Abstract
Akt isoforms play key roles in multiple cellular processes, however the roles of Akt-1 and Akt-2 isoforms in the development of T cell-mediated autoimmunity are poorly defined. In this study, we showed that Akt1–/– mice develop ameliorated experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS), whereas Akt2–/– mice develop exacerbated EAE compared to WT mice. At the cellular level, Akt-1 appears to inhibit proliferation of thymus-derived regulatory T cells (tTregs), which facilitates antigen-specific Th1/Th17 responses. In a sharp contrast to Akt-1, Akt-2 potentiates tTreg proliferation in vitro and in vivo, and suppresses antigen-specific Th1/Th17 responses. Furthermore, treating mice with established EAE with a specific Akt-1 inhibitor suppressed disease progression. Our data demonstrate that Akt-1 and Akt-2 differentially regulate the susceptibility of mice to EAE by controlling tTreg proliferation. Our data also indicate that targeting Akt-1 is a potential therapeutic approach for MS in humans.
INTRODUCTION
Akt, also known as protein kinase B (PKB), is serine/threonine-specific protein kinase that plays an important role in multiple cellular processes (1, 2). Akt consists of Akt-1, Akt-2, and Akt-3 isoforms, which all possess a catalytic domain, a pleckstrin homology (PH) domain, and a regulatory domain (1, 3). Akt-1 has a wide tissue distribution and is implicated in cell growth and survival, whereas Akt-2 is highly expressed in muscle and adipocytes and contributes to insulin-mediated regulation of glucose homeostasis (3). Akt-3 is most highly expressed in the brain and testis, and plays an important role in brain development (3).
Akt has been shown to regulate T cell activation, proliferation, glucose uptake, cytokine expression, and cell survival in response to CD28 costimulation and cytokines (4). Akt is also essential for tolerance induction (5). Akt activity in effector T cells (Teffs) and CD4+CD25+Foxp3+ regulatory T cells (Tregs) determines the fate of the response of Teffs to Tregs, and the suppressive activity of Tregs (6) as well as inducible Treg (iTreg) development (7–9). Strikingly, we have previously shown that Akt-2, but not Akt-1, is crucial for the inhibition of iTreg development via a Foxo1/Foxo3a-dependent manner (9), suggesting that different isoforms of Akt in T cells have distinct effects on T cell functions. However, the precise role of Akt isoforms in T cell responses remains to be further established.
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system (CNS), destroying the myelin and the axon in variable degrees and producing significant physical disability within 20–25 years in more than 30% of patients (10, 11). Experimental autoimmune encephalomyelitis (EAE) is a mouse model of MS in humans. EAE, and possibly MS, are believed to be mediated at least in part by Th1 and Th17 responses (10). Dysregulated CD4+CD25+Foxp3+ Tregs have also been shown to contribute to the pathogenesis of EAE and possibly MS (10, 12–16). Although Akt-3 signaling may contribute to the protection of mice from EAE (17) the relative contributions of Akt-1 and Akt-2 to autoimmune T cell responses and EAE are completely unknown. In this study, we showed that Akt1–/– mice develop ameliorated EAE, while Akt2–/– mice display exacerbated EAE. These distinct effects of Akt-1 and Akt-2 on the EAE development are achieved by their differential control of proliferation of thymus-derived Tregs (tTregs), which tightly regulate Th1 and Th17 responses during EAE induction. Our data also indicate that targeting Akt-1 may be a therapeutic approach for EAE, and possibly MS in humans.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), Akt1–/–, Akt2–/–, and Tcrb–/– mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All experimental protocols followed NIH guidelines and were approved by the institutional animal care and use committees of the Ohio State University and the University of Iowa. All mice were used for experiments at ages of 8 to 12 weeks.
Reagents
Recombinant mouse IL-2 (rmIL-2), purified anti-CD3 (Clone 145–2C11), anti-mouse CD28 (37.51), and hamster IgG isotypic controls were obtained from BD Biosciences (San Jose, CA). The following fluorescence-conjugated Abs and ELISA kits were purchased from BioLegend (San Diego, CA): PerCP-anti-mouse CD4 (Cat no:100432), FITC-anti-mouse CD4 (Cat no:100406), APC/Cy7-anti-mouse CD4 (Cat No:100414), PE/Cy7-anti-mouse CD25 (Cat No:101916), PE-anti-mouse Foxp3 (Cat no:320008), PB-anti-mouse Foxp3 (Cat no:126409), PB-anti-mouse Helios (Cat no:137220), PE-cy7-anti-neurophilin (Cat no:145212), PE-anti-mouse IL-17A (Cat no:506904), APC-anti-mouse IFN-γ (Cat no:505810), and ELISA kits for mouse IL-17A (Cat no:432505), IFN-γ (Cat no:430805), IL-12p40 (Cat no:433605), GM-CSF (Cat no:432205), IL-6 (Cat no:431105), and IL-10 (Cat no:431414). FITC-conjugated goat-anti-rabbit IgG and Alexa Flour 488 donkey anti-rabbit IgG (Cat no: A21206) purchased from ThermoFisher Scientific (Waltham, MA). The BrdU kit, carboxyfluorescein succinimidyl ester (CFSE) and APC-anti-mouse CD25, PE-anti-mouse TNF-α (Cat no:12-7321-82), and FITC-anti-mouse IL-6 (Cat no: 11-7061-41) were purchased from eBioscience (San Diego, CA). The Mouse Tregs isolation kit was purchased from Miltenyi Biotec (Auburn, CA). A-674563 (Akt-1 inhibitor), and IFA were purchased from Sigma-Alderich (St. Louis, MO). Mycobacterium tuberculosis (Mtb) strain H37Ra was obtained from Difco (Detroit, MI). Myelin oligodendrocyte glycoprotein peptide (35–55) (MOG35–55) was purchased from GL Biochem (Shanghai, China). Pertussis toxin was purchased from List Biological Laboratories; Campbell, CA). Abs against phospho-GSK-3β (S9) (Cat no: 9336), phospho-Foxo-1 (T24)/3a (T32) (Cat no: 9464), phospho-Akt-1 (S473) (D7F10; Cat no: 9018), phospho-Akt-2 (S474) (D3H2; Cat no:8599), Akt-1 (C73H10; Cat no: 2938S) and Akt-2 (D6G4) (cat no: 3063) were purchased from Cell Signaling, Inc. (Danvers, MA).
EAE induction, histopathology of spinal cords, and ex vivo recall response
WT, Akt1–/–, and Akt2–/– mice (8–12 wk of age) were immunized by s.c. injection over four sites in the flank with 100 μl of emulsified IFA supplemented with 100 μg MOG33–55 and 500 μg heat-inactivated Mtb as described (18, 19). 300 ng pertussis toxin per mouse in PBS was injected intraperitoneally at the time of immunization and 48 h later. Mice were scored on scale of 0 to 5 (18): 0, no clinical disease; 1, limp/flaccid tail; 2, moderate hind limb weakness; 3, severe hind limb weakness; 4, complete hind limb paralysis; 5 quadriplegia or premoribund state. For spinal cord pathology, mice were anethesized, and perfused with 4% paraformaldehyde (Fisher Scientific) to remove the blood from internal organs on day 18 after immunization. The spinal cords were removed and dissected as described (17). The tissues were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), and luxol fast blue (LFB). The CNS histological score was assessed on an average of 6 CNS tissue sections/mouse at 100 x magnification and expressed as mean ± SD as previously descibed (20): 0, no inflammatory cells; 1, a few scattered inflammatory cells; 2, organization of inflammatory infiltrates around blood vessels; 3, extensive perivascular cuffing with extension into adjacent parenchyma, or parenchymal infiltration without obvious cuffing. Spinal cord demyelination was scored as previously described (21): 1, traces of subpial demyelination; 2, marked subpial and perivascular demyelianation; 3, confluent perivascular or subpial demyelination; 4, massive perivascular and subpial demyelination involving one half of the spinal cord with presence of cellular infiltrates into CNS parenchyma; 5, extensive perivascular and subpial demyelination involving the whole cord with presence of cellular infiltrates into CNS parenchyma.
