Abstract
Background:
Dissociative phenomena are frequently experienced by psychologically traumatized people. However, little is known about the cognitive profiles of highly-dissociative traumatized individuals, and corresponding patterns of neural connectivity when attentional networks are engaged in the context of emotion.
Method:
One hundred seventeen traumatized women completed the multiscale dissociation inventory (MDI) and neuropsychological testing; MDI scores were used to classify high and low-dissociative participants. Forty-six participants also underwent fMRI during performance of an attentional control task that incorporates emotionally-distracting images (Affective Number Stroop; ANS).
Results:
Compared to low-dissociative participants, high-dissociative participants demonstrated better performance on an executive functioning task (F1,111=4.64,p=.03), worse performance on a task of visual memory (F1,111=9.52, p=.003), and similar performance on all other neuropsychological measures. In addition, dissociative symptoms were negatively correlated with functional connectivity between the amygdala and right anterior insula in response to trauma-related ANS trials.
Conclusion:
These findings indicate that highly-dissociative traumatized people experience difficulties with attentional control in the context of emotionally-evocative stimuli, but in a neutral context, their overall cognitive profiles are similar to low-dissociative people. Highly dissociative participants also demonstrated weaker connectivity between the amygdala and insula in response to trauma-relevant images. Evocative, trauma-relevant stimuli appear to disrupt neutral networks involved with attention to salient cues and interoception in highly-dissociative traumatized individuals.
Keywords: Cognition, emotion, dissociation, fMRI, insula, amygdala
Dissociation is a psychological defense mechanism that involves disruptions in consciousness. Dissociation has myriad manifestations, ranging from alterations in perceptions of time and memory to a fragmented sense of identity. These defenses are often deployed when escape from a threat or stressor is thought to be either impossible or highly risky, and thus, are commonly observed in those who have experienced childhood maltreatment (Stein et al., 2013; Steuwe, Lanius, & Frewen, 2012). Dissociative symptoms emerge in a subset of traumatized individuals, and have been associated with significant psychiatric comorbidities (Stein et al., 2013). The frequent observation of dissociative responses in traumatized people (Briere, 2006) led to the creation of the first subtype of posttraumatic stress disorder (PTSD), the dissociative subtype, which is characterized by symptoms of derealization and/or depersonalization (American Psychological Association, 2013). However, there is increasing acknowledgement that many people with PTSD who do not meet criteria for this subtype experience an array of dissociative phenomena that impair their functioning (Dorahy & van der Hart, 2015). Dissociation can occur in healthy individuals in non-disruptive ways (e.g., absorption in a task, daydreaming). However, in the context of trauma and PTSD, dissociative symptoms tend to be more severe and impairing, interfering with the conduct of routine tasks as well as treatment engagement (Bae, Kim, & Park, 2016; Corrigan & Hull, 2015; Kleindienst et al., 2011; Najavits, 2015). For these individuals, dissociative phenomena are experienced regularly, in response to ostensibly minor stressors.
Dissociation has a distinct neurobiological signature that reflects increased parasympathetic tone and a blunted physiological response to stressors (Sack, Cillien, & Hopper, 2012; Zaba et al., 2015). Similarly, highly dissociative traumatized people demonstrate neural response patterns that do not fit with the fear extinction model of PTSD (Jovanovic & Ressler, 2010). This model indicates that PTSD is generally characterized by deficient prefrontal inhibition of exaggerated fear-related responses elicited by the amygdala, a brain region involved with rapid evaluation of, and response to, perceived threat. However, another model has been proposed to accommodate the differing patterns of neural response that characterize dissociative PTSD (Lanius et al., 2010); here, highly dissociative traumatized people as “overmodulators” of affect, who tend to demonstrate increased neural response in medial prefrontal brain areas, which are involved with control of emotional responses, and dampened amygdala response as they attempt to control distressful emotional states.
Curiously, little is known about patterns of cognition in dissociative traumatized people, leaving a large gap in the current neurobiological understanding of this problem. The extant cognitive data on traumatized dissociative people has been mixed, and largely limited to tasks that do not include emotionally-salient information (i.e., traditional neuropsychological measures). Some data suggests poorer performance on a range of cognitive domains, including attention for visual material (Reitan, 1992) and verbally-presented material, verbal list-learning (Roca, Hart, Kimbrell, & Freeman, 2006), visual-spatial and verbal memory (Parlar, Frewen, Oremus, Lanius, & McKinnon, 2016; Rivera-Velez, Gonzalez-Viruet, Martinez-Taboas, & Perez-Mojica, 2014),as well as event memory (Chae, Goodman, Eisen, & Qin, 2011). However, some studies indicate enhanced or equivalent cognitive functioning, particularly with respect to non-emotional material (DePrince & Freyd, 2004; DePrince, Weinzierl, & Combs, 2008). For example, (DePrince and colleagues 2004) presented trauma-relevant, neutral and positive words under variable attentional demands, and observed that high-dissociative participants recalled more neutral words than low-dissociative participants in the moderate attentional demand condition (DePrince & Freyd, 2004).
There is some evidence to suggest that enhanced executive functioning in these high-dissociative traumatized individuals is associated with increased recruitment of brain regions involved with cognitive control. (Elzinga and colleagues 2007) found that, compared to controls, enhanced performance (i.e., fewer errors and shorter reaction times) on a non-emotional working memory (N-back) task in highly dissociative patients with borderline personality disorder (BPD) was accompanied by significantly greater increases in anterior prefrontal (Brodmann area 10) and dorsolateral prefrontal (DLPFC) function (Elzinga et al., 2007). This increase in BOLD response in high-dissociative participants has been observed in brain networks responsible for attentional control, salience detection and interoceptive functioning; (Krause-Utz and colleagues 2014) found that, compared to healthy controls, BPD patients showed increased connectivity between the amygdala and parahippocampal gyri, as well as with the dorsomedial prefrontal cortex (BA10), and between the dorsal ACC and the insula, to emotionally negative distractor images in the N-back (Krause-Utz et al., 2014). Furthermore, increased dissociation in the sample as a whole corresponded with greater amygdala-ACC and insula activation to emotional distractors. However, the BPD participants had poorer working memory performance compared to healthy controls (2012). The data from these studies indicate that dissociation in traumatized individuals may be linked to increased activation in brain areas related to cognitive control, but it remains unclear whether cognitive performance is similarly enhanced or impaired.
Given the limited available data on emotion and cognition in traumatized dissociative people, the present study examined how individual differences in dissociation correspond with performance on traditional measures of attention, executive functioning and memory in a sample of all-traumatized women, many of whom also exhibited symptoms of PTSD. We also examined patterns of behavioral and neural response on an attentional control task that incorporates emotionally-salient distractors, (trauma-relevant, positive and neutral images) in a subset of our larger sample. In keeping with the “overmodulator” model of dissociation in PTSD, we hypothesized that participants with more dissociative symptoms would perform equivalently on tasks of executive functioning and visual attention and memory than participants with fewer dissociative symptoms, but would demonstrate poorer performance in the context of emotional information.
Also consistent with the overmodulator model, we hypothesized that those with higher levels of dissociative symptoms would demonstrate increased connectivity between brain regions involved with emotion processing and inhibitory control. We identified the amygdala as a seed region for connectivity analyses, given its overall relevance to emotional processing and its involvement in earlier dissociation research (Krause-Utz et al., 2014). We thus examined temporal associations between amygdala activation and activation across other brain regions, hypothesizing that higher levels of dissociation would correspond with greater connectivity between the amygdala and emotional/cognitive control regions (the dorsal and ventromedial ACC, orbitofrontal cortex and DLPFC) and lesser connectivity between the amygdala and interoceptive regions, namely, the insula.
