Abstract
Background:
Long-term trends in the incidence rates (IRs) and hospital case-fatality rates (CFRs) of ventricular tachycardia (VT) and ventricular fibrillation (VF) among patients hospitalized with acute myocardial infarction (AMI) have not been recently examined.
Methods and Results:
We used data from 11,825 patients hospitalized with AMI at all 11 medical centers in central Massachusetts on a biennial basis between 1986 and 2011. Multivariable adjusted logistic regression modeling was used to examine trends in hospital IRs and CFRs of VT and VF complicating AMI. The median age of the study population was 71 years, 57.9% were men, and 94.7% were white. The hospital IRs declined from 14.3% in 1986/1988 to 10.5% in 2009/2011 for VT and from 8.2% to 1.7% for VF. The in-hospital CFRs declined from 27.7% to 6.9% for VT and from 49.6% to 36.0% for VF between 1986/1988 and 2009/2011, respectively. The IRs of both early (<48 hours) and late VT and VF declined over time, with greater declines in those of late VT and VF. The incidence rates of VT declined similarly for patients with either a STEMI or NSTEMI, while they only declined in those with VF and a STEMI.
Conclusion:
The hospital incidence and case-fatality rates of VT and VF complicating AMI have declined over time, likely due to changes in acute monitoring and treatment practices. Despite these encouraging trends, efforts remain needed to identify patients at risk for these serious ventricular arrhythmias so that preventive and treatment strategies might be implemented as necessary.
Keywords: Trends, incidence, case-fatality rates, ventricular tachycardia, myocardial infarction
INTRODUCTION
Ventricular tachycardia (VT) and ventricular fibrillation (VF) frequently complicate the clinical course of patients hospitalized with an acute myocardial infarction.1, 2 The development of these serious ventricular arrhythmias in patients hospitalized with an acute myocardial infarction (AMI) is often associated with a larger infarct, ventricular wall dyskinesia and dysfunction, more extensive underlying coronary artery disease, and ST segment-elevation myocardial infarction (STEMI).3–6 The development of VT and VF in patients hospitalized with AMI is associated with increased in-hospital and post discharge morbidity and mortality.7, 8
National data on either the incidence rates (IRs) or case-fatality rates (CFRs) of VT and VF complicating AMI in the U.S. are not presently available. In a medical records linkage study of 2,280 patients who were admitted to all hospitals in Olmsted County, Minnesota, for coronary heart disease between 1979 and 1998, the annual IRs of sustained VT remained stable at 1.8%, while the IRs of VF declined from 7.7% in the initial study years to 5.0% during the most recent years under study.9 However, this study was limited by its small sample size and by the lack of more contemporary data. A number of changes in the more optimal management of patients hospitalized with acute coronary disease during recent years may have led to favorable changes in the magnitude and/or outcomes of serious ventricular arrhythmias among patients hospitalized for an AMI.10, 11
Using data from a population-based surveillance study of patients hospitalized with confirmed AMI at all central Massachusetts medical centers, we examined 25-year trends (1986–2011) in the hospital IRs and CFRs of VT and VF, and compared differences in the in-hospital CFRs of patients who developed, with those who did not develop, VT or VF complicating their AMI.
METHODS
Study design and data collection methods
Data from the Worcester Heart Attack Study (WHAS), an ongoing population-based investigation that is examining long-term trends in the incidence, in hospital, and post-discharge CFRs of AMI among residents of the Worcester, Massachusetts, metropolitan area were used for this investigation. The details of this study have been described previously.12–16 In brief, the medical records of residents of central Massachusetts hospitalized for possible AMI at the 11 medical centers serving residents of this large central New England metropolitan area were individually reviewed. The diagnosis of AMI was independently validated according to criteria developed by the World Health Organization with further sub-classification into those with an ST-segment elevation AMI (STEMI) or Non-ST segment elevation AMI (NSTEMI).17, 18 This investigation was approved by the Institutional Review Board at the University of Massachusetts Medical School.
Trained nurses and physicians abstracted demographic and clinical data from the medical records of residents of the Worcester metropolitan area hospitalized with a confirmed AMI. Quality control activities were routinely conducted on all nurse and physician data abstractors. Abstracted information included patient’s age, sex, medical history, physiologic factors, laboratory test results at the time of hospital admission, and length of hospital stay. Information about the in-hospital use of important cardiac medications, coronary angiography, percutaneous coronary intervention (PCI), and coronary artery bypass graft (CABG) surgery was collected. Development of several significant clinical complications (e.g., atrial fibrillation, cardiogenic shock, stroke, heart failure, survival status) during the patient’s index hospitalization was defined according to standardized criteria.18–21
Study population
Among the 12,804 residents of the Worcester metropolitan area who were hospitalized with an independently confirmed AMI on an approximate biennial basis between 1986 and 2011, we excluded patients with missing data on age (n=281), race (n=476), serum potassium levels (n=212), and length of hospital stay (n=10). The final study sample consisted of 11,825 patients with an AMI.
Ventricular arrhythmias
The occurrence of VT and VF was based on physicians’ progress notes. To reduce the possible misclassification of VT and VF due to the underreported occurrence of these arrhythmias in physicians’ notes, the study research physicians also reviewed patients’ ECG strips in their hospital medical records. Ventricular tachycardia was defined as a cardiac arrhythmia of three or more consecutive complexes originating in the ventricles at a rate of greater than 100 beats per minute (cycle length less than 600 milliseconds).2 Information was also collected about the date of initial VT occurrence since the time of hospital admission. Ventricular fibrillation was defined as a rapid, usually more than 300 beats per minute (cycle length 200 millisecond or less), grossly irregular ventricular rhythm with marked variability in QRS cycle length, morphology, and amplitude.2
Following definitions used in prior investigations,9, 14, 22 we further classified VT and VF as either occurring early (within 48 hours after hospital admission) or late (after the first 48 hours) and primary (occurring without concomitant heart failure or cardiogenic shock) or secondary to concurrent heart failure or cardiogenic shock. For patients who had multiple episodes of VT (582/1,731 patients, 33.6%) and VF (158/574 patients, 27.5%), only the first episode was counted. We classified the 348 patients who developed both VT and VF as having VF, since VF is more clinically serious than VT.