For the ex vivo response, draining lymph node cells from WT, Akt1–/–, or Akt2–/– mice were collected on day 8 after immunization with MOG35–55 in CFA. The cells were labeled with CFSE, and cultured in the presence of MOG35–55 peptide (20 μg/ml) for 72 h. MOG35–55-specific T cell proliferation was determined by CFSE dilution.
CNS leukocyte isolation
Mice were perfused as above with PBS, and the spinal cords were dissected, separated, and cut into small pieces and placed in 2 ml of digestion solution containing 10 mg/ml Collagenase D (Roche Diagnostics) in HBSS. Digestion was performed for 45 min at 37°C with brief vortexing every 15 min. At the end of the digestion, the solution was mixed thoroughly and passed through a 40 μm cell strainer. The cells were washed once in PBS, placed in 6 ml of 38% Percoll solution, and pelleted for 20 min at 2,000 rpm. Pellets were resuspended in buffer for subsequent analysis.
Detection of Th1 and Th17 responses during EAE induction
For detection of Th1 and Th17 responses, the draining lymph node cells from WT, Akt1–/–, and Akt2–/– mice or Tcrb–/– mice receiving CD4+ T cells from WT, Akt1–/–, and Akt2–/– mice immunized with MOG35–55 in CFA for 8 or 10 days were stimulated with 20 μg/ml MOG35–55 in the culture medium for 3 days. The supernatants collected from these cultures were subjected for ELISA for IL-17A, IFN-γ, IL-12p40, GM-CSF, IL-6, and IL-10 using the sandwich ELISA kits (BioLegend). All the procedures were performed according to the manufacturer’s instructions. The cells were restimulated with 50 ng/ml PMA and 750 ng/ml ionomycin for 4 h. The cells were surface-stained with anti-CD4, and intracellularly stained with anti-IFN-γ or anti-IL-17, respectively. The CD4+IFN-γ+ and CD4+IL-17+ cells were determined by flow cytometry as previously described (19, 22, 23).
To determine the pro-inflammatory cytokines contributed by Tregs and non-Tregs of WT, Akt1–/–, and Akt2–/– mice, we immunized WT, Akt1–/–, and Akt2–/– mice with MOG35-55/CFA. On day 8, mice were euthanized, and draining lymph node cells were stimulated with PMA/ionomycin, and surface-stained with Abs against CD4 and CD25, together with intracellular stained with Abs against Foxp3, IL-17, IFN-γ, IL-6, and TNF-α.
Detection of Tregs, and tTreg proliferation in vivo
For detection of Tregs, the draining lymph node cells and spleen cells from above mice were surface-stained with anti-CD4 and CD25, and intracellularly stained with anti-Foxp3 and Helios. To measure in vivo tTreg proliferation, mice were injected with BrdU by i.p. at 1 mg every 12 h for three consecutive days at day 5 after immunization with MOG35–55 in CFA. The BrdU incorporation within CD4+CD25+Foxp3+Helios+ or CD4+CD25+Foxp3+neurophilin+ cells in the draining lymph nodes or infiltrating immune cells in the spinal cords was determined by flow cytometry according to the BrdU staining kit’s instruction.
Treg isolation and in vitro Treg proliferation
Tregs from WT, Akt1–/–, and Akt2–/– mice were isolated by The CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). The Tregs were labeled with CFSE, and cultured in the presence of plate-bound anti-CD3 and anti-CD28 for 72 h. The proliferation rates of Tregs was determined by CFSE dilution.
In vitro Treg suppression assay
WT CD4+CD25− T cells (Teffs) were labeled with CFSE, and incubated with CD4+CD25+ T cells (Tregs) isolated from WT, Akt1–/–, and Akt2–/– mice at different ratios in the presence of anti-CD3 and anti-CD28 for 96 h. T cell proliferation was determined by CFSE dilution.
In vivo Treg suppression assay
Tcrb–/– mice were adoptively transferred by i.v. injection with naïve WT CD4+CD44lowCD62LhiCD25− T cells (4.5 × 106) together with CD4+CD25+ T cells (0.5 × 106) from WT, Akt1–/–, or Akt2–/– mice. As controls, Tcrb–/– mice were adoptively transferred by i.v. injection with naïve WT CD4+CD44lowCD62LhiCD25− T cells (4.5 × 106) together with CD4+CD25− T cells (0.5 × 106) from WT, Akt1–/–, or Akt2–/– mice. 30 days later, the recipients were immunized with MOG33–55 in CFA. Pertussis toxin per mouse in PBS was injected intraperitoneally at the time of immunization and 48 h later. The severity of EAE was monitored for 25 days. Th1/Th17 and Tregs were detected by flow cytometry as previous described (9, 23, 24)
Expression of phospho- and non-phospho-Akt-1 and -Akt-2 in Tregs at the steady and immunization states
WT mice were immunized with MOG35–55 in CFA. Mice were sacrificed on day 3 and 7 after immunization. The draining lymph node cells were surface-stained with anti-CD4, and anti-CD25, and intracellularly stained with anti-Foxp3 and anti-Helios, and either anti-phospho-Akt-1, anti-phospho-Akt-2, anti-Akt-1 or anti-Akt-2 followed by FITC-conjugated goat-anti-rabbit IgG or Alexa Flour 488 donkey anti-rabbit IgG. The expression of phospho-Akt-1 and phospho-Akt-2, as well as Akt-1 and Akt-2 was determined in the CD4+CD25+Foxp3+Helios+ and CD4+CD25+Foxp3+Helios− Tregs, or CD4+CD25+Foxp3+ Tregs. WT mice without immunization were used as controls.
Treatment of WT mice with established EAE with A-674563
WT B6 mice were immunized with MOG35–55 in CFA as described above. When the mice reached an average EAE score of 2 they were treated with A-674563, a selective Akt-1 inhibitor, at a dose of 20 mg/kg, by i.p injection or oral gavage. The EAE severity was monitored for 25 days.
Western Blot analysis
CD4+CD25+ Tregs were isolated from WT B6 mice, pretreated with A-674563 (0.5 μM) for 30 min, incubated with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) mAbs for 30 min on ice, washed with pre-warmed RPMI1640 twice, and crosslinked with rabbit anti-hamster IgG (5 μg/ml), and lysed in 1% NP-40 lysis buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 1 mM dithiothreitol (DTT), 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin] as described (25). The cell lysates were blotted with anti-phospho-GSK-3β and anti-phospho-Foxo-1/3a. The membranes were stripped, and then blotted with anti-phospho-Akt-1 and anti-phospho-Akt-2, and reprobed with anti-Akt-1 and anti-Akt-2, respectively.
Statistical Analysis
A two-tailed Student’s t-test was applied for statistical comparison of two groups or, where appropriate and a Mann-Whitney U test for nonparametric data (EAE scoring). A P value of 0.05 or less was considered significant.
