Abstract
Advances in stem cell technology and regenerative medicine have underscored the need for effective banking of living cells. Cryopreservation, using very low temperatures to achieve suspended animation, is widely used to store or bank cells for later use. This process requires the use of cryoprotective agents (CPAs) to protect cells against damage caused by the cooling and warming process. However, current popular CPAs like DMSO can be toxic to cells and must be thoroughly removed from cells before they can be used for research or clinical applications. Trehalose, a nontoxic sugar found in organisms capable of withstanding extreme cold or desiccation, has been explored as an alternative CPA. The disaccharide must be present on both sides of the cellular membrane to provide cryo-protection. However, trehalose is not synthesized by mammalian cells nor has the capability to diffuse through their plasma membranes. Therefore, it is crucial to achieve intracellular delivery of trehalose for utilizing the full potential of the sugar for cell banking. In this review, various methods that have been explored to deliver trehalose into mammalian cells for their banking at both cryogenic and ambient temperatures are surveyed. Among them, the nanoparticle-mediated approach is particularly exciting. Collectively, studies in the literature demonstrate the great potential of using trehalose as the sole CPA for cell banking, to facilitate the widespread use of living cells in modern medicine.
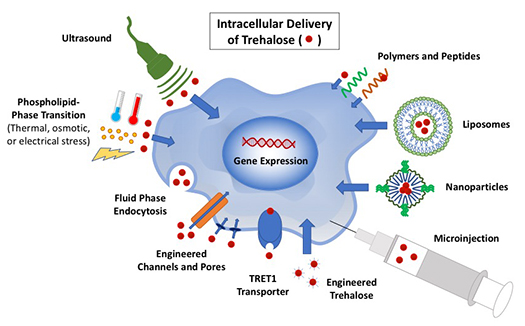
Introduction
As cell-based medicine continues to grow and advance, so does the need for effective and safe methods for long-term storage or banking of living cells1. Currently, the most widely used method for banking cells is cryopreservation, using very low temperatures to halt any metabolic and damaging activities. Typical cryopreservation requires the use of cell-membrane penetrating cryoprotective agents or cryoprotectants (CPAs) to keep the cells safe from injury during the cooling and warming processes. Common penetrating CPAs include dimethyl sulfoxide (DMSO) and glycerol. However, these agents can be toxic to mammalian cells. DMSO has shown toxicity to cells at 37 °C (body temperature), and requires rigorous and careful removal from the cells before they can be used therapeutically2. Glycerol, although it exhibits a lower toxicity than DMSO without any significant mutagenic effect, must be carefully removed using a specialized deglycerolization machine when used to cryopreserve red blood cells for transfusion3. These washing steps not only add additional time and labor but may also cause the loss of some of the precious cells: approximately 10% of the total number with each wash. Therefore, there is a need for nontoxic agents capable of achieving comparable viability of cells post-cryopreservation without the need for washing.
Trehalose, a disaccharide (342 Da) of glucose, has been found to enable organisms in nature to withstand extreme environmental conditions. High concentrations of the small molecule have been found in yeast, nematodes, tardigrades, and even insects (Polypedilum vanderplanki) that allow them to survive extreme cold and desiccation4–7. This sugar is hypothesized to facilitate survival through two capabilities during dehydration: (1) forming hydrogen bonds with and/or promoting hydration of biomacromolecules, acting as water to allow cellular components to retain functional conformation and (2) suspend metabolic activity by forming a glassy matrix with extremely low molecular mobility8.
Studies have shown promise using trehalose as an extracellular protective agent in the media of cells for cryopreservation but failed to match the performance of DMSO. Crowe noted that successful cryopreservation requires trehalose to be present on both sides of the membrane, however, trehalose is naturally impermeable to the mammalian cell membrane5,9. Here we review the different strategies that have been explored to load trehalose into mammalian cells for effective cryopreservation, desiccation, and lyopreservation, as summarized in Table 1.
Table 1.
Summary of methods for delivery of trehalose into mammalian cells and their corresponding advantages and disadvantages.
| Method | Advantages | Disadvantages |
|---|---|---|
| Phospholipid-Phase Transition via thermal, osmotic, or electric stress6–17 | Control of trehalose loading by manipulating concentration gradient Increasing membrane permeability |
Non-specific membrane permeability Cytotoxicity and membrane stability concerns due to thermal, osmotic, and electric shock to cells |
| Fluid-Phase Endocytosis22–24 | Taking advantage of the natural cell uptake process | Long incubation time Loading only small amounts of trehalose |
| Gene expression25,26 | Endogenous expression of trehalose | Adenoviral vector cytotoxicity Requiring genetic modification of cells |
| Engineered pores and channels27–32 | Reversible based on external stimuli Controllable influx of trehalose by manipulating concentration gradient |
Non-specific membrane permeability Use of exogenous, bacterial derived-protein Overstimulation of ATP receptors linked to apoptosis, necrosis, neoplasia |
| TRET1 Transporter33,34 | Selective transport of trehalose across membrane | Requiring genetic modification of cells |
| Microinjection35–38 | Direct insertion of controlled amount of trehalose into large cells such as oocytes and early embryos | Not practical for small somatic cells or large number of cells |
| Polymers and peptides39–44 | Loading high amounts of intracellular trehalose Increasing membrane permeability |
Non-specific membrane permeability Long incubation time Cytotoxicity concerns |
| Liposomes45–47 | Biocompatible with cell membrane | Loading only small amounts of trehalose Poor stability |
| Nanoparticles48,49 | Specific transport of high amounts of trehalose Utilizing endocytosis that is the natural process of cell eating No modification to cells |
Long (up to 1 day) incubation time |
| Ultrasound50 | Increased membrane permeability |
Affects cell morphology Non-specific membrane permeability |
| Engineered trehalose51 | Trehalose-derivative becomes permeable to cells Natural machinery can digest derivative into trehalose |
Possible build-up of trehalose-derivative due to limiting esterases |
Phospholipid Phase Transition
One of the first approaches developed to introduce trehalose into mammalian cells was to increase the permeability of the cell membrane through induction of a phospholipid phase transition10. Thermal, osmotic, and electrical stress have been studied as induction methods to elevate membrane permeability and allow the transport of trehalose into the cells.
Thermal Stress Induced
Concentrated efforts to introduce trehalose into the cytoplasm of mammalian cells began with the efforts of Beattie et al. to cryopreserve pancreatic islets10. The group took advantage of the lipid phase transition of the islet cell membrane, cooling the cells before freezing in a solution with high-concentration of trehalose. As the islet cells pass through the thermotropic lipid phase transition, their membranes experience elevated permeability to allow an influx of trehalose diffusing down its concentration gradient from the surrounding environment. Trehalose entered the cells increasingly as an inverse function of temperature. After cryopreservation, islets show good post-thaw viability and function just as well as freshly transplanted islets post-implantation in a rat model.
