Abstract
Purpose:
To characterize and compare the ganglion cell complex (GCC) thickness and macula vessel density in pre-perimetric and early primary open angle glaucoma (POAG) eyes.
Design:
Cross-sectional study.
Methods:
57 healthy eyes, 68 pre-perimetric and 162 early POAG eyes enrolled in the Diagnostic Innovations in Glaucoma Study. Optical coherence tomography angiography (OCT-A) based superficial macula vessel density and OCT based GCC thickness were evaluated simultaneously. Percent loss from normal of GCC thickness and macula vessel density was compared. Area under the receiver operating characteristic curves was used to describe the diagnostic utility.
Results:
Both GCC thickness and vessel density were significantly lower in pre-perimetric and early POAG eyes compared to healthy eyes. Compared to the pre-perimetric POAG group, the early POAG group showed larger GCC thickness percent loss (whole image 4.72% vs. 9.86%; all P<0.01) but similar vessel density percent loss (whole image 4.97% vs. 6.93%; all P>0.05). In pre-perimetric POAG, GCC thickness and vessel density percent losses were similar (all P>0.1). In contrast, in early POAG, GCC thickness percent loss was larger than that of vessel density (all P≤ 0.001). To discriminate pre-perimetric or early glaucoma eyes from healthy eyes, GCC thickness and macula vessel density showed similar diagnostic accuracy (all P> 0.05).
Conclusions:
Both GCC thinning and macula vessel density dropout were detectable in pre-perimetric and early POAG eyes. GCC loss was greater than macula vessel density loss in early perimetric POAG. However, OCT-A and OCT measurements showed similar efficiency to detect early glaucoma.
Keywords: Early primary open angle glaucoma, macula, optical coherence tomography angiography, vessel density, SD-optical coherence tomography
INTRODUCTION
Primary open angle glaucoma (POAG) is characterized by progressive loss of retinal ganglion cells (RGCs) and their axons, and accompanying damage to the visual field (VF).1,2 Although the pathophysiology of glaucoma is not well understood, there is growing evidence that the vascular system, and particularly the retinal microvasculature, has an important role in the process.3–6 Microvascular dropout is well recognized in patients with glaucoma, however it is not known whether it is a primary event or is the result of loss of retinal nerve fibers. 4–6
Although numerous technologies have been used to document the impairment of ocular blood flow and alterations of the retinal microvasculature in glaucoma, they have had limited success in elucidating the role of the vascular system.7 The recent introduction of optical coherence tomography angiography (OCT-A), a technique of non-invasive imaging of the blood vessels of the ONH and retina in-vivo, offers the potential for enhancing our understanding of the role of microvasculature integrity in the pathophysiology of glaucoma.8 Studies using OCT-A have provided evidence of microvascular dropout, measured as a decrease of vessel density within the ONH, the peripapillary retina and the macula in POAG eyes.3,9 Moreover, decreased vessel density is associated with the severity of VF damage.10 However, it is still unclear if microvasculature impairment is the primary causative event or secondary to loss of neural tissue,6 and whether the cascade could vary in different patients.10–12
Early detection and close monitoring of glaucomatous damage are important for advancing ocular hypotensive treatment to minimize irreversible vision loss. Early glaucomatous damage involves the macula,13,14 where there are more than 30% of the total RGCs.15 RGCs in the macula depend on regional capillary networks to meet their high metabolic requirements. If insufficient ocular blood flow has a central role in apoptotic RGC death, as has been suggested,16 assessment of macular vessel density might detect early glaucomatous damage.
It is notable, however, that inner retina thickness has been reported in some studies to have better diagnostic performance than inner macula vessel density for detection of glaucoma.17–19 While it also has been reported that there are no significant differences between macula thickness and vessel density to discriminate eyes with glaucoma from healthy eyes,20,21 Investigations of vessel density to date have largely evaluated the full continuum of glaucoma from early to advanced cases. Moreover, few studies have focused on the early detection of glaucoma,11,19,22 particularly by evaluating the macula. The purpose of the current study was to characterize and compare macula vessel density and GCC thickness in pre-perimetric and early glaucoma.
METHODS
This was a cross-sectional observational study. Participants were recruited from the Diagnostic Innovations in Glaucoma Study (DIGS).23 Written informed consent was obtained from all participants. The Institutional Review Boards of the University of California, San Diego approved the protocol, and the methodology adheres to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. This study was registered at http://clinicaltrials.gov (no. NCT00221923) on September 14, 2005.
Participants
All participants underwent an extensive ophthalmological examination, including assessment of best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, gonioscopy, central corneal thickness (CCT) measured with ultrasound pachymetry (DGH Technology, Inc, Exton, PA), dilated fundus examination, simultaneous stereophotography of the optic disc, VF testing by standard automated perimetry (SAP, Humphrey Field Analyzer; 24-2 Swedish interactive threshold algorithm; Carl Zeiss Meditec, Jena, Germany), and OCT-A imaging (Avanti AngioVue; Optovue, Inc, Fremont, CA). Perimetry and all imaging tests were conducted within a 6-month period.
Overall inclusion criteria were age≥18 years, open angles on gonioscopy, a best-corrected visual acuity of 20/40 or better, a spherical refraction within ±5.0 diopters (D), and cylinder correction within ±3.0 D. Systemic measurements included systolic and diastolic blood pressure (BP) measured at the height of the heart with an Omron Automatic BP instrument (model BP791IT; Omron Healthcare, Inc, Lake Forest, IL). Mean arterial pressure was calculated as one-third systolic BP + two-thirds diastolic BP. Mean ocular perfusion pressure (MOPP) was defined as the difference between two-thirds of mean arterial pressure and IOP. Other information such as race, age, systemic disease history, non-ocular medication, and heart rate was also collected. Exclusion criteria were (1) history of intraocular surgery (except uncomplicated cataract or glaucoma surgery), coexisting retinal pathology, nonglaucomatous optic neuropathy, uveitis, or ocular trauma; (2) diagnosis of Parkinson’s disease, Alzheimer’s disease, dementia, or history of stroke; (3) diabetic or hypertensive retinopathy; (4) unreliable VFs; and (5) poor-quality OCT-A or spectral domain OCT (SD-OCT) scans. Participants with systemic hypertension or diabetes mellitus were included unless they met exclusion criterion number 3.
