Abstract
Background:
Allocation for pediatric deceased-donor kidney transplantation (pDDKT) in the United States now deemphasizes HLA matching to improve equality in access to transplantation, but other national systems still consider HLA matching due to concerns about graft survival. We hypothesized that the impact of HLA mismatching has decreased over time due to advances including improved immunosuppression.
Methods:
Using SRTR data, we analyzed whether the association between the number of HLA mismatches and outcomes of first-time pDDKTs changed between 2 eras: 1995–2004 (N=2854) and 2005–2014 (N=4643).
Results:
Between eras, the median number of mismatches increased from 4 to 5 (p<0.001). Overall graft failure risk was higher among HLA-mismatched versus HLA-matched transplants (adjusted HR 1.211.431.69 for 3–6 vs. 0–2 mismatches, p<0.001), and this association was similar pre-2005 and post-2005 (pinteraction=0.5). Median PRA change at relisting increased from 79 to 85 (p=0.01), but the association between number of HLA mismatches and PRA change was similar between eras (pinteraction=0.6).
Conclusions:
Our finding that increased HLA mismatching continues to impact graft survival, with 43% higher risk of graft failure, highlights the tradeoff between transplant access equity and outcomes and calls into question the deemphasis on HLA matching in pDDKT allocation in the United States.
INTRODUCTION
For transplant candidates, acceptance of a deceased-donor organ offer must balance the benefit of avoiding time on dialysis with the lost opportunity for a better human leukocyte antigen (HLA)-matched organ offer.1,2 For pediatric kidney transplant candidates in particular, HLA matching had historically been an allocation priority given the likelihood of retransplantation as an adult.3,4 However, policies prioritizing HLA matching have led to racial disparities in waitlist times and access to transplantation.5 To address these disparities in the United States, successive policy changes have deemphasized HLA matching with hopes of improving equity in access to transplantation and waitlist time.6
Studies of pediatric kidney transplants performed in the 1980s through the early 2000s found that HLA mismatching was associated with inferior pediatric graft survival,7–10 as well as a 37% higher risk of sensitization.11 Since then, advances in posttransplant care, including new immunosuppressive therapies,12–14 have improved overall transplant outcomes and might have decreased the impact of HLA mismatching on graft survival. Such a decrease in the impact of HLA mismatching would be increasingly important, as policy changes deemphasizing HLA matching have led to an increase in the number of HLA-mismatched transplants.3,15 An understanding of the current impact of HLA mismatching on transplant outcomes is crucial to ensure that allocation policies are not putting pediatric candidates at risk of inferior transplant outcomes.
Therefore, we sought to explore changes over time in the impact of HLA mismatching on pediatric deceased-donor kidney transplant (pDDKT) outcomes using national registry data. Based on previous findings relating HLA matching to kidney transplant outcomes, we evaluated changes in the impact of HLA matching on post-pDDKT graft survival, sensitization at relisting, and time to retransplantation for recipients whose experienced graft failure.
METHODS
Study population
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.16 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
We included all pediatric kidney transplant recipients (pKTRs) between 1995 and 2014. We excluded recipients who had previously received a transplant, received a multiorgan transplant, were 18 years or older at the time of transplant, were missing data for both antigens for an HLA locus (HLA-A, HLA-B, or HLA-DR) (N=8), or did not have a PRA reported at the time of their first transplant (N=14). HLA information was available in SRTR for HLA-A, HLA-B, and HLA-DR loci; therefore, mismatches at these 3 loci were used to calculate the number of HLA mismatches, with a maximum of 6 possible mismatches. All data were administratively censored at June 30, 2016. This study was approved by the Johns Hopkins Institutional Review Board.
Patient and graft survival
We studied the association between the number of HLA mismatches at first kidney transplant (categorized as 0–2 vs. 3–6 based) and death-censored graft failure (DCGF) using Cox regression and used interaction term analysis to determine if this association differed by transplant era (1995–2004 vs. 2005–2014). This time period was chosen to provide sufficient sample size and encompasses multiple changes in care, including the Share-35 allocation change in December 2017.15 We performed these analyses both unadjusted and adjusted for donor and recipient sex, age, race, height, and weight, recipient ESRD etiology, pretransplant PRA, cold ischemia time, years on dialysis, and year of transplant. Multiple imputation was used for donor and recipient height and cold ischemia time.