Thermal shock has also been investigated as a means for trehalose entry by several groups. Human foreskin fibroblasts, both adherent and in suspension, were incubated on ice for 5 minutes along with 50 mM trehalose solution11. Internalization or binding of trehalose to the cell membrane was shown, but the method was associated with high levels of cytotoxicity. Hyperthermic temperatures have been explored to load suspended primary rat hepatocytes with trehalose, with cell suspension incubated on ice for 10–15 minutes, supplied with poration solution containing medium and trehalose, and then placed alternately in 0 °C (ice) and 39 °C (water) every 10 minutes for up to 2 hours14. Hepatocytes showed a significant amount of trehalose in the cytoplasm (~0.13 M), high viability (83%), and normal morphology and function.
Studies have also shown that freezing can promote cellular uptake of trehalose and have a beneficial impact on cryopreservation15–18. In a study on cryopreservation of red blood cells (RBCs) with trehalose, post-thaw survival was found to increase with increasing intracellular trehalose. The sugar likely passes through the membrane during a membrane phase transition during cooling18. Wolkers et al. suggested a freezing-induced membrane phase transition in the study of cryopreservation of human platelets using solely trehalose as the CPA served to allow the entry of trehalose into the cells15. The Wolkers group further studied this behavior in fibroblasts, showing that trehalose was taken up by the cells during freezing and thawing with a loading efficiency of close to 50% and this loading conferred cryosurvival during a narrow range of cooling rates16. The research group then studied the uptake of trehalose by fibroblasts and how it affected their survival after freeze-drying17. Although no viable cells were recovered after freeze-drying trehalose-laden fibroblasts, they did observe that the sugar molecule reduces damage caused to DNA.
Osmotic Stress Induced
Satpathy et al. used the phospholipid phase transition of RBC membranes during both cooling and osmotic imbalance to load up to 40 mM of trehalose into RBC cytoplasms19. They first attempted to load RBCs with trehalose via incubation in isotonic solution at 37 °C but found that intracellular trehalose concentration greatly increased after incubation in extracellular solution with a trehalose concentration well above the isotonic condition. The loading conditions were found to be both time and temperature dependent, and an incubation time of 7 hours was sufficient to reach 40 mM intracellular trehalose. This exposure to hypertonic solution resulted in a morphology change of RBCs compared to freshly isolated RBCs; trehalose-loaded RBCs were not of equal size and abnormally shaped.
Zhou et al. sought to improve loading of trehalose into RBCs via manipulation of the difference in osmotic pressure and concentration between the inside and outside of the cells20. To increase osmotic pressure inside, RBCs were dehydrated in hypertonic glucose. Then after, to allow trehalose to enter the cells, RBCs were incubated in hypotonic trehalose solutions. Trehalose uptake by the cells increased with increasing osmotic pressure difference, with intracellular trehalose concentration reaching 43.2 mM when the osmotic pressure difference was 1369.8 mOsm. Although this method allowed for successful trehalose uptake, a hematology study showed that dehydrated RBCs had a 13.7% MCH, or mean cell hemoglobin, compared to fresh RBCs. This means that some hemoglobin leaked out of the RBCs during the loading process. These modifications, as well as the small amounts of trehalose introduced from high levels of osmotic stress, may make this strategy ill-suited for RBC cryopreservation.
Osmotic manipulation has been studied to load trehalose into cell types other than RBCs; Reuss et al. explored the use of osmotically caused volume changes in human T-lymphocytes to load sugars, including trehalose, into the cytoplasm21. They took advantage of the cell’s ability to regulate its volume, through a process known as regulatory volume decrease (RVD), where initial cell swelling induces activation of membrane channels that allow the influx/efflux of water and ions. These volume sensitive channels can also be leveraged to introduce small molecules into the cell cytoplasm, leading the group to explore how exposure to hypotonic solutions could allow for sugar uptake by the cells. They found when cells were exposed to 200 mOsm media, the membrane permeability to carbohydrates remained poor. However, when the osmolality was decreased to 100 mOsm, the membrane permeability dramatically increased, but only to monomeric carbohydrates. Trehalose influx, therefore, was very poor, and the cells did not reach high intracellular trehalose concentrations. This method could be used to introduce other sugars, like inositol and sorbitol, into the cytoplasm.
Electrical Stress Induced
Electropermeabilization, also known as electroporation or electroinjection, has been used to elevate membrane permeability and introduce foreign molecules into cell cytoplasm that are otherwise impermeable52. Through the application of a short, external electrical field pulse, the cell membrane can be transiently and reversibly increased53,54. The exact structures and molecular mechanisms responsible for this observed elevated permeability are still being studied. Cell viability could be enhanced by manipulating pulse parameters, like field strength, duration, and number of pulses.
Shirakashi et al. loaded mouse myeloma cells with trehalose via electropermeabilization with applications for cryo- and lyopreservation12. With mild field-pulse conditions, membrane permeability was indeed elevated, and cells could be safely loaded with up to 100 mM trehalose when incubated in a trehalose-laden medium. Studies showed that higher amounts of trehalose could be loaded into the cells via higher field strength and pulses. However, this came at the expense of cell viability, which showed a sharp decrease when the field strength increased to 3.5 kV/cm. This method has the advantage of loading speed—only 10–15 minutes of post-pulse incubation was necessary to load trehalose into the cells. However, as with other methods that target membrane permeability, the resulting trans-membrane molecule transport is not restricted to trehalose. Cell membranes may become osmotically fragile afterwards, which would affect the cellular responses during freezing and thawing.
Later, Dovgan et al. explored the use of electroporation to load trehalose into human-adipose derived stem cells (hADSCs) and how it affected cryosurvival13. Cells were electroporated in buffer containing either 250 or 400 mM trehalose. They evaluated molecule uptake by studying the uptake of propidium iodide (PI), a fluorescence molecule with a molecular weight similar to trehalose. Similar to results of Shirakashi et al., they found that PI uptake increased with increasing electric field. The uptake of PI reached a plateau at 2 kV/cm and increasing the electric field further only decreased cell recovery post-electroporation. Cells cryopreserved after electroporation at 1.5 kV/cm and incubation in 250 or 400 mM trehalose showed 83 or 78% post-thaw recovery, respectively. The hADSCs cryopreserved via the conventional DMSO method (10% DMSO in 90% fetal bovine serum), showed a post-thaw recovery of 91%. Although this value was not statistically significantly different from that obtained from the trehalose-laden cells, this method comes with cytotoxicity concerns from the electric field that rival those found with DMSO. This study did not measure intracellular concentration of trehalose, so the amount of trehalose able to be loaded into the cells via this method and its cryoprotective effect on hADSCs is unknown.