Statistical analysis
Patient characteristics, including demographic factors, clinical presentation, physiologic findings, and laboratory test results were compared between patients who developed VT or VF, and those who did not, using chi-square tests for categorical variables and the t-test or Kruskal– Wallis test for continuous variables. Changes in these characteristics between our respective comparison groups were also examined across study years using chi square tests for trends.
The hospital IRs (e.g., frequencies) of VT and VF complicating AMI were calculated as the proportion of new cases of VT and VF which occurred among all patients hospitalized with AMI.22 The in-hospital CFRs for each condition were calculated as the number of in-hospital deaths/total number of cases with a particular arrhythmia.23 The IRs and CFRs were graphed in a continuous manner between 1986 and 2011 using locally weighted least-square smoothing.24
We controlled for the effects of changes in patients’ demographic characteristics, clinical presentation, and medical therapies and coronary reperfusion practices on trends in hospital IRs and CFRs using multivariable adjusted logistic regression modelling. Study year was treated as an integer-valued variable in examining linear trends in these endpoints. Potentially confounding factors were identified based on differences in the baseline characteristics of hospitalized patients according to the presence or absence of VT or VF using a p-value of <0.05 as a cutoff. These variables were successively introduced into the multivariable adjusted logistic regression model in blocks to a full model. Since length of hospital stay may affect the possibility of capturing VT or VF during the patient’s acute hospitalization, or serve as a proxy for disease severity, length of stay was also included in the fully adjusted models as a potentially confounding factor.
We examined the overall magnitude, and changes over time therein, in the frequencies of VT and VF based on the presence of concomitant heart failure/cardiogenic shock or not. Since the occurrence of VT may differ between patients with a STEMI and NSTEMI, trends in the hospital IRs and CFRs of VT were also examined for these two groups, separately in patients admitted to all central Massachusetts hospitals between 2001 and 2011, when information about the type of AMI was collected. We examined trends in the hospital IRs and CFRs of VT and VF according to whether or not the patient received coronary reperfusion therapy, namely thrombolytic therapy or a PCI, during the years under study. In addition, we examined trends in hospital IRs of early (during the first 2 days after hospital admission) versus late VT in patients further classified by type of AMI (e.g., STEMI vs. NSTEMI).
Given the large size of this study, almost all differences that were observed were highly statistically significant. Thus, all reported between group differences without an explicit p value had a p value <0.05.
RESULTS
Baseline patient characteristics
The median age of the study population was 71 years old, most patients were white (94.7%), and 57.9% were men. Patients whose clinical course was complicated by either VT (n=1,731) or VF (n=574) were several years younger than patients who did not develop these serious ventricular arrhythmias and were more likely to be male (Table 1). Patients who developed VT or VF were less likely to have a medical history of diabetes, hypertension, or a stroke/transient ischemic attack. On the other hand, patients in whom these arrhythmias were diagnosed were more likely to have developed important clinical complications (e.g., atrial fibrillation, heart failure, cardiogenic shock) during their acute hospitalization. Compared with patients who did not develop these ventricular arrhythmias, patients who developed VT or VF were more likely to have been treated with antiarrhythmic agents or thrombolytic therapy, but were less likely to have received aspirin, angiotensin converting enzyme inhibitor/angiotensin II receptor blockers (ACE-I/ARBs), beta blockers, and lipid lowering agents.
Table 1:
Baseline characteristics of patients admitted to the hospital for an acute myocardial infarction according to the presence of ventricular tachycardia or ventricular fibrillation
| Characteristics | No VT/VF (n= 9,520) | VT (n= 1,731) | VF (n= 574) | p1 | p2 |
|---|---|---|---|---|---|
| Age (median [IQR], years) | 72 [60–81] | 70 [58–79] | 68 [58–77] | <0.001 | <0.001 |
| Age (years, %) | <0.001 | <0.001 | |||
| <55 | 15.3 | 19.5 | 19.0 | ||
| 55–64 | 18.3 | 18.9 | 20.4 | ||
| 65–74 | 24.5 | 25.0 | 28.9 | ||
| ≥75 | 42.0 | 36.6 | 31.7 | ||
| Men (%) | 55.7 | 66.7 | 67.8 | <0.001 | <0.001 |
| White (%) | 94.4 | 96.0 | 95.1 | 0.010 | 0.49 |
| History of disease (%) | |||||
| Coronary artery disease | 36.5 | 36.1 | 35.2 | 0.75 | 0.53 |
| Heart failure | 20.8 | 21.1 | 14.8 | 0.83 | 0.001 |
| Diabetes | 32.8 | 27.8 | 23.9 | <0.001 | <0.001 |
| Hypertension | 65.2 | 60.2 | 57.5 | <0.001 | <0.001 |
| Angina | 21.4 | 20.8 | 17.3 | 0.56 | 0.018 |
| Stroke/transient ischemic attack | 12.6 | 10.3 | 8.7 | 0.009 | 0.006 |
| Admission potassium (median [IQR], mmol/L) | 4.2 [3.9–4.6] | 4.2 [3.8–4.6] | 4.0 [3.5–4.4] | 0.017 | <0.001 |
| Length of stay (median [IQR], day) | 5 [3–9] | 7 [4–11] | 7 [2–13] | <0.001 | <0.001 |
| In hospital complications (%) | |||||
| Atrial fibrillation | 15.8 | 24.7 | 27.9 | <0.001 | <0.001 |
| Heart failure | 35.7 | 43.9 | 47.6 | <0.001 | <0.001 |
| Pulmonary edema | 12.4 | 17.0 | 20.7 | <0.001 | <0.001 |
| Cardiogenic shock | 4.3 | 9.2 | 26.7 | <0.001 | <0.001 |
| Treatment (%) | |||||
| Aspirin | 82.2 | 81.2 | 67.6 | 0.29 | <0.001 |
| ACE-I/ARBs | 47.4 | 48.6 | 38.0 | 0.33 | <0.001 |
| Antiarrhythmic agents | 12.9 | 27.8 | 40.6 | <0.001 | <0.001 |
| Beta blockers | 77.0 | 77.9 | 62.5 | 0.41 | <0.001 |
| Lipid lowering agents | 40.3 | 39.3 | 24.6 | 0.40 | <0.001 |
| Thrombolytic therapy | 11.3 | 21.4 | 22.0 | <0.001 | <0.001 |
| Percutaneous coronary intervention | 25.6 | 27.7 | 28.1 | 0.06 | 0.19 |
| CABG surgery | 4.6 | 6.9 | 5.8 | <0.001 | 0.19 |
VT: ventricular tachycardia, VF: ventricular fibrillation (with/without VT), IQR: interquartile range, ACEI/ARBs: Angiotensin converting enzyme inhibitor/Angiotensin II receptor blockers, CABG: coronary artery bypass graft
p-value from Chi-square test and Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables; p1: no VT/VF vs. VT, p2: no VT/VF vs. VF.
