUSSION
This study on the effects of t-PBM on sexual dysfunction demonstrated a significant therapeutic effect, with reversal of sexual dysfunction in patients with multifactorial causation.
It is noteworthy that sexual dysfunction was neither a primary nor secondary outcome in the original ELATED-2 study. It was indeed serendipitous that three of the study subjects reported improvement in sexual function, which were unusual in terms of magnitude and fast onset. These three clinical cases—briefly reported in Table 3—led us to conduct the exploratory analyses described in this paper to test a putative effect of t-PBM on sexual functioning in the overall sample. We relied on the use of a composite score, the SAFTEE sex total score, to describe the sexual impairment. The SAFTEE scale had been included in the protocol to measure potential new-onset side-effects related to t-PBM; in this context, we used the SAFTEE scale to track the overtime change in sexual functioning, the latter was impaired on average in this cohort of MDD patients.
TABLE 3.
Case Vignettes From the Study Cohort
| Case #1 was a 65-year-old divorced, retired man, father of two grown-up children. At the time of the study, he was in a romantic relationship but lived independently. At baseline, he rated his loss of sexual interest as “severe” and his problems with sexual arousal (erection) as “moderate.” After two t-PBM sessions (1 week), the patient reported only “mild” loss of sexual interest and “mild” problems with sexual arousal. Despite some waxing and waning of his ratings on sexual functioning from week to week, after completion of 15 t-PBM sessions, the patient consistently reported no problems with erection and only minimal decrease in sexual interest. During study visits, the patient joked that he had been more sexually active with his girlfriend to the point that she had asked him to return to his own apartment and spend the following nights there. |
| Case #2 was a 44-year-old married woman, a professional and mother of two young children. Before entering the study she had been treated for depression with Venlafaxine 75 mg every day for 6 weeks and continued this medication during the study. While she did not experience pronounced impairment with her sexual function before starting her antidepressant medication (despite being depressed), she then complained of both decreased libido and anorgasmia on Venlafaxine. Before starting her t-PBM sessions, she rated her loss of sexual interest as “severe,” her problems with sexual arousal “mild,” and her delayed or absent orgasm as “moderate.” After the first two sessions (1 week), her loss of sexual interest was rated as “moderate,” her issues with lubrication resolved, and her delayed or absent orgasm was “mild.” After 10 t-PBM sessions her sexual dysfunction had completely resolved. At the end of the study, the patient was perplexed that t-PBM was not a popular intervention and she equated her sexual functioning to her adolescence. |
| Case #3 was a 27-year-old single man, in a romantic relationship. At baseline, he reported “mild” loss of sexual interest and problems with sexual arousal (erection). At week 1, both sexual interest and arousal were normal and at week 2 only loss of sexual interest was present and rated as “mild.” During the study (week 1), the patient reported that his sexual interest and activity had nearly doubled: he went from having sex 4–5 times per week to 8—9 per week. After four t-PBM sessions (2 weeks), the patient stopped coming to the study visits and eventually dropped from the study. |
The unanticipated finding that t-PBM improved sexual function in our cohort of depressed subjects was documented in the whole sample and in the completers’ sample. The finding was still significant in the subsample of women, but not in the reduced sample of men. Interestingly, while there were fewer men than women in the study, the magnitude of the decrease in severity of sexual dysfunction was similar across genders and in fact slightly higher in men (−75% in women and −80% in men), when receiving t-PBM. It is possible to assume that the lack of statistically significant findings in men might be related to lack of power.
Further testing was conducted to elucidate which dimensions of sexual dysfunction were most likely to drive the overall study findings. In the whole sample, sexual arousal improved significantly more with t-PBM than sham; however, in completers both libido and orgasm improved significantly more with t-PBM, but not arousal. After exploring the magnitude of the improvements in each sexual dimension, it is striking that similar decreases in severity of sexual dysfunction are noticed across dimensions. Therefore, we interpreted the lack of statistical significance of some of the comparisons as likely related to lack of power; in fact, the measure for each dimension of sexual dysfunction might have low sensitivity due to the four point Likert scale (score range 0–3). The magnitude of decrease in severity for each sexual dimension appeared slightly greater in completers (impaired libido −90%; impaired arousal and impaired orgasm −80%), compared to the whole sample (impaired libido −79%; impaired arousal −78%; impaired orgasm −72%). Completers might have benefited from more treatment sessions and from longer exposure time to t-PBM, since exposure was increased from 20 to 30 minutes over the course of the study.