Fluid-Phase Endocytosis
One way that cells can probe and monitor the environment around them is through a non-specific “cellular drinking” method known as fluid-phase endocytosis. This mechanism is not prompted by specific binding of membrane receptors and involves the cell intaking some of its environment and internalizing it with a vesicle pinched off from the plasma membrane. This cellular process has been taken advantage of to load trehalose into mammalian cells, by simply incubating cells in the presence of a high extracellular concentration of trehalose.
Wolkers et al. studied the loading of trehalose into human platelets via fluid-phase endocytosis and subsequent effect on survival after lyophilization22. When incubated in a high extracellular trehalose concentration at 37 °C, human platelets were able to internalize trehalose, measured using the anthrone reaction. Intracellular trehalose concentration increased with the length of incubation, with a loading efficiency reaching almost 60% after 4 hours of incubation. Maximum intracellular concentration reached 15 mM, which allowed high recovery rates of platelets post-lyophilization, reaching up to 88%. However, a good portion of these recovered cells, at the highest rate, were partially lysed or balloon-shaped. Researchers found that lyophilization parameters (prehydration of lyophilizate over water vapor) and initial platelet concentration had large effects on platelet recovery. Platelets loaded with trehalose, lyophilized, and rehydrated showed a thrombin response almost identical to fresh platelets. This study showed that low amounts of intracellular trehalose may be able to confer protection during lyophilization. Oliver et al. investigated loading human mesenchymal stem cells (MSCs) with trehalose via fluid-phase endocytosis and saw that the cells were able to uptake the sugar molecule, dependent on temperature, incubation time, and extracellular trehalose concentration, loading approximately 20–30 mM trehalose into the cytoplasm23. They did not further assess cryo- or lyopreservation of the trehalose-laden cells.
Zhang et al. followed up on Oliver et al.’s work, loading MSCs with trehalose via fluid-phase endocytosis and adding PVP40 as an additional protectant for lyophilization24. The intracellular trehalose concentration reached roughly 14 mM after 24 hours of incubation in 100 mM extracellular trehalose solution. The cells were then lyophilized, and recovery rates were examined 12 hours after rehydration and plating. The highest recovery rate achieved was almost 70%. However, the MSCs recovered from lyophilization had significantly impaired abilities to adhere and proliferate and all died within one week. These preliminary studies suggest that lower concentrations of intracellular trehalose may be sufficient for cellular recovery after lyophilization but did not confer sufficient lyoprotection in these cases for healthy, proliferating cells post-rehydration. This loading method requires long incubation times that result in very low intracellular trehalose concentrations, seeming more time-consuming to offer a small payoff compared to other methods. This approach would most likely not be suitable for cryopreserving mammalian cells, as a much higher intracellular concentration of trehalose is needed to provide protection during cryopreservstion without removing water from a sample..
It is also important to note that this method of delivering trehalose into the cells involves the confinement of trehalose in vesicles through the endocytotic pathway. This means that not all trehalose inside the cell may be available to exercise its protective capabilities during cryopreservation and lyopreservation. The determination of intracellular trehalose using bulk quantification techniques may not be able to recognize this confinement, inflating the amount of available trehalose in cells for cryo/lyoprotection.
Gene Expression
Some groups have taken a genetic engineering approach to achieving high concentrations of intracellular trehalose by enabling mammalian cells to endogenously synthesize trehalose. This was achieved by transfecting them with genes found in anhydrobiotic organisms. In Escherichia coli, trehalose synthesis is controlled by the otsA/B locus. This genetic sequence encodes otsA (trehalose 6-phosphate synthase), which catalyzes the synthesis of trehalose-6-phosphate, and otsB (trehalose 6-phosphate phosphatase), which catalyzes the formation of trehalose55. The referenced trehalose synthesis pathway can be seen in Figure 1. To achieve trehalose expression in mammalian cells, Guo et al. transfected human primary foreskin fibroblasts with an adenoviral vector expressing otsA and otsB25. Results from their study showed trehalose expression increased with increasing adenoviral vector multiplicity of infection (MOI), reaching 1–1.5 nmol trehalose per 106 cells. However, as the MOI increased, cell viability decreased due to toxicity from the adenoviral infection. After transfection with different MOI’s, the group explored the effect of trehalose expression on desiccation tolerance and found cells with a MOI of 200 could maintain 60% viability post rehydration after 24 hours. Viability dropped as the length of time increased, and no cells were viable past 5 days of desiccation.
Figure 1.
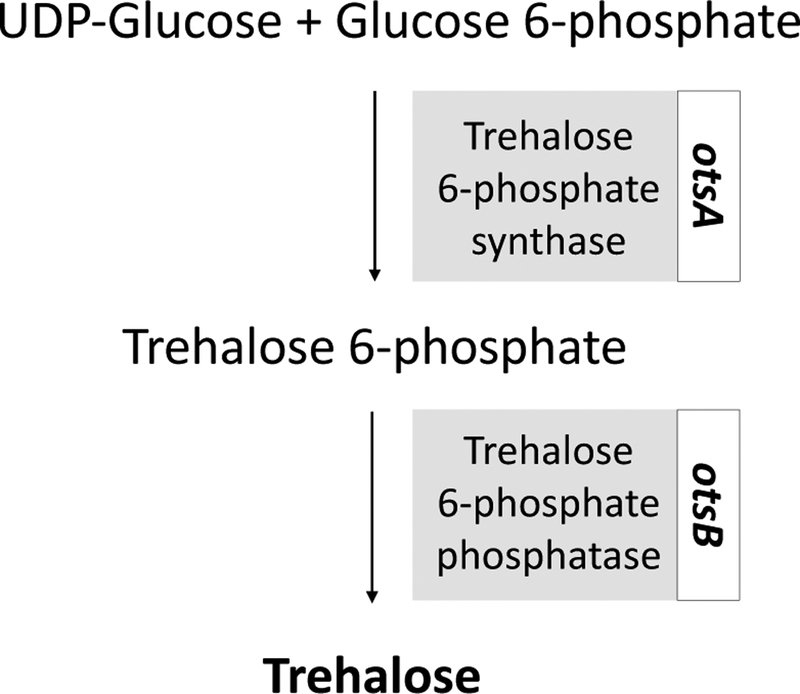
Metabolic pathway for synthesis of trehalose. UDPG, Uridine diphosphate. Guo et al.25 refer to trehalose 6-phosphate synthase and phosphatase as otsA and otsB, respectively.