Between 1986 and 2011, the prevalence of several preexisting comorbidities (e.g., heart failure, diabetes, hypertension, stroke) increased (Table 2), as did the prescribing of aspirin, ACE-I/ARBs, beta blockers, lipid lowering agents, PCI, and CABG surgery. During this period, there were declines in the frequency of several important in-hospital complications including atrial fibrillation, heart failure, and cardiogenic shock. Receipt of thrombolytic therapy and the average hospital length of stay also decreased over time (Table 2).
Table 2:
Trends in the characteristics of patients admitted to the hospital for an acute myocardial infarction
| Characteristics | 1986–1990 (n=2,012) | 1997–2001 (n=3,074) | 2007–2011 (n=2,157) | P for trend |
|---|---|---|---|---|
| Age (median [IQR], years) | 70 [60–78] | 73 [61–82] | 68 [57–80] | 0.12 |
| Age (years, %) | ||||
| <55 | 14.0 | 15.5 | 19.8 | |
| 55–64 | 21.8 | 16.1 | 21.1 | |
| 65–74 | 30.3 | 22.9 | 22.3 | |
| ≥75 | 33.9 | 45.5 | 36.9 | |
| Men (%) | 60.1 | 57.3 | 61.0 | 0.99 |
| White (%) | 97.6 | 95.4 | 91.7 | <0.001 |
| History of disease (%) | ||||
| Coronary artery disease | 19.1 | 52.4 | 32.0 | <0.001 |
| Congestive heart failure | 14.8 | 23.5 | 20.6 | <0.001 |
| Diabetes | 25.7 | 32.0 | 38.3 | <0.001 |
| Hypertension | 49.9 | 65.0 | 74.9 | <0.001 |
| Stable angina | 27.6 | 23.3 | 6.6 | <0.001 |
| Stroke/transient ischemic attack | 9.1 | 13.5 | 13.1 | <0.001 |
| Admission potassium (median [IQR], mmol/L) | 4.1 [3.7–4.4] | 4.2 [3.9–4.7] | 4.2 [3.8–4.6] | <0.001 |
| Length of stay (median [IQR], day) | 10 [7–13] | 5 [3–8] | 3 [2–6] | <0.001 |
| In hospital complications (%) | ||||
| Atrial fibrillation | 19.3 | 16.9 | 17.2 | 0.047 |
| Heart failure | 41.5 | 36.1 | 32.4 | <0.001 |
| Pulmonary edema | 18.3 | 13.3 | 8.2 | <0.001 |
| Cardiogenic shock | 6.9 | 6.3 | 5.1 | <0.001 |
| Treatment (%) | ||||
| Aspirin | 43.7 | 88.4 | 94.5 | <0.001 |
| ACE-I/ARBs | 8.6 | 54.2 | 72.4 | <0.001 |
| Antiarrhythmic agents | 20.3 | 14.6 | 11.1 | 0.11 |
| Beta blockers | 48.4 | 81.1 | 92.6 | <0.001 |
| Lipid lowering agents | 2.9 | 38.2 | 89.2 | <0.001 |
| Thrombolytic therapy | 17.4 | 16.2 | 0.6 | <0.001 |
| Percutaneous coronary intervention | 2.8 | 22.6 | 53.6 | <0.001 |
| CABG surgery | 1.7 | 6.6 | 7.5 | <0.001 |
Note: Years 1991–1995 and 2003–2005 were omitted for easy presentation of data.
p-values for trend were from logistic regression analysis for categorical variables and linear regression analysis for continuous variables with study year included as a linear predictor.
IQR: interquartile range, CK: creatinine kinase, ACE-I/ARBs: Angiotensin converting enzyme inhibitor/Angiotensin II receptor blockers, CABG: coronary artery bypass graft.
Trends in hospital IRs of VT and VF
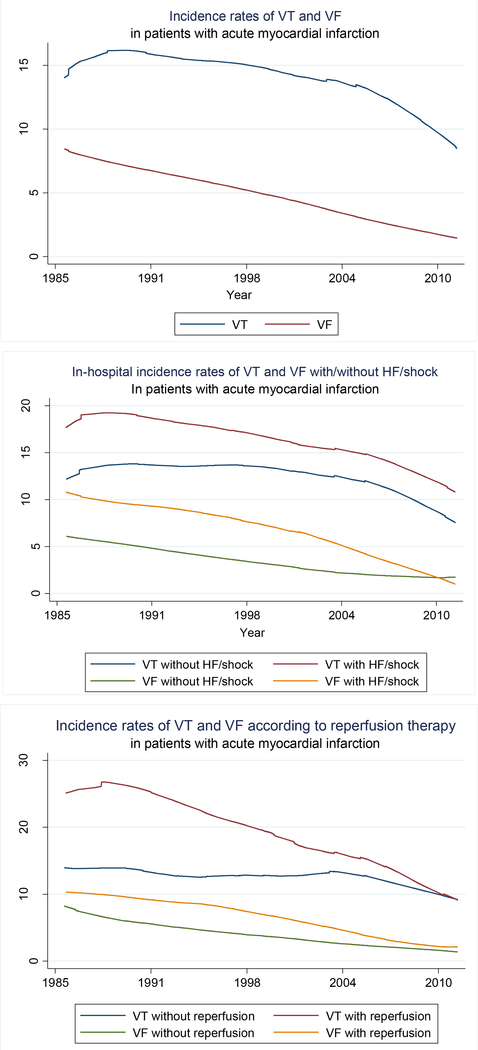
Between 1986 and 2011, VT developed in 14.6% and VF in 4.9% of all patients admitted to central Massachusetts hospitals for an AMI. During this period, the proportion of patients with AMI who developed VT declined from 14.3% in 1986/1988 to 10.5% in 2009/2011 while the proportion of patients who developed VF declined from 8.2% in 1986/1988 to 1.7% in 2009/2011 (Figure 1, top panel).
