Abstract
Auditory-nerve fibers are lost steadily with age and as a possible consequence of noise-induced glutamate excitotoxicity. Auditory-nerve loss in the absence of other cochlear pathologies is thought to be undetectable with a pure-tone audiogram while degrading real-world speech perception (hidden hearing loss). Perceptual deficits remain unclear, however, due in part to the limited behavioral capacity of existing rodent models to discriminate complex sounds. The budgerigar is an avian vocal learner with human-like behavioral sensitivity to many simple and complex sounds and the capacity to mimic speech. Previous studies in this species show that intracochlear kainic-acid infusion reduces wave 1 of the auditory brainstem response by 40–70%, consistent with substantial excitotoxic auditory-nerve damage. The present study used operant-conditioning procedures in trained budgerigars to quantify kainic-acid effects on tone detection across frequency (0.25–8 kHz; the audiogram) and as a function of duration (20–160 ms; temporal integration). Tone thresholds in control animals were lowest from 1–4 kHz and decreased with increasing duration as in previous studies of the budgerigar. Behavioral results in kainic-acid-exposed animals were as sensitive as in controls, suggesting preservation of the audiogram and temporal integration despite auditory-nerve loss associated with up to 70% wave 1 reduction. Distortion-product otoacoustic emissions were also preserved in kainic-acid exposed animals, consistent with normal hair-cell function. These results highlight considerable perceptual resistance of tone-detection performance with selective auditory-nerve loss. Future behavioral studies in budgerigars with auditory-nerve damage can use complex speech-like stimuli to help clarify aspects of auditory perception impacted by this common cochlear pathology.
Keywords: audiogram, auditory nerve, budgerigar, distortion product otoacoustic emissions hidden hearing loss, kainic acid excitotoxicity, operant conditioning, temporal integration
1. Introduction
Of approximately 30,000 auditory-nerve (AN) afferent fibers innervating each human cochlea at birth, an average of 1000–2000 fibers are lost each decade of life (Otte et al., 1978). While some baseline AN loss occurs as part of the normal aging process (i.e., ~25% reduction of AN ganglion cells over the full lifespan; Makary et al., 2011; Sergeyenko et al., 2013), additional neurodegeneration occurs as a secondary consequence of inner hair cell loss (McFadden et al., 2004; Spoendlin, 1984) and possibly due to noise-induced glutamate excitotoxicity (Kujawa and Liberman, 2009; Lin et al., 2011; Young, 2013). Primary AN degeneration in the absence of other cochlear pathologies is thought to be undetectable with a clinical audiogram (Makary et al., 2011; Schuknecht and Woellner, 1953), but nonetheless profoundly alters sensory input to the ascending auditory pathway. AN loss has been proposed to cause deficits in real-world perception of speech and other complex sounds, known as “hidden hearing loss” (Bharadwaj et al., 2014; Schaette and McAlpine, 2011), but support for this hypothesis is presently unclear.
Several studies in human subjects with normal audiograms have found no consistent associations between putative metrics of AN health and behavioral performance on a range of psychoacoustic tasks including speech-in-noise perception (Prendergast et al., 2017a, 2017b; Yeend et al., 2017). Metrics of AN health were based on wave I of the auditory brainstem response (ABR; wave I is AN component) and self-reported previous noise exposure. In contrast, Liberman and colleagues found significant speech-in-noise perceptual deficits in young subjects identified as at risk for AN damage based on self-reported noise exposure (Liberman et al., 2016). The amplitude ratio of the hair-cell summating potential to ABR wave I was also elevated in at-risk individuals, consistent with AN loss, though the effect was primarily due to higher summating potential amplitude rather than reduced wave I for unclear reasons. Conflicting results between studies may reflect limitations of existing AN health metrics in humans. Wave I amplitude varies considerably across individuals and rarely exceed 1 μV, even with the use of tympanic membrane electrodes (Harris et al., 2017).
Animal models can help clarify the impact of AN loss on perception because neural lesions can be directly controlled through exposure to noise or neurotoxic agents (Hickox et al., 2017). Behavioral effects are nearly unexplored in animal models, but in rodents include changes in the sensitivity of acoustic startle reflexes that are positive or negative depending on the severity of neural loss (Chambers et al., 2016; Hickox and Liberman, 2014; Lobarinas et al., 2017). In contrast, behavioral thresholds for tone detection based on operant conditioning remain unaffected by even profound AN loss (Chambers et al., 2016; Schuknecht and Woellner, 1953). Further insight into the perceptual consequences of AN loss may be gained through the development of new animal models for this pathology with the ability to learn, discriminate, and mimic speech and other complex sounds.
The budgerigar (Melopsittacus undulatus) is a highly vocal avian species with human-like behavioral sensitivity to many simple and complex sounds and the capacity to mimic speech. Budgerigars have sensitive hearing from 0.25–6 kHz (Dooling and Saunders, 1975) and behavioral thresholds similar to humans on numerous psychoacoustic tasks including tone-in-noise detection (Okanoya and Dooling, 1987), frequency discrimination of tones and vowel formants (Dent et al., 2000; Henry et al., 2017b, 2017a), amplitude modulation detection (Carney et al., 2013; Dooling and Searcy, 1981; Henry et al., 2016), and gap detection (Dooling et al., 2000). We recently developed new methods in the budgerigar to induce permanent AN damage with kainic acid (KA) (Henry and Abrams, 2018). KA is a glutamate analog that damages AN afferent neurons due to excitotoxicity at their synapse with cochlear hair cells (Bledsoe et al., 1981; Juiz et al., 1989; Sun et al., 2001; Zheng et al., 1999). KA infusion in budgerigars causes long-term reduction of ABR wave 1 without impairing ABR thresholds or the amplitude of centrally generated ABR waves (Henry and Abrams, 2018). Wave 1 reduction ranges from 40–70% across animals and is similar across test frequencies, consistent with diffuse AN loss of substantial clinical significance. Normal amplitude of later ABR waves suggests a compensatory increase in central gain following AN damage (Caspary et al., 2008; Chambers et al., 2016; Hickox and Liberman, 2014; Salvi et al., 2017; Wang et al., 2011).
As part of a larger effort aimed at identifying aspects of auditory perception impacted by selective AN damage, the first goal in this new system was to determine whether KA exposure in budgerigars affects behavioral thresholds for tone detection in quiet. Thresholds were assessed using operant-conditioning procedures as a function of stimulus frequency from 0.25–8 kHz to quantify the behavioral audiogram. Second, we measured behavioral thresholds for detection of a 2-kHz tone as a function of stimulus duration from 20–160 ms to quantify temporal integration. Recent cochlear implant studies in trained guinea pigs show that temporal integration of electrical pulse-train stimuli may decrease with lower AN survival (Pfingst et al., 2017; Zhou and Pfingst, 2014), but whether temporal integration of acoustic hearing also depends on AN survival is unclear. Finally, distortion product otoacoustic emissions (DPOAEs) were recorded to determine whether KA causes any impairment of hair-cell function. The results show that AN damage from KA does not adversely impact hair cell function or the behavioral audiogram, and hence remains undetectable by these common clinical measures of auditory function. Temporal integration was also unaffected, even in animals with evidence of substantial AN loss.
2. Methods
2.1. Animals
Experimental procedures were performed in 14 budgerigars of either sex. Animals were less than two years of age at the time of testing. All procedures were approved by the University Committee on Animal Resources at the University of Rochester.
2.2. Kainic acid infusions
KA infusions were performed bilaterally in five animals using surgical procedures described previously in detail (Henry and Abrams, 2018). Infusions of the left and right ear were performed during separate recovery surgeries separated by 4 weeks to avoid prolonged periods of anesthesia. Each ear was infused once, except in one animal (K1) where an extra right-ear infusion was performed based on preliminary ABR results showing minimal impact of the first procedure. Animals were anesthetized (3–5 mg/kg ketamine; 0.08–0.1 mg/kg dexmedetomidine; subcutaneous injection) and implanted with a head post to facilitate positioning during subsequent ABR/DPOAE recording sessions and KA infusions. Additional subcutaneous anesthesia was given as needed to maintain an areflexic state. Body temperature was monitored (Physitemp BAT-12; Clifton, NJ, USA) and maintained at 39–41 degrees Celsius using a heating pad (Adroit HTP-1500; Loudon, TN, USA). Infusions were performed into the base of the cochlea by first bluntly separating the neck muscles in the region overlying the crossing of the horizontal and posterior semicircular canals. The muscles were retracted using fine wire hooks to reveal the semicircular canals through the partly transparent skull. A bone flap was made in the area ventral to the horizontal canal and anterior to the posterior canal, and lifted up to expose the underlying middle ear space and dome shaped bony prominence of the cochlear base.