Outcomes after graft failure
For pDDKT recipients who experienced graft failure, we calculated the change in PRA as the difference between their PRA at the time of transplant and their PRA at relisting. Time to retransplantation was calculated from the time of relisting. We analyzed the association between the number of HLA mismatches at first DDKT and the median change in PRA from original pretransplant to relisting using rank-sum tests. We analyzed the association between the era of first DDKT and the time to retransplantation using Cox regression.
Confidence intervals are reported as per the method of Louis and Zeger.17 All analyses were performed using Stata 14.1/MP for Windows (College Station, Texas).
RESULTS
Study population
Of 7497 pKTRs who underwent their first DDKT transplant between 1995–2015, 2854 were pre-2005 (between 1994–2004) and 4643 were post-2005 (between 2005–2014) (Table 1). Compared to pKTRs pre-2005, those transplanted post-2005 were less likely to be African American (25.7% vs. 28.9%, p=0.003) and have non-FSGS glomerular disease causing their ESRD (11.6% vs. 16.5%, p<0.001) (Table 1). Recipients post-2005 also had a higher weight (median (IQR) 44 (24–59) vs. 42 (25–56), p=0.003) and a lower pretransplant PRA (median (IQR) 0 (0–0) vs. 0 (0–3), p<0.001). The deceased donors of pKTRs post-2005 were less likely to be female (32.1% vs. 38.2%, p<0.001), have a history of smoking (5.5% vs. 27.2%, p<0.001) or hypertension (2.5% vs. 7.5%, p<0.001), or die from stroke (12.0% vs. 24.9%, p<0.001) and were more likely to be African American (17.5% vs. 12.5%, p<0.001), be