Figure 1:
Trends in hospital incidence rates of ventricular tachycardia (VT) and ventricular fibrillation (VF) in patients with acute myocardial infarction, overall (top panel), according to the presence of heart failure/shock (middle panel), and reperfusion therapy (bottom panel).
After adjusting for changes in the demographic and clinical characteristics of our patient population over time, the proportion of patients who developed VT declined by approximately 11% (95%CI=6–17%) while the proportion of patients who developed VF declined by 37% (95%CI=30–43%) after each decade, respectively (Table 3). After we adjusted for changes over time in the receipt of various cardiac medications and reperfusion therapies received at the time of hospital admission during the years under study, the proportion of patients who developed VT did not change over time while adjustment for the receipt of these therapies attenuated the magnitude of the decline in the hospital IRs of VF after each decade to 20% (95%CI=7–32%).
Table 3:
Trends in hospital incidence rates and case-fatality rates of ventricular tachycardia (VT) and ventricular fibrillation (VF) for each decade among patients with acute myocardial infarction
| VT | VF | |||
|---|---|---|---|---|
| Incidence rates | (n= 11,251) | (n= 10,094) | ||
| In years 1986/1988 | 14.3 | 8.2 | ||
| In years 2009/2011 | 10.5 | 1.7 | ||
| Trends regression models | IRR | 95%CI | IRR | 95%CI |
| Unadjusted | 0.85 | 0.81–0.91 | 0.57 | 0.51–0.64 |
| Demographic factors (1) | 0.86 | 0.81–0.91 | 0.58 | 0.52–0.65 |
| History of disease (2) & (1) | 0.87 | 0.82–0.92 | 0.60 | 0.53–0.67 |
| Clinical presentation (3) & (1), (2) | 0.89 | 0.83–0.94 | 0.63 | 0.57–0.70 |
| In-hospital treatments (4) & (1), (2), (3) | 0.89 | 0.81–0.97 | 0.80 | 0.68–0.93 |
| Case-fatality rates | (n= 1,731) | (n= 574) | ||
| In years 1986/1988 | 27.7 | 49.5 | ||
| In years 2009/2011 | 6.9 | 37.5 | ||
| Trends regression models | IRR | 95%CI | IRR | 95%CI |
| Unadjusted | 0.64 | 0.53–0.77 | 0.87 | 0.76–0.99 |
| Demographic (1) | 0.59 | 0.49–0.71 | 0.84 | 0.74–0.95 |
| History of disease (2) & (1) | 0.57 | 0.47–0.68 | 0.80 | 0.71–0.90 |
| Clinical presentation (3) & (1), (2) | 0.59 | 0.50–0.71 | 0.76 | 0.67–0.86 |
| In-hospital treatments (4) & (1), (2), (3) | 1.09 | 0.86–1.39 | 1.07 | 0.91–1.27 |
IRR: incidence rate ratio, 95%CI: 95% Confidence interval
Note: each block of variables was added to precedent blocks in the successive models.
Block 1: Age, sex, and race.
Block 2: History of heart failure, diabetes, hypertension, and stroke/transient ischemic attack.
Block 3: In-hospital serum potassium levels and hospital complications of atrial fibrillation, heart failure, and cardiogenic shock.
Block 4: In hospital treatment with aspirin, angiotensin converting enzyme inhibitors, antiarrhythmic agents, beta-blockers, lipid-lowering agents, thrombolytics, percutaneous coronary intervention, and coronary artery bypass surgery.
Trends in hospital IRs of VT and VF according to concomitant heart failure or cardiogenic shock
Approximately 41% of our 11,825 study patients developed either heart failure or cardiogenic shock during their hospitalization for AMI. The development of ventricular arrhythmias in these patients was more common than in patients who did not develop either heart failure or cardiogenic shock (17.2% vs. 12.9% for VT and 7.3% vs. 3.2% for VF, respectively). The proportion of patients who developed VT among those with concomitant heart failure or cardiogenic shock decreased from 17.7% in 1986/1988 to 13.6% in 2009/2011 while the hospital IRs for VT for those who did not develop these hemodynamic disturbances did not significantly decline (p=0.45). The IRs of VF among those with concomitant heart failure or cardiogenic shock decreased from 10.7% in 1986/1988 to 2.1% in 2009/2011 compared with a decrease from 6.0% in 1986/1988 to 1.6% in 2009/2011 among patients without concomitant heart failure or cardiogenic shock (Figure 1, middle panel).
Trends in hospital IRs of VT and VF according to time of occurrence
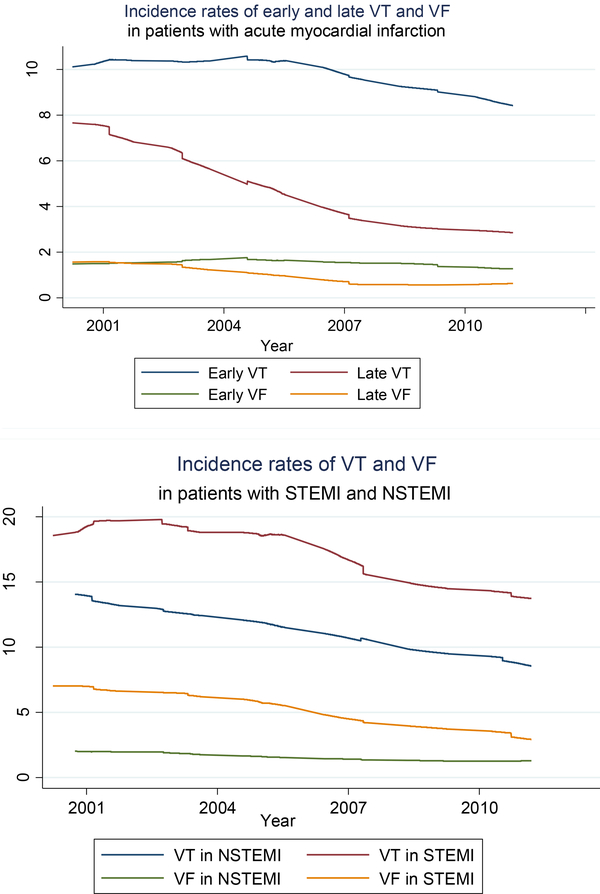
Data on the time of occurrence of VT and VF and type of AMI were collected in 5,764 patients admitted to all greater Worcester hospitals on a biennial basis with AMI between 2001 and 2011. During this period, VT occurred in 13.2% while VF occurred in 2.8% of these patients, respectively. The frequency of VT declined from 15.8% in 2001/2003 to 9.9% in 2009/2011 and from 3.5% to 1.6% for VF. More than two thirds of all VT and VF episodes occurred during the first 48 hours after hospital admission. The hospital IRs of both early and late VT declined over time, with greater declines noted for those with late VT in both relative and absolute terms (Figure 2, top panel). Similarly, the incidence rates of early and late VF significantly declined during the most recent decade under study, with a larger decline observed for late VF (Figure 2).