RESULTS
T cell-intrinsic Akt-1 or Akt-2 regulates the susceptibility to EAE
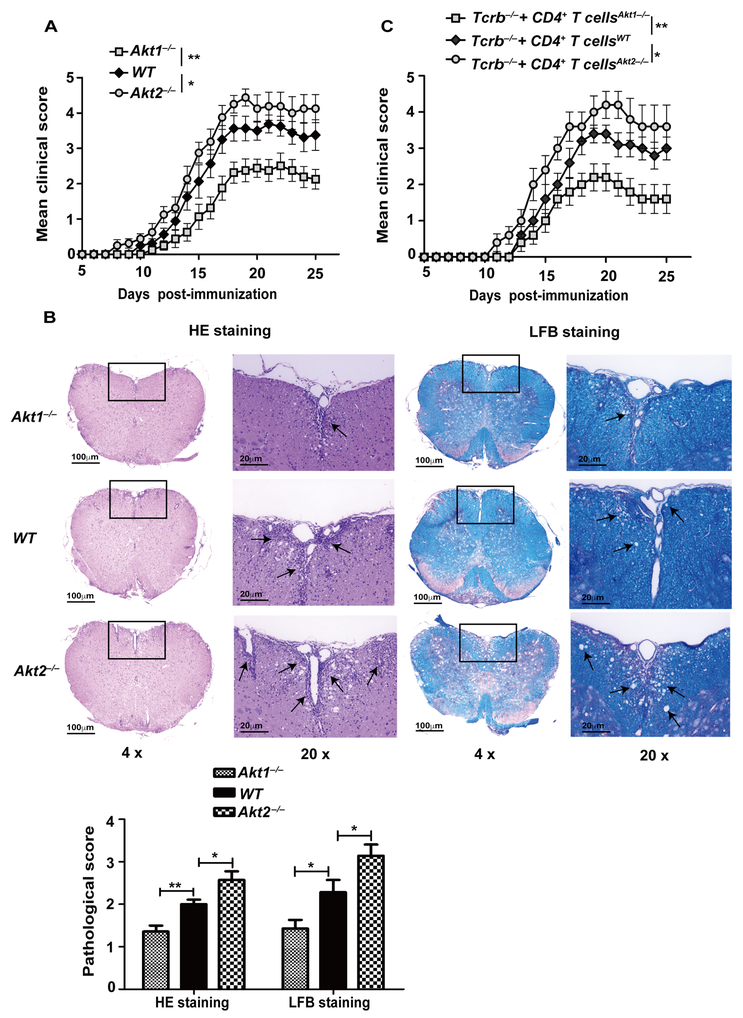
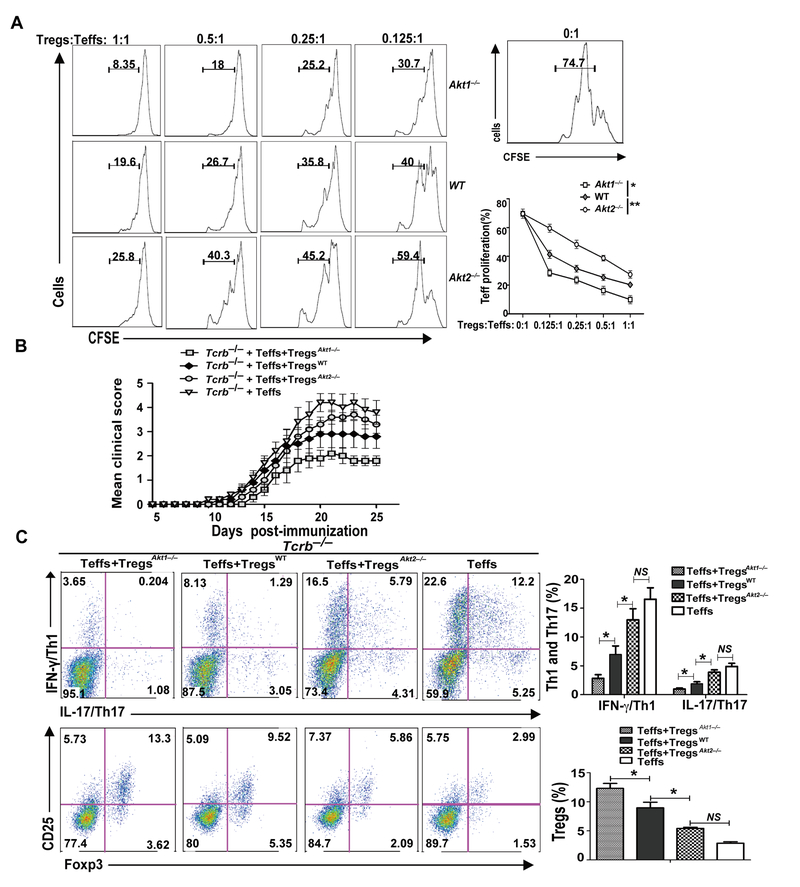
Previously, we and others have shown that Akt-1 and Akt-2, but not Akt-3, are expressed in primary naive T cells in mice (9, 26), and that Akt-2 but not Akt-1 is crucial for the inhibition of iTreg development (9). It was reported that Akt-3 suppresses central nervous system (CNS) inflammatory responses in MOG35–55-induced EAE via both CNS cell- and hematopoietic cell-dependent manners (17), but the roles of Akt-1 and Akt-2 to autoimmune T cell responses and autoimmunity remains to be defined. To determine this, we chose EAE as a model, which is mediated by both Th1 and Th17 cells (10, 27), and utilized mice lacking Akt-1 or Akt-2. We immunized WT, Akt1–/–, and Akt2–/– mice with MOG35–55 in CFA by s.c. injection. Surprisingly, the disease was ameliorated in Akt1–/– mice, whereas Akt2–/– mice developed severe disease activity compared to WT mice as revealed by EAE clinical scores and inflammation of spinal cords (Fig. 1A and B). To confirm whether T cells lacking Akt-1 or Akt-2 are responsible for the phenotype observed, we adoptively transferred CD4+ T cells from WT, Akt1–/–, and Akt2–/– mice into Tcrb–/– mice which lack α/β T cells. After 30 days of equilibration, the Tcrb–/– mice receiving CD4+ T cells from WT, Akt1–/–, and Akt2–/– mice were immunized with MOG35–55 in CFA as described (19). Consistent with the data shown in Figure 1A, Tcrb–/– mice receiving Akt1–/– CD4+ T cells were relatively resistant to EAE induction, whereas Tcrb–/– mice receiving Akt2–/– CD4+ T cells developed severe disease (Fig. 1C). Taken together, our data indicate that T cell-intrinsic Akt-1 and Akt-2 differentially regulate the susceptibility to EAE in mice.
FIGURE 1.
Akt-1 and Akt-2 differentially regulate the susceptibility to EAE in a T cell-intrinsic manner. (A) WT, Akt1–/–, and Akt2–/– mice (n = 8) were immunized by s.c. injection with MOG35–55 in CFA. The EAE severity was monitored for 25 days. *p < 0.05, **p < 0.01; Mann-Whitney U test. (B) WT, Akt1–/–, and Akt2–/– mice (n = 7) were immunized by s.c. injection with MOG35–55 in CFA. Mice were euthanized on day 18 after immunization, and spinal cords were removed, sections of fixed spinal cords were stained with H&E or Luxol fast blue (LFB) to assess inflammation and demyelination. *p < 0.05, **p < 0.01; Mann-Whitney U test. (C) 5 × 106 CD4+ T cells from WT, Akt1–/–, and Akt2–/– mice were transferred by i.v. injection into Tcrb–/– mice (n = 5), which were permitted to equilibrate 30 days after transfer to avoid effects of homeostatic proliferation. The mice were immunized with MOG35–55 in CFA. The disease development was monitored. *p < 0.05, **p < 0.01; Mann-Whitney U test. The data shown are one representative of three independent experiments.