Healthy eyes had (1) IOP < 21 mmHg with no history of elevated IOP; (2) normal appearing optic disc, intact neuroretinal rim and retinal nerve fiber layer (RNFL); and (3) a minimum of two reliable normal visual fields, defined as a pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test (GHT) result within normal limits.3 Pre-perimetric glaucoma was defined as eyes having optic discs appearance suspicious of glaucoma but without evidence of repeatable glaucomatous VF damage.11,24,25 A suspicious appearing optic disc was defined as a disc with observable excavation, neuroretinal rim narrowing or notching, or a localized or diffuse RNFL defect suggestive of glaucoma with stereophotographs.23 RNFL thickness measurement by OCT was not considered as a criterion of pre-perimetric glaucoma. Glaucomatous VF damage was defined as a GHT outside normal limits and a PSD outside 95% normal limits, which were confirmed on at least 2 consecutive, reliable (fixation losses and false-negatives≤ 33% and false-positives≤15%) tests. POAG eyes had reliable and repeatable glaucomatous VF damage.10,26 Early glaucoma was defined as 24-2 mean deviation (MD) >-6 dB.10,27 The three groups were age-matched.
Optical Coherence Tomography Angiography and Spectral-Domain Optical Coherence Tomography
All subjects underwent OCT-A and SD-OCT imaging using the AngioVue imaging system (Optovue, Inc., Fremont, CA, USA, software version 2017, 1, 0, 144). This system has been described previously.3 In brief, the AngioVue is an angiographic platform implemented on an existing commercially available SD-OCT platform which provides both thickness and vascular measurements. With the simultaneously acquired OCT and OCT-A volume of the AngioVue scan, and automatic segmentation by the AngioVue software (version 2017.1.0.144), thickness and vascular analyses can be derived from the same scan with exact registration of the analyzed regions.
Macula 3 × 3 mm2 scans center on the fovea were acquired with OCT-A AngioVue system. OCT-A based GCC vessel density and OCT based GCC thickness measures were calculated from the same macula scan as follows. The split- spectrum amplitude-decorrelation angiography method was used to capture the dynamic motion of the red blood cells and provide a high-resolution 3D visualization of perfused retinal vasculature. Macula vessel density was calculated as the percent area occupied by flowing blood vessels in the selected region. The retinal layers of each scan were automatically segmented by the AngioVue software in order to visualize the superficial retinal capillary plexuses in a slab from the internal limiting membrane (ILM) to the inner plexiform layer (IPL) −10 μm. For this study, whole en-face image vessel density (wiVD) was derived from the entire 3×3 mm2 scan and perifoveal vessel density (pfVD) was measured in an annular centered on the fovea with an inner diameter of 1 mm and outer diameter of 3 mm. Sectoral analysis was also completed by calculating GCC thickness and vessel density in the superior and inferior hemifields separately and 4 sectors of 90° each (nasal, inferior, superior, and temporal sectors) in the perifoveal regions.
The macula cube scanning protocol measured the GCC thickness of the same scan slab as OCT-A scan. GCC thickness analysis regions of whole image (wiGCC), perifoveal (pfGCC), two hemifields and four sectors of SD-OCT images were the same as that in the OCT-A vessel density analysis.
Only good-quality images were included. OCT-A and SD-OCT images quality review was completed according to the Imaging Data Evaluation and Analysis (IDEA) Reading Center standard protocol on all scans processed with standard AngioVue software (version 2017.1.0.144). Poor quality images, defined as images with (1) low scan quality as scan quality score (SQ) less than 4, (2) poor clarity, (3) residual motion artifacts visible as irregular vessel pattern or disc boundary on the enface angiogram, (4) image cropping or local weak signal due to vitreous opacity, or (5) segmentation errors, were excluded.
Although their dynamic range is different, direct comparison of GCC vessel density and thickness values was obtained by normalizing the GCC vessel density and thickness values as percent loss.28 Percent loss of GCC thickness and vessel density was calculated as [1− (raw measurement / mean value of the same measurement of healthy eyes)] × 100 (unit, %).
In addition, all subjects also underwent Spectralis SD-OCT imaging (Spectralis HRA+OCT; Heidelberg Engineering Inc., Heidelberg, Germany, software version 5.4.7.0) to calculate the peripapillary RNFL thickness from a high resolution RNFL circle scan in a 10-pixel-wide band along a circle of 12 degrees centered on the ONH. All images were processed and reviewed by the IDEA Center graders. Images with noncentered scans, inaccurate segmentation of the RNFL that could not be manually corrected, or quality scores of ~15 dB or less were excluded.
Statistical Analysis
The distribution of continuous variables was assessed by inspecting histograms and using Shapiro-Wilk W tests of normality. The demographic data were expressed as the mean ± standard deviation (SD) for continuous variables and frequencies (percentages) for categorical variables. Mean and 95% confident interval (CI) were computed for other normally distributed variables.
Categorical variables were compared using the chi-square test. Analysis of variance (ANOVA) and post-hoc Tukey’s honest significant differences were calculated to compare demographic numeric parameters among healthy, pre-perimetric glaucoma, and early glaucoma subjects. Mixed-effects modeling was used to compare ocular parameters among groups. Models were fit with ocular measurements as response variable and diagnostic group as fixed effects. Measurements of bilateral eyes were nested within subject to account for the fact that eyes from the same individual are more likely to have similar measurements.29,30 To estimate the difference in percent loss between pre-perimetric glaucoma and early glaucoma eyes, mixed effects modeling was used. Linear mixed effects models were used to compare the percent loss of GCC thickness and vessel density within one certain diagnostic group, i. e. in pre-perimetric glaucoma group or early glaucoma group. Multivariable models included the following potential confounding factors, age, gender, race, SQ, and any other demographics or ophthalmic characteristics if the P value was <0.1 in univariate analysis. Linear and quadratic regression models were used to evaluate the association of percent loss between thickness and vessel density. Area under the receiver operating characteristic (ROC) curves were used to describe the diagnostic utility.