Figure 2:
Trends in hospital incidence rates of ventricular tachycardia (VT) and ventricular fibrillation (VF) in patients with ST segment-elevation myocardial infarction and non-ST segment -elevation myocardial infarction (top panel) and by timing of VT and VF (early: in the first 48 hours of hospital stay, late: after the first 48 hours of hospital stay, bottom panel).
Trends in hospital IRs of VT and VF according to type of AMI
During the most recent decade (2001–2011) under study in which data were collected on the type of AMI patients experienced, 32.0% of study patients (n=1,161) were diagnosed with a STEMI while the remainder (n= 3,930) were diagnosed with an NSTEMI. The occurrence of both VT and VF were more common in patients with a STEMI than for those with an NSTEMI (17.3% vs. 11.3% for VT and 5.3% versus 1.6% for VF). Among patients with a STEMI, about 80% of episodes of VT and VF occurred within the first 48 hours after hospital admission, compared with only 60% of such episodes for those with an NSTEMI. The hospital IRs of VT and VF declined between 2001 and 2011 in patients with a STEMI (Figure 2, bottom panel). The IRs of VT also declined in patients with an NSTEMI, whereas the IRs of VF in these patients did not change significantly over time (p=0.25, Figure 2).
Trends in hospital IRs of VT and VF according to the receipt of coronary reperfusion therapy
We also examined trends in the hospital IRs of VT and VF during the years under study according to the receipt of coronary reperfusion therapy (e.g., thrombolytic therapy or a PCI) during the patient’s acute hospitalization.
Patients who received either form of coronary reperfusion therapy were more likely to have developed either VT (17.8% vs. 12.8%) or VF (5.9% vs. 4.3%) than those who were not treated with coronary reperfusion therapy (p<0.001). Encouragingly, there was a marked decline over time in the likelihood of developing either VT or VF for patients who did or did not receive coronary reperfusion therapy (Figure 1, bottom panel). A greater decline was observed in the IRs of VT among patient who received coronary reperfusion therapy compared with patients who did not receive this treatment modality (Figure 1, bottom panel).
Trends in hospital CFRs of VT and VF
The overall in-hospital CFRs were 48.3% among patients who developed VF, 13.9% in patients who developed VT, and 8.9% in patients who did not develop either of these ventricular arrhythmias during their acute hospital stay. The in-hospital CFRs decreased from 27.7% in our initial 2 study years (1986/1988) to 6.9% in our 2 most recent study years of 2009/2011 for patients who developed VT, from 49.6% to 36.0% for patients who developed VF, and from 11.5% to 4.6%for those whose hospital course was not complicated by either VT or VF (Figure 3). After adjusting for changes in patient’s demographic characteristics and clinical presentation during the years under study, the hospital CFRs decreased by 41% (95%CI=3950%) for patients who developed VT and by 24% (95%CI=14–32%) for those who developed VF after each study decade (Table 3). However, the declining trends in the hospital CFRs of VT and VF were markedly attenuated after adjusting for the receipt of various cardiac medications and reperfusion treatments during the patient’s index hospitalization for AMI.
Figure 3:
Trends in hospital case-fatality rates of ventricular tachycardia (VT) and ventricular fibrillation (VF) in patients with acute myocardial infarction, overall (top panel) and according to receipt of coronary reperfusion therapy (bottom panel)
Trends in hospital CFRs of VT and VF according to the receipt of coronary reperfusion therapy
Patients who were treated with coronary reperfusion therapy in the form of either thrombolytic therapy or a PCI experienced markedly lower in-hospital death rates than those who did not receive this form of therapy, irrespective of whether they did not develop either VT or VF (3.5% vs. 11.7%), or if they developed VT (7.7% vs. 18.9%) or VF (29.4% vs. 63.3%), respectively. The in-hospital CFRs declined over time for all patients, regardless of whether they received reperfusion therapy or if they developed VAs, with more significant declines observed in patients who developed VT who were treated with either conventional or coronary reperfusion therapy (Figure 3, bottom panel). However, caution needs to be exercised in the interpretation of the in-hospital death rates among patients who developed VT, and especially VF, given the declining frequency of these serious VAs among patients with AMI which resulted in relatively small sample sizes of several patient groups during the most recent years under study.
DISCUSSION
In this community-based study of approximately twelve thousand patients hospitalized with AMI at all central Massachusetts medical centers, we found that the incidence rates of both VT and VF, especially among patients with concomitant heart failure or cardiogenic shock, declined between 1986 and 2011. While the in-hospital death rates for patients whose hospital course was complicated by VT or VF have declined during the study period, mortality remained high, at approximately 40% in patients who developed VF in 2009/2011. Between 2001 and 2011, trends in the magnitude of early vs. late VT differed, while trends in the frequency of VF complicating AMI differed according to the type of AMI patients developed.
Trends in the hospital incidence rates of VT complicating AMI
We observed declining trends in the frequency of sustained and nonsustained VT among patients admitted to all central Massachusetts hospitals for an AMI between 1986 and 2011. Published data examining long-term or recent trends in the magnitude or death rates associated with VT complicating AMI are extremely limited. In a study of 2,280 patients who were admitted to all hospitals in Olmsted County, Minnesota, for an AMI between 1979 and 1998, sustained VT occurred in 1.8% of hospitalized patients.9 Among the more than 3,000 patients enrolled in the Primary Angioplasty in Myocardial Infarction (PAMI) trials who underwent a primary PCI, VT/VF occurred in 4.3% of studied patients.25 In the large multicenter randomized HORIZONS-AMI trial of patients with a STEMI who underwent a primary PCI,26 5.2% of patients developed VT/VF after undergoing their PCI, with most episodes (85%) occurring during the first 48 hours of their procedure. In a subgroup analysis of data from the multi-center, multi-country, APEX AMI trial,27 among the 5,745 patients with a STEMI who underwent a primary PCI, 5.7% of patients developed VT/VF.