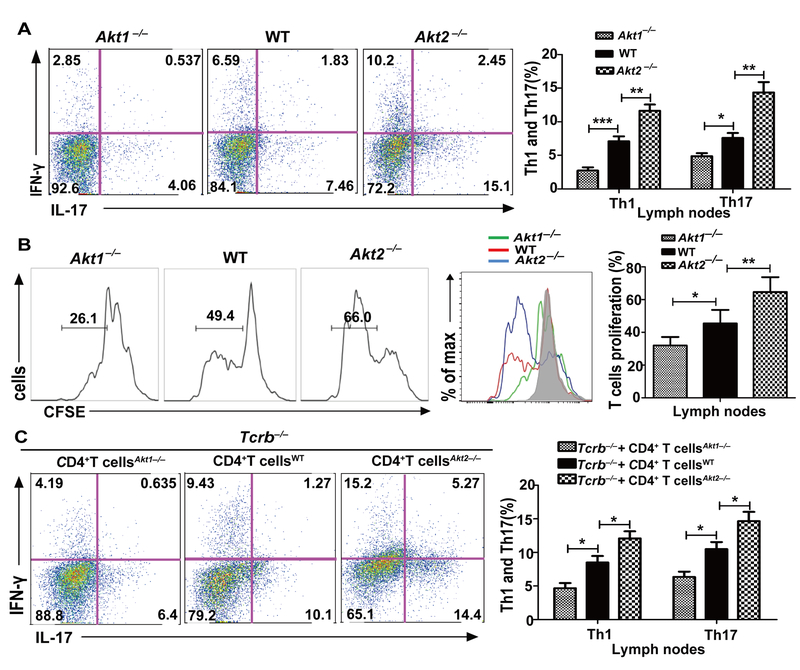
Akt-1 and Akt-2 differentially regulate Th1/Th17 responses during EAE induction
The development of EAE is mediated by both Th1 and Th17 cells (10). To determine the cellular basis of differential disease susceptibility in Akt1–/– and Akt2–/– mice, we immunized WT, Akt1–/–, and Akt2–/– mice with MOG35–55 in CFA and 8 days later the mice were sacrificed. The draining lymph node cells were stained for Th1 (CD4+IFN-γ+) and Th17 (CD4+IL-17+). These cells were also cultured in the presence of MOG35–55 (20 μg/ml) for 72 h to determine MOG35–55-specific T cell proliferation and cytokine production. Consistent with the differential disease susceptibility observed in Akt1–/– and Akt2–/– mice, both Th1/Th17 cell populations and Th1 and Th17 inflammatory cytokines such as IL-17, IFN-γ, IL-6, and GM-CSF were significantly reduced in Akt1–/– mice immunized with MOG35–55 in CFA (Fig. 2A and Supplemental Fig. 1A). In contrast, Akt2–/– mice immunized with MOG35–55 in CFA showed heightened Th1/Th17 cell populations and production of IL-17, IFN-γ, IL-6, and GM-CSF (Fig. 2A and Supplemental Fig. 1A). These data correlated with differential MOG35–55-specific T cell proliferation observed in Akt1–/– and Akt2–/– mice (Fig. 2B). To confirm whether T cell-intrinsic Akt-1 or Akt-2 regulates MOG35–55-specific Th1/Th17 responses, we analyzed the Tcrb–/– mice receiving Akt1–/– or Akt2–/– CD4+ T cells immunized with MOG35–55 in CFA at day 10. In keeping with the data described above, Tcrb–/– mice receiving Akt1–/– CD4+ T cells displayed reduced Th1/Th17 responses, while Tcrb–/– mice receiving Akt2–/– CD4+ T cells developed aberrant Th1/Th17 responses (Fig. 2C and Supplemental Fig. 1B). Note that there was no difference in IL-6 production by WT, Akt1–/–, and Akt2–/– T cells upon ex vivo MOG35–55 stimulation, suggesting that the effect of Akt-1 and Akt-2 on IL-6 production in these lymph node cell cultures may derive from Akt-1 or Akt-2 deficiency in other cell types. Therefore, the differential susceptibility of Akt1–/– and Akt2–/– mice to EAE induction appears to be mediated by their differential capacity to develop pathogenic Th1/Th17 responses in a T cell-intrinsic manner.
FIGURE 2.
Akt-1 potentiates, while Akt-2 inhibits, Th1/Th17 responses during EAE induction. (A) WT, Akt1–/–, and Akt2–/– mice (n = 5) were immunized by s.c. injection with MOG35–55 in CFA. At 8 days after immunization, mice were sacrificed. The draining lymph node cells were stimulated with MOG35–55 (20 μg/ml) for 72 h. After collecting the culture medium, these cells were restimulated with PMA/ionomycin, and surface-stained with anti-CD4, and intracellularly stained with anti-IFN-γ and anti-IL-17, respectively. *p < 0.05, **p < 0.01; Student t test. (B) The draining lymph node cells obtained from the above mice described in A were labeled with CFSE, and cultured with MOG35–55 (20 μg/ml) for 72 h. T cell proliferation was determined by CFSE dilution. *p < 0.05, **p < 0.01; ***p < 0.001; Student t test. (C) Tcrb–/– mice (n = 5) receiving Akt1–/– CD4+ T cells and Akt2–/–CD4+ T were immunized by s.c injection with MOG35–55 in CFA. At 10 days after immunization, mice were sacrificed. The draining lymph node cells were stimulated with MOG35–55 (20 μg/ml) for 72 h, after collecting the culture medium, these cells were restimulated with PMA/ionomycin, and surface-stained with anti-CD4, and intracellularly stained with anti-IFN-γ and anti-IL-17, respectively. *p < 0.05, **p < 0.01; Student t test. The data shown are one representative of three independent experiments.
To determine whether the relative contributions of Th17 cytokines by CD4+Foxp3− effector T cells (Teff) vs. CD4+CD25+Foxp3+ Tregs, we immunized WT, Akt1–/–, and Akt2–/– mice with MOG35–55 in CFA. At day 8, draining lymph node cells were surface-stained with anti-CD4 and CD25 Abs, intracellularly stained with Abs against Foxp3 and INF-γ, IL-17, TNF-α, and IL-6. Although we detected very small populations of Tregs from WT, Akt1–/–, and Akt2–/– mice that produced INF-γ, IL-17, TNF-α, and IL-6, there was no difference observed among the three groups of mice (Supplemental Fig. 2). Consistent with the data presented in Figure 2A, we observed a significant increase in CD4+Foxp3− T cells lacking Akt-2 that were positive for INF-γ and IL-17, but few CD4+Foxp3− T cells lacking Akt-1 were positive for INF-γ and IL-17 (Supplemental Fig. 2). We did not observe any differences in TNF-α+ or IL-6+CD4+Foxp3− T cells among WT, Akt1–/–, and Akt2–/– mice, consistent with the data shown in Supplemental Figure 1. Therefore, the major T cells that contribute to pro-inflammatory cytokines are Teffs, and not Tregs.
Akt-1 inhibits thymus-derived Treg (tTregs), but Akt-2 potentiates tTregs during EAE induction
Tregs have emerged as the crucial players in peripheral T cell tolerance, and that dysregulated Tregs are involved in EAE development, and possibly MS (10, 28). Indeed, a Th1-Treg phenotype which displays defective suppressive capacity was observed in untreated relapsing-remitting MS patients, possibly due to heightened activation of the PI3-K/Akt-1/Foxo1/3 pathway (29). To determine the role of Akt-1 and Akt-2 in Tregs during EAE induction, we first assessed the expression of Akt-1 and Akt-2 isoforms in WT mice at the steady and immunization states. We also assessed the expression of Akt-1 and Akt-2 in Tregs isolated from thymuses from WT mice at the steady state by immunoblotting. As shown in Supplemental Figure 3A, both Akt-1 and Akt-2 were expressed in WT CD4+CD25+Foxp3+ Tregs at the steady state. Although there is a lack of markers to reliably distinguish tTregs from iTregs (30), we did however measure the expression of Helios or neurophilin within CD4+CD25+Foxp3+ population since they are still widely used as specific determination markers of tTregs. To determine whether Akt isoforms are differentially activated in tTregs vs iTregs during EAE induction, we immunized WT mice with MOG35–55 in CFA. The mice were sacrificed at days 3 and 7 and the draining lymph node cells were surface-stained with anti-CD4 and CD25, and intracellularly stained with anti-Foxp3, Helios, and anti-phospho-Akt-1 or phospho-Akt-2. The expression of phospho-Akt-1 vs phospho-Akt-2 within tTregs (CD4+CD25+Foxp3+Helios+) and iTregs (CD4+CD25+Foxp3+Helios−) was determined by flow cytometry. We observed only a slight increase in phospho-Akt-1 in tTregs on day 3 after immunization. However, a marked increase in phospho-Akt-2 was observed in both tTregs and iTregs on day 3 after immunization (Supplemental Fig. 3B). No detectable expression of both phospho-Akt-1 and phospho-Akt-2 was observed in both tTregs and iTregs on day 7 after immunization (Supplemental Fig. 3B). We also note that while the expression of Akt-1 was relatively stable in Tregs from the draining lymph nodes after MOG35–55/CFA immunization at day 3 and 7, Akt-2 expression was markedly increased upon immunization (Supplemental Fig. 3C). These data suggest a possible role of these two Akt isoforms in the expression and function of Tregs during EAE induction.