Methods
Participants
A sample of 117 African-American women aged 18–63 (M=38.5, SD=10.6) was recruited from an ongoing NIH funded study examining genetic and environmental risk for post-traumatic psychopathology. A subset of this sample, 46 individuals aged 23–59 (M=37.7, SD=10.1), also received functional MRI. Individuals were approached in general medical clinics of a publicly-funded hospital serving low income individuals in inner-city Atlanta, Georgia. On average, participants had completed 13 years of schooling, had monthly incomes between $1000-$2000, and had experienced at least 4 traumatic events throughout their lifetime (see Table 1). Rates of trauma exposure were similar to our earlier studies within this population (e.g., (Fani, Bradley, Ressler, & McClure-Tone, 2010; Fani et al., 2016; Gillespie et al., 2009). All participants had experienced at least one type of trauma. A majority of participants (73%) reported having incomes ≤$1999 a month, indicating limited economic resources for most people in this sample.
Table 1.
Demographic and Clinical Characteristics
| Low Dissociation | High Dissociation | ||
|---|---|---|---|
| (n=59) | (n=58) | ||
| Mean (SD) | Mean (SD) | F | |
| Age | 38.5 (11) | 38.2 (10.4) | 0.03 |
| Education (in years) | 13 (2) | 12.6 (2) | 1.2 |
| TEI lifetime trauma (excluding abuse) | 4 (2.7) | 5 (2.6) | 3.3 |
| CTQ total score | 37.2 (15.2) | 46.7 (20.5) | 8.1** |
| CTQ sexual abuse | 7.4 (4.8) | 9.9 (6.3) | 6* |
| CTQ physical abuse | 7.6 (3.7) | 8.9 (4.6) | 2.8 |
| CTQ emotional abuse | 7.5 (3.8) | 10.9 (5.7) | 14.2** |
| MDI total score | 35.4 (4) | 59.4 (16.3) | 120.4** |
| MDI disengagement | 7.2 (1.8) | 12.7 (4.3) | 81.6** |
| MDI depersonalization | 5.2 (.7) | 8.1 (3.4) | 41** |
| MDI derealization | 5.8 (1.1) | 10.2 (3.8) | 76.4** |
| MDI emotional constriction | 6.3 (2.2) | 12 (4.2) | 83.6** |
| MDI memory disturbance | 5.9 (1.1) | 9.5 (3.1) | 72.8** |
| MDI identity dissociation | 5 (.3) | 6.8 (3.2) | 17** |
| Yes | Chi-square | ||
| Current PTSD (CAPS diagnosis; n=) | n=8, 21.6% | n=29, 78.4% | 16.5** |
|
No (%) Yes (%) |
Mann-Whitney U U UU | ||
| Currently employed | 34 (57.6) | 38 (65.5) | .51 |
| Monthly Income | N (%) | .38 | |
| $0 – 249 | 7 (12.3) | 7 (12.5) | |
| $250 – 499 | 3 (5.3) | 2 (3.6) | |
| $500 – 999 | 13 (22.8) | 16 (28.6) | |
| $1000–1999 | 18 (31.6) | 20 (35.7) | |
| $2000+ | 16 (28.1) | 11 (19.6) | |
TEI = Traumatic Events Inventory
CTQ = Childhood Trauma Questionnaire
CAPS = Clinician Administered PTSD Scale
p<.05
p<.01
The eligibility criterion for participation in the current study was intentionally kept broad and included the ability to understand English (assessed by a study researcher) and willingness to provide informed consent. Participants were excluded from the larger cognitive study if they had current neurological conditions or psychosis. Participants were excluded from MRI on the basis of: claustrophobia; contra-indications to MRI scanning (e.g., metal implants); current bipolar disorder or psychotic disorder (as assessed with the MINI interview (Sheehan et al., 1998); prior head injury with loss of consciousness (>5 minutes) or current substance or alcohol dependence (past 12 months). Participants were not required to have a specified period of abstinence from substances/alcohol to participate, but were given a urine drug test on the day of the scan. A total of five participants met criteria for substance abuse or tested positive for the following substances on the scan day: amphetamines (1), cannabis (3), polysubstance (1, opioid and cannabis). Four of these five participants (four participants in the total sample) endorsed psychotropic medication use: tricyclic antidepressants (2), benzodiazepine (1), citalopram (1). Participants receiving MRI were asked to refrain from caffeine consumption on the day of the scan. The Institutional Review Board of Emory University and Grady Hospital Research Oversight Committee approved all study procedures.
Neuropsychological Assessment
The Penn Computerized Neuropsychological Battery (CNP: http://penncnp.med.upenn.edu/) (Gur et al., 2010) was administered to the larger sample. This battery takes approximately 1.5 hours to administer, and comprises measures of attention, abstraction/cognitive flexibility, working memory, verbal and visual memory, and facial emotion recognition. The Penn CNP has demonstrated good reliability and construct validity (Gur et al., 2010), with use in both healthy and patient populations (Sachs, Steger-Wuchse, Kryspin-Exner, Gur, & Katschnig, 2004). Measures of attention, executive functioning, and memory were included in analyses, as detailed below.
Penn Conditional Exclusion Task (PCET).
The PCET(Kurtz, Ragland, Moberg, & Gur, 2004) is a measure of executive functioning, specifically assessing abstraction and cognitive flexibility, similar to the Wisconsin Card Sorting Test (WCST; (Heaton, 1981)). This measure requires participants to select which of four presented objects does not belong with the other three. Three strategies may be used to “sort” the objects, and participants receive feedback after each trial (“correct” or “incorrect”) to determine whether they have selected the correct sorting criteria. After 10 consecutively correct responses, the sorting strategy changes. Errors on the PCET have been highly correlated with WCST errors, and thought to reflect cognitive flexibility. Number of correct and erroneous responses (including perseverative errors), were recorded and analyzed for this study. Errors were counted as perseverative if the participant made three successive incorrect responses that matched a single sorting category. The PCET demonstrates small-moderate correlations with the Penn Letter N-back task (r=.4), Penn List Learning Task (immediate recall, r=.3) and Penn Short Visual Object Learning Task (immediate recall, r=.4, delayed recall, r=.3) according to a validation study (Gur et al., 2010).
Penn Continuous Performance Test (PCPT).
The PCPT (Kurtz, Ragland, Bilker, Gur, & Gur, 2001) is similar in format to other continuous performance tests, such as Conners’ Continuous Performance Test II (Conners, 2002), and measures facets of sustained attention, including errors of omission (inattention) and commission (impulsivity). In this task, the participant is presented with a series of numbers/non-number distractors or letters/non-letter distractors (each presented at 1s duration); the participant is asked to press a button when they see an actual number or letter. Participants must successfully complete a brief practice round before moving on to the actual task. Number of correct responses were recorded and analyzed for each of the three conditions. The PCPT demonstrates a moderate correlation with the Penn Letter N-back task (CPT number condition, r=.5, CPT letter condition, r=.6), according to a validation study (Gur et al., 2010).
Penn Letter N-Back Task (PLNB).
The PLNB is a measure that assays attention as well as working memory. In this task, participants are presented with a series of letters (at 2.5s durations) and are asked to press a button to indicate their response under three different rules: the 0-back condition requires participants to press a button when they see the letter “X”; under the 1-back and 2-back conditions, participants press a button when they view a letter that appeared 1-back or 2-back from the current letter, respectively. Participants must successfully complete a practice round for each condition before moving on to the actual task. Number of correct responses were recorded and analyzed for each of the three conditions. In addition to the PCPT, the PLNB demonstrates moderate correlations with Penn Short Visual Object Learning Test (r=.4), according to a validation study (Gur et al., 2010)
Penn List Learning Task (PLLT).
The PLLT assesses verbal learning and memory. Similar to the California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 2000), a list of 16 words is orally presented to participants across five learning trials; after each trial, participants are asked to recall the words. Interference trials and semantic cue trials are included after the learning trials are completed, followed by a short-delay recall. A long-delay recall (approximately 20 minutes) recall trial is also administered. Number of correct responses for each learning trial, as well as both recall trials and slope of learning were recorded and analyzed. Immediate recall trials have been moderately correlated with the Penn Short Visual Object Learning Test (immediate recall, r=.5, delayed recall, r=.5) according to a validation study (Gur et al., 2010).