Notably, our observed declines in the frequency of VT were primarily driven by declines in VT among patients who had concomitant heart failure or cardiogenic shock. The declines we observed in the frequency of VT in patients with heart failure or cardiogenic shock might be due to increases in the use of coronary reperfusion therapies, including thrombolytic therapy and PCI, which have been shown to improve cardiac function and lower the risk of developing heart failure.28 Indeed, in our study, the proportion of patients who developed heart failure after AMI decreased from 44% in 1986 to 29% in 2011.
While the use of coronary reperfusion therapies might reduce the frequency of occurrence of primary and secondary VT after AMI, reperfusion arrhythmias, including VT, might offset these benefits.6, 29 An increasing occurrence of reperfusion arrhythmias might also explain the smaller decrease in the incidence of early VT, where reperfusion VTs are more prevalent, compared with the incidence rates of late VT that were observed between 2001 and 2011.
While evidence-based treatment modalities may have an impact on preventing early onset VT, which is often induced by transient abnormalities of automaticity or triggered activity following acute myocardial ischemia, these treatments can reduce infarct size and remodeling of the arrhythmic substrate or reentry pathways, thereby reducing the risk of late VT.30
Discontinuation of the use of prophylactic lidocaine and other antiarrhythmic agents in the mid1990s might also have contributed to declines in the frequency of VT.31,32 The trends we observed are consistent with these hypotheses; however, disentangling the interplay of these factors on trends in the incidence rates of VT is complex, and remains a topic for further study.
Trends in the hospital incidence rates of VF complicating AMI
The frequency of primary VF (i.e., VF which occurs in patients without heart failure or cardiogenic shock) and secondary VF among patients with an AMI also decreased between 1986 and 2011, with a larger decrease in the frequency of secondary VF. This finding was in contrast to our prior analysis of data from this community-wide study which failed to find a significant change in the incidence rates of primary VF complicating AMI between 1975 and 1997,14, 15 or with the results of the Rochester Epidemiology study between 1979 and 1998.9
Declining trends in the magnitude of VF that we observed may be due, in part, to the favorable effects of increased utilization of evidence-based treatments for AMI during the most recent years under study. In contrast with relatively stable trends in the frequency of VT between 1986 and 2011, declines in the occurrence of primary VF might be explained by the fact that reperfusion-induced VF occurred in a considerably smaller proportion of treated patients compared with the frequency of VT.33
Trends in hospital incidence rates of VT and VF according to type of AMI
Consistent with prior reports,7, 34, 35 we found that VT and VF occurred more commonly in patients with a STEMI than in patients with an NSTEMI. Most instances of VT and VF tended to occur early within the first 48 hours of hospitalization in patients with a STEMI while slightly more than one half of all episodes of VF occurred during this high-risk period among patients with an NSTEMI. The greater frequency of ventricular arrhythmias in patients with a STEMI is possibly due to the more common occurrence of electrical or inflammatory disturbances that result after the complete blockage of a coronary artery compared to less common disturbances due to residual coronary flow in patients with an NSTEMI.36 In addition, emergent PCI is more common in patients with a STEMI, which may result in a greater frequency of reperfusion-induced VT as compared to patients with an NSTEMI, where a “watchful waiting” strategy is more commonly employed. Differences in the timing of these ventricular arrhythmias may have implications for when to discharge high risk patients with an NSTEMI. However, later discharge and/or increased post-discharge surveillance for patients with an NSTEMI needs to be considered from a cost-benefit perspective given the fairly low incidence rates of VF (1.6%) among these patients.
Patients with either a STEMI or a NSTEMI experienced a decline in the hospital frequency of VT between 2001 and 2011. Similar trends were observed with regards to the development of VF among patients with a STEMI while the occurrence of VF among patients with an NSTEMI did not change during this period. It is unclear why the frequency of VF did not decrease in patients with a NSTEMI during the study period. However, the low frequency of VF (1.6%) among these patients might have been difficult to detect in our study. Differences in the trends of VT and VF among patients with an NSTEMI may also suggest distinct underlying mechanisms and risk factors for the development of these serious arrhythmias among these patients.
Trends in hospital case-fatality rates of VT and VF complicating AMI
We observed an encouraging decline in the hospital death rates of patients who did not develop VT or VF, as well as in patients who developed either VT or VF, between 1986 and 2011. Our prior work also found a lower odds of dying among patients who developed primary VF in the late 1990s and early 2000s compared with the late 1970s.22
Improvements in the timing and use of in-hospital cardiopulmonary resuscitation might have contributed to the decreased in-hospital CFRs of VT and VF that we observed during the years under study. Data from 17,490 patients with in-hospital VT or VF who were studied in the Get With The Guidelines–Resuscitation registry showed that immediate survival after resuscitation increased from 58% in 2001 to 72% in 2009.37–40 Several factors, including early recognition and rapid defibrillation, greater availability of trained personnel, better chest compressions, and therapeutic hypothermia likely contributed to improved survival rates.34–37 For patients with hemodynamically stable VT, changes in patient management, including correction of electrolyte and acid/base abnormalities and treatment of important clinical complications during the period under study may have further reduced the risk of deterioration into pulseless VT or VF and death.
It is important to note that in the present study, changes in hospital management practices, namely the increased use of aspirin, ACE-I/ARBs, beta blockers, and lipid-lowering agents, as well as increased use of PCI and CABG surgery during the period under study, primarily accounted for the improvement in the short-term survival rates we observed among patients who developed either VT or VF. Our findings provide encouragement for the continued high use of effective hospital treatments for AMI to reduce the risk of dying from these ventricular arrhythmias.