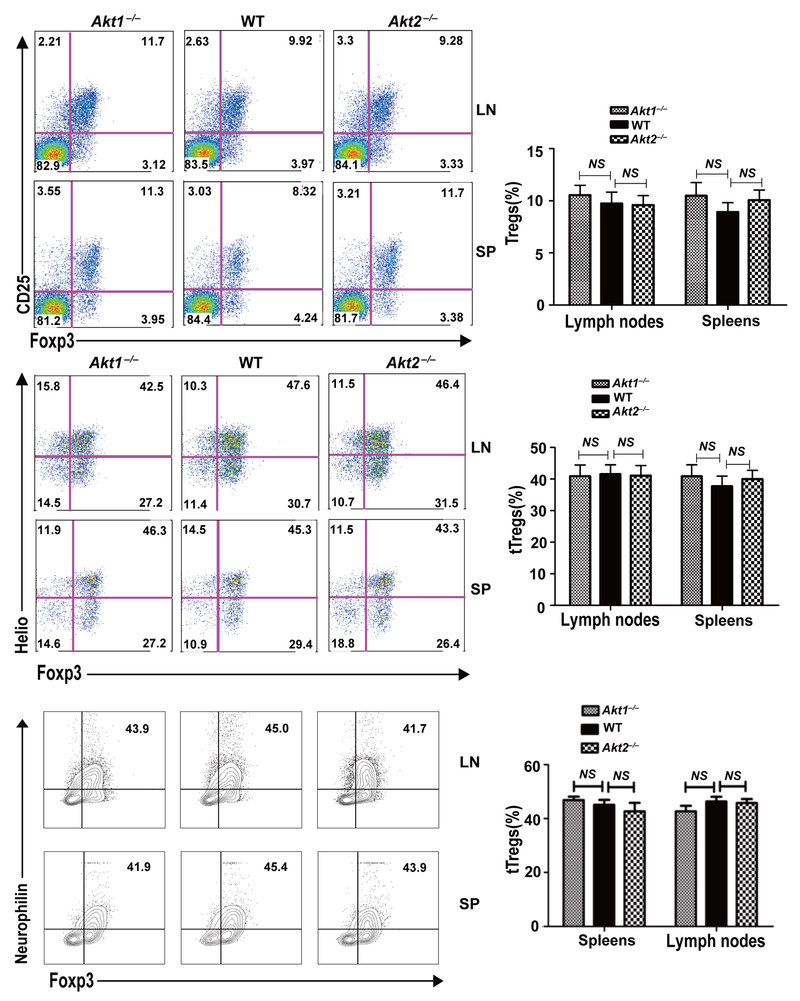
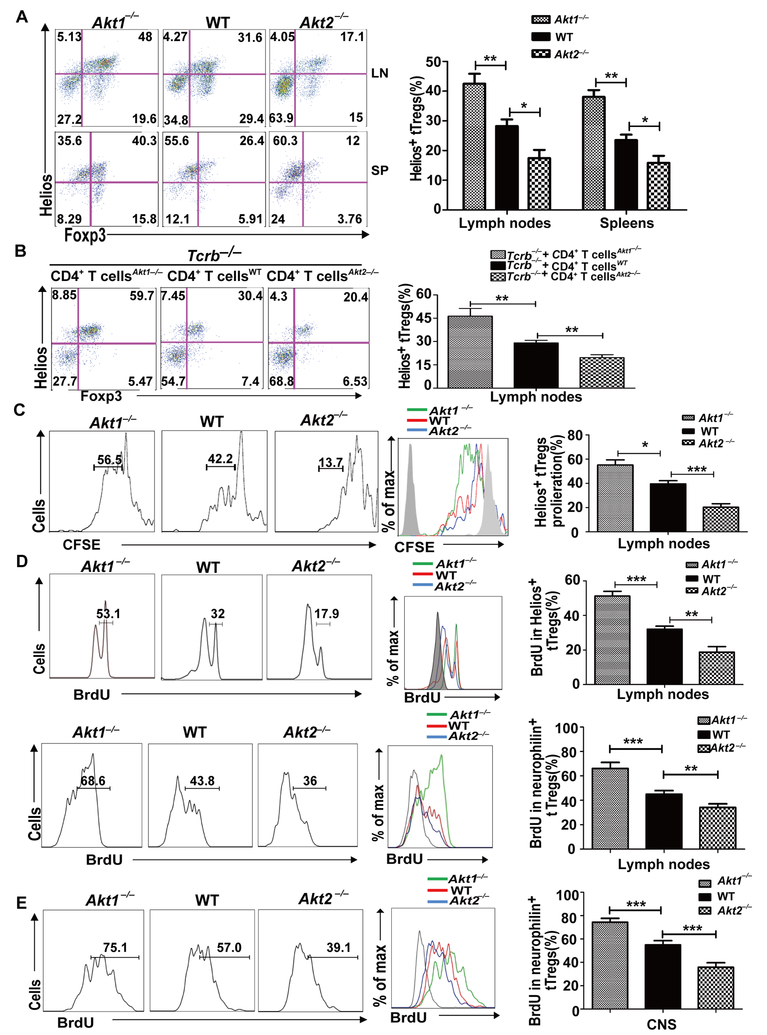
We then asked whether the absence of Akt-1 or Akt-2 affects iTreg development or tTreg proliferation during EAE induction. To this end, WT, Akt1–/–, and Akt2–/– mice were immunized with MOG35–55 in CFA. At day 8, the levels of Tregs and tTregs in draining lymph nodes and spleens were determined. Strikingly, although Tregs and tTregs in Akt1–/– and Akt2–/– mice displayed similar levels in the steady state (Fig. 3), Akt1–/– mice had significantly increased tTregs, whereas tTregs were significantly reduced in Akt2–/– mice during EAE induction (Fig. 4A). Consistent with this data, Tcrb–/– mice reconstituted with Akt1–/– CD4+ T cells had dramatically increased tTregs, while Tcrb–/– mice reconstituted with Akt2–/– CD4+ T cells showed few tTregs at day 10 after immunization with MOG35–55 (Fig. 4B). Our data suggest that Akt-1 and Akt-2 have opposite roles in promoting their proliferation during EAE induction.
FIGURE 3.
Akt1–/– and Akt2–/– mice have similar amounts of Tregs at the steady state. Spleens and lymph nodes of WT, Akt1–/– and Akt2–/– mice (n = 3) were surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3 and anti-Helios or anti-neurophilin. Student t test. The data shown are one representative of three independent experiments.
FIGURE 4.