Short Visual Object Learning Test (SVOLT).
The SVOLT assesses visual learning and memory (Glahn, Gur, Ragland, Censits, & Gur, 1997). Participants view a series of 10 complex shapes, one at a time, presented at a rate of 5 seconds per image. Immediately following the presentation they view 20 shapes (10 novel and 10 previously-viewed shapes) and must indicate whether or not the shape was previously presented. A similar recall trial is presented following an approximately 20 minute delay. Number of correct responses on immediate and delayed recall trials were recorded and analyzed.
fMRI task: Affective Number Stroop.
The Affective Number Stroop (ANS; see Figure 2) is a measure of attentional control that has been detailed in earlier studies (Blair et al., 2007; Blair et al., 2012; White, Costanzo, Blair, & Roy, 2015). Participants are instructed to rapidly identify, via button-press, the number of numbers in a given display (3, 4, 5, or 6) while ignoring distractor images (trauma-relevant, positive, and neutral scenes) that appear prior to and following each number stimulus. In some of these trials, the number of numbers is inconsistent with the actual number displayed, posing a heightened cognitive challenge. In addition, the task includes trials with no cognitive demands (“view only” trials). The task comprises 16 trials for each of three conditions: number congruent, number incongruent, and passive viewing of distractor images. A total of 40 fixation trials (2500ms duration) are also randomly presented. Distractor and number stimuli are each presented for 400ms, followed by a blank screen (1300ms), as shown in Figure 1. Each trial includes two identical distractor stimuli. The distractor images are unique for each trial condition. We modified the task to include images that are relevant to the experiences of our study population, based off of our earlier studies, which indicate a high amount of interpersonal trauma and assault involving a weapon (Gillespie et al., 2009). These trauma-relevant images included scenes of assault and gun violence—all but one of the trauma-relevant scenes included people in the images. Nearly half of the positive stimuli were pictures that included people (43%), a minority included animals (17%), activities (theme park, 6%; sports, 23%) or nature scenes (12%). Neutral stimuli included a similar proportion of person-related images (36%) and nature scenes (14%) but also included object stimuli (50%). A majority of the images were obtained from the International Affective Picture System (IAPS; (Lang, Bradley, & Cuthbert, 2005)). Error rates and response times were recorded analyzed for all trials. Prior to task administration, participants are given practice trials (with non-task images), and task proficiency (accuracy on a majority of trials) is required before administration of the actual task.
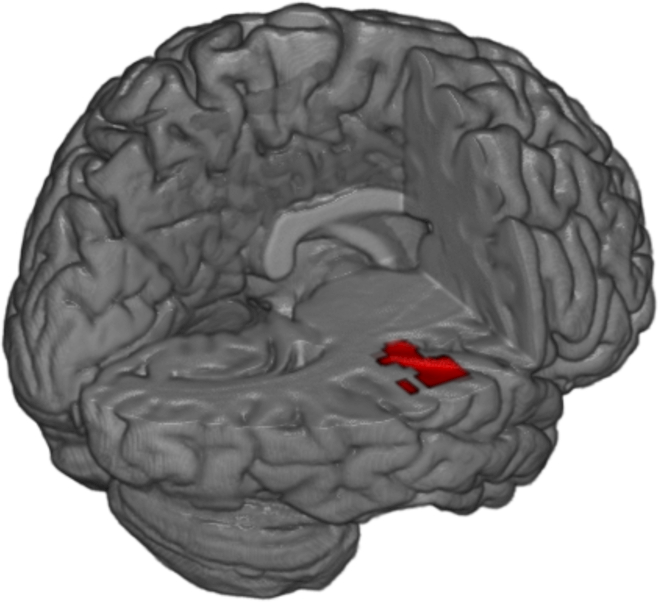
Figure 2.
Regions demonstrating decreased functional connectivity with the amygdala shown in red, following cluster-level family-wise error correction (p<.05).
Figure 1. Schematic representation of affective number stroop trials.
A) number congruent, B) number incongruent and C) view only trials.
Clinical Assessments
The Traumatic Events Inventory (TEI) was administered as part of the parent study to measure the frequency and type of traumatic experiences and was used as a measure of environmental adversity in this analysis, as in prior studies (Fani et al., 2012; Gillespie et al., 2009). An index of lifetime trauma frequency (excluding child abuse) was calculated based on self-reported frequency of trauma exposure to 15 types of trauma. For this sample, mean TEI non-abuse trauma was 5.10 (SD=3.20, range 0–13). Child abuse exposure was measured using the Childhood Trauma Questionnaire (CTQ; (Bernstein & Fink, 1998), a 25-item self-report measure of childhood abuse and neglect; overall severity scores of exposure were calculated and submitted to analyses. CTQ total score was highly correlated with maltreatment (physical, sexual, emotional abuse) on the TEI (Spearman’s rho=.8, p<.001), indicating consistent reporting of maltreatment history. The Multiscale Dissociation Inventory (Briere, Weathers, & Runtz, 2005) was used to assess current (past month) dissociative symptoms. MDI total score ranged from 30–110, with a mean and median score of 53 (SD=19.8) for this sample. A median split was used to classify participants with low versus high current dissociative symptoms, in keeping with earlier studies of trauma and dissociation (Sack et al., 2012; Seng et al., 2013). Our categorization of participants is also consistent with a model that recognizes the prevalence and wide spectrum of dissociative features in traumatized people (Dorahy & van der Hart, 2015). The Clinician Administered PTSD Scale for DSM-IV (Blake et al., 1995) was administered to determine presence and severity of PTSD symptoms. Based on the CAPS, 37 participants met diagnostic criteria for PTSD, whereas 79 did not meet criteria for the disorder (data for one participant was not available).
MRI acquisition, image processing and statistical analysis
Scanning was conducted on a research-dedicated Siemens 3-Tesla TIM-Trio scanner (12 channel head coil) at Emory University Hospital. A high-resolution T1-weighted structural scan was acquired for co-registration purposes using an MPRAGE sequence (176 slices, FOV= 256 mm cubic voxels; 1mm isotropic slices; repetition time (TR)= 2600 msec; echo time (TE) = 3.02 msec; inversion time (TI)= 900msec; flip angle=8°). Functional images (190 volumes) were acquired during task administration using a T2-weighted gradient echo planar sequence (40 interleaved transaxial slices; TR= 2500msec; TE= 30 msec; FOV= 220 mm; 3 mm isotropic voxel). Statistical Parametric Mapping, version 8 (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used for fMRI file conversion and image pre-processing.
Image pre-processing included: realignment of functional images to the first volume in the series; the mean realigned functional image was co-registered with the T-weighted scan; images were normalized to standard space (International Consortium for Brain Mapping 152-subject template) then spatially smoothed (6mm Gaussian kernel). To examine blood-oxygen-level dependent (BOLD) signal change to task stimuli, a first-level, fixed-effects analysis was conducted. Onset times for each task condition were entered into a general linear model, convolved with a hemodynamic response function and linear contrasts between conditions were estimated. Subject-specific motion parameters were also included in the model as effects of non-interest. The primary t-contrasts for examining BOLD signal change were trauma-relevant versus neutral images; positive vs neutral images; task-related versus passive viewing trials. Events included the distractor and number stimuli.
Connectivity analyses were conducted with the CONN toolbox, version 16 (Whitfield-Gabrieli & Nieto-Castanon, 2012) implemented in SPM8. This toolbox permits computation of temporal correlations between BOLD signals from selected ROIs to other voxels in the brain, and has been used in earlier functional connectivity studies of emotion processing and learning (Pecina et al., 2013; Powers, Hevey, & Wallace, 2012; Stevens et al., 2013). We utilized this toolbox to examine individual differences in dissociation (MDI total score) and psychophysiological interactions (PPIs) with connectivity for the bilateral amygdala for the abovementioned contrasts, in keeping with earlier studies of amygdala connectivity in dissociation (Nicholson et al., 2017). Amygdala masks were defined using the amygdala probability map in the SPM Anatomy Toolbox (http://www.fz-juelich.de/inm/inm-1/EN/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html). MDI total scores were entered as subject-level regressors. Signal from subject motion was removed from the data. Seed-to-voxel bivariate correlations were computed for each contrast. Results were considered significant at a whole brain statistical threshold of p<.005, with cluster-level FWE correction at a threshold of p<.05 conducted with a priori specified regions of interest, including the ACC, orbitofrontal cortex (OFC), bilateral dorsolateral prefrontal cortex (DLPFC) and insula.