Study strengths and limitations
The present study has several strengths. It is among the very few studies that have provided more generalizable community-based epidemiologic data that can furnish insights into the magnitude and impact of the major ventricular arrhythmias in patients hospitalized with AMI. All patients admitted to the coronary care unit were telemonitored for their entire stay, making it unlikely that episodes of asymptomatic and nonsustained VT were missed.
However, our study also has several limitations. Due to considerable missing data on duration of prehospital delay, we were unable to systematically classify the time of onset of VT or VF according to acute symptom onset. However, in an analysis of 1,501 patients with information available about duration of pre-hospital delay, the classification of early from late VT or VF based on acute symptom onset agreed well with that based on the timing of hospital admission. Due to our methods of data collection, we were unable to disentangle patients who developed sustained VT from those who developed nonsustained VT, which may, in part, explain the relatively high IRs of VT that we observed in the present study compared with prior investigations. Finally, caveats need to be placed on the interpretation of our data that examined trends in the hospital CFRs of VT and VF according to the receipt of coronary reperfusion therapy during the years under study given the sample sizes involved and unclear temporarily of administration of this therapy and development of the serious VAs under study.
CONCLUSIONS
The results of this community-wide study found declining multi-decade long trends in the incidence and mortality of VT and VF in a community-wide population of patients hospitalized with AMI. The encouraging trends that we observed are likely due to changes in hospital treatment practices during the years under study. However, efforts remain needed to identify patients at risk for these serious ventricular arrhythmias so that effective preventive and treatment strategies could be implemented as necessary.41
Acknowledgments
FUNDING SUPPORT AND DISCLOSURES
Partial support for R.J.G was provided by NIH/NHLBI grants 1R01HL126911–01A1, 5R01HL125089–02, and 5R01HL115295–05. Partial support for C.I.K. was provided by Patient-Centered Outcomes Research Institute (PCORI) ME-1310–07682, NIH/NCRR U54 RR 026088, and National Heart, Lung, and Blood Institute (NHLBI) R01 HL126911. Partial support for A.S.A was provided by NIH grant 5UL1TR001453.
Footnotes
All authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Newby KH, Thompson T, Stebbins A, Topol EJ, Califf RM and Natale A. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy. Circulation. 1998;98:2567–2573. doi: 10.1161/01.CIR.98.23.2567 [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Myerburg RJ and Quinones MA. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Khairy PP. Prognostic significance of ventricular arrhythmias post-myocardial infarction. Can J Cardiol. 2003;19:1393–1404. [PubMed] [Google Scholar]
- 4.Scirica BM, Braunwald E, Belardinelli L, Hedgepeth CM, Spinar J, Wang W, Qin J, Karwatowska-Prokopczuk E, Verheugt FW and Morrow DA. Relationship Between Nonsustained Ventricular Tachycardia After Non–ST-Elevation Acute Coronary Syndrome and Sudden Cardiac Death. Circulation. 2010;122:455–462. doi: 10.1161/CIRCULATIONAHA.110.937136 [DOI] [PubMed] [Google Scholar]
- 5.Timmer J, Breet N, Svilaas T, Haaksma J, Van Gelder I and Zijlstra F. Predictors of ventricular tachyarrhythmia in high-risk myocardial infarction patients treated with primary coronary intervention. Neth Heart J. 2010;18:122–128. doi: 10.1007/bf03091750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta RH, Starr AZ, Lopes RD and et al. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009;301:1779–1789. doi: 10.1001/jama.2009.600 [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Granger CB, Huang Y, Lee KL, Califf RM, Simoons ML, Armstrong PW, Van de Werf F, White HD and Simes RJ. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation. Circulation. 2002;106:309–312. doi: 10.1161/01.CIR.0000022692.49934.E3 [DOI] [PubMed] [Google Scholar]
- 8.Volpi A, Cavalli A, Turato R, Barlera S, Santoro E and Negri E. Incidence and short-term prognosis of late sustained ventricular tachycardia after myocardial infarction: Results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-3) Data Base. American Heart Journal. 2001;142:87–92. doi: 10.1067/mhj.2001.115791 [DOI] [PubMed] [Google Scholar]
- 9.Henkel DM, Witt BJ, Gersh BJ, Jacobsen SJ, Weston SA, Meverden RA and Roger VL. Ventricular arrhythmias after acute myocardial infarction: A 20-year community study. Am Heart J. 2006;151:806–812. doi: 10.1016/j.ahj.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 10.Bradley EH, Nallamothu BK, Stern AF, Cherlin EJ, Wang Y, Byrd JR, Linnander EL, Nazem AG, Brush JE and Krumholz HM. The door-to-balloon alliance for quality: who joins national collaborative efforts and why? Jt Comm J Qual Patient Saf. 2009;35:93–99. doi: 10.1016/S1553-7250(09)35012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang JJ. Temporal Evolution and Implications of Ventricular Arrhythmias Associated With Acute Myocardial Infarction. Cardiol Rev. 2013;21:289–294. doi: 10.1097/CRD.0b013e3182a46fc6 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RJ, Yarzebski J, Lessard D and Gore JM. Decade-long trends and factors associated with time to hospital presentation in patients with acute myocardial infarction: the Worcester Heart Attack study. Arch Intern Med. 2000;160:3217–3223. doi: 10.1001/archinte.160.21.3217 [DOI] [PubMed] [Google Scholar]
- 13.Crowley A, Menon V, Lessard D, Yarzebski J, Jackson E, Gore JM and Goldberg RJ. Sex differences in survival after acute myocardial infarction in patients with diabetes mellitus (Worcester Heart Attack Study). Am Heart J. 2003;146:824–831. doi: 10.1016/S0002-8703(03)00406-X [DOI] [PubMed] [Google Scholar]
- 14.Chiriboga D, Yarzebski J, Goldberg RJ, Gore JM and Alpert JS. Temporal trends (1975 through 1990) in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction. A communitywide perspective. Circulation. 1994;89:998–1003. doi: 10.1161/01.CIR.89.3.998 [DOI] [PubMed] [Google Scholar]