Akt-1 inhibits, but Akt-2 facilitates, the proliferation of tTregs during EAE induction. (A) The spleen cells and lymph node cells from the mice (n = 5) described in Figure 2 were surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3 and anti-Helios. *p < 0.05, **p < 0.01; Student t test. (B) 5 × 106 CD4+ T cells from WT, Akt1–/–, and Akt2–/– mice were transferred by i.v. injection into Tcrb–/– mice (n = 5). After 30 days equilibration, the recipient mice were immunized with MOG35–55 in CFA. 10 days after immunization, the mice were sacrificed. The draining lymph node cells were surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3 and Helios. **p < 0.01; Student t test. (C) WT, Akt1–/–, and Akt2–/– mice (n = 5) were immunized with MOG35–55 in CFA. At day 8, we collected the draining lymph node cells from each group, labeled with CFSE, and cultured them in the presence of 20 μg/ml MOG35–55 for three days. The cells were then surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Helios and anti-Foxp3. The expression of Helios within CD4+CD25+Foxp3+ T cells was determined. *p < 0.05, ***p < 0.001; Student t test. (D and E) WT, Akt1–/–, and Akt2–/– mice (n = 5) were immunized with MOG35–55 in CFA. On day 5, the mice were injected with BrdU at 1 mg/mouse every 12 h for three consecutive days. The mice were sacrificed on day 8. The BrdU incorporation in CD4+CD25+Foxp3+Helios+ or CD4+CD25+Foxp3+neurophilin+ T cells in the draining lymph nodes (D) and CNS (E) of WT, Akt1–/–, and Akt2–/– mice was determined. **p < 0.01, ***p < 0.001; Student t test. The data shown are one representative of three independent experiments.
To further test this, we immunized WT, Akt1–/–, and Akt2–/– mice with MOG35–55 in CFA. At day 8, we collected the draining lymph node cells from each group, labeled with CFSE, and cultured them in the presence of MOG35–55 for 72 h. As expected, Akt1–/– tTregs were highly proliferative, whereas Akt2–/– tTregs proliferated poorly in response to MOG35–55 stimulation ex vivo (Fig. 4C). To confirm whether Akt-1 and Akt-2 differentially control tTreg proliferation in vivo, we immunized WT, Akt1–/–, and Akt2–/– mice with MOG35–55 in CFA, and 5 days later we injected BrdU at 1 mg/day for three consecutive days. In support of our in vitro data, CD4+CD25+Helios+Foxp3+ or CD4+CD25+neurophilin+Foxp3+ tTregs from MOG35–55-immunized Akt1–/– mice proliferated more effectively than those of WT mice. In sharp contrast, Akt-2 deficiency impaired tTreg proliferation in vivo (Fig. 4D). In keeping with this, tTreg proliferation in the CNS was significantly higher in Akt1–/– mice compared to WT and Akt2–/– mice, with tTreg proliferation from Akt2–/– mice being the lowest upon MOG35–55/CFA immunization (Fig. 4E). These findings suggest that Akt isoforms not only control the proliferative capacity of tTregs in the periphery but also in the CNS. Our data also suggest that differential tTreg proliferation mediated by Akt-1 and Akt-2 may underlie the different susceptibility of Akt1–/– and Akt2–/– mice to EAE.
To directly determine whether Akt-1 or Akt-2 deficiency has an opposite effect on Treg function, we first performed an in vitro Treg suppression assay. To this end, we isolated CD4+CD25+ Tregs from WT, Akt1–/– and Akt2–/– mice, and co-cultured them with CD4+CD25− Teff from WT mice in the presence of plate-bound anti-CD3 and anti-CD28. As shown in Figure 5A, Akt1–/– Tregs effectively suppressed Teff proliferation, whereas Akt2–/– Tregs failed to suppress Teff proliferation. To further confirm this finding in an in vivo setting, we reconstituted Tcrb–/– mice with naïve CD4+CD25− T cells from WT mice together with CD4+CD25+ Tregs from WT, Akt1–/– or Akt2–/– mice. We then immunized these Tcrb–/– mice with MOG35–55 in CFA. Tcrb–/– mice receiving Akt1–/– Tregs and naïve WT CD4+CD25− T cells developed ameliorated disease, whereas Tcrb–/– mice receiving Akt2–/– Tregs and naïve WT CD4+CD25− T cells developed severe disease (Fig. 5B). We observed a significant increase in Tregs of Tcrb–/– mice receiving Akt1–/– Tregs, but a marked decrease in Tregs of Tcrb–/– mice receiving Akt2–/– Tregs (Fig. 5C). In contrast, Tcrb–/– mice receiving naïve WT CD4+CD25−CD44lowCD62hi T cells together with WT, Akt1–/–, or Akt2–/– CD4+CD25− T cells developed comparable EAE (Supplemental Fig. 4). These data collectively demonstrate that Akt-1 and Akt-2 have opposing roles in the suppressive activity of Tregs in vivo, possibly due to their capacity to control the proliferation of Tregs under an inflammatory environment. The molecular mechanism by which Akt-1 and Akt-2 differentially control the proliferation of Tregs is currently under investigation.
FIGURE 5.
Akt1–/– and Akt1–/– Tregs display differential suppressive activity in vitro and in vivo. (A) CD4+CD25+ Tregs of WT, Akt1–/– and Akt1–/– mice were cultured with CFSE-labeled naïve CD4+CD25− Teffs in the presence of plate-bound anti-CD3 and anti-CD28 for 96 h. The proliferation of Teffs was determined by flow cytometry. (B) Naïve CD4+CD25− T cells from WT mice together with CD4+CD25+ T cells from WT, Akt1–/–, or Akt1–/– mice were adoptively transferred into Tcrb–/– mice (n = 5 per group) which were then immunized with MOG35–55 in CFA. The development of EAE was monitored. * p < 0.05; Mann-Whitney U test. (C) The CD4+IFN-γ+, CD4+IL-17+ T cells, and CD4+CD25+Foxp3+ T cells within the draining lymph node cells of the recipients were determined. * p< 0.05; Student t test. The data shown are one representative of three independent experiments.