Garcia de Castro et al. transfected a mouse fibroblastoid cell line (LMTK-) with heat-shock inducible otsA and otsB genes amplified from E. coli26. Intracellular trehalose increased steadily in LMTK-s after heat shock, reaching a maximum of approximately 80 mM. Trehalose concentration rapidly declined after 30 hours post heat shock, attributed to the limited period that the synthesis genes are active after heat shock. Transfected cells showed improved osmotolerance when subjected to hypotonic conditions, but could not survive desiccation despite containing a much higher amount of intracellular trehalose than the fibroblasts studied by Guo et al.
Although both groups successfully engineered mammalian cells to express trehalose endogenously, this approach to achieving an intracellular trehalose concentration does not translate well to cryo- and lyopreservation of cells. Editing the genome of cells intended for clinical or therapeutic applications may cause issues with cell function and raise safety concerns with stem cell regulatory agencies, further hindering or halting use of the preserved cells to treat diseases. The concentration of intracellular trehalose achieved in these studies also would most likely not be high enough to provide for sufficient cell protection from freezing damage during cryopreservation.
Engineered Pores and Channels
Trehalose has also been introduced into mammalian cytoplasm by creating pores and channels in the cell membrane. Toner et al. took advantage of a cell membrane porating agent derived from Staphyloccus aureus, a genetically engineered endotoxin called α-hemolysin, to generate pores in the lipid bilayers of both fibroblasts and keratinocytes to allow for trehalose influx27,28. These membrane pores, known as H5, allowed reversible elevated membrane permeability—they were able to be switched on or off by the removal or addition of small concentrations of Zn2+, preventing cell lysis from too much solute influx. This method generated intracellular trehalose concentrations of up to 0.5 M within one hour of exposure to extracellular trehalose solution of the same molarity29. Loading low concentration (0.2 M) of trehalose into fibroblasts and keratinocytes via the H5 technology allowed for cryopreservation with trehalose as the sole CPA. Long-term post-thaw survivals were 80% for fibroblasts and 70% for keratinocytes28. Buchanan et al. expanded upon this work with H5 technology to use intra- and extracellular trehalose as the sole CPA for a human hematopoietic cell line (acting as a surrogate for stem cells). They found that colony-forming units generated from trehalose-frozen cells were not significantly different from those generated from DMSO-frozen cells30. Although H5 allowed for cell membrane permeabilization and cryopreservation, the exogenous, bacteria-derived pore protein would need to be removed before the cells could be used for any cell-based therapy or clinical application, as it could generate an undesired immune response. This may limit the clinical translation of the technology.
Several groups have investigated the activation of a transmembrane cell receptor, P2X7 (synonymous with P2Z), in order to form non-selective pores in the plasma membrane that allow the influx of small molecules, namely trehalose31,32. P2X7 is a purinergic receptor channel, opening in the presence of extracellular ATP and closing upon addition of magnesium31. P2X7 is best known for its expression on antigen-presenting immune cells, but has ubiquitous distribution in almost all tissues and organs of the body, allowing for poration of a great number of cell types56. Buchanan et al. activated the P2Z receptor to achieve 200 mM trehalose in hematopoietic progenitor cells and attempted cryopreservation31. Trehalose-preserved cells generated 90% of colonies produced by the untreated control cells, compared to DMSO-preserved cells that generated only 70% of colonies produced by the untreated control. Elliot et al. explored the use of the P2X7 channel to safely achieve up to 50 mM intracellular trehalose concentration in mouse macrophages32. Cells were subsequently desiccated over a range of moisture contents and showed better next-day survival than control cells. While this strategy is advantageous in that it leverages native cell machinery to load trehalose, increasing poration times showed large decreases in cell survival, and maximizing intracellular trehalose concentration meant sacrificing cell viability. Both nonselective membrane openings, H5 and P2Z/P2X7, could allow not only the influx of trehalose but simultaneously the influx and efflux of undesired molecules. Concerns have also been raised due to evidence that stimulation of the P2X7 receptor could lead to apoptosis, necrosis, or neoplasia57,58.
TRET1 Transporter
Organisms that use trehalose to survive extreme conditions possess the necessary machinery to transport the small molecule across their cell membranes. Polypedilum vanderplanki larvae are anhydrobiotic rock pool dwellers that survive desiccation and rehydration. During environmental stresses, approximately 20% of their dry mass is accumulated as trehalose7,59,60. Based on these observations, researchers isolated a gene from these organisms that encodes a facilitated trehalose transporter, known as TRET161. When expressed in mouse oocytes, TRET1 allowed the cells the specific, bidirectional, and high-capacity transport of trehalose across the plasma membrane. Chakraborty et al. used TRET1 to load trehalose into Chinese hamster ovary (CHO) cells and saw great improvement in desiccation tolerance compared to control CHO cells33. Uchida et al. further studied CHO-TRET1 cells in the context of cryopreservation and found that their post-thaw viabilities were much improved compared to CHO cells transfected with an empty vector34. This use of TRET1 enables achievement of high intracellular trehalose concentration, specific transport of solely trehalose without influx or efflux of other small molecules, control over intracellular concentration by changing the gradient created by extracellular trehalose, and ease of transgenesis into cells due to its single gene product nature. However, genetically modifying cells for improved cryo- and lyopreservation might not translate well into clinical applications for cell-based therapies.
Microinjection
The most direct approach studied for loading of trehalose into mammalian cells is to microinject the small molecule directly into the cytoplasm of each individual cell. Eroglu et al. successfully delivered trehalose into discarded human oocytes and froze them in the presence of extracellular trehalose35. Subsequent study of the trehalose-laden oocytes revealed good survival after cryopreservation. Because of the large size of oocytes (~100 μm in diameter) and typical small sample quantity (only tens to hundreds), these cells are great candidates for the microinjection approach and the delivery was successful36–38. However, this approach has limitations for applications to other types of eukaryotic mammalian cells, which are typically much smaller (less than 20 μm in diameter) and needed in much greater quantity (over millions).