A 150-μm cochleostomy was made using gentle, rotating pressure on a small hand drill, thus allowing access to the underlying scala vestibuli. Infusions were of 2.5 μL of 1-mM KA (Abcam ab 144490; Cambridge, United Kingdom) in Hanks’ balanced salt solution (Sigma-Aldrich H8264; Saint Louis, MO, USA). Higher KA concentration (2 mM) was used in one animal (K13) in an attempt to produce greater AN damage. Infusions were performed over 90 s through a 35-gauge needle with blunted tip. The needle was coupled to a 10-μL syringe with flexible tubing and advanced ~0.2 mm into the cochleostomy using a micromanipulator. Excess solution was absorbed with paper points following the infusion. The bony flap and neck muscles were returned to their original position, and overlying skin was closed with tissue adhesive.
2.3. Free-field ABRs
Free-field ABRs were recorded repeatedly before and after the KA exposure, and for the full duration of behavioral experiments, to quantify change over time in wave 1 amplitude (the gross AN response; see Henry and Abrams, 2018 for detailed methods and preliminary results). Animals were anesthetized as described above and placed in a stereotaxic apparatus located in a double-walled acoustic chamber (9.2 m3; Industrial Acoustics). Stimuli were generated in MATLAB (The MathWorks, Natick, MA, USA) using custom programs and converted to analog with a data acquisition card (National Instruments PCIe-6251, Austin, TX, USA). Stimuli were amplified (Crown D-75A, Elkhart, IN, USA) and presented from a loudspeaker (Polk Audio MC60, Baltimore, MD, USA) located 45 cm from the animal. Sound level was calibrated based on the output of a ¼-inch precision microphone (Brüel and Kjaer type 4938, Marlborough, MA, USA) in response to pure tones, placed at the location of the animal’s head.
Electrophysiological activity was recorded differentially between an implanted vertex electrode (M0.6 stainless steel screw) and a subdermal needle electrode (Grass F-E2, Natus Manufacturing, Gort, Co. Galway, Ireland) inserted 2–3 mm posterior to the external occipital protuberance. A second needle electrode was inserted into the nape of the neck for ground. Electrophysiological activity was amplified by 50k and band-pass filtered from 30–10,000 Hz (Grass IP511, West Warwick, RI, USA) prior to digital sampling (50 kHz; National Instruments PCIe-6251) and storage on a PC hard drive.
ABRs were obtained in response to 0.1-ms clicks presented 19.2/s with alternating polarity. Sound level ranged from 30–80 dB peak-equivalent (p.e.) SPL in 10 dB steps. Each ABR waveform was calculated as the average response to 300 stimuli of each polarity (600 presentations total). Wave 1 amplitude was quantified as the voltage difference between the first major positive peak and the preceding baseline (Brittan-Powell et al., 2002), to minimize the contribution of early brainstem activity to the measurement. For monaurally presented closed-field stimuli, this method also limits the contribution of the contralateral AN due to ~0.5-ms greater latency of the contralateral response (see Discussion).
2.4. Closed-field ABRs and DPOAES
Closed-field ABRs and DPOAEs were recorded once in each KA exposed animal at the conclusion of behavioral testing (5 animals), and in a second group of unexposed controls (ABRs: 7 animals; DPOAEs: 8 animals). Procedures were similar to those described above for free-field ABRs, except that a probe assembly was coupled to each ear canal through a short length of plastic tubing. Each probe assembly consisted of two earphones (Etymotic ER2, Elk Grove Village, IL, USA) and a low noise microphone (Etymotic ER10-B+). Earphones were driven by a headphone buffer (Tucker Davis Technologies HB7, Alachua, FL, USA) and calibrated in the ear canal based on the output of the microphone in response to pure tones. In cases of low microphone output at test frequencies below ~500 Hz, the probe assembly was reseated to ensure a tight seal to the ear canal and additional calibrations repeated until a satisfactory closed acoustic system was achieved. ABRs were recorded as described above, except that clicks ranged in level up to 90 dB p.e. SPL and were presented in random sequences of left-ear, right-ear, and diotic stimulation.
DPOAEs were recorded in response to linearly swept primary tones with frequencies F1 and F2. F1 increased from 0.5–6 kHz over 4 s. F2 was 1.25*F1. Primary tones were presented with equal sound level of 35–70 dB SPL in 5 dB steps. Stimuli were presented with a 4.35-ms repetition period and 25-ms cosine-squared onset and offset ramps. Total stimulus duration was 4.05 s. Each DPOAE was calculated as the average response to 20 stimuli. The amplitude of the distortion product at 2F1-F2 was extracted using a least-squares fitting procedure that minimizes the sum of squared residuals between a model of the time varying distortion product and the recorded data (Long et al., 2008). Least-squares analyses were conducted in MATLAB using standard matrix operations, a 100-ms Hann window, and step size of 20 ms. The same least-squares analysis procedure was applied to a null response to estimate the noise floor. The null response was calculated as the average of responses to even-order stimulus presentations and inverted responses to odd-order presentations
2.5. Behavioral experiments
Behavioral experiments were conducted in the five animals exposed bilaterally to KA and in three unexposed controls over the course of 3–5 months. Experiments in the KA exposed group began five or more weeks after the final KA infusion. Note that reduction of ABR wave 1 is stable after 3–4 weeks following KA exposure (Henry and Abrams, 2018). The number of weeks between the final KA exposure and behavioral training was 17 in K1, 9 in K4, 40 in K6, 6 in K9; and 5 in K13.
Experiments were performed in four matching acoustic test chambers (Industrial Acoustics; 0.30 m3) lined with sound-absorbing foam (see Henry et al., 2017b, 2017a, 2016, for detailed behavioral methods). Each chamber contained an overhead loudspeaker (Polk Audio MC60), house light, and a video camera for monitoring the animal’s behavior. Animals perched in a wire-mesh cage, centered under the loudspeaker, with access to three horizontally arranged response switches and the feeding trough of a seed dispenser. Behavioral experiments were controlled by custom MATLAB programs running on a PC. Hardware included a data acquisition card (National Instruments PCI-6151), programmable attenuator (Tucker Davis Technologies PA5), Crown D75 amplifier, microcontroller (Arduino Leonardo; Turin, Italy), and custom electronic circuits for control of the house light, response switches, and seed dispenser. Stimuli were calibrated based on the output of a ½” precision microphone (Brüel and Kjaer type 4134) in response to pure tones. The microphone was placed at the location of the animal’s head during behavioral tests.
Animals were trained to perform a single-interval, two-alternative, non-forced choice task (Henry et al., 2017b). A trial was initiated by pecking the center switch, which resulted in the presentation of either a single tone stimulus (target) or no tone (standard). Following each trial, animals had three seconds to respond. The correct response to the target tone was to peck the right switch (hit). The correct response to the standard (no sound) was to peck the left switch (correct rejection). Correct responses initiated a reward of one or two seeds depending on the animal’s response bias towards the right or left switch (see below). Incorrect responses (left switch: misses; right switch: false alarms) resulted in a 5-second timeout, during which the house light was turned off and the animal was prevented from starting another trial. Responses during the timeout period extended the timeout (i.e. the timeout timer was restarted). A shorter two-second timeout was imposed in rare instances when the animal did not respond within three seconds of initiating a trial, or responded by pecking the center switch (less than 0.22% of trials in all animals).
Response bias toward the right or left switch was calculated as −0.5 times the sum of the Z-score of the hit rate and the Z-score of the false alarm rate (Macmillan and Creelman, 1991). Bias was calculated for each block of 50 trials, and controlled by adjusting the percentage of trials on each side for which two-seed reinforcement was delivered. If the animal presented persistent bias over at least two tracks despite 100% two-seed reinforcement against the biased side, the timeout duration was increased for the biased side, to a maximum of 15 seconds, and the contralateral timeout period was decreased to a minimum of 0.5 seconds. Only sessions with overall absolute values of bias less than 0.3 were included in subsequent analyses.
Animals were initially trained to discriminate between a supra-threshold tone (60–90 dB SPL depending on frequency; see below) and quiet (i.e., no sound). The sound level of the target tone was held constant during these early non-tracking sessions. After reaching discrimination performance of 90% correct or higher on this task, animals were then moved on to tracking sessions during which the sound level of the tone was systematically varied within the session according to a two-down, one-up adaptive staircase method (Levitt, 1970). This procedure efficiently converges on the stimulus level associated with ~70.7% correct responses and provides one threshold per test session. During tracking sessions, the initial step size of 10 dB was reduced to five dB after two reversals in the step direction (up vs. down) and to two dB after four track reversals (Fig. 1). Each track continued until (1) at least 15 reversals occurred, (2) the absolute difference between the mean of the last four reversals and the mean of the previous four reversals was less than three dB, and (3) the standard deviation of the last eight reversals was less than four dB. The threshold for each track was estimated as the mean of the last eight reversals. Behavioral experiments were conducted seven days per week. Animals performed a total of four to six tracking sessions per day within two ~30-minute testing blocks separated by four hours.