younger (median (IQR) 21 (17–26) vs. 23 (16–38) years, p<0.001) and taller (173 (163–180) vs. 170 (160–178) cm, p<0.001), and have a higher weight (72 (60–84) vs. 69 (55–82) kg, p>0.001). The median (IQR) number of HLA mismatches increased from 4 (4–5) pre-2005 to 5 (4–5) post-2005 (p<0.001).
Table 1.
Characteristics of recipients and donors, by time period.
| Year of Kidney Transplant | |||
|---|---|---|---|
| Characteristics | 1995–2004 (N=2854) | 2005–2014 (N=4643) | P |
| Recipient, N (%) | |||
| Female | 1208 (42.3%) | 1962 (42.3%) | >0.9 |
| African American | 824 (28.9%) | 1195 (25.7%) | <0.001 |
| Age in years, median (IQR) | 13 (9–16) | 14 (8–16) | 0.7 |
| Height in cm, median (IQR) | 147 (120–160) | 148 (119–163) | 0.07 |
| Weight in kg, median (IQR) | 42 (25–56) | 44 (24–59) | 0.003 |
| Diagnosis | <0.001 | ||
| FSGS | 429 (15.0%) | 710 (15.3%) | |
| Other glomerular disease | 470 (16.5%) | 537 (11.6%) | |
| CAKUT | 700 (24.5%) | 1218 (26.2%) | |
| Other | 1255 (44.0%) | 2178 (46.9%) | |
| Public insurance | 1877 (66.1%) | 3165 (68.2%) | 0.1 |
| Years on dialysis, median (IQR) | 1.2 (0.3–2.2) | 1.1 (0.3–2.1) | 0.007 |
| Pretransplant PRA, median (IQR) | 0 (0–3) | 0 (0–0) | <0.001 |
| Donor | |||
| Female | 1091 (38.2%) | 1490 (32.1%) | <0.001 |
| African American | 356 (12.5%) | 797 (17.2%) | <0.001 |
| Age in years, median (IQR) | 23 (16–38) | 21 (17–26) | <0.001 |
| Height in cm, median (IQR) | 170 (160–178) | 173 (163–180) | <0.001 |
| Weight in kg, median (IQR) | 69 (55–82) | 72 (60–84) | <0.001 |
| Diabetes | 31 (1.1%) | 43 (0.9%) | 0.5 |
| Smoker | 766 (27.2%) | 255 (5.5%) | <0.001 |