Statistical analyses were performed using statistical software JMP Pro 12 (SAS Institute Inc, Cary, NC) and Stata 14.2 (StataCorp LLC, College Station, TX). P values less than 0.05 were considered statistically significant. Bonferroni correction (0.05/n) with n as the number of statistical tests was used to adjust for multiple comparisons.
RESULTS
A total of 213 subjects (287 eyes), consisting of 37 healthy subjects (57 eyes), 55 pre-perimetric subjects (68 eyes) and 121 early glaucoma subject (162 eyes) were included in this report. Demographic and ophthalmic characteristics of the study subjects are summarized in Table 1. There was no significant difference among the groups in terms of age, race, BP, mean arterial pressure, heart rate, MOPP, axial length, CCT, and IOP (all P values > 0.1), and the prevalence of self-reported diabetes (P values > 0.05). The groups differed by gender (P= 0.017), self-reported history of hypertension (P= 0.005), VF indices (all P values< 0.0001), and usage rate of topical glaucoma medications (P< 0.0001). Compared to healthy eyes, the pre-perimetric glaucoma and early glaucoma group had a higher prevalence of self-reported hypertension. Although the prevalence of self-reported hypertension in the healthy group is lower, there was no difference of the BP among the groups (P= 0.324 for diastolic BP and P= 0.734 for systolic BP). The healthy group and pre-perimetric group had similar MD and PSD values which, as expected, were better than the values in the early glaucoma group. Peripapillary RNFL showed significant differences among the three groups with the thickest mean RNFL measurement in the healthy group and thinnest mean measurement in the early glaucoma group.
Table 1.
Demographics and Ocular Characteristics of Study Population
| A. Healthy | B. Pre-perimetric Glaucoma |
C. Early Glaucoma |
P value | Post Hoc | |
|---|---|---|---|---|---|
| By subject (No.) | 37 | 55 | 121 | ||
| Age (years) | 65.7±8.7 | 68.4±10.8 | 68.4±8.6 | 0.271 | A=B=C |
| Gender (M/F) | 9/28 | 25/30 | 61/60 | 0.017 | |
| Race, no. (%) | 0.706 | ||||
| Caucasian | 26 (70.3%) | 37 (67.3%) | 72 (59.5%) | ||
| African American | 8 (21.6%) | 14 (25.5%) | 39 (32.2%) | ||
| Other | 3 (8.1%) | 4 (7.3%) | 10 (8.3%) | ||
| Self-reported history of Diabetes, no (%) | 2 (5.4%) | 5 (9.1%) | 21 (17.4%) | 0.081 | |
| Anti-diabetes medications, no (%) | 2 (5.4%) | 5 (9.1%) | 20 (16.5%) | 0.111 | |
| Self-reported history of Hypertension, no (%) | 10 (27.0%) | 31 (56.4%) | 68 (56.2%) | 0.005 | |
| Anti-hypertensive medications, no (%) | 9 (24.3%) | 25 (45.5%) | 54 (44.6%) | 0.060 | |
| Diastolic BP (mmHg) | 77.1±10.8 | 79.3±1.5 | 80.2±11.1 | 0.324 | A=B=C |
| Systolic BP (mmHg) | 127.4±13.6 | 130.5±18.6 | 128.9±19.4 | 0.734 | A=B=C |
| Mean arterial pressure (mmHg) | 93.6±10.5 | 96.3±1.7 | 96.4±1.1 | 0.517 | A=B=C |
| Heart rate ( /min.) | 67.6±8.9 | 68.2±1.7 | 67.8±1.1 | 0.972 | A=B=C |
| By Eye (No.) | 57 | 68 | 162 | ||
| MOPP (mmHg) | 52.6 (50.9, 54.4) | 52.4 (50.3, 54.5) | 53.9 (52.6, 55.3) | 0.361 | A=B=C |
| Axial Length (mm) | 23.7 (23.5, 24.0) | 24.1 (23.8, 24.4) | 24.1 (23.9, 24.3) | 0.123 | A=B=C |
| CCT ((μm) | 555.3 (546.9, 563.6) | 547.5 (537.1, 558.0) | 541.3 (534.9, 547.6) | 0.102 | A=B=C |
| IOP (mmHg) | 15.2 (14.6, 15.8) | 16.3 (15.3, 17.3) | 15.2 (14.6, 15.9) | 0.126 | A=B=C |
| MD (dB) | 0.05 (−0.3, 0.4) | −0.3 (−0.6, 0.1) | −2.1 (−2.4, −1.8) | <.0001 | A=B>C |
| PSD (dB) | 1.7 (1.5, 1.9) | 1.9 (1.7, 2.1) | 3.6 (3.2, 4.0) | <.0001 | A=B<C |
| Peripapillary RNFL thickness μm) | 95.2 (92.5, 97.9) | 83.6 (80.5, 86.7) | 77.5 (75.1,79.8) | <.0001 | A<B<C |
| Topical glaucoma medications, no (%) | 0 (%) | 45 (66.2%) | 120 (74.1%) | <.0001 |
Normally distributed variables by subject, results are shown in mean ± standard deviation. Normally distributed variables by eye are shown in mean (95% confident interval). Categorical variables were compared using the chi-square test. Other demographic parameters were compared with ANOVA and post hoc Tukey’s honest significant difference test. Linear mixed model was used for comparison of ocular parameters. Values with statistical significance are shown in bold.
Abbreviations: M, male; F, female; BP, blood pressure; MOPP, mean ocular perfusion pressure; CCT, central corneal thickness; IOP, intraocular pressure; MD, mean deviation; dB, decibels; PSD, pattern standard deviation; RNFL: retinal nerve fiber layer.
Table 2 summarizes the GCC thickness and vessel density values for the three diagnostic groups. The mean (95% CI) SQ was significantly higher in healthy eyes compared to the pre-perimetric glaucoma eyes and early glaucoma eyes. In univariate analysis of GCC vessel density and GCC thickness, statistically significant differences were found in wiGCC thickness and pfGCC thickness (all P values< 0.05, Table 2). Specifically, significantly thicker GCC was found in pre-perimetric eyes compared to early glaucoma eyes in the inferior hemifield of the whole image and perifoveal region (all P values< 0.05), as well as temporal and inferior sectors (all P values< 0.05), but not in superior hemifield and superior and nasal sectors (Table 3). For vessel density indices, no significant difference was found between the pre-perimetric glaucoma and early glaucoma eyes (all P values> 0.05). However, the healthy eyes had higher global and regional GCC vessel density compared with either pre-perimetric eyes or early glaucoma eyes (all P values≤ 0.001, Table 2 and Table 3). After Bonferroni correction with a cutoff P value of 0.005, most of the significances remained, except those of the differences of GCC thickness between healthy eyes and pre-perimetric eyes.