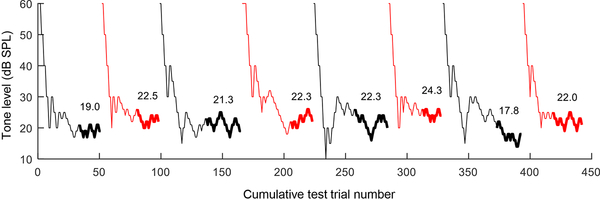
Fig. 1.
Representative behavioral results from two-down, one-up, adaptive tracking sessions in the budgerigar. Tone level is plotted as a function of cumulative test trial number across eight sessions. Results of successive sessions are drawn in different colors for clarity. Thick lines indicate the region of the track used to calculate threshold, which is indicated in dB SPL above the track. Data are from animal K6 in response to 80-ms tones with a frequency of 2 kHz.
The first behavioral experiment in each animal determined audiometric thresholds as a function of frequency from 0.25–8 kHz. Different frequencies were tested in the same order in all animals: 2, 0.5, 4, 1, 3, 6, 8, and 0.25 kHz. After reaching 90% correct on non-tracking sessions, animals performed a minimum of 20 tracking sessions at each test frequency until two stability criteria were met: (1) the standard deviation of the last six threshold estimates was less than four dB and (2) the absolute difference between the mean of the last three thresholds and the previous three thresholds was less than three dB. The last six thresholds were then used to calculate a mean threshold for the tone frequency. The starting point of tracking sessions was 75 dB SPL at the 0.25 kHz test frequency, 90 dB SPL at 8 kHz, and 60 dB SPL for the remaining frequencies. Tones were 300 ms in duration with 10-ms, cosine-squared onset and offset ramps.
The second experiment used the same procedures to determine detection thresholds as a function of stimulus duration from 20–160 ms, to investigate temporal integration. All animals were initially tested with 40-ms stimuli followed by durations of 160, 20, and 80 ms. Stimuli were presented 50 ms after animals pressed the center switch and were gated with 1.3-ms cosine-squared onset and offset ramps. Stimulus frequency was held constant at 2 kHz. The starting point of tracking sessions was 60 dB SPL for all stimulus durations. Note that thresholds are not directly comparable between the two experiments because in the audiogram experiment, the ramp duration was longer and animals often responded before the end of the 300-ms stimulus.
2.6. Statistical Analyses
Statistical analyses were conducted using linear mixed effects models (Bates et al., 2015) in R (version 3.4.1) and t-tests in MATLAB (2017a). For the DPOAE mixed-model analysis, the dependent variable was the median DPOAE amplitude within the half-octave frequency band from 1–1.41, 1.41–2, 2–2.83, 2.83–4, or 4–5.66 kHz. Fixed, within-subject effects were frequency band (5 bands total) and primary sound level (45–70 dB SPL in 5-dB steps). KA status (exposed vs. control) was included as a between-subject effect. Subject intercepts were modeled as a random effect. Interactions were included between fixed effects and dropped when not significant (p>0.05) in order of decreasing p value. Degrees of freedom for F tests and pairwise comparisons of least-squares means were calculated based on the Satterwaite approximation. Visual inspection of model results showed that residuals were normally distributed. Statistical analyses for behavioral results were similar except that the dependent variable was the tone detection threshold, and within-subject fixed effects included test frequency and duration.
3. Results
3.1. Physiological characterization of auditory-nerve damage
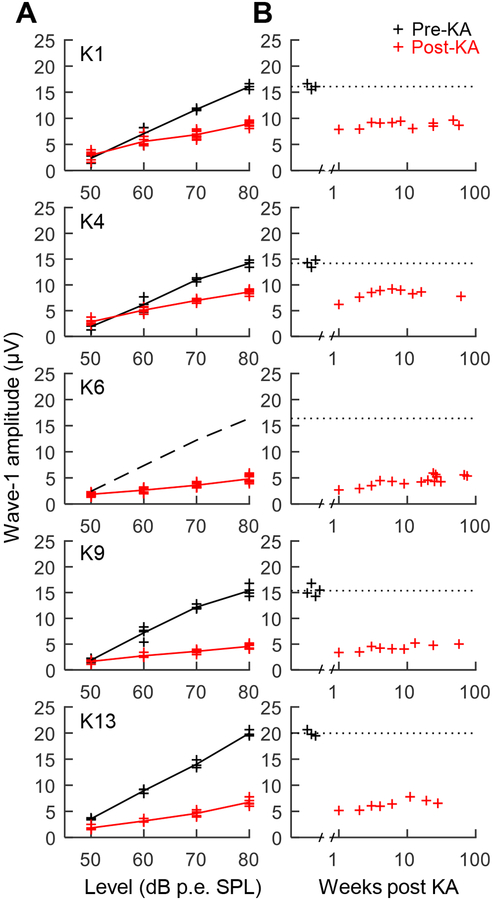
Wave-1 amplitude in response to free-field click stimuli increased with sound level and ranged from 15–20 μV at 80 dB p.e. SPL prior to KA infusion (Fig. 2A). Bilateral KA exposure caused a pronounced reduction of wave 1 at moderate to high click levels. Wave 1 reduction varied in severity across animals and showed modest recovery over the first three to four weeks following infusion (see Henry and Abrams, 2018). No further change in wave 1 was observed after four weeks, and for the full duration of behavioral experiments (i.e., for up to 75 weeks post exposure; Fig. 2B). Based on ABRs evoked at 80 dB p.e. SPL four or more weeks after KA exposure, percent reduction of wave 1 was 44.3% in K1, 39.1% in K4, 70.6% in K6, 70.3% in K9, and 66.2% in K13 (t4=−6.74, p=0.0025; paired t-test). Note that wave-1 reduction in K6 was estimated based on pre-exposure ABRs of the other four animals because control data for K6 were unavailable. Persistent reduction of wave 1 in these animals is consistent with permanent AN damage from KA.
Fig. 2.
Auditory brainstem response (ABR) wave 1 amplitude as a function of click presentation level (A). Data are from before (black) and ≥4 weeks following (red) bilateral exposure to kainic acid (KA). KA reduces wave 1 amplitude at moderate to high sound levels by 40–70% across animals. The dashed black line drawn for K6 indicates mean wave-1 amplitude prior to KA in the other animals because pre-exposure control data were unavailable. Change over time in ABR wave 1 amplitude following KA exposure (B). Click level was 80 dB peak equivalent (p.e.) SPL. KA exposure causes long term reduction of wave 1, consistent with permanent auditory nerve (AN) injury.
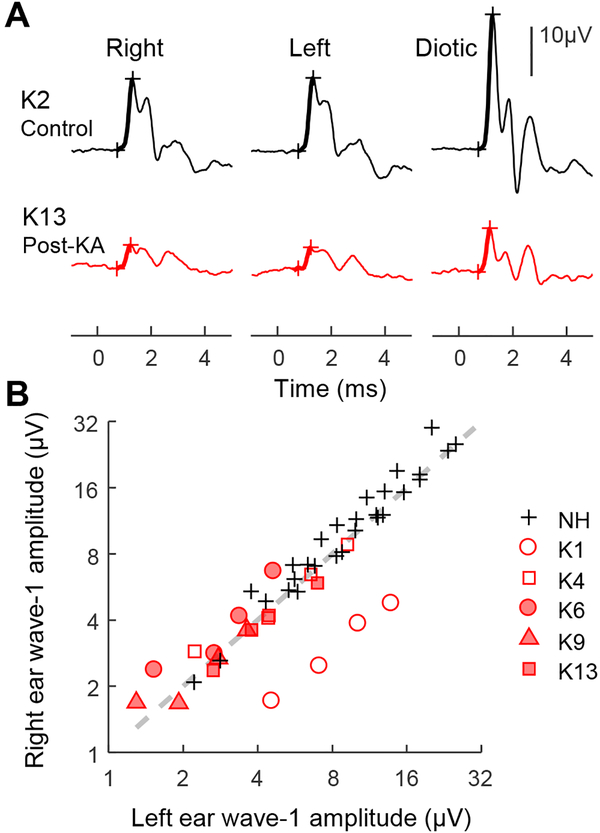
ABRs were also recorded once in each KA exposed animal using a closed-field acoustic system to compare impairment from KA between the left and right ear. Click responses were recorded for monaural stimulation of each ear as well as for diotic stimulation. Wave-1 amplitude in a control group of seven animals was similar for monaural stimulation of either ear, and approximately equal between diotic stimulation and the sum of the two monaural conditions (Fig. 3). The same pattern was observed in the five KA exposed animals, except in K1 for which wave-1 amplitude was 2.78 times greater for left-ear stimulation compared to right. Note that K1 received an extra KA infusion of the right ear. Thus, in animals that received balanced KA treatments, physiological evidence of AN damage was similar between the left and right ear.