Polymers and peptides
Synthetic polymers have been investigated as facilitators of trehalose into cytoplasm by interacting with the cell membrane and temporarily increasing permeability39–44. Amphipathic polymers with weakly ionizable carboxyl acid side groups and hydrophobic side chains have garnered interest because of their high affinity for lipid membranes and ability to elevate membrane permeability by mimicking viral peptides as well as promote endosomal escape62,63. The most popular biopolymer investigated for trehalose-loading has been PP50, composed of biodegradable poly(L-lysine iso-phthalamide) (PLP) grafted with L-phenylalanine64. PP50 has been greatly explored as a method for loading trehalose into RBCs for desiccation tolerance and cryosurvival39,41,65. This method allowed for a cytoplasmic trehalose concentration of 123 mM in erythrocytes that, along with the presence of extracellular trehalose, resulted in a 20% increase in post-thaw viability compared to erythrocytes without cytoplasmic trehalose39. Elevated membrane permeability was able to be reversed by washing in PBS. Longer incubation times increased intracellular trehalose concentration, however, they also increased the incidence of hemolysis. Although this method increased erythrocyte post-thaw survival to roughly 80%, membrane permeabilization was not specific to solely trehalose and could allow for both the influx and efflux of other small molecules. The hemolysis observed in reaction to PP50 was undesirable, and the biopolymer would need to be removed from the cells before any clinical application, necessitating a tedious washing procedure.
Sharp et al. and Mercado et al. extended the use of PP50 for trehalose loading from red blood to a nucleated cell line derived from human osteosarcoma (SAOS-2)40,42. PP50 was able to safely and effectively load trehalose into the nucleated human cell line. Cells cryopreserved with a combination of PP50 and extracellular trehalose showed improved post-thaw survival compared to cells preincubated in trehalose without the polymer42. However, cells preserved in the standard cryopreservation protocol with DMSO showed significantly higher post-thaw viability than PP50-cryopreserved cells (80% versus 60%)40.
Wei et al. explored the use of a cell penetrating peptide (CPP) to deliver trehalose into mouse embryonic fibroblasts44. They designed a new CPP based on molecular dynamics simulations, with conformations shown in Figure 2, and the resulting sequence contained many amino acids that allowed the coupling of trehalose through non-covalent bonding. This cargo-coupling allowed specific transport of trehalose into the cell, different from the non-specific membrane permeability induced by PP50. This peptide enabled the delivery of trehalose into the cytoplasm, up to a concentration of 20 mM after 2 hours of incubation. The novel CPP did not exhibit significant toxicity to mammalian cells even at high concentrations. The intracellular trehalose concentration achieved here would probably not be sufficient for effective cryopreservation of mammalian cells. Cell types more stress-sensitive than fibroblasts may also not react well to the possibly cytotoxic CPP.
Figure 2.
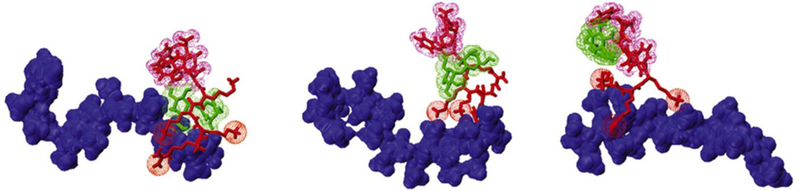
The molecular simulations for the binding conformations of trehalose-heparin-CPP to deliver trehalose into mammalian cells. Trehalose is indicated in green, heparin in blue, and the peptides in red. Adapted from Ref 44. Copyright 2014 Elsevier.
Liposomes
Used in disciplines ranging from medicine, biology, and pharmaceutical science, liposomes are spherical vesicles composed of a lipid bilayer that encloses an aqueous compartment. The liposomes have applications as a delivery vehicle for drugs and other bioactive substances into cells45. Because of their structure and biocompatibility, liposomes have been investigated as a carrier for trehalose into the cytoplasm of mammalian cells18,45,46. Fluorescence and spectrophotometry studies of labelled trehalose-containing liposomes conducted by Holovati et al. suggested that liposomes could permeabilize human RBC membranes and safely deliver trehalose into the cytosol45. However, this method could only deliver micromolar concentrations of trehalose inside the cells. Holovati et al. further extended the study of these liposomes to examine their effect on RBC response to freezing and post-thaw membrane quality. Interestingly, they found improved post-thaw recovery of RBCs due to the application of liposomes, not their delivery of trehalose into the cytosol46. Liposomes loaded with trehalose and liposomes loaded with saline showed similar post-thaw recovery for the RBCs. It is important to note, however, that these trehalose-laden liposomes were only able to deliver approximately 15 mM trehalose into the cytosol of the RBCs, which may not have been enough to provide sufficient protection from cryoinjury.
Trehalose-laden liposomes have also been used to cryopreserve stem cells obtained from human umbilical cord blood47. Motta et al. tested different conditions of intracellular and extracellular trehalose along with DMSO47. They found that intra- and extracellular trehalose, with addition of a small amount of DMSO, improved cryopreservation of HSCs in terms of post-thaw viability and stemness similar to DMSO alone. Intra- and extracellular trehalose delivered by the liposomes alone did not improve cryopreservation compared to the control. Although these results are promising, liposomes may not be able to load high amounts of trehalose into other types of mammalian cells, and resulting membrane permeabilization may be nonspecific, allowing entry or escape of other small molecules into or out of the cells. In addition, liposomes are not stable in aqueous solutions and they may coalesce to form large vesicles.
Nanoparticles
Research into the field of nanotechnology has garnered much interest, with applications of nanoplatforms ranging from food and water safety to regenerative medicine66,67. Nanoparticles have been investigated as drug vehicles for delivery of chemotherapy, enabling protected delivery of small molecules across the cancer cell membranes68. Researchers have used this approach to deliver trehalose across cell membranes to achieve high intracellular trehalose concentrations48,49. Zhang et al. developed a thermally responsive polymeric hydrogel nanocapsule for the encapsulation and delivery of trehalose into fibroblasts48. Nanocapsules were synthesized from Pluronic F127 (PF127), a triblock copolymer that can self-assemble in aqueous solution in a temperature-dependent manner, and polyethyleneimine (PEI), an endosomolytic cationic polymer found useful in cytosolic gene delivery,69–71. Trehalose was successfully encapsulated in the nanocapsules; the capsules remained stable and held the trehalose inside during cellular uptake at 37 °C and then remained in the cytoplasm. Quick release of trehalose was enabled by thermally cycling the nanoparticle between 37 °C and 22 °C and fibroblasts showed an intracellular trehalose concentration of up to 0.3 M after a 40-minute incubation. Neither the nanoparticles nor thermal cycling (cold shock at 22 °C) showed significant toxicity to the cells. Although this method achieved a high intracellular trehalose concentration in a short incubation time, the nanocapsule synthesis process was very complex and required many steps, following a tedious soaking-freezing-drying-heating procedure to encapsulate the trehalose. The fact that room temperature can induce release of trehalose from the nanocapsules makes it very difficult to handle the trehalose-laden nanocapsules for further use.