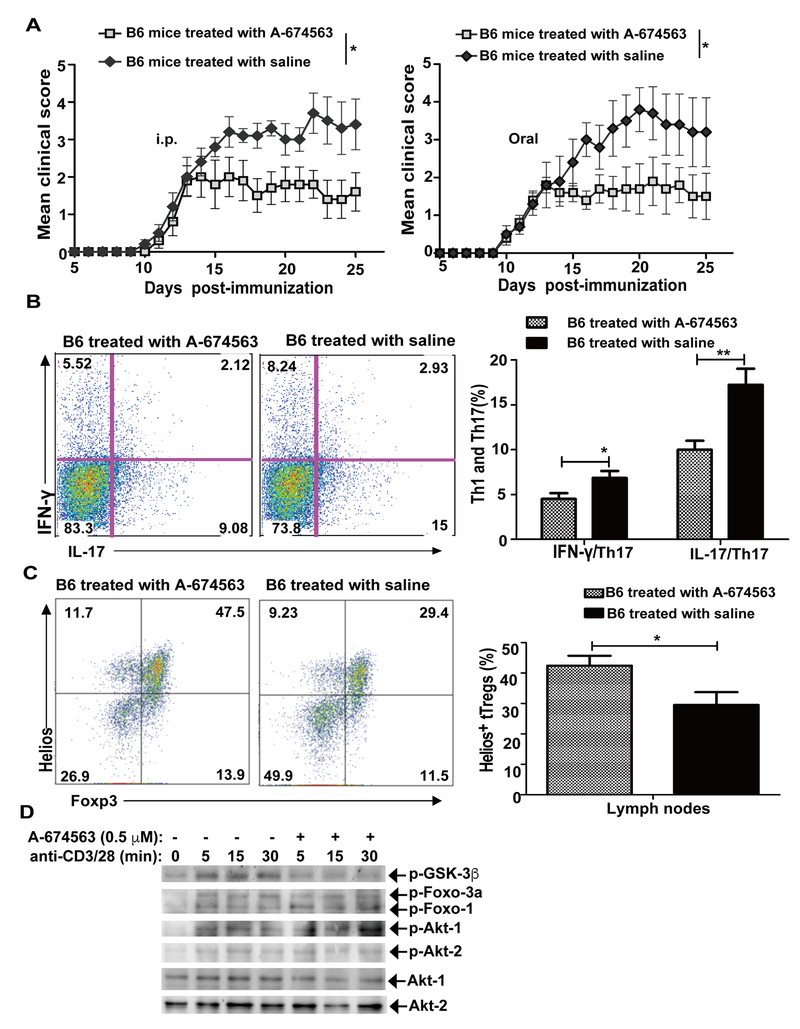
Inhibition of Akt-1 may be a potential therapeutic approach for MS
Our data clearly indicate that Akt-1 inhibits tTreg proliferation, but Akt-2 potentiates tTreg proliferation during EAE induction. These data suggest that inhibition of Akt-1 may have a therapeutic effect on EAE, and possibly MS. To test this idea, we first immunized WT mice with MOG35–55 in CFA. Once WT mice reached the disease score of 2, we treated WT mice with A-674563, a potent and selective Akt1 inhibitor, by daily i.p. injection or oral gavage (Fig. 6A). In support of our observations described above, treating WT mice with the A-674563 ameliorated the disease, which correlated with reduced Th1 and Th17 cells and increased tTregs (Fig. 6B and C). To determine the specific inhibition of A-674563 on Akt-1, we examined the phosphorylation status of GSK-3β, a well-characterized substrate of Akt isoforms (25), since A-674563 does not inhibit Akt-1 phosphorylation, but blocks the downstream targets in a dose-dependent manner (31). We also examined the phosphorylation status of Foxo-1/3a which is phosphorylated by Akt-2 in T cells (9). Pretreating WT Tregs with a 0.5 μM dose of A-674563 markedly inhibited phosphorylation of GSK-3β, but had no effect on phosphorylation Foxo-1/3a (Fig. 6D). Therefore, our data indicate that inhibition of Akt-1 may represent a novel approach to treat MS in humans. It should be noted that we cannot exclude the possible effect of A-674563 on cells other than Tregs, however the significant increase in Tregs after in vivo A-674563 treatment suggests that this mechanism contributes to the attenuation of EAE in WT mice.
FIGURE 6.
Akt-1 is a potential therapeutic target for EAE. (A) WT mice (n = 8) were immunized with MOG35–55 in CFA and when the mice reached an average EAE score of 2 they were treated with A-674563, a selective Akt-1 inhibitor, at dose of 20 mg/kg, by i.p injection (left panel) or oral gavage (right panel). The EAE severity was monitored for 25 days. *p < 0.05; Mann-Whitney U test. (B and C) WT mice (n = 5) were immunized with MOG35–55 in CFA and treated with A-674563 by oral gavage. The mice were sacrificed on day 18. The draining lymph node cells were with MOG35–55 (20 μg/ml) for 72 h, and restimulated with PMA and ionomycin for 4 h. The cells were surface-stained with anti-CD4, and intracellularly stained with anti-IFN-γ and anti-IL-17. *p< 0.05, **p< 0.01; Student t test. (B) The CD4+IFN-γ+ and CD4+IL-17+ T cells within the draining lymph node cells were determined. (C) Some draining lymph node cells were directly surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3 and anti-Helios. (D) CD4+CD25+ Tregs were isolated from WT spleens, pretreated with A-674563 (0.5 μM) for 30 min, stimulated with anti-CD3 and anti-CD28 for 5 min, and lysed in RIPA lysis buffer. The cell lysates were blotted with anti-phospho-GSK-3β and anti-phospho-Foxo-1/3a, respectively. The membranes were stripped, then blotted with anti-phospho-Akt-1 and anti-phospho-Akt-2, and reprobed with anti-Akt-1 and anti-Akt-2, respectively. *p < 0.05; Student t test. The data shown are one representative of three independent experiments.
DISCUSSION
Although Akt has been extensively studied in the immune system, the role of Akt isoforms in T cell biology is poorly defined. Furthermore, the involvement of Akt isoforms in Th17-mediated autoimmunity is unknown. In this study we surprisingly found that, although both Akt-1 and Akt-2 are expressed in T cells, they have opposite roles in the development of EAE. Mice lacking Akt-1 are resistant to EAE induction, which correlates with robust proliferation of Tregs that inhibits Th1/Th17 responses. In a sharp contrast, Akt2–/– mice develop severe EAE, which correlates with defective tTreg proliferation and heightened Th1/Th17 responses. To our knowledge, our study is the first to indicate that Akt-1 and Akt-2 exert opposing roles in tTreg proliferation under an inflammatory environment.
The role of CD4+CD25+ Tregs in the development and in the course of MS has been in the focus of intensive basic and clinical research during the last decade. Although there has been some controversy as to whether patients with MS have a reduced number of Tregs, the majority of studies suggest that the defect is not in their number but in their function (12, 16, 32, 33). Consistent with the data of human MS patients, several groups have shown that Tregs accumulate in the CNS, but these accumulated Tregs fail to effectively suppress effector T cells during the peak of EAE (13, 34). Additional concern regarding the importance of Tregs in EAE development comes from mice with a frame-shift mutation of Foxp3, Scurfy mice. These mice have massive lymphoproliferation and severe inflammatory infiltration of the skin and liver, but many organs including the CNS, the joints, and the small intestine remained unaffected in Scurfy mice (35, 36). However, crossing Scurfy mice onto Faslpr or Il2–/– backgrounds clearly extends the inflammation to joints, lung, stomach, small intestines, and colon (37). It is highly possible that the breakdown of peripheral T cell tolerance in different organs/tissues may require specific antigen triggers, and the thresholds for the breakdown of tolerance vary in different organs/tissues. The importance of Tregs in EAE is further supported by the fact that depletion of Tregs by an anti-CD25 mAb has been shown to result in a significant enhancement of EAE disease severity (38, 39) in an IL-10-dependent manner (38). Consistent with these reports, depleting Tregs in DEREG mice by treating them with diphtheria toxin (DTx) exacerbates EAE severity which is accompanied by increased pro-inflammatory cytokine production and proliferation of effector T cells (40). Furthermore, transfer of Tregs is able to suppress clinical signs of either EAE or colitis (41). It is important to note that Tregs are not only limited to CD4+CD25+Foxp3+ T cells, but also include IL-10+LAP+ type 1 regulatory T cells (Tr1). It has been shown that nasal administration of anti-CD3 induces the generation of IL-10+LAP+ Tr1-like T cells that also suppress the progressive model of MS in an IL-10-dependent manner by attenuating CNS innate immunity (42). These studies indicate that under certain circumstances, IL-10+LAP+ Tr1-like T cells can be induced, and can suppress EAE. However, CD4+CD25+Foxp3+ Tregs are the major Tregs involved in controlling disease development during the natural process of EAE induction. Our data indicate that WT CD4+CD25+ Tregs are able to suppress EAE development in an adaptive transfer model (Fig. 5), supporting a crucial role of CD4+CD25+ Tregs in controlling EAE development.