| Hypertension | 212 (7.5%) | 115 (2.5%) | <0.001 |
| Death from stroke | 24.9% | 12.0% | <0.001 |
| Transplant | |||
| Number of HLA mismatches, median (IQR) | 4 (4–5) | 5 (4–5) | <0.001 |
| Time on waitlist before first transplant (months), median (IQR) | 7 (2–16) | 5 (2–13) | <0.001 |
| Cold ischemia time (hours), median (IQR) | 16.5 (12–22) | 12 (8.4–17) | <0.001 |
Risk of DCGF
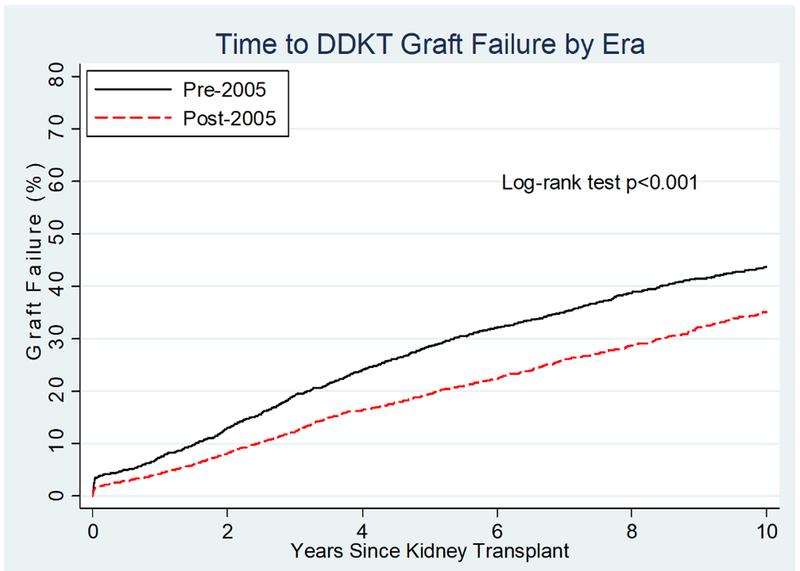
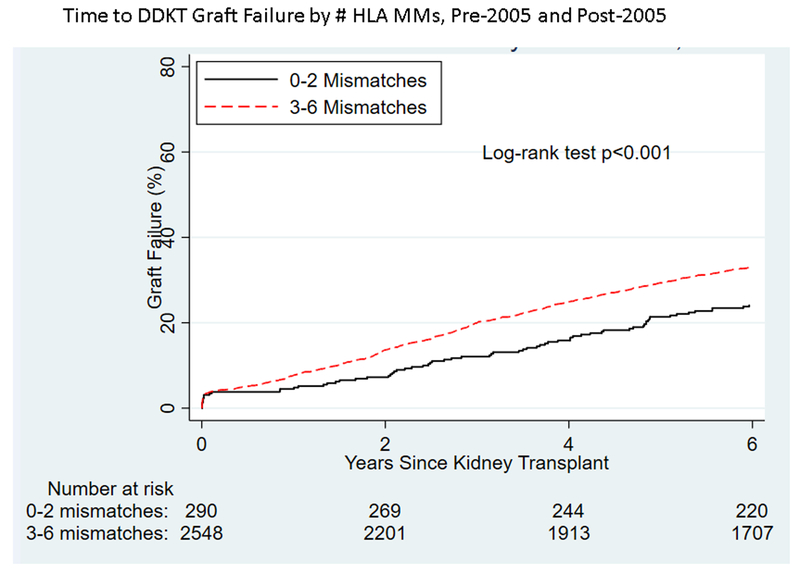
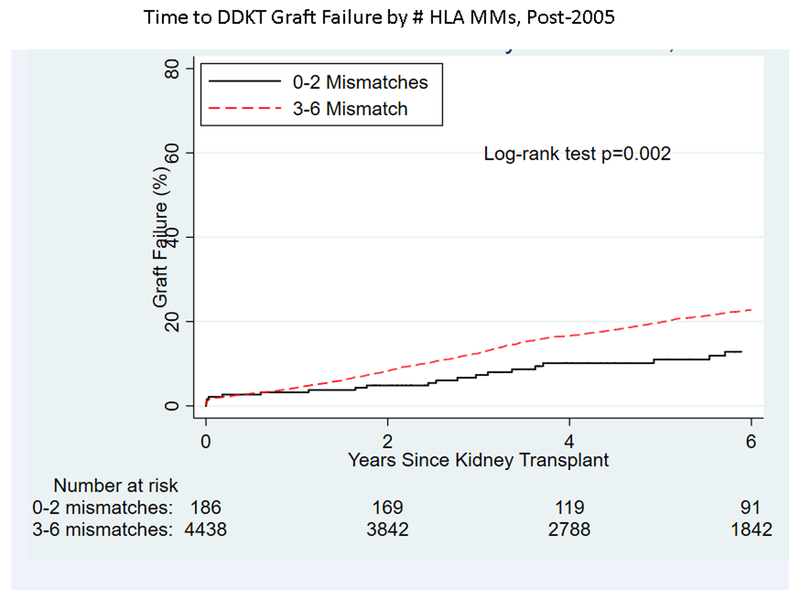
Among pKTRs whose transplants were pre-2005, 1,597 experienced graft failure during the follow-up period, while 1,107 of the pKTRs post-2005 experienced graft failure. DCGF among pKTRs was significantly different by era (Figure 1), decreasing 30% post-2005 versus pre-2005 (HR 0.650.700.76). Unadjusted analysis showed a significantly higher risk of graft failure with a greater number of HLA mismatches both pre-2005 (p<0.001, Figure 2A) and post-2005 (p=0.002, Figure 2B). However, on adjusted analysis, the association between the number of HLA mismatches and graft failure was similar between eras (interaction term: 0–2 vs. 3–6 mismatches, p=0.5). The risk of graft failure for a given number of HLA mismatches over the entire time period (1995–2014) was 1.43-fold (95% CI 1.21–1.69) higher for transplants with 3–6 mismatches compared to those with 0–2 mismatches.
Figure 1.
Time to deceased-donor kidney transplant graft failure for pediatric recipients pre-2005 vs. post-2005.
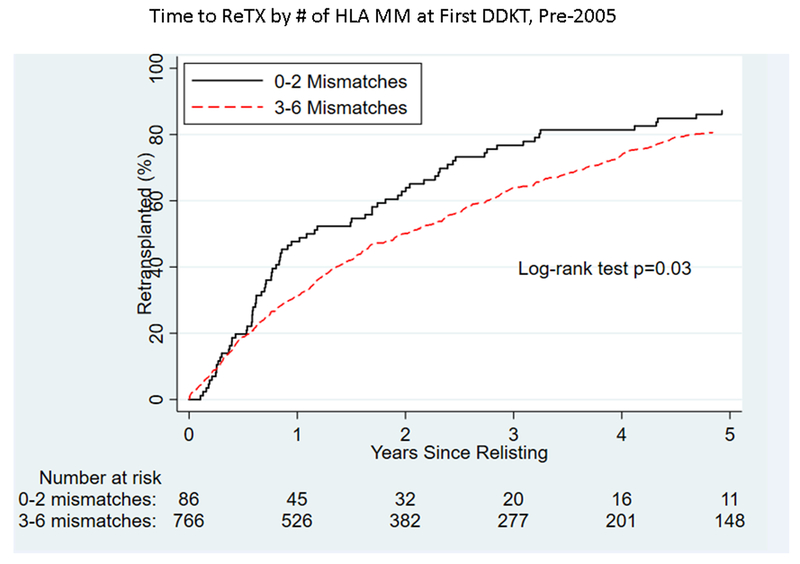
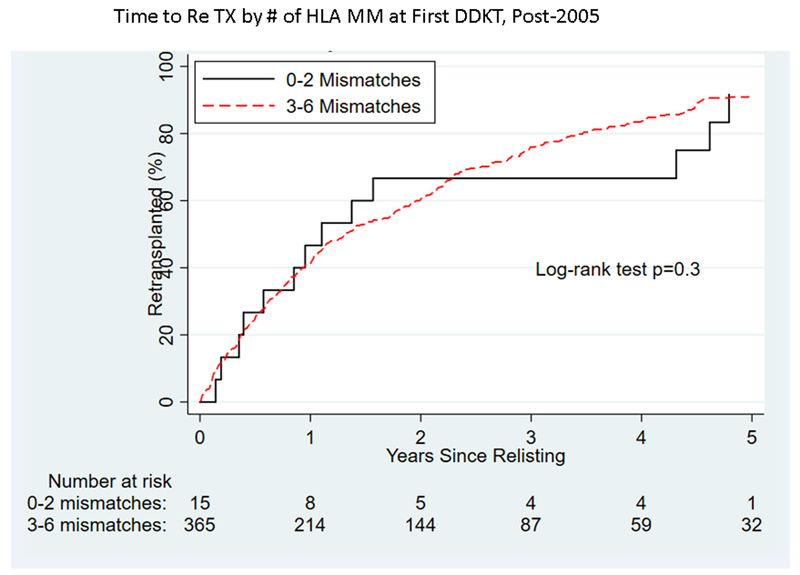
Figure 2.