Fig. 3.
ABR waveforms evoked by clicks presented monaurally to the right and left ears and diotically to both ears (A). Representative responses are shown for one control animal (K2; black) and one animal following bilateral KA exposure (K13; red). Click presentation level was 90 dB p.e. SPL. Monaural responses are approximately half the amplitude of the diotic response. Comparison of ABR wave-1 amplitude between monaural click stimulation of the left and right ears (B). Click presentation level ranged from 60–90 dB p.e. SPL in 10-dB steps. Wave 1 amplitude is similar between the left and right ears in control animals (black crosses) and in all KA exposed animals except for K1 (red).
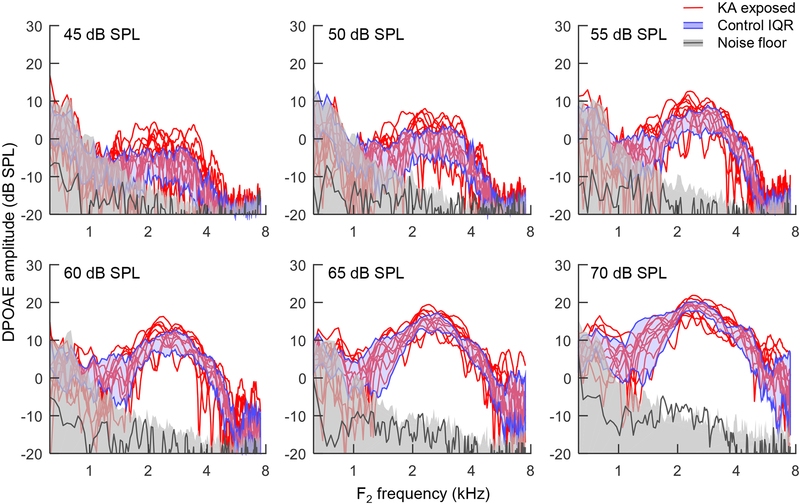
DPOAEs were recorded using the same closed-field system and a swept-tone paradigm to evaluate effects of KA exposure on hair-cell status. DPOAEs at 2F1-F2 in the control group (eight animals) were generally above the noise floor at primary levels of 45 dB SPL and above, and increased in amplitude with increasing stimulus level (Fig. 4). Maximum DPOAE amplitude was observed at F2 frequencies from 2–3.5 kHz, with deep notches sometimes present in individual ears. No difference in DPOAE amplitude was observed between control animals and those exposed to KA, consistent with normal hair-cell function despite substantial wave 1 reduction for both ears.
Fig. 4.
Distortion product otoacoustic emission (DPOAE) amplitude at 2F1-F2 as a function of F2 frequency. Stimuli were swept tone pairs with F1 increasing linearly from 0.5–6 kHz over four seconds and F2 equal to 1.25*F1. Each panel shows data for a different primary sound level from 45–70 dB SPL (top). Red lines show DPOAE responses from 10 KA exposed ears in 5 animals. Blue shaded regions show the interquartile range (IQR) of 15 control ears in in eight animals. Gray shaded regions show the 90th percentile of the noise estimate across all 25 ears. Dark gray lines show mean DPOAE amplitude in two cadaver ears. KA exposure does not affect the amplitude of DPOAEs.
A mixed model analysis showed no effect of KA exposure on DPOAE amplitude (F1,11=0.66, p=0.43) and no interaction of KA exposure with test frequency (F4,324.1=2.01, p=0.09) or sound level (F5,324.3=0.53, p=0.75). Effects of frequency (F4,324.1=289.26, p<0.0001), sound level (F5,324.3=518.92, p<0.0001), and the frequency by sound level interaction (F20,324.1=6.74, p<0.0001) were significant factors in the model. An alternative statistical model including percent wave-1 reduction as a continuous measure of KA damage produced equivalent results.
3.2. Behavioral audiogram
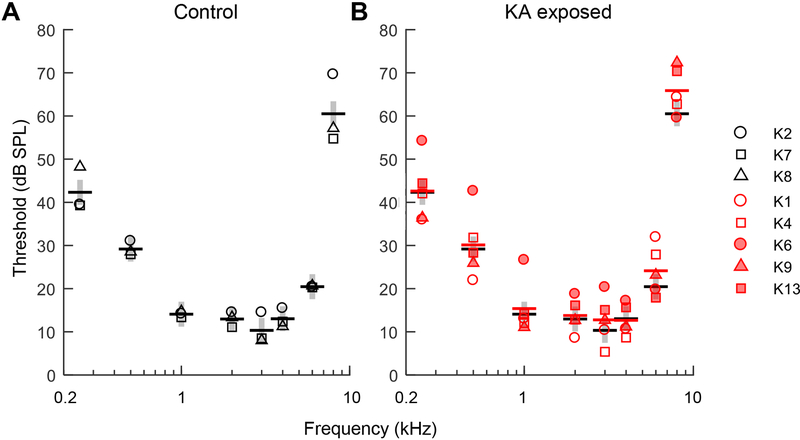
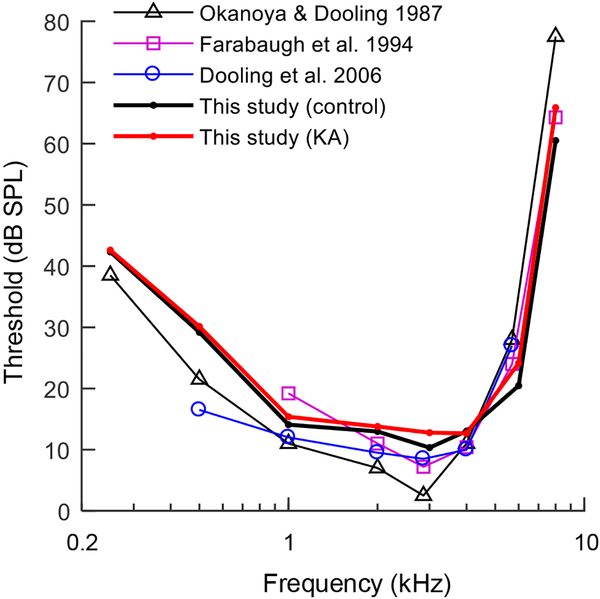
The first behavioral experiment quantified thresholds for pure tone detection as a function of frequency, to evaluate the impact of KA damage on the audiogram. Tone frequencies ranged from 0.25–8 kHz. Stimulus duration was held constant at 300 ms. Thresholds were most sensitive from 1–4 kHz and increased at higher and lower test frequencies (Fig. 5). Thresholds were generally similar between KA exposed animals and the control group, though one exposed animal (K6) showed ~10 dB threshold elevation compared to other test subjects at frequencies ≤1 kHz. Thresholds from both groups in the present study were consistent with thresholds reported previously in this species (Fig. 6) (Dooling et al., 2006; Farabaugh et al., 1998; Okanoya and Dooling, 1987).
Fig. 5.
Behavioral audiograms plotting tone detection threshold as a function of frequency in three control animals (A) and five KA exposed animals (B). Stimulus duration was 0.3 s. Thick horizontal bars are mean thresholds of each group (control: black; KA exposed: red). Gray vertical bars indicate the average standard deviation of the control group across test frequencies. KA induced AN damage has no apparent impact on the behavioral audiogram.
Fig. 6.
Behavioral audiograms of KA exposed and control groups from the present study (thick red and black lines, respectively) compared to previous behavioral audiograms in the budgerigar. Audiograms are similar across studies.
A mixed model analysis of audiometric thresholds showed a significant main effect of frequency (F7,42=109.8, P<0.0001) due to lower thresholds from 1–4 kHz. The effect of KA exposure (F1,6=0.78, P=0.41) and the interaction between KA exposure and frequency (F7,42=0.30, P=0.95) were not significant factors in the model. An alternative model including wave 1 reduction as a continuous metric of AN damage produced the same conclusions. These results show that AN damage associated with up to 70% wave-1 reduction was essentially undetectable from the behavioral audiogram.