It has been shown that Akt inhibits Foxp3 expression by phosphorylating Foxo-1/Foxo-3a, which results in the translocation of Foxo-1/Foxo-3a in the nucleus to the cytoplasm (43, 44). Therefore, Akt is crucial for inhibition of iTreg development. However, the exact isoform of Akt that is responsible for this inhibition was not defined. Several years ago, we showed that Akt-2, but not Akt-1, is involved in the inhibition of iTreg generation via a Foxo1/Foxo3a-dependent manner (9). Furthermore, patients with MS were reported to display an increased frequency of Tregs secreting IFN-γ (Th1-Tregs), which fail to exert their immune regulation (16, 45). The generation of these Th1-Tregs is mediated by heightened PI3-K/Akt/Foxo1/3 signaling cascade activity (29). Similarly, the PI3K/Akt/mTORC1/S6K1/2 axis has been shown to control Th17 differentiation, although the involvement of specific Akt isoforms in this process is not known (46). Our data showed that although Akt-2 inhibits Foxp3 expression by iTregs (9), Akt-1 seems to potentiate the proliferation of tTregs under an inflammatory environment using Helios as the tTreg markers, whereas Akt-2 suppresses this process (Fig. 4). The expression of Akt-1 and Akt-2 is also differentially required for their suppressive activity. tTregs lacking Akt-1 effectively suppress Teff response in vitro and in vivo, while tTregs lacking Akt-2 fail to suppress Teff response (Fig. 5). However, the molecular mechanism by which the structurally similar Akt isoforms behave differently in tTreg proliferation and suppressive activity remains to be determined.
In summary, our data demonstrate that Akt-1 inhibits, but Akt-2 potentiates, tTreg proliferation during EAE induction, which differentially regulate Th1 and Th17 responses and the susceptibility of mice to EAE. The molecular mechanisms by which Akt-1 and Akt-2 differentially control the proliferation of Tregs are currently under investigation.
Supplementary Material
Acknowledgements
We thank Drs. Amy Lovett-Racke and Michael Racke for their advice on the EAE mouse model; and Drs. Nitin Karandikar and Ashutosh Mangalam for their critical reading of the manuscript.
Footnotes
The project described was supported by grants from the National Institutes of Health (NIH) (R01 AI090901, R01 AI121196, R01AI123253, and R21 AI117547 to J.Z.) and from the American Heart Association (16GRNT26990004 to JZ).
References
- 1.Manning BD, and Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao Y, and Hung MC. 2010. Physiological regulation of Akt activity and stability. Am J Transl Res 2: 19–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez E, and McGraw TE. 2009. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8: 2502–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane LP, and Weiss A. 2003. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev 192: 7–20. [DOI] [PubMed] [Google Scholar]
- 5.Mercadante ER, and Lorenz UM. 2016. Breaking Free of Control: How Conventional T Cells Overcome Regulatory T Cell Suppression. Front Immunol 7: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crellin NK, Garcia RV, and Levings MK. 2007. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood 109: 2014–2022. [DOI] [PubMed] [Google Scholar]
- 7.Haxhinasto S, Mathis D, and Benoist C. 2008. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med 205: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, and Merkenschlager M. 2008. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A 105: 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, and Zhang J. 2013. T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells. J Immunol 191: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dendrou CA, Fugger L, and Friese MA. 2015. Immunopathology of multiple sclerosis. Nat Rev Immunol 15: 545–558. [DOI] [PubMed] [Google Scholar]
- 11.Lovett-Racke AE 2016. Contribution of EAE to understanding and treating multiple sclerosis. J Neuroimmunol. [DOI] [PubMed] [Google Scholar]
- 12.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, and Stinissen P. 2008. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol 180: 6411–6420. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, and Kuchroo VK. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costantino CM, Baecher-Allan C, and Hafler DA. 2008. Multiple sclerosis and regulatory T cells. J Clin Immunol 28: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor RA, and Anderton SM. 2008. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol 193: 1–11. [DOI] [PubMed] [Google Scholar]
- 16.Viglietta V, Baecher-Allan C, Weiner HL, and Hafler DA. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 199: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsiperson V, Gruber RC, Goldberg MF, Jordan A, Weinger JG, Macian F, and Shafit-Zagardo B. 2013. Suppression of inflammatory responses during myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis is regulated by AKT3 signaling. J Immunol 190: 1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller SD, and Karpus WJ. 2007. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol Chapter 15: Unit 15 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao Y, Qiao G, Tang J, Tang R, Guo H, Warwar S, Langdon WY, Tao L, and Zhang J. 2015. Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation. J Immunol 195: 4218–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill EJ, Day MJ, and Wraith DC. 2006. IL-10 is essential for disease protection following intranasal peptide administration in the C57BL/6 model of EAE. J Neuroimmunol 178: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, and Uccelli A. 2005. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755–1761. [DOI] [PubMed] [Google Scholar]
- 22.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, Li Z, Solway J, Tao E, Chiang YJ, Lipkowitz S, Penninger JM, Langdon WY, and Zhang J. 2014. E3 Ubiquitin Ligase Cbl-b suppresses proallergic T cell development and allergic airway inflammation. Cell Rep 6: 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying HY, Yang LF, Qiao GL, Li ZP, Zhang L, Yin F, Xie D, and Zhang JA. 2010. Cutting Edge: CTLA-4-B7 Interaction Suppresses Th17 Cell Differentiation. Journal of Immunology 185: 1375–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Guo H, Qiao G, Zucker M, Langdon WY, and Zhang J. 2015. E3 Ubiquitin Ligase Cbl-b Regulates Thymic-Derived CD4+CD25+ Regulatory T Cell Development by Targeting Foxp3 for Ubiquitination. J Immunol 194: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, and Zhang J. 2008. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol 28: 2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Phong B, Wilson DC, Hirsch R, and Kane LP. 2011. Akt fine-tunes NF-kappaB-dependent gene expression during T cell activation. J Biol Chem 286: 36076–36085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarland HF, and Martin R. 2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8: 913–919. [DOI] [PubMed] [Google Scholar]
- 28.Zozulya AL, and Wiendl H. 2008. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol 4: 384–398. [DOI] [PubMed] [Google Scholar]
- 29.Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, and Dominguez-Villar M. 2016. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep 17: 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, and Ziegler SF. 2013. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 14: 307–308. [DOI] [PubMed] [Google Scholar]
- 31.Zhu QS, Ren W, Korchin B, Lahat G, Dicker A, Lu Y, Mills G, Pollock RE, and Lev D. 2008. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha. Cancer Res 68: 2895–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, and Vandenbark AA. 2005. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 81: 45–52. [DOI] [PubMed] [Google Scholar]
- 33.Kumar M, Putzki N, Limmroth V, Remus R, Lindemann M, Knop D, Mueller N, Hardt C, Kreuzfelder E, and Grosse-Wilde H. 2006. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol 180: 178–184. [DOI] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Stephens LA, and Anderton SM. 2005. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol 175: 3025–3032. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey VL, Wilkinson JE, and Russell LB. 1991. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 138: 1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 36.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, and Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 37.Ju ST, Sharma R, Gaskin F, Kung JT, and Fu SM. 2012. The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells. Biology (Basel) 1: 18–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, and Weiner HL. 2004. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol 16: 249–256. [DOI] [PubMed] [Google Scholar]
- 39.Stephens LA, Gray D, and Anderton SM. 2005. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci U S A 102: 17418–17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koutrolos M, Berer K, Kawakami N, Wekerle H, and Krishnamoorthy G. 2014. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun 2: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPherson RC, Turner DG, Mair I, O’Connor RA, and Anderton SM. 2015. T-bet Expression by Foxp3(+) T Regulatory Cells is Not Essential for Their Suppressive Function in CNS Autoimmune Disease or Colitis. Front Immunol 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo L, Cunha AP, Madi A, Beynon V, Yang Z, Alvarez JI, Prat A, Sobel RA, Kobzik L, Lassmann H, Quintana FJ, and Weiner HL. 2016. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 139: 1939–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, and Li MO. 2010. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 11: 618–627. [DOI] [PubMed] [Google Scholar]
- 44.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, and Liu YC. 2010. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 207: 1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venken K, Hellings N, Hensen K, Rummens JL, Medaer R, D’Hooghe M B, Dubois B, Raus J, and Stinissen P. 2006. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res 83: 1432–1446. [DOI] [PubMed] [Google Scholar]
- 46.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, and Koyasu S. 2012. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep 1: 360–373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.