Rao et al. built upon the work with thermally responsive nanoparticles to deliver trehalose into hADSCs and cryopreserve them49. In a previous study, Zhang et al. improved upon the PF127 nanoconstruct by using chitosan, a naturally derived, biocompatible polysaccharide commonly found in seafood72, as a crosslinker73. Rao et al. further improved upon this work by further cross-linking the nanoparticles with genipin to prevent release of trehalose from the nanocapsules at room or lower temperature. Instead, a pH responsive release of trehalose from the nanocapsules at 37 °C could be achieved49. The schematic for encapsulation of trehalose in the nanoparticle is shown in Figure 3.
Figure 3.
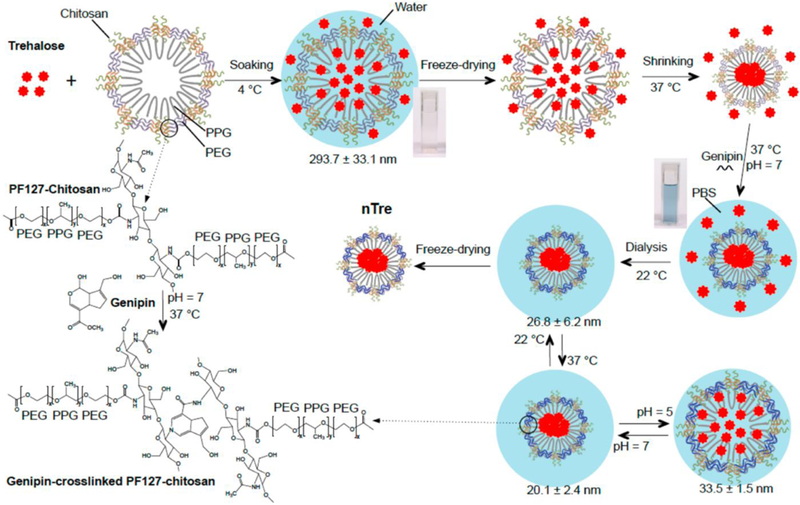
A schematic illustration of the procedure for encapsulating hydrophilic (i.e., water-soluble) trehalose in genipin-crosslinked Pluronic F127-chitosan nanoparticles (GNPs) to obtain the nanoparticle-encapsulated trehalose (nTre). After genipin crosslinking, the solution turns from light brown for the aqueous solution of Pluronic F127-chitosan nanoparticles (NPs) into blue for the aqueous solution of GNPs. The GNPs are pH responsive in size at 37 °C although they are not as thermally responsive in size at pH 7 as the Pluronic F127-chitosan NPs. The size (diameter) shown at various temperatures and pH values is for nTre made with a feeding ratio of 1:10 (trehalose:Pluronic F127-chitosan NPs) in weight. PPG: poly(propylene glycol); PEG: poly(ethylene glycol); and PBS: phosphate buffered saline (1x by default). Reprinted with permission from Rao et al49. Copyright 2015 American Chemical Society.
Cumulative release of trehalose from the pH-responsive nanoparticles was increased at a pH of 5 compared to a pH of 7. Late endosomes are known to have an acidic pH, so the faster release of trehalose from the nanoparticles at pH 5 facilitates trehalose release in mammalian cells after cellular uptake. hADSCs were shown to uptake the nanoparticles and their viability was not affected by the nanoparticles themselves. Subsequent cryopreservation of trehalose-laden hADSCs showed comparable post-thaw viability to those cryopreserved with the conventional DMSO treatment. hADSCs also retained their stemness, shown by no significant difference in expression of stem cell genes and markers and differentiation into osteo-, adipo-, and chondrogenic lineages. Nanoparticle-mediated delivery of trehalose shows great promise for cryopreservation of cell-based therapies, as no possibly harmful modification must be made to the cells or their membranes. Delivery of trehalose is specific—this method does not involve influx or efflux of other small molecules. Possible limitations could be the incubation time needed for sufficient intracellular trehalose concentration—in this study, hADSCs were incubated with the trehalose-laden nanoparticles for 24 hours before cryopreservation. However, nanoparticles designed with cell-targeting modalities and quick payload releases could shorten the incubation time needed and make this method even more attractive. Although this technique involves internalization of trehalose into subcellular structures (similar to the confinement faced in fluid-phase endocytosis), nanoparticles can be designed to actively release the trehalose payload and facilitate endosomal escape.
Other Methods
Zhang et al. explored an experimental method of using ultrasound to load trehalose into human platelets50. They used different frequencies and exposure times to optimize a procedure to load ~30 mM of trehalose into the platelets by applying 0.8 W/cm2, 25 kHz ultrasound for 30 minutes. Although platelet number and average volume were not greatly affected by the ultrasound procedure, radiated platelets showed a significantly different morphology from the controls. This method is undesirable for cell preservation applications, as the membrane permeability is non-selective for trehalose, and ultrasound may also compromise activity or change morphology of different cell types as well.
Abazari et al. took a different approach to trehalose loading—instead of focusing on manipulating the cell or its membrane, they engineered trehalose itself to become permeable to mammalian cells51. Conjugating the small molecule with six acetyl groups facilitated trehalose uptake into primary rat hepatocytes more efficiently than unmodified trehalose. Once inside the cell, the trehalose derivative could be deacetylated by non-specific esterases located inside the cell to yield trehalose. Although this method allowed for extremely efficient trehalose delivery, it raises some concerns about undesired cellular effects. Because the deacetylation of the modified trehalose occurs by a limited number of cell esterases, an accumulation of the trehalose derivative could occur that may produce harmful effects or not confer cryoprotection. Introduction of this modified molecule into cells intended for clinical use may also incur some risk.
Outlook and Conclusions
If cells are to be banked for research and clinical applications, it is desired that protective agents and the preservation process not greatly affect their structure and functionality. Additionally, ideally preserved cells would be ready for use immediately upon warming back to superzero temperature, without the need to remove any agents from the cells or perform other processing steps. Protective agents and the methods for applying them to the cells also should not cause significant cytotoxicity or damage to the cells. If trehalose is to be used as an effective cryo- or lyoprotectant for preserving cells and tissues, its loading method should not significantly alter the cell structure or function, or cause cell damage or death. Methods that allow for selective entry of trehalose are also preferred, as the influx or efflux of other undesired small molecules could have a negative impact on the cells. Approaches that allow for quick trehalose uptake are preferred, as cryopreservation of cells is usually desired within one day of procurement. Nanoparticles or other encapsulation methods appear to be promising for intracellular delivery of trehalose, as they allow for specific delivery of trehalose without modification of significant cell structures. Future directions for improving this approach include modifying the nanoparticle design to allow for faster and more efficient cellular uptake. Nanoparticles designed with a fast payload release and endo/lysosomal escape could improve intracellular trehalose concentration/homogeneity and cut down on incubation time. Cold-responsive nanoparticles capable of releasing trehalose upon exposure to cold temperatures (i.e., lower than room temperature) naturally experienced by the cells during the cooling process would be well suited for cryopreservation applications. Overall, trehalose shows much promise as a nontoxic cryoprotectant that does not need to be removed from cells prior to applications in research or clinical settings. Much advance has been made in developing approaches for delivering the sugar across the plasma membrane into cells. The nanoparticle-mediated approach is particularly attractive. With further development, it might revolutionize the field by offering an organic solvent (e.g., DMSO)-free strategy for cell banking to facilitate the widespread use of living cells in modern medicine.