Table 2.
Ganglion Cell Complex Thickness and Vessel Density in Healthy, Pre-perimetric Glaucoma and early Glaucoma Eyes: Univariate and Multivariate Analysis
| Mean (95% Confidence Interval) |
P value (univariate, multivariate) |
|||||
|---|---|---|---|---|---|---|
| A. Healthy | B. Pre-perimetric Glaucoma |
C. Early Glaucoma | A vs. B | B vs. C | A vs. C | |
| Scan Quality | 7.3 (7.0, 7.6) | 6.9 (6.6, 7.2) | 6.6 (6.4, 6.8) | 0.045 | 0.121 | <.0001 |
| Ganglion Cell Complex Thickness (μm) | ||||||
| Whole image | 103.5 (101.8, 105.1) | 98.6 (96.2, 101.1) | 93.3 (91.5, 95.1) | 0.011, 0.008 | 0.004, 0.002 | <.0001, <.0001 |
| Perifoveal | 108.9 (107.3, 110.6) | 104.0 (101.3, 106.8) | 98.2 (96.3, 100.2) | 0.016, 0.011 | 0.003, 0.002 | <.0001, <.0001 |
| Vessel density (%) | ||||||
| Whole image | 47.91 (47.32, 48.51) | 45.53 (44.70, 46.36) | 44.59 (43.96, 45.22) | <.0001, 0.002 | 0.089, 0.196 | <.0001, <.0001 |
| Perifoveal | 50.59 (50.00, 51.18) | 48.13 (47.32, 48.93) | 47.27 (46.60, 47.93) | <.0001, 0.001 | 0.113, 0.242 | <.0001, <.0001 |
Univariate and multivariate analysis, which controlled for age, gender, race, self-reported diabetes and hypertension, and scan quality, used mixed effects model. Only univariate analysis was used for scan quality comparison. Values with statistical significance are shown in bold.
Table 3.
Regional Ganglion Cell Complex Thickness and Vessel Density in Healthy, Pre-perimetric Glaucoma and Early Glaucoma Eyes: Univariate and Multivariate Analysis
| Mean (95% Confidence Interval) |
P value (univariate, multivariate) |
|||||
|---|---|---|---|---|---|---|
| A. Healthy | B. Pre-perimetric Glaucoma |
C. Early Glaucoma |
A vs. B | B vs. C | A vs. C | |
| Ganglion Cell Complex Thickness (μm) | ||||||
| Superior Hemifield of Whole Image | 103.2 (101.7, 104.8) | 98.0 (95.4, 100.6) | 94.4 (92.7, 96.1) | 0.006, 0.008 | 0.055, 0.066 | <.0001, <.0001 |
| Inferior Hemifield of Whole Image | 103.5 (101.8, 105.2) | 99.1 (96.5, 101.6) | 91.8 (89.5, 94.0) | 0.021, 0.012 | <.0001, <.0001 | <.0001, <.0001 |
| Superior Hemifield of perifoveal | 108.7 (107.0, 110.3) | 103.4 (100.6, 106.2) | 99.5 (97.7, 101.3) | 0.011, 0.013 | 0.051, 0.059 | <.0001, <.0001 |
| Inferior Hemifield of perifoveal | 109.22 (107.51, 110.94) | 104.7 (102.0, 107.4) | 96.9 (94.5, 99.3) | 0.027, 0.013 | <.0001, <.0001 | <.0001, <.0001 |
| Temporal perifoveal | 102.1 (100.4, 103.7) | 97.9 (95.3, 100.4) | 90.1 (88.1, 92.0) | 0.028, 0.007 | <.0001, <.0001 | <.0001, <.0001 |
| Superior perifoveal | 112.0 (110.2, 113.9) | 105.7 (102.6, 108.7) | 102.4 (100.4, 104.5) | 0.004, 0.006 | 0.131, 0.169 | <.0001, <.0001 |
| Nasal perifoveal | 109.7 (108.0, 111.4) | 105.8 (103.0, 108.6) | 101.8 (99.8, 103.7) | 0.066, 0.070 | 0.053, 0.054 | <.0001, <.0001 |
| Inferior perifoveal | 111.9 (110.0, 113.8) | 106.8 (104.0, 109.6) | 98.5 (95.7, 101.2) | 0.018, 0.013 | <.0001, <.0001 | <.0001, <.0001 |
| Vessel density (%) | ||||||
| Superior Hemifield of Whole Image | 48.0 (47.4, 48.7) | 45.6 (44.9, 46.4) | 44.9 (44.3, 45.5) | <.0001, 0.004 | 0.161, 0.404 | <.0001, <.0001 |
| Inferior Hemifield of Whole Image | 47.8 (47.2, 48.4) | 45.4 (44.5, 46.3) | 44.2 (43.5, 45.0) | <.0001, 0.002 | 0.058, 0.103 | <.0001, <.0001 |
| Superior Hemifield of perifoveal | 50.7 (50.0, 51.3) | 48.1 (47.3, 49.0) | 47.6 (47.0, 48.2) | <.0001, 0.003 | 0.312, 0.663 | <.0001, <.0001 |
| Inferior Hemifield of perifoveal | 50.5 (49.9, 51.1) | 48.1 (47.2, 49.0) | 46.9 (46.1, 47.7) | <.0001, 0.002 | 0.056, 0.098 | <.0001, <.0001 |
| Temporal perifoveal | 49.5 (48.9, 50.2) | 46.9 (46.1, 47.7) | 46.0 (45.3, 46.7) | <.0001, 0.001 | 0.098, 0.143 | <.0001, <.0001 |
| Superior perifoveal | 51.6 (50.8, 52.4) | 49.0 (48.1, 49.9) | 48.6 (48.0, 49.3) | <.0001, 0.007 | 0.517, 0.961 | <.0001, 0.002 |
| Nasal perifoveal | 49.9 (49.2, 50.7) | 47.9 (47.0, 48.7) | 47.0 (46.3, 47.7) | 0.001, 0.038 | 0.124, 0.298 | <.0001, 0.001 |
| Inferior perifoveal | 51.3 (50.6, 52.0) | 48.7 (47.7, 49.7) | 47.5 (46.6, 48.4) | <.0001, 0.001 | 0.065, 0.132 | <.0001, <.0001 |
Age, gender, race, self-reported diabetes and hypertension, and scan quality were adjusted for multivariate analysis. Values with statistical significance are shown in bold.