By number of HLA matches, time to (A) graft failure pre-2005, (B) graft failure post-2005), (C) retransplantation pre-2005, and (D) retransplantation post-2005 for pediatric deceased-donor kidney transplant recipients.
Change in sensitization
Among pKTRs who experienced graft failure, the median PRA change from pretransplant to graft failure increased from 79 pre-2005 to 85 post-2005 (p=0.001). Recipients of organs with more HLA mismatches had a larger increase in PRA in both time periods (Figure S1). After adjusting for donor, recipient, and transplant characteristics, the PRA change from transplant to relisting was significantly associated with the number of HLA mismatches. Post-2005, the overall change in PRA from transplant to relisting increased by an average of 29.5 (95% CI 22.1–37.0, p<0.001) more for 3–6 vs. 0–2 mismatches. In other words, on average, patients who received a transplant post-2005 and experienced graft failure experienced a greater increase in sensitization than comparable patients who received a transplant pre-2005 and later experienced graft failure. However, the increase in PRA change for a particular number of HLA mismatches was similar between eras (p=0.9).
Time to retransplantation
Among pKTRs who experienced graft failure, time to retransplantation decreased overall between the 2 eras (p<0.001, Figure S2). Recipients whose grafts failed had a 1.39-fold (95% VI 1.23–1.58) higher likelihood of receiving a re-transplantation post-2005 versus pre-2005 (p<0.001). Pre-2005, recipients of an organ with 1–3 or 4–6 HLA mismatches had a longer time to transplantation than those who received an organ with 0 HLA mismatches (p=0.03, Figure 2C). Post-2005, there was no evidence of difference in time to retransplantation by the number of HLA mismatches at first kidney transplant for pKTRs (p=0.3, Figure 2D).
DISCUSSION
In this national study of 7497 pediatric deceased-donor kidney transplants, we found that the negative impact of HLA mismatching on transplant outcomes remains strong and has not been impacted by improvements in posttransplant care over the last decade. The continued association of HLA mismatching with increased graft failure risk is concerning in light of the increased median number of HLA mismatches from pre-2005 to post-2005 vs. pre-2005 (p<0.001). Additionally, the change in PRA for a particular number of HLA mismatches remained similar post-2005, resulting in a greater median increase in sensitization post-2005 (85 vs. 79, p=0.001). In summary, HLA mismatching has increased in frequency and continues to have a strong negative impact on kidney transplant outcomes for pediatric recipients.
Our findings of an association between HLA mismatch and inferior posttransplant outcomes is consistent with a report from 1985–2004 in a combined cohort of adult and pediatric DDKT recipients that even though graft survival improved over time, the association between the degree of HLA match and graft survival remained strong and highly significant.18 Our findings extend this report to the era post-2005, with continued importance of HLA match despite any improvements in immunosuppression in the last decade. Additionally, some authors have noted the same association between greater HLA mismatching and inferior posttransplant outcomes among living donor kidney transplantation, recommending that living donor kidney transplants to pediatric recipients should be performed with donors with fewer than 4 HLA mismatches.19
Our finding that the change in sensitization, as measured by PRA, increased with an increased number of mismatches is also consistent with prior literature.11 The amount by which PRA increased for a transplant with a given number of mismatches was similar between the 2 eras in our study (Figure S1), suggesting that the increase in median PRA change observed between the 2 eras was driven by the increase in the degree of HLA mismatching in pDDKTs. However, it is important to note that methods of detecting PRA increased in sensitivity between these 2 time periods,20 meaning that PRA measurements in the second era (2005–2014) might be higher simply due to improved technology. The similar PRA change that we observed between the 2 eras for a given number of HLA mismatches might therefore represent a decrease in actual PRA change that is confounded by increased sensitivity in the PRA measurement used. This increase in measurement sensitivity cannot be accounted for in our models.