- 15.Thompson CA, Yarzebski J, Goldberg RJ, Lessard D, Gore JM and Dalen JE. Changes over time in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: Perspectives from the Worcester heart attack study. Am Heart J. 2000;139:1014–1021. doi: 10.1067/mhj.2000.106160 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RJ, Gore JM, Alpert JS and Dalen JE. Recent changes in attack and survival rates of acute myocardial infarction (1975 through 1981): The worcester heart attack study. JAMA. 1986;255:2774–2779. doi: 10.1001/jama.1986.03370200076031 [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFftUDoMI, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ and Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 18.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ and Zhao DX. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 19.Bonow RO, Mann DL, Zipes DP and Libby P. Braunwald’s heart disease: a textbook of cardiovascular medicine: Elsevier Health Sciences; 2011. [Google Scholar]
- 20.Goldberg RJ, Gore JM, Alpert JS, Osganian V, De Groot J, Bade J, Chen Z, Frid D and Dalen JE. Cardiogenic shock after acute myocardial infarction: incidence and mortality from a community-wide perspective, 1975 to 1988. N Engl J Med. 1991;325:1117–1122. doi: 10.1056/NEJM199110173251601 [DOI] [PubMed] [Google Scholar]
- 21.Saczynski JS, Spencer FA, Gore JM, Gurwitz JH, Yarzebski J, Lessard D, Goldberg RJ. Twenty-year trends in the incidence of stroke complicating acute myocardial infarction: Worcester heart attack study. Arch Intern Med. 2008;168:2104–2110. doi: 10.1001/archinte.168.19.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg RJ, Yarzebski J, Spencer FA, Zevallos JC, Lessard D and Gore JM. Thirty-Year Trends (1975–2005) in the Magnitude, Patient Characteristics, and Hospital Outcomes of Patients With Acute Myocardial Infarction Complicated by Ventricular Fibrillation. Am J Cardiol. 2008;102:1595–1601. doi: 10.1016/j.amjcard.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly H and Cowling BJ. Case fatality: rate, ratio, or risk? Epidemiology. 2013;24:622–623. doi: 10.1097/EDE.0b013e318296c2b6 [DOI] [PubMed] [Google Scholar]
- 24.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. doi: 10.1080/01621459.1979.10481038 [DOI] [Google Scholar]
- 25.Mehta RH, Harjai KJ, Grines L, Stone GW, Boura J, Cox D, O’Neil W, and Grines CL. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention: incidence, predictors, and outcomes. J Am Coll Cardiol. 2004;43:1765–1772. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RH, Yu J, Piccini JP, Tcheng JE, Farkouh ME, Reiffel J, Fahy M, Mehran R, and Stone GW. Prognostic significance of postprocedural sustained ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention (from the HORIZONS-AMI Trial). Am J Cardiol. 2012;109:805–812. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KS, Armstrong PW, and Granger CB for the APEX AMI Investigators. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009;301:1779–1789. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg RJ, Spencer FA, Okolo J, Lessard D, Yarzebski J and Gore JM. Long-term trends in the use of coronary reperfusion strategies in acute myocardial infarction: a community-wide perspective. J Thromb Thrombolysis. 2007;23:163–171. doi: 10.1007/s11239-0069029-0 [DOI] [PubMed] [Google Scholar]
- 29.Aufderheide TP. Arrhythmias Associated with Acute Myocardial Infarction And Thrombolysis. Emerg Med Clin North Am. 1998;16:583–600. doi: 10.1016/S0733-8627(05)70019-5 [DOI] [PubMed] [Google Scholar]
- 30.Thomas D, Jex N and Thornley A. Ventricular arrhythmias in acute coronary syndromes—mechanisms and management. Cont Cardiol Educ. 2017;3:22–29. doi: 10.1002/cce2.51 [DOI] [Google Scholar]
- 31.Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith Iii EE, Weaver WD, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Gibbons RJ, Lewis RP and O’Rourke RA. ACC/AHA Guidelines for the Management of Patients with Acute Myocardial Infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol. 1996;28:1328–1428. doi: 10.1016/S0735-1097(96)00392-0 [DOI] [PubMed] [Google Scholar]
- 32.Antman EM. Declining incidence of ventricular fibrillation in myocardial infarction. Implications for the prophylactic use of lidocaine. Circulation (New York, NY). 1992;86:764–773. doi: 10.1161/01.CIR.86.3.764 [DOI] [PubMed] [Google Scholar]
- 33.Gressin V, Louvard Y, Pezzano M and Lardoux H. Holter recording of ventricular arrhythmias during intravenous thrombolysis for acute myocardial infarction. Am J Cardiol. 1992;69:152–159. doi: 10.1016/0002-9149(92)91295-F [DOI] [PubMed] [Google Scholar]
- 34.Steg PG, Goldberg RJ, Gore JM, Fox KAA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton-Mellor SK and Anderson FA. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol. 2002;90:358–363. doi: 10.1016/S0002-9149(02)02489-X [DOI] [PubMed] [Google Scholar]
- 35.Gheeraert PJ, De Buyzere ML, Taeymans YM, Gillebert TC, Henriques JPS, De Backer G and De Bacquer D. Risk factors for primary ventricular fibrillation during acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2006;27:2499–2510. doi: 10.1093/eurheartj/ehl218 [DOI] [PubMed] [Google Scholar]
- 36.Di Stefano R, Di Bello V, Barsotti MC, Grigoratos C, Armani C, Dell’Omodarme M, Carpi A and Balbarini A. Inflammatory markers and cardiac function in acute coronary syndrome: Difference in ST-segment elevation myocardial infarction (STEMI) and in non-STEMI models. Biomed Pharmacother. 2009;63:773–780. doi: 10.1016/j.biopha.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM and Chan PS. Trends in Survival after In-Hospital Cardiac Arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jentzer JC, Clements CM, Wright RS, White RD and Jaffe AS. Improving Survival From Cardiac Arrest: A Review of Contemporary Practice and Challenges. Ann Emerg Med. 2016;68:678–689. doi: 10.1016/j.annemergmed.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 39.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM and Zimmerman JL. Part 8: Post–Cardiac Arrest Care. 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–S482. doi: 10.1161/cir.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan PS, Berg RA, Tang Y, Curtis LH, and Spertus JA. Association between therapeutic hypothermia and survival after in-hospital cardiac arrest. JAMA. 2016;316:1375–1382. doi: 10.1001/jama.2016.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhar-Amato J, Davies W, and Agarwal S. Ventricular arrhythmia after acute myocardial infarction: the perfect storm. Arrhythm Electrophysiol Rev. 2017;6:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]