Table 4 and Table 5 summarizes the calculated percent loss of GCC thickness and vessel density in pre-perimetric glaucoma and early glaucoma eyes. In pre-perimetric glaucoma, the extent of thickness and vessel density percent losses were similar (all P values >0.1). However, in early glaucoma, global (Table 4, all P values≤ 0.001) and regional (Table 5, all P values< 0.05) thickness percent losses were significantly greater than corresponding percent loss of vessel density, except in the nasal perifoveal region, which showed similar percent loss of GCC thickness and vessel density (P= 0.196). After adjusting for multiple comparisons, with a Bonferroni corrected P value 0.005, the differences between the percent loss of thickness and vessel density in superior hemifields of whole image and perifoveal region, and superior perifoveal no longer reached statistical significant. Other significances remained. Figure 1 illustrates the distribution of the percent loss of vessel density and GCC thickness in pre-perimetric glaucoma and early glaucoma eyes.
Table 4.
Percent Loss of Ganglion Cell Complex Thickness and Vessel Density in Pre-perimetric Glaucoma and Early Glaucoma Eyes: Univariate and Multivariate Analysis
| Percent loss (%) |
P value (univariate, multivariate) |
||
|---|---|---|---|
| Thickness | Vessel Density | ||
| Whole image | |||
| Pre-perimetric Glaucoma | 4.72 (2.27, 7.18) | 4.97 (3.24, 6.70) | 0.855, 0.856 |
| Early Glaucoma | 9.86 (8.14, 11.57) | 6.93 (5.61, 8.24) | <.001, 0.001 |
| P value (univariate, multivariate) | 0.004, 0.002 | 0.089, 0.218 | |
| Perifoveal | |||
| Pre-perimetric Glaucoma | 4.50 (2.01, 6.99) | 4.87 (3.27, 6.46) | 0.801, 0.805 |
| Early Glaucoma | 9.83 (8.06, 11.60) | 6.57 (5.25, 7.88) | <.001, <.0001 |
| P value (univariate, multivariate) | 0.004, 0.001 | 0.113, 0.265 | |
Percent loss, which was calculated as [1- (raw measurement / mean value of healthy eyes)]×100 (%), are shown in mean (95% confidence interval). Values with statistical significance are shown in bold. Multivariate analysis adjusted for age, gender, race, self-reported diabetes and hypertension, and scan quality.
Table 5.
Percent Loss of Regional Ganglion Cell Complex Thickness and Vessel Density in Pre-perimetric Glaucoma and Early Glaucoma Eyes: Univariate and Multivariate Analysis
| Percent Loss | Thickness (%) | Vessel Density (%) |
P value (univariate, multivariate) |
|---|---|---|---|
| Superior Hemifield of Whole Image | |||
| Pre-perimetric Glaucoma | 5.1 (2.5, 7.6) | 5.0 (3.3, 6.6) | 0.943, 0.945 |
| Early Glaucoma | 8.6 (6.9, 10.2) | 6.5 (5.3, 7.8) | 0.026, 0.028 |
| P value (univariate, multivariate) | 0.055, 0.065 | 0.161, 0.446 | |
| Inferior Hemifield of Whole Image | |||
| Pre-perimetric Glaucoma | 4.3 (1.9, 6.8) | 5.0 (3.1, 6.9) | 0.637, 0.645 |
| Early Glaucoma | 11.4 (9.2, 13.6) | 7.5 (5.9, 9.0) | <.0001, <.0001 |
| P value (univariate, multivariate) | <.001, <.0001 | 0.058, 0.113 | |
| Superior Hemifield of Perifoveal | |||
| Pre-perimetric Glaucoma | 4.8 (2.2, 7.4) | 5.0 (3.4, 6.6) | 0.908, 0.910 |
| Early Glaucoma | 8.4 (6.7, 10.1) | 6.1 (4.9, 7.3) | 0.015, 0.016 |
| P value (univariate, multivariate) | 0.051, 0.057 | 0.312, 0.708 | |
| Inferior Hemifield of Perifoveal | |||
| Pre-perimetric Glaucoma | 4.2 (1.7, 6.6) | 4.7 (2.9, 6.5) | 0.703, 0.710 |
| Early Glaucoma | 11.3 (9.1, 13.5) | 7.1 (5.5, 8.7) | <.0001, <.0001 |
| P value (univariate, multivariate) | <.001, <.0001 | 0.056, 0.108 | |
| Temporal Perifoveal | |||
| Pre-perimetric Glaucoma | 4.2 (1.6, 6.7) | 5.3 (3.7, 6.9) | 0.398, 0.409 |
| Early Glaucoma | 11.8 (9.9, 13.7) | 7.2 (5.7, 8.6) | <.0001, <.0001 |
| P value (univariate, multivariate) | <.001, <.0001 | 0.098, 0.150 | |
| Superior Perifoveal | |||
| Pre-perimetric Glaucoma | 5.7 (3.0, 8.4) | 5.0 (3.2, 6.8) | 0.634, 0.642 |
| Early Glaucoma | 8.6 (6.8, 10.4) | 5.7 (4.4, 7.0) | 0.005, 0.006 |
| P value (univariate, multivariate) | 0.131, 0.167 | 0.517, 0.998 | |
| Nasal Perifoveal | |||
| Pre-perimetric Glaucoma | 3.5 (1.0, 6.1) | 4.1 (2.4, 5.8) | 0.731, 0.737 |
| Early Glaucoma | 7.2 (5.5, 9.0) | 5.9 (4.2, 7.4) | 0.196, 0.201 |
| P value (univariate, multivariate) | 0.053, 0.053 | 0.124, 0.327 | |
| Inferior Perifoveal | |||
| Pre-perimetric Glaucoma | 4.5 (2.0, 7.1) | 5.1 (3.1, 7.0) | 0.733, 0.739 |
| Early Glaucoma | 12.0 (9.6, 14.4) | 7.5 (5.7, 9.3) | <.0001, <.0001 |
| P value (univariate, multivariate) | <.001, <.0001 | 0.065, 0.145 | |
Percent loss, which was calculated as [1-( raw measurement / mean value of healthy eyes)]×100 (%), are shown in mean (95% confidence interval). Values with statistical significance are shown in bold. Multivariate analysis adjusted for age, gender, race, self-reported diabetes and hypertension, and scan quality.