The nature or cause of the sexual dysfunction in our cohort is multifactorial; while the underlying depressive syndrome was common to all included patients, multiple other potential factors coexisted such as comorbid medical conditions and pharmacotherapies. Given that we had previously demonstrated an antidepressant effect for t-PBM in the same cohort, we attempted to untangle the effect of t-PBM on sexual dysfunction from its effect on depression. In the current study, the timing and the magnitude of the effect of t-PBM on sexual dysfunction were much faster and far greater than its effect on depression; this contradicts any speculation that sexual function improved because of the lessening of the depressive syndrome. On the contrary, t-PBM is likely to benefit sexual function independently from the outcome of depression.
If so, the intriguing question is “how does t-PBM work on sexual function?” Two rather different hypotheses about mechanisms can be considered: (i) the effect is mediated by direct neuromodulation of the central nervous system, more specifically of the targeted prefrontal cortex (i.e., enhanced EEG activity) [21] and (ii) the effect is mediated by an increase in signaling molecules, such as neuronal nitric oxide (NO). Not only NO is increased by t-PBM with NIR [22] but also neuronal, intra- or extra-cellular NO in the hypothalamus is postulated to be essential to onset of puberty and to fertility, and regulates GnRH and LH release [23].
Our study has three main limitations: (i) the small sample-size precludes generalizability and warrants replication in larger cohorts of patients with sexual dysfunction; (ii) the SAFTEE scale, while exploring sexual functioning, is not commonly used in studies on sexual dysfunction; and (iii) the study was not designed to investigate sexual dysfunction and it is, therefore, subject to type I error. The counterargument to the last point is that this study was driven by a clinical observation and, therefore, tested an informed hypothesis. During the conduct of the clinical trial, neither investigators nor study subjects expected an effect of t-PBM on sexual functioning, this might have increased our chance to detect a signal by decreasing the placebo effect.
A larger double-blind, randomized study of t-PBM in depression (NCT02959307) is currently underway, and the effects of t-PBM on sexual functioning will be more rigorously tested by using a validated scale on sexual dysfunction.
ACKNOWLEDGMENTS
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. We thank Dr. Lee Hang from Massachusetts General Hospital for his consultations on statistical analyses. His service was supported by the Harvard Catalyst Program. https://catalyst.harvard.edu/services/biostatsconsult/.
Contract grant sponsor: Brain and Behavior Research Foundation; Contract grant sponsor: Harvard Catalyst Program; Contract grant sponsor: Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award); Contract grant number: UL1 TR001102.
Footnotes
CONFLICTS OF INTEREST
Dr. P. Cassano
Dr. Cassano’s salary was supported by the Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award), by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and by the Photothera Inc. unrestricted grant. Drug donation from TEVA. Travel reimbursement from Pharmacia-Upjohn. Dr. Cassano has received consultation fees from Janssen Research and Development. Dr. Cassano has filed several patents related to the use of near-infrared light in psychiatry. PhotoMedex, Inc. supplied four devices for this clinical study. Since completion of the study and of the manuscript, Dr. Cassano has (i) Received unrestricted funding from Litecure Inc. to conduct an additional study on transcranial photobiomodulation for the treatment of major depressive disorder and to conduct a study on healthy subjects; (ii) Co-founded a company (Niraxx Light Therapeutics) focused on the development of new modalities of treatment based on near-infrared light; he is also a consultant for the same company; (iii) Received funding from Cerebral Sciences to conduct a study on transcranial photobiomodulation for generalized anxiety disorder. Dr. C. Dording, Mr. G. Thomas, Dr. S. Foster, Dr. A. Yeung, Dr. M. Uchida, and Dr. E Bui had no potential conflict of interest to disclose related to the paper under consideration.
Dr. D. Mischoulon
Dr. Mischoulon has received research support from the FisherWallace, Nordic Naturals, Methylation Sciences, Inc. (MSI), Blackmores, and PharmoRx Therapeutics. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
Dr. M. Hamblin
Vielight Inc, Scientific Advisory Board; Global Photon, Scientific Advisory Board; Lexington Inc, Scientific Advisory Board; LaserCap Scientific Advisory Board; USHIO Inc, Consultant.
Dr. M. Fava
Research support. Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithK-line; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc.; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Takeda Pharmaceuticals;Tal Medical; VistaGen); Wyeth-Ayerst Laboratories.
Advisory board/consultant. Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG;Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Puretech Ventures; Psycho-Genics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; VistaGen.
Speaking/publishing. Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories.
Stock/other financial options.
Equity holdings. Compellis; PsyBrain, Inc.
Royalty/patent, other income. Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven.
Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd.