Neuropsychological and Affective Number Stroop Data Analysis
Given that the tests measured distinct cognitive constructs, separate univariate analyses of covariance (ANCOVAs) were conducted to examine between-group differences in performance on neuropsychological measures after accounting for the effects of age and trauma exposure (childhood and adult), given that these factors have demonstrated clear associations with test performance (Ganguli et al., 2010; Gould et al., 2012; Malarbi, Abu-Rayya, Muscara, & Stargatt, 2017; Palmer et al., 1997). High- and low-dissociation groups were the grouping variables, outcome variables were performance on the various neuropsychological measures; we also conducted correlational analyses with these variables. Separate ANCOVAs were performed with ANS data, with childhood maltreatment, non-maltreatment trauma exposure during adulthood, and age used as covariates. Where significant between-group differences were observed, we performed partial correlational analyses (Pearson’s r) to explore associations between cognitive performance and the various dissociative subscales, controlling for age and trauma exposure. The threshold for statistical significance was set at p<.05; Sidak correction was used to address potential inflation of Type I error due to multiple comparisons. Homogeneity of variance for the two groups was tested with Levene’s statistic. The sample size for the present study is similar to or larger than other recent studies of dissociation in trauma (Bichescu-Burian, Steyer, Steinert, Grieb, & Tschoke, 2017; Krause-Utz, Frost, Winter, & Elzinga, 2017; Rabellino, Densmore, Theberge, McKinnon, & Lanius, 2018)
Results
Dissociation
Compared to a normative population from an earlier study of the MDI (Briere et al., 2005), the following percentages represent the amount of participants within the entire sample who had scores that were two standard deviations or above for each MDI subscale; 27% for disengagement; 17% for depersonalization; 27% for derealization; 28% for emotional constriction; 25% for memory disturbance; 12% for identity disturbance. Within the high-dissociation group, the following percentages represent the amount of participants who had scores that were two standard deviations or above for each MDI subscale; 50% for disengagement; 30% for depersonalization; 53% for derealization; 51% for emotional constriction; 50% for memory disturbance; 24% for identity dissociation.
Neuropsychological Measures
Levene’s tests indicated equivalent error variance for the two diagnostic groups for all analyses (ps>.05); thus, these data were subjected to parametric analyses. Compared to low-dissociative participants, high-dissociative participants had significantly more childhood abuse exposure overall (F1,116=8.1, p<.01), particularly emotional abuse, but the groups were equivalent with respect to age, education, and non-childhood abuse-related trauma exposure over the lifetime (see Table 1).
On average, on a measure of abstraction and cognitive flexibility (PCET), participants successfully completed over two sorting categories (M=2.4, SD=.8), as compared to a mean of 1.7 for a normative population (Gur et al., 2010). Although high participants had comparable correct responses on the PCET compared to low-dissociative participants [(M=36.7, SD=6.5 vs (M=37.7, SD=7.8)], respectively), high-dissociative participants had fewer incorrect responses (see Table 2)(Gur et al., 2010) (F1,111=4.6,p=.03; Cohen’s d=3.1) and marginally fewer perseverative errors (F1,111=3.5, p=.07), after covarying age, childhood trauma and lifetime trauma exposure. Partial correlational analyses revealed that PCET perseverative errors (r=−.24, p=.01) and incorrect responses (r=−.23, p=.01) were inversely correlated with MDI total score. These findings remained significant when trauma exposure was not covaried. Specifically, PCET incorrect responses were also inversely correlated with the disengagement (r=−.3, p=.01) and emotional constriction (r=−.2, p=.04) MDI subscales.
Table 2.
Neuropsychological Test and Affective Number Stroop Performance of Low- and High-Dissociative Participants
| Low Dissociation | High Dissociation | ||
|---|---|---|---|
| Mean (SE) | Mean (SD) | F | |
| Penn Conditional Exclusion Task | |||
| Correct responses | 37.8 (1.3) | 37.7 (1.4) | .97 |
| Incorrect responses | 41 (2.3) | 33.9 (2.3) | 4.63* |
| Perseverative errors | 21.1 (1.4) | 17.2 (1.5) | 3.46 |
| Penn Continuous Performance Test | |||
| Correct responses | 112.9 (1.1) | 112.1 (1.1) | .25 |
| Penn Letter N-Back Task | |||
| 0-Back | 14.8 (.1) | 14.6 (.1) | 1.04 |
| 1-Back | 14.2 (.2) | 14.1 (.2) | .09 |
| 2-Back | 12.6 (.3) | 12 (.3) | 1.7 |
| Penn List Learning Task | |||
| Correct responses on first learning trial | 5.3 (.3) | 5 (.2) | .9 |
| Correct responses on last learning trial | 11.5 (.4) | 11.3 (.3) | .11 |
| Correct responses on interference trial | 5.7 (.3) | 5.1 (.2) | 3.2 |
| Correct responses on short-delay recall | 10.4 (.4) | 9.8 (.4) | .78 |
| Correct responses on long-delay recall | 10.8 (.4) | 10.5 (.4) | .34 |
| Slope of learning | 1.5 (.1) | 1.5 (.1) | .00 |
| Short Visual Object Learning Test | |||
| Correct responses overall | 15.3 (.3) | 13.8 (.3) | 9.93** |
| Correct responses on immediate recall trials | 8.2 (.22) | 7.6 (.22) | 4.6* |
| Correct response on long-delay recall trial | 13.8 (.3) | 13.3 (.3) | 1.1 |
| Affective Number Stroop Task | |||
| Trial type | Percent error (SD) | ||
| Trauma-relevant, number congruent | 7.5 (.0) | 11 (.0) | 1.2 |
| Positive, number congruent | 7.3 (.0) | 10 (.0) | .59 |
| Neutral, number congruent | 6.7 (.0) | 6.2 (.0) | .03 |
| Trauma-relevant, number incongruent | 6.6 (.0) | 15.2 (.0) | 4.67* |
| Positive, number incongruent | 8.9 (.0) | 14 (.0) | 1.5 |
| Neutral, number incongruent | 7.5 (.0) | 11 (.0) | 1.00 |
| Overall errors | 7.4 (.0) | 11.2 (.0) | 1.72 |
p<.05
p<.01
Covariates= age, CTQ total, TEI lifetime total (excluding abuse)
TEI = Traumatic Events Inventory
CTQ = Childhood Trauma Questionnaire
CAPS = Clinician Administered PTSD Scale
There were no significant between-group differences for correct responses on measures of working memory, attention, and list-learning (PLNB, PCPT, and PLLT, respectively). With regard to recall of visual material (SVOLT), low-dissociative (M=15.3, SE=.3) participants performed similarly to a normative population (M=15.6, SD=2.5), but high-dissociative participants performed more poorly (M=13.8; SE=.3; F1,111=9.5, p=.03) after accounting for age, childhood and lifetime trauma exposure. These findings were influenced by performance on immediate recall trials, which was significantly worse for high-dissociative participants (M=7.6, SD=.2) as compared to low-dissociative participants (M=8.2, SD=.2) after accounting for age and trauma exposure (F1,111=4.6, p=.03). No significant between-group differences were observed with delayed recall of visual material. Partial correlational analyses revealed that SVOLT correct responses were inversely correlated with MDI total score (r=−.19, p=.04). Specifically, these correct responses were correlated with MDI depersonalization (r=−.2, p=.01), derealization (r=−.2, p=.01) and emotional constriction (r=−.2, p=.04) subscales.