The benefit of allocation policies deemphasizing HLA matching have been questioned in the context of a “lifetime approach” to pediatric kidney transplantation.11 Specifically, previous authors have expressed the concern that the risks of immunologic differences between donors and recipients under policies deemphasizing HLA matching might outweigh the benefits of shorter wait times and allografts from younger donors that are allocated to pKTR under these policy changes.5 A study by Gralla et al of 12 000 pediatric kidney transplants performed 1990–2008 found an association between 5-year retransplantation graft survival and the number of HLA MM at the first and second kidney transplants.21 Similarly, a study by Foster et al of 8400 pKTRs between 1988 and 2009 found that, compared to recipients of DDKTs with 2–3 mismatches, patients with 4–6 mismatches spent 12% less time with a functioning graft after the first transplant (p<0.001).22 While we observed a significant association between the number of HLA matches and graft failure that was similar in both eras (pre-2005 and post-2005), we observed that overall graft survival continued to improve. While we did observe a greater median increase in PRA among pKTRs whose grafts failed in the post-2005 era, there was no evidence of a change in the magnitude of PRA increase for a particular number of HLA mismatches. There were insufficient data on outcomes following retransplantation to assess the association between increased HLA mismatching at first transplant or increased PRA at relisting on the outcomes of retransplants. As more data on outcomes of post-2005 re-transplants become available, the association between HLA matching at first DDKT and longer-term patient outcomes should be carefully evaluated to assess the “lifetime” impact on pKTRs.
Limitations of all national registry studies include the observational nature, the varying protocols of centers from which data are collected, and the potential presence of unmeasured confounding. Our results are also vulnerable to residual confounding, although we have adjusted for available confounders in the OPTN database. We hypothesize that the improvement in graft survival over time, a finding that has previously been described in other studies of registry data,18 is likely due to improvements in immunosuppression and other transplant protocols during that time period, however we are unable to investigate this using the available registry data. Given this gradual improvement in posttransplant outcomes over time, we adjusted for available confounders, included year of transplant, in an attempt to isolate the association between HLA mismatching and DCGF.
Though graft survival has continued to improve overall, increased HLA mismatching continues to be associated with poorer outcomes, including a 43% higher risk of graft failure. This is concerning in light of the increasing frequency and degree of HLA mismatching among pediatric deceased-donor kidney transplant recipients in the last decade. Our findings highlight the tradeoff between transplant access equity and outcomes and underscore the need for continued assessment of the impact of HLA mismatching on outcomes in pediatric deceased-donor kidney transplant recipients in the United States.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding:
This work was supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The first author was supported by a Doris Duke Clinical Research Foundation grant.