Figure 1.
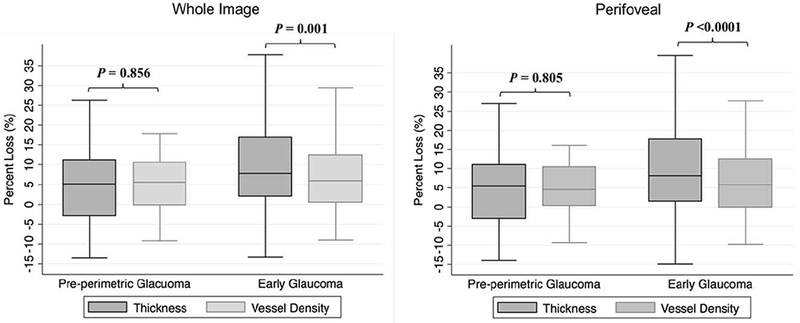
Boxplots illustrating the distribution of whole image (left) and perifoveal (right) percent loss of ganglion cell complex (GCC) vessel density and thickness in pre-perimetric glaucoma and early glaucoma eyes. The medians are represented by horizontal lines in the gray boxes. Error bars denote interquartile range. In early glaucoma, percent loss of GCC thickness is greater than vessel density. P-values are based on multivariable analysis controlling for age, gender, race, self-reported diabetes and hypertension, and scan quality.
In addition, the percent loss of GCC thickness and vessel density in pre-perimetric glaucoma and early glaucoma eyes were compared. In univariate analyses, all global, inferior and temporal regional thickness indices in the early glaucoma group had higher percent loss than the pre-perimetric glaucoma eyes (all P values< 0.01). Although the early glaucoma group also showed higher percent loss of vessel density than pre-perimetric glaucoma eyes, the difference did not reach statistical significance (all P values>0.05, Table 4 and Table 5). In multivariate analysis, after adjustment for age, gender, race, self-reported diabetes and hypertension, and SQ, the difference between pre-perimetric and early glaucoma eyes in percent loss of GCC thickness remained significant (all P values< 0.01), while percent loss of vessel density was similar in the two groups (all P values> 0.1). Figure 2 illustrates OCT-A and OCT images and corresponding percent loss in a representative pre-perimetric glaucoma eye and an early glaucoma eye.
Figure 2.
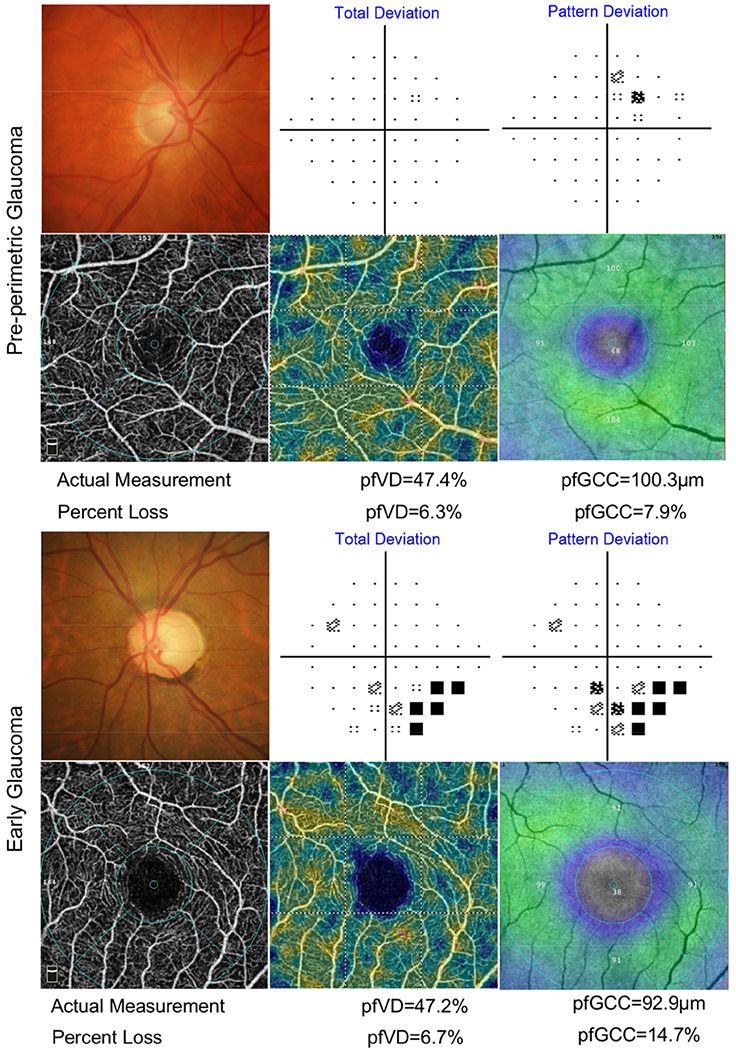
Microvasculature and thickness measurements in a pre-perimetric glaucoma eye (top 2 rows) and an early glaucoma eye (bottom 2 rows). Top and third row: optic disc photograph (left) and 24-2 standard automated perimetry (SAP) results. Second and forth row: optical coherence tomography angiography (OCT-A) macula scan showing the superficial vascular plexus (left); corresponding color-coded flow density map of the superficial vascular plexus flow density (middle; the warmer the color, the greater the flow); and color-coded thickness map of ganglion cell complex (GCC) (right; the warmer the color, the greater the thickness) deriving from spectral domain optical coherence tomography (SD-OCT) macula scan of the identical slab. The mean deviation and pattern standard deviation of the pre-perimetric eye are −0.07 dB and 1.62 dB. This pre-perimetric case shows similar severity of vessel density and GCC thickness percent loss of 6.3% and 7.9% respectively. While the early glaucoma case, which with mean deviation and pattern standard deviation as −1.63 dB and 3.43 dB, shows greater loss in GCC thickness (14.7%) comparing to vessel density (6.7%).