Acknowledgment
This work is partially supported by grants from NSF (CBET-1831019) and NIH (R01EB023632 and R43HL142371).
References
- 1.Pancrazio JJ, Wang F & Kelley CA Enabling tools for tissue engineering. Biosens. Bioelectron 22, 2803–2811 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Cox MA, Kastrup J & Hrubiško M Historical perspectives and the future of adverse reactions associated with haemopoietic stem cells cryopreserved with dimethyl sulfoxide. Cell Tissue Bank 13, 203–215 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Cregan P, Donegan E & Gotelli G Hemolytic transfusion reaction following transfusion of frozen and washed autologous red cells. Transfusion 31, 172–175 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Crowe JH & Crowe LM Preservation of mammalian cells—learning nature’s tricks. Nat. Biotechnol 18, 145–146 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Crowe JH, Hoekstra FA & Crowe LM Anhydrobiosis. Annu. Rev. Physiol 54, 579–599 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Potts M Desiccation tolerance of prokaryotes. Microbiol. Rev 58, 755–805 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe M, Kikawada T, Minagawa N, Yukuhiro F & Okuda T Mechanism allowing an insect to survive complete dehydration and extreme temperatures. J. Exp. Biol 205, 2799–802 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Crowe JH, Carpenter JF & Crowe LM THE ROLE OF VITRIFICATION IN ANHYDROBIOSIS. Annu. Rev. Physiol 60, 73–103 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Crowe JH et al. Stabilization of Dry Mammalian Cells: Lessons from Nature. Integr. Comp. Biol 45, 810–820 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Beattie GM et al. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes 46, 519–23 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Puhlev I, Guo N, Brown DR & Levine F Desiccation Tolerance in Human Cells. Cryobiology 42, 207–217 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Shirakashi R et al. Intracellular Delivery of Trehalose into Mammalian Cells by Electropermeabilization. (2002). doi: 10.1007/s00232-002-1003-y [DOI] [PubMed]
- 13.Dovgan B, Barlič A, Knežević M & Miklavčič D Cryopreservation of Human Adipose-Derived Stem Cells in Combination with Trehalose and Reversible Electroporation. J. Membr. Biol 250, 1–9 (2017). [DOI] [PubMed] [Google Scholar]
- 14.He X, Amin AA, Fowler A & Toner M Thermally Induced Introduction of Trehalose into Primary Rat Hepatocytes. CELL Preserv. Technol 4, (2006). [Google Scholar]
- 15.Gläfke C, Akhoondi M, Oldenhof H, Sieme H & Wolkers WF Cryopreservation of platelets using trehalose: The role of membrane phase behavior during freezing. Biotechnol. Prog 28, 1347–1354 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Oldenhof H, Sieme H & Wolkers WF Freezing-induced uptake of trehalose into mammalian cells facilitates cryopreservation. Biochim. Biophys. Acta - Biomembr 1858, 1400–1409 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Zhang M et al. Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity. Sci. Rep 7, 6198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoll C, Holovati JL, Acker JP & Wolkers WF Synergistic effects of liposomes, trehalose, and hydroxyethyl starch for cryopreservation of human erythrocytes. Biotechnol. Prog 28, 364–371 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Satpathy GR et al. Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology 49, 123–136 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, He H, Liu B & Hua T Loading Trehalose into Red Blood Cells by Improved Hypotonic Method. Cell Preserv. Technol 6, 119–124 (2008). [Google Scholar]
- 21.Reuss R et al. Intracellular Delivery of Carbohydrates into Mammalian Cells through Swelling-activated Pathways. J. Membr. Biol 200, 67–81 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Wolkers WF, Walker NJ, Tablin F & Crowe JH Human Platelets Loaded with Trehalose Survive Freeze-Drying. Cryobiology 42, 79–87 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Oliver AE, Jamil K, Crowe JH & Tablin F Loading Human Mesenchymal Stem Cells with Trehalose by Fluid-Phase Endocytosis. CELL Preserv. Technol 2, (2004). [Google Scholar]
- 24.Zhang S et al. Preliminary study on the freeze-drying of human bone marrow-derived mesenchymal stem cells. J. Zhejiang Univ. Sci. B 11, 889–94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo N, Puhlev I, Brown DR, Mansbridge J & Levine F Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol 18, 168–171 (2000). [DOI] [PubMed] [Google Scholar]
- 26.García de Castro A & Tunnacliffe A Intracellular trehalose improves osmotolerance but not desiccation tolerance in mammalian cells. FEBS Lett 487, 199–202 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Russo MJ, Bayley H & Toner M Reversible permeabilization of plasma membranes with an engineered switchable pore. Nat. Biotechnol 15, 278–282 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Eroglu A et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol 18, 163–167 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Acker JP et al. Measurement of trehalose loading of mammalian cells porated with a metal-actuated switchable pore. Biotechnol. Bioeng 82, 525–532 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Buchanan SS et al. Cryopreservation of Stem Cells Using Trehalose: Evaluation of the Method Using a Human Hematopoietic Cell Line. Stem Cells Dev 13, 295–305 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Buchanan SS, Menze MA, Hand SC, Pyatt DW & Carpenter JF Cryopreservation of Human Hematopoietic Stem and Progenitor Cells Loaded with Trehalose: Transient Permeabilization via the Adenosine Triphosphate-Dependent P2Z Receptor Channel. Cell Preserv. Technol 3, 212–222 (2005). [Google Scholar]
- 32.Elliott GD et al. Trehalose uptake through P2X7 purinergic channels provides dehydration protection. Cryobiology 52, 114–127 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty N et al. Trehalose transporter from African chironomid larvae improves desiccation tolerance of Chinese hamster ovary cells. Cryobiology 64, 91–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchida T, Furukawa M, Kikawada T, Yamazaki K & Gohara K Intracellular trehalose via transporter TRET1 as a method to cryoprotect CHO-K1 cells. Cryobiology 77, 50–57 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Eroglu A, Toner M & Toth TL Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil. Steril 77, 152–158 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Eroglu A, Bailey SE, Toner M & Toth TL Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol. Reprod 80, 70–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eroglu A, Lawitts JA, Toner M & Toth TL Quantitative microinjection of trehalose into mouse oocytes and zygotes, and its effect on