Affective Number Stroop Behavioral Response
In the entire sample, percent error rates for trauma-related distractor trials were highest (M=20.5, SD=21.5) followed by positive (M=17.9, SD=21) and neutral distractor trials (M=15.6, SD=17.8). Error rates were proportionally higher for number congruent trials (trauma-relevant distractors, M=9.2, SD=9.4; positive distractors M=8.6, SD=11.2, neutral distractors M=6.3, SD=8) as compared to number incongruent trials (trauma-relevant distractors, M=11.3, SD=12.1; positive distractors M=11.5, SD=12.6, neutral distractors M=9.4, SD=9.9). A similar pattern was observed with response times (in milliseconds, ms) for number congruent trauma-relevant distractors (M=896, SD=169.4; positive distractors M=889, SD=161.1, neutral distractors M=871.2, SD=146.3) and incongruent trials (trauma-relevant distractors, M=953.7, SD=154.1; positive distractors M=946, SD=156.1, neutral distractors M=939.1, SD=155.1).
After accounting for trauma exposure (CTQ total, TEI total) as well as age, highly dissociative participants demonstrated significantly more errors on number-incongruent trials with trauma-relevant image distractors, as compared to low-dissociative participants (F1,36=4.8, p=.04). No other statistically significant differences in performance or response time were observed between low- and high-dissociative participants (see Table 2).
Functional Connectivity
In response to trauma-relevant vs neutral images, a significant negative correlation between dissociation severity (MDI total score) and functional connectivity was observed between the amygdala and a large cluster of activation in the right anterior insula (k=287, MNIx,y,z=42, −22, 6; see Figure 2). No other regions survived error correction for all other contrasts and conditions; Table 3 details these findings. Given these significant findings, these analyses were repeated, excluding the five participants who had endorsed substance and/or psychotropic medication use. Although no findings survived cluster-size correction, a negative correlation between MDI total score and amygdala connectivity to the anterior insula remained (k=81, MNIx,y,z=−56, −16, 0).
Table 3.
Anatomical locations of amygdala to brain-wide functional connectivity in response to affect number stroop distractor trials (p<.005, uncorrected)
| Region | Brodmann Area | x | y | z | Cluster size (mm3) |
|---|---|---|---|---|---|
| Trauma-relevant vs Neutral Distractor Trials | |||||
| Negative Correlation | |||||
| Insula | 13 | 42 | -22 | 6 | 287 |
| Superior Temporal Gyrus | 22 | -56 | -16 | 4 | 170 |
| Superior Temporal Gyrus | 22 | 70 | -38 | 16 | 110 |
| Claustrum | -36 | 8 | -2 | 79 | |
| Superior Temporal Gyrus | 22 | -56 | 8 | 4 | 76 |
| Cuneus; Occipital Lobe | 19 | -2 | -94 | 24 | 55 |
| Insula | 13 | 34 | -32 | 18 | 38 |
| Inferior Parietal Lobe | 40 | 60 | -32 | 36 | 36 |
| Parietal Lobe, Sub-Gyral | 40 | -26 | -46 | 52 | 34 |
| Posterior Lobe, Pyramis | -24 | -70 | -36 | 33 | |
| Inferior Parietal Lobe | 40 | -66 | -26 | 24 | 32 |
| Positive Correlation | |||||
| Parahippocampal Gyrus | 36 | 22 | -32 | -14 | 60 |
| Middle Frontal Gyrus | 9 | 48 | 34 | 40 | 43 |
| Precuneus | 31 | 18 | -56 | 34 | 43 |
| Middle Temporal Gyrus | 21 | -66 | -50 | -8 | 38 |
| Parietal Lobe, Angular Gyrus | 39 | -30 | -58 | 34 | 35 |
| Superior Temporal Gyrus | 39 | -34 | -58 | 30 | 33 |
| Anterior Lobe | 16 | -58 | -26 | 33 | |
| Claustrum | 30 | 4 | 18 | 31 | |
| Positive vs Neutral Distractor Trials | |||||
| Negative Correlation | |||||
| Posterior Lobe, Uvula | 24 | -88 | -32 | 67 | |
| Posterior Lobe, Declive | 34 | -80 | -24 | 62 | |
| Posterior Lobe, Declive | -24 | -88 | -30 | 49 | |
| Putamen | -20 | 16 | 12 | 38 | |
| Inferior Temporal Gyrus | 20 | 36 | -2 | -50 | 37 |
| Occipital Lobe, Lingual Lobe | 18 | 4 | -94 | -20 | 35 |
| Positive Correlation | |||||
| Inferior Temporal Gyrus | 37 | -66 | -52 | -14 | 121 |
| Superior Temporal Gyrus | 38 | 28 | 16 | -36 | 69 |
| Cingulate Gyrus | 31 | 10 | -32 | 46 | 67 |
| Inferior Parietal Lobe | 40 | 34 | -58 | 42 | 60 |
| Brainstem, Red Nucleus | 6 | -20 | -2 | 58 | |
| Inferior Temporal Lobe | 37 | 48 | -56 | -2 | 57 |
| Parahippocampal Gyrus | 30 | -18 | -44 | 0 | 43 |
| Parahippocampal Gyrus | 19 | -24 | -56 | -8 | 43 |
| Posterior Cingulate | 29 | 10 | -40 | 12 | 34 |
| Posterior Lobe, Precuneus | 7 | 22 | -62 | 36 | 32 |
| Posterior Lobe, Precuneus | 7 | 22 | -70 | 52 | 30 |
| Task-related vs Passive View Trials | |||||
| Negative Correlation | |||||
| Postcentral Gyrus | 40 | -56 | -22 | 22 | 90 |
| Posterior Lobe, Inferior Semi-Lunar | -18 | -68 | -42 | 64 | |
| Precentral Gyrus | 44 | 58 | 10 | 6 | 45 |
| Orbital Gyrus | 11 | -6 | 46 | -20 | 43 |
| Positive Correlation | |||||
| Parietal Lobe, Precuneus | 7 | 16 | -68 | 30 | 529 |
| Posterior Cingulate | 23 | 4 | -44 | 24 | 355 |
| Precentral Gyrus | 9 | 36 | 14 | 42 | 211 |
| Cingulate Gyrus | 23 | 4 | -14 | 24 | 122 |
| Superior Parietal Lobe | 7 | 30 | -56 | 42 | 88 |
| Anterior Cingulate | 25 | 0 | 8 | -12 | 70 |
| Middle Temporal Gyrus | 39 | 54 | -70 | 22 | 69 |
| Superior Frontal Gyrus | 8 | 10 | 46 | 58 | 65 |
| Occipital Lobe | 19 | -54 | -82 | 12 | 60 |
| Supramarginal Gyrus | 40 | 42 | -46 | 30 | 45 |
| Fusiform Gyrus | 20 | 46 | -22 | -18 | 44 |
| Cingulate Gyrus | 31 | -14 | -22 | 36 | 42 |
| Caudate | -12 | -22 | 28 | 40 | |
| Parahippocampal Gyrus | 35 | -24 | -22 | -20 | 37 |
| Middle Temporal Gyrus | 37 | -66 | -56 | -6 | 36 |
| Anterior Lobe, Culmen | 22 | -32 | -20 | 33 | |
| Cingulate Gyrus | 31 | -14 | -34 | 42 | 31 |
| Middle Occipital Gyrus | 18 | 22 | -88 | -14 | 31 |
Discussion
The present study compared the performance of traumatized participants with high and low levels of dissociation on measures of executive functioning, memory, and attention, both in a neutral context (standard neuropsychological measures) and in the context of emotion; we administered an attentional control task during fMRI that included trauma-salient, positive, and neutral images. Our findings indicated that, compared to participants with fewer dissociative symptoms, highly dissociative participants had better performance on a measure of abstraction and cognitive flexibility, but poorer performance on a measure of immediate visual memory. Highly dissociative participants also performed more poorly on the ANS during fMRI trials that presented trauma-relevant distractors, and had comparatively lesser functional connectivity between the amygdala and insula in response to these distractors.