Both linear and quadratic regression models showed statistically significant associations between percent loss of GCC thickness and vessel density in pre-perimetric glaucoma eyes and early glaucoma eyes (all P≤ 0.01), but the associations were weak to modest with R2 values ranging from 12% to 32% (Figure 3).
Figure 3.
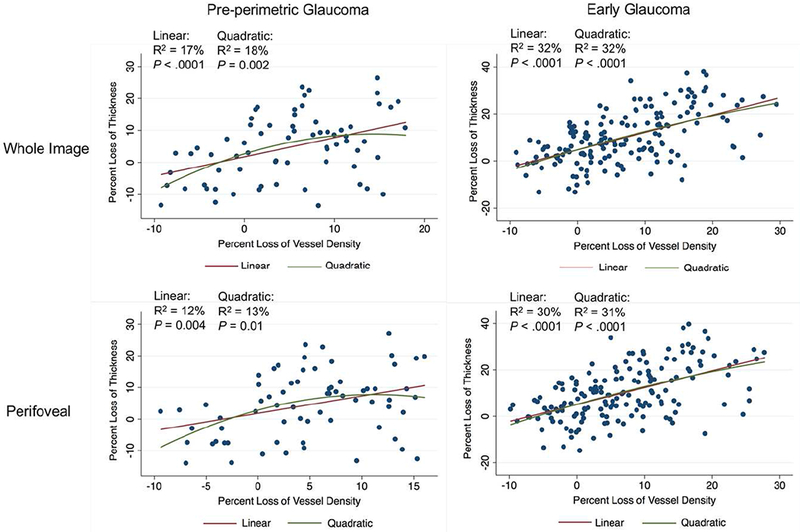
Scatterplots illustrating the linear and quadratic association between percent loss of GCC thickness and vessel density in pre-perimetric glaucoma eyes and early glaucoma eyes.
Table 6 summarizes the diagnostic accuracy of macula vessel density and GCC thickness to discriminate 1) pre-perimetric glaucoma from healthy eyes and 2) early glaucoma from healthy eyes. The AUC for differentiating between pre-perimetric glaucoma and healthy eyes was highest for pfVD, followed by wiVD, wiGCC and pfGCC. For discriminating early glaucoma from healthy eyes, GCC thickness parameters showed higher AUC than macula vessel density parameters. However, none of the differences of AUC were statistically significant.
Table 6.
Diagnostic Performance of Ganglion Cell Complex Thickness and Macula Vessel Density in Healthy and Glaucoma Eyes
| Healthy vs Pre-perimetric Glaucoma Discrimination AUC |
Healthy vs Early Glaucoma Discrimination AUC | |||||
|---|---|---|---|---|---|---|
| Vessel Density | Thickness | P Value | Vessel Density | Thickness | P Value | |
| Whole Image | 0.71 (0.62, 0.80) | 0.65 (0.55, 0.75) | 0.190 | 0.74 (0.68, 0.81) | 0.79 (0.72, 0.85) | 0.215 |
| Perifoveal | 0.73 (0.64, 0.82) | 0.65 (0.55, 0.75) | 0.125 | 0.73 (0.67, 0.80) | 0.78 (0.72, 0.84) | 0.198 |
Results are shown in mean (95% confident interval). AUC= area under receiver operating characteristic curve.
DISCUSSION
In this study, both GCC vessel density and thickness were significantly reduced in pre-perimetric and early POAG eyes compared with healthy eyes. Compared to pre-perimetric glaucoma eyes, those with early glaucoma showed significantly higher GCC thickness percent loss, but similar macula vessel density percent loss. In pre-perimetric glaucoma, the magnitude of the percent loss for the thickness and microvascular metrics were similar. In early POAG, percent loss for GCC thickness was greater than for vessel density.
Recently, there has been an increasing interest in the evaluation of the macula to diagnose and manage glaucoma .31 Glaucomatous damage to the macula often occurs early in the disease.13,32 Although many studies evaluated macula thickness change in glaucoma, only a few focused on the macula microvasculature, and most of them reported lower macula vessel density in glaucoma eyes.17,19–21,33–36 Similarly, only a few prior studies investigated pre-perimetric glaucoma19 and early glaucoma.36 The current study found a significant reduction of macula vessel density in pre-perimetric glaucoma eyes, suggesting that retinal vasculature attenuation may begin early in the course of the glaucoma continuum. These results differ from those of Triolo et al,19 who did not observe a significant difference in macula superficial perifoveal vessel density between pre-perimetric glaucoma and healthy eyes.
There are several possible explanations for the difference in results. First, the definition of pre-perimetric glaucoma of Triolo et al differed from others.11,24,25 They defined pre-perimetric glaucoma eyes as having average and quadrant RNFL thickness within 95% and 99% confidence limits. Such a definition may cause selection bias as only “healthier” pre-perimetric glaucoma eyes were included. Second, imaging devices often show discrepant results. The various data acquisition protocols used by the different versions of software, as well as their accuracy and reproducibility, must be taken into account.37,38 Particularly, as reported by Spaide et al, studies of the superficial vascular plexus using default settings of different devices are likely to be biased because the segmentation slab designed to isolate the superficial vascular plexus includes a variable amount of the deep vascular plexus in macula. 39 The results of the current study are consistent with an earlier study36 that showed a significant reduction of macula vessel density and GCC thickness in early glaucoma eyes compared with healthy eyes.
The adoption of the same 3×3 mm2 macula region facilitated a comparison of the same region for both OCT-A and OCT measurements18 in the current study. In order to further facilitate the comparison of the different parameters with different units and potentially different dynamic ranges, we normalized the measures by calculating the percent loss- deviation from the mean value of normal eyes.28,40 By analyzing the percent loss, we could directly compare thickness and vessel density.