development. Cryobiology 46, 121–134 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Bhowmick P, Eroglu A, Wright DL, Toner M & Toth TL Osmometric behavior of mouse oocytes in the presence of different intracellular sugars. Cryobiology 45, 183–7 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Lynch AL et al. Biopolymer mediated trehalose uptake for enhanced erythrocyte cryosurvival. Biomaterials 31, 6096–6103 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Sharp DMC et al. Amphipathic polymer-mediated uptake of trehalose for dimethyl sulfoxide-free human cell cryopreservation. Cryobiology 67, 305–311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch AL, Chen R & Slater NKH pH-responsive polymers for trehalose loading and desiccation protection of human red blood cells. Biomaterials 32, 4443–4449 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Mercado SA & Slater NKH Increased cryosurvival of osteosarcoma cells using an amphipathic pH-responsive polymer for trehalose uptake. Cryobiology 73, 175–180 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Chen A, Mercado SA & Slater NK Antioxidant modified amphiphilic polymer improves intracellular cryoprotectant delivery and alleviates oxidative stress in HeLa cells. Adv. Mater. Sci 2, 1–7 (2017). [Google Scholar]
- 44.Wei Y, Li C, Zhang L & Xu X Design of novel cell penetrating peptides for the delivery of trehalose into mammalian cells. Biochim. Biophys. Acta - Biomembr 1838, 1911–1920 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Holovati JL, Gyongyossy-Issa MIC & Acker JP Investigating Interactions of Trehalose-Containing Liposomes with Human Red Blood Cells. CELL Preserv. Technol 6, 131–142 (2008). [Google Scholar]
- 46.Holovati JL, Gyongyossy-Issa MIC & Acker JP Effects of trehalose-loaded liposomes on red blood cell response to freezing and post-thaw membrane quality. Cryobiology 58, 75–83 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Motta JPR, Paraguassú-Braga FH, Bouzas LF & Porto LC Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology 68, 343–348 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Rong J, Wang Q & He X The encapsulation and intracellular delivery of trehalose using a thermally responsive nanocapsule. Nanotechnology 20, 275101 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Rao W et al. Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl. Mater. Interfaces 7, 5017–5028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S et al. An experimental study of the use of ultrasound to facilitate the loading of trehalose into platelets. Cryobiology 59, 135–140 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Abazari A et al. Engineered Trehalose Permeable to Mammalian Cells. PLoS One 10, e0130323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rols M-P Electropermeabilization, a physical method for the delivery of therapeutic molecules into cells. Biochim. Biophys. Acta - Biomembr. 1758, 423–428 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Teissie J Electropermeabilization of the Cell Membrane. in 25–46 (Humana Press, New York, NY, 2014). doi: 10.1007/978-1-4614-9632-8_2 [DOI] [PubMed] [Google Scholar]
- 54.Neumann E & Rosenheck K Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol 10, 279–290 (1972). [DOI] [PubMed] [Google Scholar]
- 55.Kaasen I, McDougall J & Strøm AR Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase/phosphatase complex. Gene 145, 9–15 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Wiley JS, Sluyter R, Gu BJ, Stokes L & Fuller SJ The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Adinolfi E et al. P2X7 receptor: Death or life? Purinergic Signal 1, 219–227 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adinolfi E et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72, 2957–69 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Watanabe M, Kikawada T & Okuda T Increase of internal ion concentration triggers trehalose synthesis associated with cryptobiosis in larvae of Polypedilum vanderplanki. J. Exp. Biol 206, 2281–6 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Kikawada T, Minakawa N, Watanabe M & Okuda T Factors Inducing Successful Anhydrobiosis in the African Chironomid Polypedilum vanderplanki: Significance of the Larval Tubular Nest. Integr. Comp. Biol 45, 710–4 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Kikawada T et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl. Acad. Sci. U. S. A 104, 11585–90 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramadurai S et al. Dynamic studies of the interaction of a pH responsive, amphiphilic polymer with a DOPC lipid membrane. Soft Matter 13, 3690–3700 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Hoffman AS et al. Design of "Smart" Nano-Scale Delivery Systems for Biomolecular Therapeutics. J. Biomed. Nanotechnol 3, 213–217 (2007). [Google Scholar]
- 64.Chen R, Khormaee S, Eccleston ME & Slater NKH The role of hydrophobic amino acid grafts in the enhancement of membrane-disruptive activity of pH-responsive pseudo-peptides. Biomaterials 30, 1954–1961 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch AL & Slater NKH Influence of intracellular trehalose concentration and pre-freeze cell volume on the cryosurvival of rapidly frozen human erythrocytes. Cryobiology 63, 26–31 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Zhang B, Luo Y & Chen H Nanotechnology Applications to Improve Food Safety in Food Safety in China 609–635 (John Wiley & Sons, Ltd, 2017). doi: 10.1002/9781119238102.ch36 [DOI] [Google Scholar]
- 67.Vernekar VN, James R, Smith KJ & Laurencin CT Nanotechnology Applications in Stem Cell Science for Regenerative Engineering. J. Nanosci. Nanotechnol 16, 8953–8965 (2016). [Google Scholar]
- 68.Banerjee D & Sengupta S Nanoparticles in Cancer Chemotherapy. Prog. Mol. Biol. Transl. Sci 104, 489–507 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Breunig M, Bauer S & Goepferich A Polymers and nanoparticles: Intelligent tools for intracellular targeting? Eur. J. Pharm. Biopharm 68, 112–128 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Vasir JK & Labhasetwar V Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev 59, 718–728 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seung Ho Choi, Soo Hyeon Lee, and & Park* TG Temperature-Sensitive Pluronic/Poly(ethylenimine) Nanocapsules for Thermally Triggered Disruption of Intracellular Endosomal Compartment. (2006). doi: 10.1021/BM060182A [DOI] [PubMed] [Google Scholar]
- 72.Prabaharan M Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl 23, 5–36 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Zhang W et al. Synthesis and Characterization of Thermally Responsive Pluronic F127−Chitosan Nanocapsules for Controlled Release and Intracellular Delivery of Small Molecules. ACS Nano 4, 6747–6759 (2010). [DOI] [PubMed] [Google Scholar]