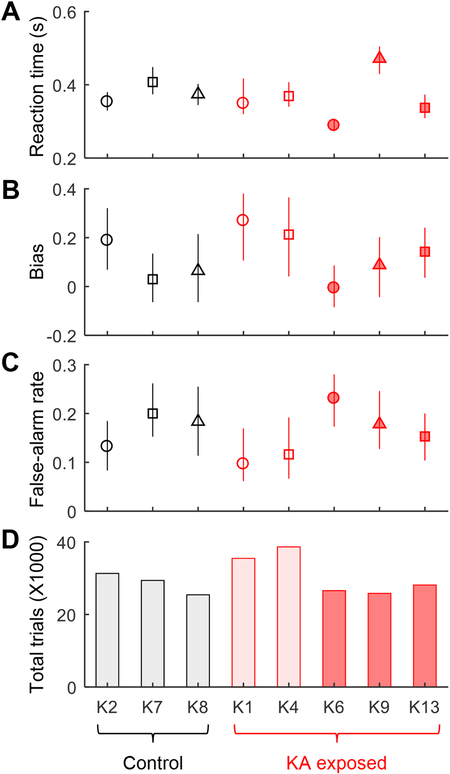
To address the possibility that AN might produce subtle behavioral deficits not evident in audiometric threshold shifts, we quantified reaction times, response bias, and false alarm rates across all tracking sessions from the audiogram experiment (Fig. 7). No significant effect of KA exposure was found on these response measures (reaction time: t6=0.37, p=0.73; response bias: t6=−0.64, p=0.54; false alarm rate: t6=0.48, p=0.65; Fig. 7A–C; two-sample t-tests) or on the cumulative number of trials needed to complete the experiment (t6=−0.60, p=0.57; Fig. 7D). These results suggest that AN damage did not impact the ability of KA-exposed animals to learn and perform the behavioral task.
Fig. 7.
Behavioral reaction time, response bias, false-alarm rate, and total number of trials to complete the audiogram experiment in all trained animals. Symbols in A-C indicate the median value for each animal across all tracking sessions; error bars are the interquartile range. These behavioral response measures are similar between KA exposed and control animals.
3.3. Temporal integration
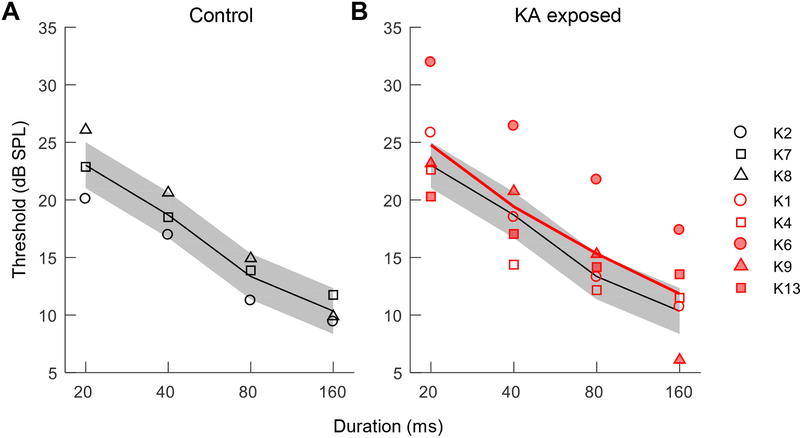
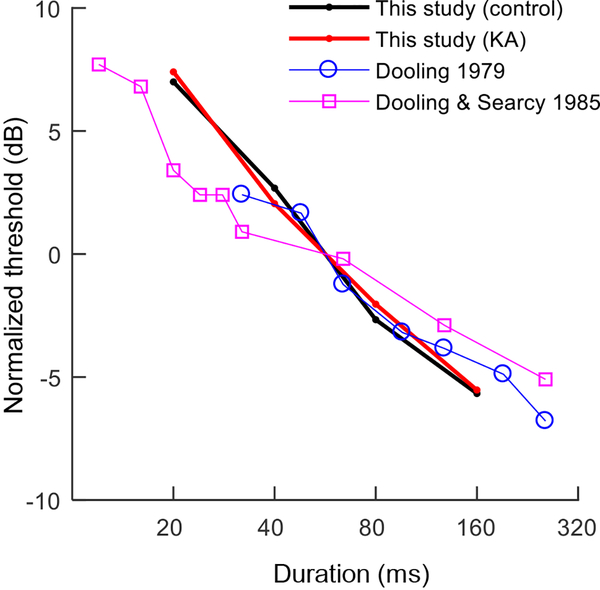
Following completion of the audiogram, the second experiment quantified thresholds for detection of a 2-kHz tone as a function of stimulus duration from 20–160 ms, to test whether KA damage impacts temporal integration. Thresholds were highest for the 20-ms tone condition and decreased by 14.4 ±1.7 dB per decade increase in stimulus duration (mean ±SE; Fig. 8), similar to two previous studies in the budgerigar conducted with 2.86-kHz tones (Dooling, 1979; Dooling and Searcy, 1985) (Fig. 9). Average thresholds and the slope of the temporal integration function were similar between KA exposed animals and the control group, though thresholds in K6 were slightly higher than in other animals for all stimulus durations, consistent with the audiogram in this animal.
Fig. 8.
Behavioral thresholds for tone detection as a function of stimulus duration in three control animals (A) and five KA exposed animals (B). Test frequency was 2 kHz. Thick lines indicate mean thresholds of each group (control: white; KA exposed: red). Gray bands show the average standard deviation of the control group across stimulus durations. KA induced AN damage has no detectable impact on the threshold by stimulus duration function.
Fig. 9.
Thresholds as a function of stimulus duration in the present study (control: black; KA exposed: red) compared to behavioral data from previous studies in the budgerigar. Previous studies used a higher test frequency of 2.86 kHz (vs. 2 kHz in the present study). Thresholds are normalized by the intercept of the observed function at 56.6 ms (i.e., the geometric mean stimulus duration of the present study). The slope of the function plotting threshold across stimulus durations is similar between the present and previous studies.
A mixed model analysis showed a significant main effect of stimulus duration (F3,18=54.91, P<0.0001) but not KA exposure (F1,6=0.408, P=0.55) or the interaction between KA exposure and stimulus duration (F3,18=0.13, P=0.94). An alternative model including wave 1 reduction as a continuous measure of AN status produced equivalent results. Thus, we find no evidence than AN damage altered temporal integration of tone stimuli over the range of stimulus durations studied.
4. Discussion
KA exposure in budgerigars caused permanent reduction of ABR wave 1 amplitude without impacting DPOAEs, consistent with selective KA damage to primary AN afferent neurons rather than sensory hair cells. Behavioral thresholds in KA exposed animals were as sensitive as in controls at audiometric test frequencies from 0.25–8 kHz and as a function of stimulus duration from 20–160 ms, even when loss of AN function was substantial (i.e., ~70% wave 1 reduction). These results suggest considerable resistance of the behavioral audiogram and temporal integration of tone stimuli following selective AN damage.
ABR wave 1 amplitude decreased by 40–70% across animals following KA exposure and remained depressed for the full duration of behavioral studies (i.e., up to 75 weeks after infusions). In contrast, DPOAEs were unaffected by KA. These results agree with previous reports in birds and mammals that KA damages AN afferent neurons/synapses without impacting sensory hair cells (Bledsoe et al., 1981; Juiz et al., 1989; Sun et al., 2001). KA exposure in guinea pig, rat, chinchilla, and chicken causes immediate swelling and breakage of AN afferent synapses due to excitotoxicity (Pujol et al., 1985; Shero et al., 1998) followed by limited recovery over the first few days following exposure (Zheng et al., 1999, 1997). More extensive exposures cause permanent loss of AN synapses and subsequent degeneration of ganglion cell bodies over weeks or months (Juiz et al., 1989; Sun et al., 2001). In contrast to the neurodegenerative effects of KA, no adverse impact has been found on hair cell morphology (Juiz et al., 1989; Sun et al., 2001) or on hair-cell dependent responses including DPOAEs and the cochlear microphonic (Bledsoe et al., 1981; Sun et al., 2000; Zheng et al., 1996). Similar, selective damage to AN afferent neurons has been found following moderate noise overexposure in rodents, except that noise damage is generally focused on a limited cochlear frequency region (Hickox et al., 2017; Kujawa and Liberman, 2009; Lin et al., 2011). The diffuse pattern of neural damage produced by KA (Henry and Abrams, 2018; Sun et al., 2000) resembles age-related AN loss (Makary et al., 2011; Otte et al., 1978; Spoendlin, 1984; Spoendlin and Schrott, 1989; Viana et al., 2015; see Ryals and Westbrook, 1988 for age-related AN degeneration in birds) and AN damage from ouabain (Chambers et al., 2016; Yuan et al., 2014).