We found that particular aspects of executive functioning—namely, the ability to form abstract concepts and think flexibly and adaptively in a novel situation—were relatively enhanced in our high-dissociative participants. Compared to low-dissociative participants, high-dissociators showed fewer incorrect responses, and marginally fewer perseverative responses, on a measure of abstraction and cognitive flexibility (PCET). These findings indicate that high-dissociative participants were better able to use task-related feedback to grasp the target concepts and adjust their responses adaptively; higher-order processes like these are intrinsic to adaptive functioning. Some earlier studies have observed enhanced executive functions, including working memory and divided attention, in both traumatized (Elzinga et al., 2007) and healthy high-dissociative individuals (see McKinnon et al., 2016 for a review). For example, high-dissociative adults have shown better performance on tasks of divided attention (DePrince & Freyd, 2004) and verbal working memory span (de Ruiter, Phaf, Elzinga, & van Dyck, 2004) (Veltman et al., 2005) compared to low-dissociative adults. Notably, in the latter study, high-dissociative participants showed more extensive recruitment of the DLPFC regions during task performance as compared to low-dissociative participants (Veltman et al., 2005), which may suggest that enhanced performance may come at the price of cognitive efficiency. In this study, dissociative disengagement and emotional constriction were most highly linked to better PCET performance. This may suggest that participants with these dissociative features were more absorbed in the task, and in the absence of a time constraint (endemic to attention tasks), could think flexibly and abstractly. These findings are similar to a study of children, which found that, despite performing more poorly on a test of cognitive inhibition, highly dissociative children showed preserved rule-learning on a more complex task as compared to low-dissociative peers (Cromer, Stevens, DePrince, & Pears, 2006). Thus, in the absence of emotion, highly dissociative participants showed intact cognitive performance compared to low-dissociative peers, and even performing somewhat better on a test of abstraction. Overall, this suggests that outside of an emotional context, people who are highly dissociative may have intact higher-order cognitive functions.
In contrast to these findings, high-dissociative participants demonstrated relative decrements in attentional control (manifest by higher error rates on ANS trauma-relevant distractor trials) in an emotional context—when they were confronted with trauma-relevant images. These findings were significant even after accounting for variance associated with childhood and adult trauma exposure. Higher levels of dissociation were also linked to lesser connectivity between the amygdala and anterior insula in response to these trauma-relevant stimuli, as we had expected. Although all participants performed somewhat more poorly in the context of the trauma-relevant images, demonstrating higher error rates and slower response times during these trials, it appears that these evocative images were even more arousing and disruptive to high-dissociative participants. In keeping with the “overmodulator model” of dissociation in PTSD (Lanius et al., 2010), our highly-dissociative participants were not impaired or deficient on tasks of cognitive control/inhibition that had no emotional component (letter n-back and continuous performance tasks), but their attentional control was significantly disrupted when trauma-salient stimuli were introduced. These findings were similar to a study of borderline personality disorder that used a different emotional stroop task, which observed that state dissociation was related to poorer performance on negative, but not positive or neutral word trials (Winter et al., 2015). For those who endorsed more frequent experiences of dissociation, being confronted with images that resembled their interpersonal trauma experiences may have generated greater physiological and emotional arousal. This arousal, in turn, could have interrupted their ability to focus their attention to the task at hand.
These findings contrast significantly with those we observed from the neuropsychological battery—highly dissociative participants demonstrated relatively equivalent performance on the latter measures in comparison to low-dissociative peers, and even showed superior performance on an executive functioning task that had no emotionally-salient features. This contrast highlights the salience of emotion in affecting cognitive processes in this population of traumatized individuals. It is possible that the relatively enhanced abstraction abilities observed in our most dissociative participants may have developed as a compensatory response to deficits in attentional control.
The decreased amygdala-insula connectivity observed in high-dissociative traumatized participants during attention to trauma images may indicate poor integration and coordination of cognitive, affective and interoceptive networks. The insula is thought of as a central hub for the central executive network, which involves control regions (like the DLPFC) and guides the processing of incoming visual and verbal stimuli, and the default mode network, which engages in self-referential processing, and involves regions such as the medial PFC and posterior cingulate cortex (Menon & Uddin, 2010). The anterior insula serves to select information and initiate attentional processes (Menon & Uddin, 2010), and has reciprocal connectivity with brain regions that engage during detection of emotionally-salient stimuli, such as the amygdala (Augustine, 1996; Hoistad & Barbas, 2008; Mufson, Mesulam, & Pandya, 1981). When a highly dissociative individual is triggered by an emotionally-salient stimulus, poorer connectivity between the amygdala and insula may lead to consequent dysregulation of stimulus- and self-referential processing networks. Participants with PTSD have shown variable patterns of amygdala-insula connectivity to trauma-relevant scripts. At rest, several researchers have observed increased amygdala-insula connectivity (Nicholson et al., 2015; Nicholson et al., 2016) in dissociative vs non-dissociative PTSD and in dissociative BPD patients vs healthy controls (Krause-utz 2014). These authors have suggested that this increased connectivity at rest in high-dissociative individuals (particularly those with BPD) represents a neural substrate for increased affective arousal. The decreased connectivity we observed between the amygdala and insula in response to trauma-relevant stimuli may be indicative of general dysfunction within networks that facilitate attention to salient cues and integrate awareness of physiological states. Given the poorer performance demonstrated in the context of aversive, and trauma-salient cues on this attentional measure, it is possible that highly-dissociative participants were less aware of the physiological signs of arousal and attentional disruption caused by these cues, and became more cognitively and affectively disengaged over time. Visceral awareness is useful in monitoring one’s cognitive performance—being aware of increased levels of arousal and potential negative emotions that accompany this arousal can allow the person to employ appropriate strategies to regulate their emotions and thus, their performance on the task.
Relatively diminished activation in the insula has been previously observed in women with PTSD in response to reward-related stimuli, including positive social videos and positive images (Aupperle et al., 2012; Moser et al., 2015). There is evidence to suggest that oxytocin, a neuropeptide that plays a role in social affiliation and attachment (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011), may improve social reward sensitivity and normalize insula response; oxytocin administration has been found to increase insula response and enhances cooperation and feelings of trust (reviewed in Wigton et al., 2015). It is thus possible that oxytocin is an avenue through which amygdala-insula response may be normalized in dissociative traumatized people.
High-dissociative participants also performed more poorly on immediate recall trials of the visual memory task. This pattern of performance was expected, given that negative correlations between dissociation and visual memory have been observed fairly consistently in traumatized adult populations (Morgan, Doran, Steffian, Hazlett, & Southwick, 2006; Parlar et al., 2016; Rivera-Velez et al., 2014). Specifically, some studies have observed associations between derealization and poorer delayed recall of visuospatial stimuli (Parlar et al., 2016). It is likely that the high-dissociative participants experienced more difficulties with initial encoding, which interfered with their ability to correctly identify these images during immediate recall trials. However, no differences were observed with delayed recall, which suggest that overall visual memory is not impaired in high dissociators relative to low dissociators, and that initial encoding/immediate recall of items may be more sensitive to dissociation-related effects.
With respect to study limitations, several factors must be considered. We did not compare our neuropsychological findings to a normative data set, limiting our ability to draw conclusions about relative “deficits” in domains of cognitive functioning. However, given that low educational attainment and minority status has been previously been associated with lower scores on neuropsychological measures (due to factors such as familiarity with test-taking and test-related cultural bias) these standard scores would likely under-estimate participants true abilities in these domains. Further, our goal was to compare low and high dissociators within a traumatized population, and no normative dataset including a highly-traumatized, lower-education is available to our knowledge; a current goal is to create these norms for future studies. In addition, we included participants who had experienced relatively high rates of trauma exposure, which is known to have an impact on neuropsychological test performance (Malarbi et al., 2017); however, our findings were significant even after controlling for these factors. All of our participants were traumatized, and a majority of the highly-dissociative participants had PTSD—thus, our results may be most generalizable to this population. However, given that trauma and dissociation can occur in the context of numerous psychiatric disorders, such as depression (Bob et al., 2008; Parlar et al., 2016), anxiety disorders (Mula, Pini, & Cassano, 2007; Sierra, Medford, Wyatt, & David, 2012), and schizophrenia (Lysaker & Larocco, 2008; Yu et al., 2010), these findings may have broader applicability.