Abbreviations:
- DCGF
death-censored graft survival
- DDKT
Deceased-donor kidney transplant
- HLA
Human leukocyte antigen
- HRSA
Health Resources and Services Administration
- OPTN
Organ Procurement and Transplantation Network
- pDDKT
Pediatric deceased-donor kidney transplant
- pKTR
Pediatric kidney transplant recipient
- PRA
Panel reactive antibody
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosures:
The authors declare no conflicts of interest
REFERENCES
- 1.Chaudhuri A, James G, Grimm P. Whether or not to accept a deceased donor kidney offer for a pediatric patient. Pediatr Nephrol. 2015;30:1529–1536. [DOI] [PubMed] [Google Scholar]
- 2.Crafter SR, Bell L, Foster BJ. Balancing Organ Quality, HLA-Matching, and Waiting Times: Impact of a Pediatric Priority Allocation Policy for Deceased Donor Kidneys in Quebec. Transplantation. 2007;83:1411–1415. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Oak N, Siddique J, Harland RC, Abbo ED. Changes in Pediatric Renal Transplantation After Implementation of the Revised Deceased Donor Kidney Allocation Policy. Am J Transplant. 2009;9:1237–1242. [DOI] [PubMed] [Google Scholar]
- 4.Hata Y, Ozawa M, Takemoto SK, Cecka JM. HLA matching. Clin Transpl. 1996:381–396. [PubMed] [Google Scholar]
- 5.Amaral S, Patzer RE, Kutner N, McClellan W. Racial Disparities in Access to Pediatric Kidney Transplantation Since Share 35. J Am Soc Nephrol. 2012;23:1069–1077. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri A, Gallo A, Grimm P. Pediatric deceased donor renal transplantation: An approach to decision making I. Pediatric kidney allocation in the USA: The old and the new. Pediatr Transplant. 2015;19:776–784. [DOI] [PubMed] [Google Scholar]
- 7.Barocci S, Valente U, Gusmano R, et al. HLA matching in pediatric recipients of a first kidney graft: a single center analysis. Transplantation. 1996;61:151–154. [DOI] [PubMed] [Google Scholar]
- 8.Stippel DL, Cingoz T, Wahbal R, Muller RU, Bauerfeind U, Dieplinger G. 25 Years of Kidney Transplantation--A Period of Change. Clin Transpl. 2014:69–76. [PubMed] [Google Scholar]
- 9.Hirata M, Terasaki PI. Pediatric renal transplantation. Clin Transpl. 1994:395–402. [PubMed] [Google Scholar]
- 10.Emonds MP, Herman J, Dendievel J, Waer M, Van Damme-lombaerts R. Evaluation of anti-human leukocyte antigen allo-immunization in pediatric cadaveric kidney transplantation. Pediatr Transplant. 2000;4:6–11. [DOI] [PubMed] [Google Scholar]
- 11.Meier-Kriesche H-U, Scornik JC, Susskind B, Rehman S, Schold JD. A Lifetime Versus a Graft Life Approach Redefines the Importance of HLA Matching in Kidney Transplant Patients. Transplantation. 2009;88:23–29. [DOI] [PubMed] [Google Scholar]
- 12.Neu AM, Ho PL, Fine RN, Furth SL, Fivush BA. Tacrolimus vs. cyclosporine A as primary immunosuppression in pediatric renal transplantation: A NAPRTCS study. Pediatr Transplant. 2003;7:217–222. [DOI] [PubMed] [Google Scholar]
- 13.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215–245. [DOI] [PubMed] [Google Scholar]
- 14.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N Engl J Med. 2006;355:1967–1977. [DOI] [PubMed] [Google Scholar]
- 15.Abraham EC, Wilson AC, Goebel J. Current Kidney Allocation Rules and Their Impact on a Pediatric Transplant Center. Am J Transplant. 2009;9:404–408. [DOI] [PubMed] [Google Scholar]
- 16.Massie AB, Kuricka LM, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. Am J Transplant. 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opelz G, Dohler B. Effect of Human Leukocyte Antigen Compatibility on Kidney Graft Survival: Comparative Analysis of Two Decades. Transplantation. 2007;84:127–143. [DOI] [PubMed] [Google Scholar]
- 19.Opelz G, Dohler B, Middleton D, Susal C. HLA Matching in Pediatric Kidney Transplantation: HLA Poorly Matched Living Donor Transplants Versus HLA Well-Matched Deceased Donor Transplants. Transplantation. 2017;101:2789–2792. [DOI] [PubMed] [Google Scholar]
- 20.Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7:626–632. [DOI] [PubMed] [Google Scholar]
- 21.Gralla J, Tong S, Wiseman AC. The Impact of Human Leukocyte Antigen Mismatching on Sensitization Rates and Subsequent Retransplantation After First Graft Failure in Pediatric Renal Transplant Recipients. Transplantation. 2013;95:1218–1224. [DOI] [PubMed] [Google Scholar]
- 22.Foster BJ, Dahhou M, Zhang X, Platt RW, Smith JM, Hanley JA. Impact of HLA Mismatch at First Kidney Transplant on Lifetime With Graft Function in Young Recipients. Am J Transplant. 2014;14:876–885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.