Audiometric thresholds obtained through operant conditioning procedures in the budgerigar were lowest from 1–4 kHz and increased at higher and lower test frequencies as in previous studies of this species (Dooling and Saunders, 1975; Farabaugh et al., 1998; Hashino et al., 1988; Heffner et al., 2016; Okanoya and Dooling, 1987). Notably, the behavioral audiogram was normal in KA exposed animals despite AN loss associated with up to 70% wave 1 reduction. Preservation of the behavioral audiogram following AN loss has been found in a small number of previous behavioral studies in mammalian animal models. In cats, surgical sectioning of the AN does not affect behavioral tone detection thresholds unless more than 80% of AN spiral ganglion neurons are lost (Schuknecht and Woellner, 1953). Similarly, in mice, diffuse AN loss due to ouabain exposure can approach 95% without substantial impact on behavioral tone detection performance (Chambers et al., 2016). Human studies also suggest that the behavioral audiogram is insensitive to AN loss since, whereas AN loss accumulates steadily with increasing age, thresholds remain normal in individuals without hair-cell loss until the last few decades of life (Makary et al., 2011; Otte et al., 1978).
Birds regenerate hair cells following ototoxic or noise-induced damage (Corwin and Cotanche, 1988; Ryals and Rubel, 1988) yet, in contrast to the present results, show permanent elevation of audiometric thresholds after exposure (Dooling et al., 1997; Hashino et al., 1988; Hashino and Sokabe, 1989; Marean et al., 1998; Ryals et al., 2013). Thus, hair-cell injury elevates audiometric thresholds in birds whereas selective AN damage appears undetectable with the audiogram. Budgerigars treated with kanamycin show profound loss of basal cochlear hair cells followed by complete regeneration of the sensory epithelium over several weeks (Dooling et al., 2006, 1997; Hashino et al., 1992). Behavioral thresholds improve concomitantly but remain elevated by up to 20–30 dB even after many months of recovery and behavioral testing (Dooling et al., 2006, 1997; Hashino and Sokabe, 1989). Incomplete recovery of the behavioral audiogram is putatively due to persistent irregularity in the orientation of regenerated hair cell bundles (Duckert and Rubel, 1993; Ryals et al., 2013). Perhaps surprisingly, kanamycin-exposed budgerigars show no lasting deficits for behavioral discrimination of frequency and intensity differences or of natural calls, though call classification after hair-cell regeneration appears to require relearning of previously familiar stimuli (Dooling et al., 2006, 1997). AN loss was not examined in kanamycin exposed budgerigars, but may have occurred to some extent given 25–30% permanent AN loss in quail with regenerated hair cells following noise overexposure (Ryals et al., 1989).
Tone detection thresholds improved with increasing stimulus duration in the budgerigar, consistent with previous results in this species (Dooling, 1979; Dooling and Searcy, 1985) and in a broad range of others including humans (reviewed by Gerken et al., 1990). The pattern can be explained by classic psychophysical models based on ‘leaky’ integration of sensory input over time (Green et al., 1957; Plomp and Bouman, 1959). Notably, threshold improvement with increasing duration was similar between control animals and those exposed to KA, suggesting no impairment of temporal integration by AN loss, at least over the range of neural damage and stimulus durations studied here. In contrast, impaired (flatter) temporal integration is commonly found in human and animal test subjects (including avians; Lauer et al., 2007) with threshold elevation due to sensorineural cochlear damage (Gerken et al., 1990, 1983; Solecki and Gerken, 1990; Watson and Gengel, 1969; Wright, 1968) and in cochlear implant users, possibly related to the number of surviving AN fibers (Donaldson et al., 1997; Zhou and Pfingst, 2014). Intriguingly, implanted guinea pigs with lower AN survival show weaker integration of electrical stimuli over time and across individual pulses, though correlation of AN status with inner hair cell survival complicates interpretation of the pattern (Pfingst et al., 2017).
Wave-1 reduction was similar for click stimulation of the left and right ears in most animals, suggesting balanced AN damage in most animals. Therefore, it seems unlikely that preservation of behavioral performance in the KA group was because animals relied on a ‘better ear’ with high AN survival. Indeed, significant asymmetry was found in a single animal (K1) that received two KA infusions on the right side, consistent with greater wave-1 reduction for that ear. ABR results in response to unilaterally presented clicks must be interpreted with caution in birds due to the open interaural canal that allows monaurally presented closed field sounds to effectively stimulate the contralateral cochlea and AN (Rosowski and Saunders, 1980). Isolation of the ipsilateral AN response only remains possible because the contralateral response is delayed by approximately 0.5 ms. This delay is mostly due to stimulus attenuation duration propagation through the interaural canal (~14 dB; Larsen et al., 2006; Rosowski and Saunders, 1980), resulting in 0.4 ms of wave-1 delay (Brittan-Powell et al., 2002; Henry and Abrams, 2018), and partly due to physical propagation time (~0.1 ms) (Calford, 1988; Larsen et al., 2006).
Behavioral thresholds from both experiments appeared more variable across KA exposed animals than in controls, with one exposed animal (K6) showing ~10 dB threshold elevation at frequencies ≤1 kHz. While this pattern could results from diminished or desynchronized AN input due to KA, threshold differences of 10–15 dB between individuals are not uncommon in previous studies of the budgerigar (Dooling et al., 2006; Farabaugh et al., 1998) and other species including humans (Heffner et al., 1994; Heffner and Heffner, 1991; Margolis et al., 2015). The pattern could therefore reflect normal inter-subject variation rather than a KA effect. Ultimately, determining whether AN loss causes ‘subclinical’ thresholds shifts (<20 dB; or small changes in complex sound perception; see Oxenham, 2016) will require methods to overcome inter-subject variation such as larger sample sizes or a paired experimental design in which behavioral thresholds are measured before and after KA exposure. Inadvertent hair-cell or middle-ear injury during the infusion procedure are unlikely to explain higher thresholds in K6 considering that DPOAE amplitude was normal in this animal (the fourth highest among 13 subjects).
Preservation of the behavioral audiogram and temporal integration in KA exposed budgerigars raises the question of what aspects of auditory perception are impacted by this common cochlear pathology. Perceptual impairment from AN loss has been suggested to reflect disproportionate loss of high-threshold AN fibers with low spontaneous rates (Bharadwaj et al., 2014; Furman et al., 2013; Liberman and Liberman, 2015), but specific predictions are uncertain due to long-standing questions regarding the role of low vs. high spontaneous rate fiber populations in normal hearing (Carney, 2018; Delgutte, 1996; Sachs and Young, 1979; Young and Sachs, 1979). Alternatively, AN loss may impair aspects of perception that depend on central gain (i.e., the net excitability of central brain nuclei to peripheral inputs). Central gain increases following AN injury in a number of model systems including the budgerigar, putatively due to downregulation of inhibitory neural signaling pathways in the CNS (Caspary et al., 2008; Chambers et al., 2016; Henry and Abrams, 2018; Hickox and Liberman, 2014; Salvi et al., 2017; Wang et al., 2011). Reduced inhibition alters envelope processing in the midbrain (Burger and Pollak, 1998; Caspary et al., 2002; Zhang and Kelly, 2003), and hence might adversely affect behavioral sensitivity to envelope fluctuations, but this hypothesis remains unexplored. Degraded midbrain response synchrony in mice with profound AN loss also suggest the possibility of impaired envelope sensitivity (Chambers et al., 2016). Future behavioral experiments in the budgerigar may provide special insight into these open questions, considering previous work on complex-sound perception in this species (Carney et al., 2013; Dent et al., 2000; Dooling et al., 2000; Dooling and Searcy, 1981; Henry et al., 2017b, 2017a, 2016; Okanoya and Dooling, 1987) and evidence of increased central gain after KA exposure (Henry and Abrams, 2018).
In conclusion, the results of the present study show that bilateral KA infusions in the budgerigar reduce ABR wave-1 amplitude without impacting DPOAEs, consistent with selective damage to AN afferent neurons. KA damage had no detectable impact on the behavioral audiogram or on temporal integration of tones, even in animals with evidence of substantial AN loss from KA. These new results suggest considerable resistance of behavioral performance on these tasks to selective AN damage. Future studies in the budgerigar can help identify aspects of complex sound perception affected by this common cochlear pathology.
Highlights.