Our findings indicate that dissociation selectively impacts cognitive function specifically, the ability to sustain focus on a given task in the presence of trauma-related (and interpersonally-relevant) cues, such as gun violence. For our participants, who have limited economic resources and who live in environments that are rife with these reminders, these attentional problems are likely to emerge on a frequent basis.
In summary, the findings of this study shed light on patterns of cognition in highly dissociative traumatized people. The results indicate that, overall, highly-dissociative traumatized people demonstrate relatively similar cognitive abilities, compared to those who experience fewer dissociative symptoms, in the absence of a provocative emotional stimulus. However, the presence of emotional stimuli (in this case, trauma-relevant reminders) adversely affects their performance, and attention to these cues is related to poorer amygdala-insula connectivity.
Treatments that emphasize emotional awareness and acceptance, such as acceptance and commitment (ACT) or mindfulness-based treatments, may be of particular benefit to these dissociative individuals, prior to engaging in trauma-focused treatment. Augmentation of treatment with oxytocin may also serve to normalize insula response, facilitate attentional control, and enhance interoceptive ability in this group of traumatized people.
Funding and Acknowledgments:
This work was primarily supported by National Institutes of Mental Health (R01 MH071537, M01RR00039 and P20RR16435 to KJR, MH101380 to NF, MH100122 to TJ). Support was also received from Howard Hughes Medical Institute, PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, the Emory Medical Care Foundation, National Center for Research Resources, and the Burroughs Wellcome Fund. We wish to thank Tim Ely for technical support, Amrita Kaimal, Michaela Desrosiers, Katrina Conrad, and Nayan Tiwary for their assistance with data collection, as well as the contributions of our research participants.
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose.
References
- Association AP (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC. [Google Scholar]
- Augustine JR (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews, 22(3), 229–244. doi: S0165017396000112 [pii] [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Stein MB (2012). Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry, 69(4), 360–371. doi: 10.1001/archgenpsychiatry.2011.153969/4/360 [pii] [DOI] [PubMed] [Google Scholar]
- Bae H, Kim D, & Park YC (2016). Dissociation predicts treatment response in eye-movement desensitization and reprocessing for posttraumatic stress disorder. J Trauma Dissociation, 17(1), 112–130. doi: 10.1080/15299732.2015.1037039 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, & Fink L (1998). Childhood Trauma Questionnaire: a retrospective self-report manual. New York: The Psychological Corporation. [Google Scholar]
- Bichescu-Burian D, Steyer J, Steinert T, Grieb B, & Tschoke S (2017). Trauma-related dissociation: Psychological features and psychophysiological responses to script-driven imagery in borderline personality disorder. Psychophysiology, 54(3), 452–461. doi: 10.1111/psyp.12795 [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Blair RJ (2007). Modulation of emotion by cognition and cognition by emotion. Neuroimage, 35(1), 430–440. doi: S1053–8119(06)01117–7 [pii] 10.1016/j.neuroimage.2006.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Blair RJ (2012). Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med, 1–11. doi: S0033291712000840 [pii] 10.1017/S0033291712000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, & Charney DS (1995). The development of a clinician-administered PTSD scale. J Trauma Stress, 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Bob P, Fedor-Freybergh P, Jasova D, Bizik G, Susta M, Pavlat J, Raboch J (2008). Dissociative symptoms and neuroendocrine dysregulation in depression. Med Sci Monit, 14(10), CR499-504. [PubMed] [Google Scholar]
- Briere J (2006). Dissociative symptoms and trauma exposure: specificity, affect dysregulation, and posttraumatic stress. J Nerv Ment Dis, 194(2), 78–82. doi: 10.1097/01.nmd.0000198139.47371.54 [DOI] [PubMed] [Google Scholar]
- Briere J, Weathers FW, & Runtz M (2005). Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. J Trauma Stress, 18(3), 221–231. doi: 10.1002/jts.20024 [DOI] [PubMed] [Google Scholar]
- Chae Y, Goodman GS, Eisen ML, & Qin J (2011). Event memory and suggestibility in abused and neglected children: trauma-related psychopathology and cognitive functioning. J Exp Child Psychol, 110(4), 520–538. doi: 10.1016/j.jecp.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Conners CK (2002). Conners’ Continuous Performance Test (CPT-II) Technical Guide and Software Manual. North Tonawanda, NY: Multi Health Systems. [Google Scholar]
- Corrigan FM, & Hull AM (2015). Neglect of the complex: why psychotherapy for post-traumatic clinical presentations is often ineffective. BJPsych Bull, 39(2), 86–89. doi: 10.1192/pb.bp.114.046995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer LD, Stevens C, DePrince AP, & Pears K (2006). The relationship between executive attention and dissociation in children. J Trauma Dissociation, 7(4), 135–153. doi: 10.1300/J229v07n04_08 [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Phaf RH, Elzinga BM, & van Dyck R (2004). Dissociative style and individual differences in verbal working memory span. Conscious Cogn, 13(4), 821–828. doi: 10.1016/j.concog.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test, 2nd edition: Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- DePrince AP, & Freyd JJ (2004). Forgetting trauma stimuli. Psychol Sci, 15(7), 488–492. doi: 10.1111/j.0956-7976.2004.00706.x [DOI] [PubMed] [Google Scholar]
- DePrince AP, Weinzierl KM, & Combs MD (2008). Stroop performance, dissociation, and trauma exposure in a community sample of children. J Trauma Dissociation, 9(2), 209–223. doi: 10.1080/15299730802048603 [DOI] [PubMed] [Google Scholar]
- Dorahy MJ, & van der Hart O (2015). DSM-5’s posttraumatic stress disorder with dissociative symptoms: challenges and future directions. J Trauma Dissociation, 16(1), 7–28. doi: 10.1080/15299732.2014.908806 [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Ardon AM, Heijnis MK, De Ruiter MB, Van Dyck R, & Veltman DJ (2007). Neural correlates of enhanced working-memory performance in dissociative disorder: a functional MRI study. Psychol Med, 37(2), 235–245. doi: 10.1017/S0033291706008932 [DOI] [PubMed] [Google Scholar]
- Fani N, Bradley RG, Ressler KJ, & McClure-Tone EB (2010). Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognitive Therapy and Research, 35(1), 57–67. [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, & Ressler KJ (2012). Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol, 90(2), 134–142. doi: S0301–0511(12)00063–4 [pii] 10.1016/j.biopsycho.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, Ressler KJ (2016). Structural and Functional Connectivity in Posttraumatic Stress Disorder: Associations with Fkbp5. Depress Anxiety, 33(4), 300–307. doi: 10.1002/da.22483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, & Chang CC (2010). Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health, 14(1), 100–107. doi: 10.1080/13607860903071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Ressler KJ (2009). Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry, 31(6), 505–514. doi: S0163-8343(09)00090-5 [pii] 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Gur RC, Ragland JD, Censits DM, & Gur RE (1997). Reliability, performance characteristics, construct validity, and an initial clinical application of a visual object learning test (VOLT). Neuropsychology, 11(4), 602–612. [DOI] [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, & Nemeroff CB (2012). The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res, 46(4), 500–506. doi: 10.1016/j.jpsychires.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Gur RE (2010). A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods, 187(2), 254–262. doi: 10.1016/j.jneumeth.2009.11.017 S0165-;0270(09)00615-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK (1981). Wisconsin Card Sorting Test manual . Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Hoistad M, & Barbas H (2008). Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage, 40(3), 1016–1033. doi: 10.1016/j.neuroimage.2007.