Dr. D. Iosifescu
In the past 5 years, Dr. Iosifescu was a consultant for Alkermes, Avanir, Axsome, Centers of Psychiatric Excellence, CNS Response, INSYS Therapeutics, Jazz Pharmaceuticals, Lundbeck, Otsuka, Servier, and Sunovion; he has received research support (through his academic institution) from Alkermes, Astra Zeneca, Brainsway, Euthymics, Litecure, Neosync, Roche, and Shire, and he has received speaker honoraria from the Massachusetts General Hospital Psychiatry Academy, Medscape, and Global Medical Education.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: All other authors have no conflict of interest to declare.
FINANCIAL DISCLOSURES
The Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award) and the Brain and Behavior Research Foundation (NARSAD Young Investigator Award; grant number 19159) supported this investigation. Two pairs of study devices (active and sham) were provided by Photomedex Inc (Orangeburg, NY, USA), and underwent independent dosimetry testing (real and sham), provided by Litecure Inc (Newark, DE, USA). None of the funding sources had any involvement in the study design; in the collection, analysis, or interpretation of study data; in the writing of the report; or in the decision to submit the article for publication.
REFERENCES
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999; 281(6):537–544. [DOI] [PubMed] [Google Scholar]
- 2.Clayton A, Ramamurthy S. The impact of physical illness on sexual dysfunction. Adv Psychosom Med 2008;29: 70–88. [DOI] [PubMed] [Google Scholar]
- 3.Angst J Sexual problems in healthy and depressed persons. Int Clin Psychopharmacol 1998;13(Suppl 6):S1–S4. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy SH, Dickens SE, Eisfeld BS, Bagby RM. Sexual dysfunction before antidepressant therapy in major depression. J Affect Disord 1999;56(2–3):201–208. [DOI] [PubMed] [Google Scholar]
- 5.Cassano P, Fava M. Depression and public health—An overview. J Psychosom Res 2002;53(4):849–857. [DOI] [PubMed] [Google Scholar]
- 6.Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: A systematic review and meta-analysis. J Affect Disord 2017;221:36–46. [DOI] [PubMed] [Google Scholar]
- 7.Clayton AH, Alkis AR, Parikh NB, Votta JG. Sexual dysfunction due to psychotropic medications. Psychiatr Clin North Am 2016;39(3):427–463. [DOI] [PubMed] [Google Scholar]
- 8.Harsh V, Clayton AH. Sex differences in the treatment of sexual dysfunction. Curr Psychiatry Rep 2018;20(3):18. [DOI] [PubMed] [Google Scholar]
- 9.Ernst E, Posadzki P, Lee MS. Complementary and alternative medicine (CAM) for sexual dysfunction and erectile dysfunction in older men and women: An overview of systematic reviews. Maturitas 2011;70(1):37–41. [DOI] [PubMed] [Google Scholar]
- 10.Corazza O, Martinotti G, Santacroce R, et al. Sexual enhancement products for sale online: Raising awareness of the psychoactive effects of yohimbine, maca, horny goat weed, and Ginkgo biloba. Biomed Res Int 2014;2014:841798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin 2016;6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett D, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013;230:13–23. [DOI] [PubMed] [Google Scholar]
- 13.Hwang J, Castelli DM, Gonzalez-Lima F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 2016;31(6):1151–1160. [DOI] [PubMed] [Google Scholar]
- 14.Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2017;11(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco NJ, Saucedo CL, Gonzalez-Lima F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 2017;139:69–75. [DOI] [PubMed] [Google Scholar]
- 16.Cassano P, Cusin C, Mischoulon D, et al. Near-infrared transcranial radiation for major depressive disorder: Proof of concept study. Psychiatry J 2015;2015:352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016;3(3):031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassano P, Petrie S, Mischoulon D, et al. Transcranial photobiomodulation for the treatment of major depressive disorder. The ELATED-2 pilot trial. Photomed Laser Surg 2018; (accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine J, Schooler NR. General versus specific inquiry with SAFTEE. J Clin Psychopharmacol 1992;12(6):448. [DOI] [PubMed] [Google Scholar]
- 21.Vargas E, Barrett DW, Saucedo CL, et al. Beneficial neuro-cognitive effects of transcranial laser in older adults. Lasers Med Sci 2017;32(5):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HI, Lee SW, Kim SY, et al. Pretreatment with light-emitting diode therapy reduces ischemic brain injury in mice through endothelial nitric oxide synthase-dependent mechanisms. Biochem Biophys Res Commun 2017;486(4):945–950. [DOI] [PubMed] [Google Scholar]
- 23.Chachlaki K, Garthwaite J, Prevot V. The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat Rev Endocrinol 2017;13(9):521–535. [DOI] [PubMed] [Google Scholar]