Intracochlear kainic-acid (KA) infusions were performed in the budgerigar
KA reduced ABR wave I by 40–70% without impacting DPOAEs
Tone perception was studied using operant-conditioning procedures
Behavioral tone detection was unaffected as a function of frequency and duration
The audiogram and temporal integration appear resistant to AN loss
Acknowledgements
This research was supported by National Institutes of Health grant R00 DC013792. Laurel Carney provided equipment for behavioral tests. Douglas Schwarz and Madeline Cappelloni assisted with software development. Lucinda Hinojosa, Britt Tingley, and Regina Yu assisted with data collection.
Abbreviations:
- ABR
auditory brainstem response
- AN
auditory nerve
- DPOAE
distortion product otoacoustic emission
- KA
kainic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67, 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG, 2014. Cochlear neuropathy and the coding of supra-threshold sound. Front. Syst. Neurosci 8, 26. doi: 10.3389/fnsys.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Bobbin RP, Chihal DM, 1981. Kainic acid: an evaluation of its action on cochlear potentials. Hear. Res 4, 109–20. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O, 2002. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus). J. Acoust. Soc. Am 112, 999–1008. doi: 10.1121/1.1494807 [DOI] [PubMed] [Google Scholar]
- Burger RM, Pollak GD, 1998. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. J. Neurophysiol 80, 1686–701. [DOI] [PubMed] [Google Scholar]
- Calford MB, 1988. Constraints on the coding of sound frequency imposed by the avian interaural canal. J. Comp. Physiol. A 162, 491–502. doi: 10.1007/BF00612514 [DOI] [Google Scholar]
- Carney LH, 2018. Supra-Threshold Hearing and Fluctuation Profiles: Implications for Sensorineural and Hidden Hearing Loss. J. Assoc. Res. Otolaryngol 19, 331–352. doi: 10.1007/s10162-018-0669-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney LH, Ketterer AD, Abrams KS, Schwarz DM, Idrobo F, 2013. Detection thresholds for amplitude modulations of tones in budgerigar, rabbit, and human. Adv. Exp. Med. Biol 787, 391–8. doi: 10.1007/978-1-4614-1590-9_43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF, 2008. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol 211, 1781–1791. doi: 10.1242/jeb.013581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF, 2002. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear. Res 168, 163–73. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB, 2016. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89, 867–879. doi: 10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA, 1988. Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–4. [DOI] [PubMed] [Google Scholar]
- Delgutte B, 1996. Physiological models for basic auditory percepts, in: Hawkins H, McMullen T, Fay R (Eds.), Auditory Computation. Springer, New York, pp. 157–220. doi: 10.1007/978-1-4612-4070-9_5 [DOI] [Google Scholar]
- Dent ML, Dooling RJ, Pierce AS, 2000. Frequency discrimination in budgerigars (Melopsittacus undulatus): effects of tone duration and tonal context. J. Acoust. Soc. Am 107, 2657–2664. doi: 10.1121/1.428651 [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Viemeister NF, Nelson DA, 1997. Psychometric functions and temporal integration in electric hearing. J. Acoust. Soc. Am 101, 3706–21. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, 1979. Temporal summation of pure tones in birds. J. Acoust. Soc. Am 65, 1058–60. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML, 2000. Hearing in Birds and Reptiles, in: Dooling RJ, Fay RR, Popper AN (Eds.), Comparative Hearing: Birds and Reptiles. Springer, New York, pp. 308–359. [Google Scholar]
- Dooling RJ, Ryals BM, Dent ML, Reid TL, 2006. Perception of complex sounds in budgerigars (Melopsittacus undulatus) with temporary hearing loss. J. Acoust. Soc. Am 119, 2524. doi: 10.1121/1.2171839 [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Manabe K, 1997. Recovery of hearing and vocal behavior after hair-cell regeneration. Proc. Natl. Acad. Sci. U. S. A 94, 14206–14210. doi: 10.1073/pnas.94.25.14206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Saunders JC, 1975. Hearing in the parakeet (Melopsittacus undulatus): absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J. Comp. Physiol. Psychol 88, 1–20. doi: 10.1037/h0076226 [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Searcy MH, 1985. Temporal integration of acoustic signals by the budgerigar (Melopsittacus undulatus). J. Acoust. Soc. Am 77, 1917–20. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Searcy MH, 1981. Amplitude modulation thresholds for the parakeet (Melopsittacus undulatus). J. Comp. Physiol. A 143, 383–388. doi: 10.1007/BF00611177 [DOI] [Google Scholar]
- Duckert LG, Rubel EW, 1993. Morphological correlates of functional recovery in the chicken inner ear after gentamycin treatment. J. Comp. Neurol 331, 75–96. doi: 10.1002/cne.903310105 [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Dent ML, Dooling RJ, 1998. Hearing and vocalizations of wild-caught Australian budgerigars (Melopsittacus undulatus). J. Comp. Psychol 112, 74–81. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC, 2013. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol 110, 577–586. doi: 10.1152/jn.00164.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken GM, Bhat VKH, Hutchison‐Clutter M, 1990. Auditory temporal integration and the power function model. J. Acoust. Soc. Am 88, 767–778. doi: 10.1121/1.399726 [DOI] [PubMed] [Google Scholar]
- Gerken GM, Gunnarson AD, Allen CM, 1983. Three Models of Temporal Summation Evaluated Using Normal-Hearing and Hearing-Impaired Subjects. J. Speech Lang. Hear. Res 26, 256. doi: 10.1044/jshr.2602.256 [DOI] [PubMed] [Google Scholar]
- Green DM, Birdsall TG, Tanner WP, 1957. Signal Detection as a Function of Signal Intensity and Duration. J. Acoust. Soc. Am 29, 523–531. doi: 10.1121/1.1908951 [DOI] [Google Scholar]
- Harris KC, Vaden KI, McClaskey CM, Dias JW, Dubno JR, 2017. Complementary metrics of human auditory nerve function derived from compound action potentials. J. Neurophysiol. jn.00638.2017. doi: 10.1152/jn.00638.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashino E, Sokabe M, 1989. Kanamycin induced low-frequency hearing loss in the budgerigar (Melopsittacus undulatus). J. Acoust. Soc. Am 85, 289–94. [DOI] [PubMed] [Google Scholar]
- Hashino E, Sokabe M, Miyamoto K, 1988. Frequency specific susceptibility to acoustic trauma in the budgerigar (Melopsittacus undulatus). J. Acoust. Soc. Am 83, 2450–3. [DOI] [PubMed] [Google Scholar]
- Hashino E, Tanaka Y, Salvi RJ, Sokabe M, 1992. Hair cell regeneration in the adult budgerigar after kanamycin ototoxicity. Hear. Res 59, 46–58. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T, 1994. Audiogram of the hooded Norway rat. Hear. Res 73, 244–7. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Koay G, Heffner RS, 2016. Budgerigars (Melopsittacus undulatus) do not hear infrasound: the audiogram from 8 Hz to 10 kHz. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol 202, 853–857. doi: 10.1007/s00359-016-1125-9 [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE, 1991. Behavioral hearing range of the chinchilla. Hear. Res 52, 13–6. [DOI] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, 2018. Persistent Auditory Nerve Damage Following Kainic Acid Excitotoxicity in the Budgerigar (Melopsittacus undulatus). J. Assoc. Res. Otolaryngol 19, 435–449. doi: 10.1007/s10162-018-0671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, Forst J, Mender MJ, Neilans EG, Idrobo F, Carney LH, 2017a. Midbrain Synchrony to Envelope Structure Supports Behavioral Sensitivity to Single-Formant Vowel-Like Sounds in Noise. JARO - J. Assoc. Res. Otolaryngol 18, 165–181. doi: 10.1007/s10162-016-0594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Amburgey KN, Abrams KS, Idrobo F, Carney LH, 2017b. Formant-frequency discrimination of synthesized vowels in budgerigars (Melopsittacus undulatus) and humans. J. Acoust. Soc. Am 142, 2073–2083. doi: 10.1121/1.5006912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Neilans EG, Abrams KS, Idrobo F, Carney LH, 2016. Neural correlates of behavioral amplitude modulation sensitivity in the budgerigar midbrain. J. Neurophysiol 115, 1905–1916. doi: 10.1152/jn.01003.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP, 2017. Translational issues in cochlear synaptopathy. Hear. Res 349, 164–171. doi: 10.1016/j.heares.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC, 2014. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol 111, 552–564. doi: 10.1152/jn.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juiz JM, Rueda J, Merchán JA, Sala ML, 1989. The effects of kainic acid on the cochlear ganglion of the rat. Hear. Res 40, 65–74. doi: 10.1016/0378-5955(89)90100-7 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2009. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci 29, 14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ON, Dooling RJ, Michelsen A, 2006. The role of pressure difference reception in the directional hearing of budgerigars (Melopsittacus undulatus). J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol 192, 1063–1072. doi: 10.1007/s00359-006-0138-1 [DOI] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR, Poling K, 2007. Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. J. Acoust. Soc. Am 122, 3615–3627. doi: 10.1121/1.2799482 [DOI] [PubMed] [Google Scholar]