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, & Ressler KJ (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry, 167(6), 648–662. doi: appi.ajp.2009.09071074 [pii] 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst N, Limberger MF, Ebner-Priemer UW, Keibel-Mauchnik J, Dyer A, Berger M, Bohus M (2011). Dissociation predicts poor response to Dialectial Behavioral Therapy in female patients with Borderline Personality Disorder. J Pers Disord, 25(4), 432–447. doi: 10.1521/pedi.2011.25.4.432 [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Frost R, Winter D, & Elzinga BM (2017). Dissociation and Alterations in Brain Function and Structure: Implications for Borderline Personality Disorder. Curr Psychiatry Rep, 19(1), 6. doi: 10.1007/s11920-017-0757-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A, Veer IM, Rombouts SA, Bohus M, Schmahl C, & Elzinga BM (2014). Amygdala and anterior cingulate resting-state functional connectivity in borderline personality disorder patients with a history of interpersonal trauma. Psychol Med, 44(13), 2889–2901. doi: 10.1017/S0033291714000324 [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, & Gur RE (2001). Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res, 48(2–3), 307–316. doi: S0920996400000608 [pii] [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Moberg PJ, & Gur RC (2004). The Penn Conditional Exclusion Test: a new measure of executive-function with alternate forms of repeat administration. Arch Clin Neuropsychol, 19(2), 191–201. doi: 10.1016/S0887-6177(03)00003-9 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2005). International Affective Picture System (IAPS): Digitized photographs, instruction manual and affective ratings (Vol. A-6). Gainesville, FL: University of Florida. [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, & Spiegel D (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry, 167(6), 640–647. doi: 10.1176/appi.ajp.2009.09081168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker PH, & Larocco VA (2008). The prevalence and correlates of trauma-related symptoms in schizophrenia spectrum disorder. Compr Psychiatry, 49(4), 330–334. doi: 10.1016/j.comppsych.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Malarbi S, Abu-Rayya HM, Muscara F, & Stargatt R (2017). Neuropsychological functioning of childhood trauma and post-traumatic stress disorder: A meta-analysis. Neurosci Biobehav Rev, 72, 68–86. doi: 10.1016/j.neubiorev.2016.11.004 [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Boyd JE, Frewen PA, Lanius UF, Jetly R, Richardson JD, & Lanius RA (2016). A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2016.07.017 [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct, 214(5–6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci, 12(9), 524–538. doi: 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Morgan CA 3rd, Doran A, Steffian G, Hazlett G, & Southwick SM (2006). Stress-induced deficits in working memory and visuo-constructive abilities in Special Operations soldiers. Biol Psychiatry, 60(7), 722–729. doi: 10.1016/j.biopsych.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Moser DA, Aue T, Suardi F, Kutlikova H, Cordero MI, Rossignol AS, Schechter DS (2015). Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Soc Cogn Affect Neurosci, 10(5), 645–653. doi: 10.1093/scan/nsu099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, & Pandya DN (1981). Insular interconnections with the amygdala in the rhesus monkey. Neuroscience, 6(7), 1231–1248. [DOI] [PubMed] [Google Scholar]
- Mula M, Pini S, & Cassano GB (2007). The neurobiology and clinical significance of depersonalization in mood and anxiety disorders: a critical reappraisal. J Affect Disord, 99(1–3), 91–99. doi: 10.1016/j.jad.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Najavits LM (2015). The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep, 7, 43. doi: 10.12703/P7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, Frewen PA, Theberge J, Neufeld RW, McKinnon MC, & Lanius RA (2015). The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology, 40(10), 2317–2326. doi: 10.1038/npp.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Lanius RA (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp, 38(1), 541–560. doi: 10.1002/hbm.23402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RW, Theberge J, Lanius RA (2016). Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res, 250, 61–72. doi: 10.1016/j.pscychresns.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Palmer LK, Armsworth M, Swank PR, Bush G, Frantz C, & Copley J (1997). The neuropsychological sequelae of chronically psychologically traumatized children. Archives of Clinical Neuropsychology, 12(4), 379–380. [Google Scholar]
- Parlar M, Frewen PA, Oremus C, Lanius RA, & McKinnon MC (2016). Dissociative symptoms are associated with reduced neuropsychological performance in patients with recurrent depression and a history of trauma exposure. Eur J Psychotraumatol, 7, 29061. doi: 10.3402/ejpt.v7.29061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Theberge J, McKinnon MC, & Lanius RA (2018). The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp. doi: 10.1002/hbm.24081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1992). Trail Making Test. Manual for Administration and Scoring . Tucson, AZ: Reitan Neuropsychological Laboratory. [Google Scholar]
- Rivera-Velez GM, Gonzalez-Viruet M, Martinez-Taboas A, & Perez-Mojica D (2014). Post-traumatic stress disorder, dissociation, and neuropsychological performance in Latina victims of childhood sexual abuse. J Child Sex Abus, 23(1), 55–73. doi: 10.1080/10538712.2014.864746 [DOI] [PubMed] [Google Scholar]
- Roca V, Hart J, Kimbrell T, & Freeman T (2006). Cognitive function and dissociative disorder status among veteran subjects with chronic posttraumatic stress disorder: a preliminary study. J Neuropsychiatry Clin Neurosci, 18(2), 226–230. doi: 10.1176/jnp.2006.18.2.226 [DOI] [PubMed] [Google Scholar]
- Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, & Katschnig H (2004). Facial recognition deficits and cognition in schizophrenia. Schizophr Res, 68(1), 27–35. doi: 10.1016/S0920-9964(03)00131-2 [DOI] [PubMed] [Google Scholar]
- Sack M, Cillien M, & Hopper JW (2012). Acute dissociation and cardiac reactivity to script-driven imagery in trauma-related disorders. Eur J Psychotraumatol, 3. doi: 10.3402/ejpt.v3i0.17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng J, Miller J, Sperlich M, van de Ven CJ, Brown S, Carter CS, & Liberzon I (2013). Exploring dissociation and oxytocin as pathways between trauma exposure and trauma-related hyperemesis gravidarum: a test-of-concept pilot. J Trauma Dissociation, 14(1), 40–55. doi: 10.1080/15299732.2012.694594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sierra M, Medford N, Wyatt G, & David AS (2012). Depersonalization disorder and anxiety: a special relationship? Psychiatry Res, 197(1–2), 123–127. doi: 10.1016/j.psychres.2011.12.017 [DOI] [PubMed] [Google Scholar]
- Stein DJ, Koenen KC, Friedman MJ, Hill E, McLaughlin KA, Petukhova M, Kessler RC (2013). Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Biol Psychiatry, 73(4), 302–312. doi: 10.1016/j.biopsych.2012.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Lanius RA, & Frewen PA (2012). Evidence for a dissociative subtype of PTSD by latent profile and confirmatory factor analyses in a civilian sample. Depress Anxiety, 29(8), 689–700. doi: 10.1002/da.21944 [DOI] [PubMed] [Google Scholar]
- Veltman DJ, de Ruiter MB, Rombouts SA, Lazeron RH, Barkhof F, Van Dyck R, Phaf RH (2005). Neurophysiological correlates of increased verbal working memory in high-dissociative participants: a functional MRI study. Psychol Med, 35(2), 175–185. [DOI] [PubMed] [Google Scholar]
- White SF, Costanzo ME, Blair JR, & Roy MJ (2015). PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage Clin, 7, 19–27. doi: 10.1016/j.nicl.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, Fusar-Poli P (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci, 40(1), E1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Krause-Utz A, Lis S, Chiu CD, Lanius RA, Schriner F, Schmahl C (2015). Dissociation in borderline personality disorder: Disturbed cognitive and emotional inhibition and its neural correlates. Psychiatry Res, 233(3), 339–351. doi: 10.1016/j.pscychresns.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Yu J, Ross CA, Keyes BB, Li Y, Dai Y, Zhang T, Xiao Z (2010). Dissociative disorders among Chinese inpatients diagnosed with schizophrenia. J Trauma Dissociation, 11(3), 358–372. doi: 10.1080/15299731003793468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schmidt U (2015). Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology, 55, 102–115. doi: 10.1016/j.psyneuen.2015.02.005 [DOI] [PubMed] [Google Scholar]