- Levitt H, 1970. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am 49, 467–477. [PubMed] [Google Scholar]
- Liberman LD, Liberman MC, 2015. Dynamics of cochlear synaptopathy after acoustic overexposure. J. Assoc. Res. Otolaryngol 16, 205–219. doi: 10.1007/s10162-015-0510-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF, 2016. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11, 1–15. doi: 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC, 2011. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO - J. Assoc. Res. Otolaryngol 12, 605–616. doi: 10.1007/s10162-011-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Spankovich C, Le Prell CG, 2017. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear. Res 349, 155–163. doi: 10.1016/j.heares.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL, Lee J, 2008. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J. Acoust. Soc. Am 124, 1613–1626. doi: 10.1121/1.2949505 [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD, 1991. Detection Theory: A User’s Guide. CUP Archive. [Google Scholar]
- Makary C. a., Shin J, Kujawa SG, Liberman MC, Merchant SN, 2011. Age-related primary cochlear neuronal degeneration in human temporal bones. JARO - J. Assoc. Res. Otolaryngol 12, 711–717. doi: 10.1007/s10162-011-0283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW, 1998. Auditory perception following hair cell regeneration in European starling (Sturnus vulgaris): frequency and temporal resolution. J. Acoust. Soc. Am 103, 3567–3580. doi: 10.1121/1.423085 [DOI] [PubMed] [Google Scholar]
- Margolis RH, Wilson RH, Popelka GR, Eikelboom RH, Swanepoel DW, Saly GL, 2015. Distribution characteristics of normal pure-tone thresholds. Int. J. Audiol 54, 796–805. doi: 10.3109/14992027.2015.1033656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding DL, Jiang H, Salvi RJ, 2004. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 997, 40–51. doi: 10.1016/j.brainres.2003.10.031 [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ, 1987. Hearing in passerine and psittacine birds: a comparative study of absolute and masked auditory thresholds. J. Comp. Psychol 101, 7–15. [PubMed] [Google Scholar]
- Otte J, Schuknecht HF, Kerr AG, 1978. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope 88, 1231–1246. doi: 10.1288/00005537-197808000-00004 [doi] [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, 2016. Predicting the Perceptual Consequences of Hidden Hearing Loss. Trends Hear. 20, 233121651668676. doi: 10.1177/2331216516686768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, Raphael Y, 2017. Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. JARO - J. Assoc. Res. Otolaryngol 18, 731–750. doi: 10.1007/s10162-017-0633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp R, Bouman MA, 1959. Relation between Hearing Threshold and Duration for Tone Pulses. J. Acoust. Soc. Am 31, 749–758. doi: 10.1121/1.1907781 [DOI] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ, 2017a. Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear. Res 344, 68–81. doi: 10.1016/j.heares.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Millman RE, Guest H, Munro KJ, Kluk K, Dewey RS, Hall DA, Heinz MG, Plack CJ, 2017b. Effects of noise exposure on young adults with normal audiograms II: Behavioral measures. Hear. Res 356, 74–86. doi: 10.1016/j.heares.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM, 1985. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear. Res 18, 145–51. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Saunders JC, 1980. Sound transmission through the avian interaural pathways. J. Comp. Physiol 136, 183–190. doi: 10.1007/BF00657532 [DOI] [Google Scholar]
- Ryals BM, Dent ML, Dooling RJ, 2013. Return of function after hair cell regeneration. Hear. Res 297, 113–120. doi: 10.1016/j.heares.2012.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW, 1988. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240, 1774–6. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Ten Eyck B, Westbrook EW, 1989. Ganglion cell loss continues during hair cell regeneration. Hear. Res 43, 81–90. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW, 1988. Ganglion cell and hair cell loss in Coturnix quail associated with aging. Hear. Res 36, 1–8. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED, 1979. Encoding of steady-state vowels in the auditory nerve: Representation in terms of discharge rate. J. Acoust. Soc. Am 66, 470–479. doi: 10.1121/1.383098 [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Sun W, Ding DL, Chen G-D, Lobarinas E, Wang J, Radziwon K, Auerbach BD, 2017. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front. Neurosci 10, 1–14. doi: 10.3389/fnins.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D, 2011. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci 31, 13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC, 1953. Hearing losses following partial sectioning of the cochlear nerve. Laryngoscope 63, 441–465. doi: 10.1288/00005537-195306000-00001 [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG, 2013. Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline. J. Neurosci 33, 13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero M, Salvi RJ, Chen L, Hashino E, 1998. Excitotoxic effect of kainic acid on chicken cochlear afferent neurons. Neurosci. Lett 257, 81–84. doi: 10.1016/S0304-3940(98)00821-0 [DOI] [PubMed] [Google Scholar]
- Solecki JM, Gerken GM, 1990. Auditory temporal integration in the normal‐hearing and hearing‐impaired cat. J. Acoust. Soc. Am 88, 779–785. doi: 10.1121/1.399727 [DOI] [PubMed] [Google Scholar]
- Spoendlin H, 1984. Factors inducing retrograde degeneration of the cochlear nerve. Ann. Otol. Rhinol. Laryngol. Suppl 112, 76–82. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A, 1989. Analysis of the human auditory nerve. Hear. Res 43, 25–38. doi: 10.1016/0378-5955(89)90056-7 [DOI] [PubMed] [Google Scholar]
- Sun H, Hashino E, Ding DL, Salvi RJ, 2001. Reversible and irreversible damage to cochlear afferent neurons by kainic acid excitotoxicity. J. Comp. Neurol 430, 172–181. doi: [DOI] [PubMed] [Google Scholar]
- Sun H, Salvi RJ, Ding DL, Hashino DE, Shero M, Zheng XY, 2000. Excitotoxic effect of kainic acid on chicken otoacoustic emissions and cochlear potentials. J. Acoust. Soc. Am 107, 2136–2142. [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC, 2015. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res 327, 78–88. doi: 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM, 2011. Inhibitory neurotransmission in animal models of tinnitus: Maladaptive plasticity. Hear. Res 279, 111–117. doi: 10.1016/j.heares.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Gengel RW, 1969. Signal Duration and Signal Frequency in Relation to Auditory Sensitivity. J. Acoust. Soc. Am 46, 989–997. doi: 10.1121/1.1911819 [DOI] [PubMed] [Google Scholar]
- Wright HN, 1968. The Effect of Sensori-Neural Hearing Loss on Threshold-Duration Functions. J. Speech Hear. Res 11, 842–852. doi: 10.1044/jshr.1104.842 [DOI] [PubMed] [Google Scholar]
- Yeend I, Beach EF, Sharma M, Dillon H, 2017. The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hear. Res 353, 224–236. doi: 10.1016/j.heares.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Young ED, 2013. Which neurons survive the glutamate storm? J. Neurophysiol 110, 575–576. doi: 10.1152/jn.00292.2013 [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB, 1979. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory- nerve fibers. J. Acoust. Soc. Am 66, 1381–1403. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC, Edge ASB, 2014. Ouabain-induced cochlear nerve degeneration: Synaptic loss and plasticity in a mouse model of auditory neuropathy. JARO - J. Assoc. Res. Otolaryngol 15, 31–43. doi: 10.1007/s10162-013-0419-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kelly JB, 2003. Glutamatergic and GABAergic regulation of neural responses in inferior colliculus to amplitude-modulated sounds. J. Neurophysiol 90, 477–90. doi: 10.1152/jn.01084.2002 [DOI] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, Hu BH, McFadden SL, 1997. Recovery of structure and function of inner ear afferent synapses following kainic acid excitotoxicity. Hear. Res 105, 65–76. doi: 10.1016/S0378-5955(96)00188-8 [DOI] [PubMed] [Google Scholar]
- Zheng XY, Salvi RJ, Fadden SLMC, Ding DL, Henderson D, 1999. Recovery of Kainic Acid Excitotoxicity in Chinchilla Cochlea. Ann. N. Y. Acad. Sci 884, 255–269. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Wang J, Salvi RJ, Henderson D, 1996. Effects of kainic acid on the cochlear potentials and distortion product otoacoustic emissions in chinchilla. Hear. Res 95, 161–167. doi: 10.1016/0378-5955(96)00047-0 [DOI] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE, 2014. Relationship between multipulse integration and speech recognition with cochlear implants. J. Acoust. Soc. Am 136, 1257. doi: 10.1121/1.4890640 [DOI] [PMC free article] [PubMed] [Google Scholar]