According to the vascular theory of glaucoma, optic nerve damage is a consequence of reduced ocular blood flow that can lead to axonal ischemia.4,6 In contrast, the destruction of the neural tissue in glaucoma may lead to secondary microvascular changes.16 In the current study, macula vessel density percent loss was significantly less than that for GCC thickness in early glaucoma eyes. Therefore, this thickness/microvascular mismatch indicates that neurodegeneration may be faster than vascular damage in early glaucoma. In a previous study7 in which most participants had moderate glaucoma, macula vessel density changed without GCC thinning. Along with our current results, this suggests that the rate of GCC thinning and vessel density loss differs across different stages of glaucoma. Although both GCC thickness and vessel density loss can be detected in early glaucoma, thickness parameters may be better for evaluating early glaucoma. This also suggests that macula microvasculature dropouts may be secondary events after structural thinning in early glaucoma, similar to what has been reported in angle closure glaucoma.41 However, it should be noted that about one third of the early glaucoma eyes showed greater percent loss of vessel density than GCC thickness. The above inference cannot be generalized to all glaucoma cases. Vascular change as the primary event in pathogenesis of glaucoma cannot be excluded due to the cross-sectional design of the study and a difference in test-retest variability between thickness and vessel density measurements.
Nevertheless, no matter whether neural tissue loss or vessel loss is the primary event, vascular abnormality and thickness change can be interdependent. Previous studies have demonstrated significant association between ONH vessel density with peripapillary RNFL thickness in glaucoma eyes.10,35 The current study also found significant association between GCC thickness loss and vessel density loss in both pre-perimetric glaucoma and early glaucoma.
A previous study22 reported that macula vessel density had better diagnostic accuracy compared with GCC thickness for differentiating pre-perimetric glaucoma and healthy eyes. In contrast, thickness parameters better differentiated glaucoma and healthy eyes. The current study found similar trends. However, the AUC difference did not reach statistical significance. In addition to the difference of glaucoma severity in the current study (only early glaucoma), the inconsistency may be related to that the interest region for vessel density measurement did not directly correspond with the region for thickness measurement in the former study. The earlier study employed a 7mm×7mm macula cube for GCC thickness but a 3mm×3mm scan for macula vessel density measurements. In contrast, the current study had more recent software that allowed an identical scan volume and analysis for both measurements. The current findings suggest that, although macula vessel density loss was less than GCC thickness loss in early glaucoma, OCT-A measurement is still relevant for early detection of glaucoma.
There are some limitations to the current study. There is evidence that ocular hypotensive eye drops might affect ocular blood flow.42,43 Although the number of patients using topical glaucoma medications were similar in the two studied groups, some patients were receiving multiple eye drops and the overall use of topical medications in the two groups was different. Therefore, we cannot entirely exclude the possibility that the ocular hypotensive drops could be responsible for the vascular changes. Similarly, it is unknown if there is an effect on macula vessel density dropout by systemic medications. Since the three groups differed by the proportion of subjects with self-reported history of hypertension (P= 0.005) and diabetes (borderline P= 0.081), self-reported diabetes and hypertension were included as confounders in all multivariable analyses to adjust for the possible effect of these medications. It has been shown that the mean vessel density and macula thickness is significantly correlated with age and image quality.7,44,45 Although age of study groups was matched in the current study and age had been included in the multivariable models, we still cannot completely exclude the influence of age on the results. Further, a sample of 57 healthy eyes was examined to acquire the normal mean value. A larger number of healthy eyes would provide a more reliable reference. In addition, it has been reported that 6×6 mm2 macula scans showed higher diagnostic accuracy compared to 3×3 mm2 scans for differentiating between healthy and glaucoma eyes20 because the most vulnerable macula areas to glaucoma lie mostly outside the central 3×32 mm.13,46 However, the concomitant reduction in scan resolution would decrease the signal-to-noise ratio of the OCT-A images and underestimate vessel density measurement. Moreover, measurement of inner macula vessel density over the 3×3 mm2 area has a low test-retest variability.18 Analysis of vascular density from a high-resolution 6×6 mm2 field might better address this issue. Besides, in the current study, vessel density of deep retina layer (IPL-10 μm~OPL+10 μm) was not significantly different among healthy, pre-perimetric glaucoma and early glaucoma eyes (whole image 48.9 (48.1, 49.7)% vs. 48.3 (47.4, 49.2)% vs. 49.1 (48.5, 49.7)%; perifoveal 50.7 (49.9, 51.5)% vs. 50.0 (49.1, 50.9)% vs. 50.8 (50.3, 51.4)%) , respectively; all P values> 0.05), a result that is consistent with previous report.20 Corresponding thickness measurement of the deep retinal layer, which mainly composed of the inner nuclear layer and OPL are not available. Future exploration of relevant thickness and vessel changes in the deep retina layer may provide more information about glaucoma pathophysiology. Finally, since this was a cross-sectional study, we are not able to comment on the effectiveness of vessel density measurements in assessing disease progression. Longitudinal studies will help clarify the pattern of glaucomatous microvasculature damages.
In conclusion, both macula GCC thinning and macula vessel density dropout were detectable in pre-perimetric and early POAG. Although GCC loss was greater than macula vessel density loss in early perimetric POAG, OCT-A and OCT measurements similarly detected early glaucoma.
Acknowledgments/Disclosure
a. Funding/Support:
National Institutes of Health/National Eye Institute Grants R01EY029058, R01EY011008, R01 EY14267, and R01 EY027510, Core Grant P30EY022589, an unrestricted grant from Research to Prevent Blindness (New York, NY), and grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen.
b. Financial Disclosures:
Huiyuan Hou: none; Sasan Moghimi: none; Linda Zangwill: Research support- National Eye Institute, Carl Zeiss Meditec, Heidelberg Engineering, Topcon, and Optovue; Consultant- Merck; Honoraria Topcon, Regeneron; Takuhei Shoji: Financial support-Alcon; Rafaella C. Penteado: none; Elham Ghahari: none; Patricia Isabel C. Manalastas: none; Robert N. Weinreb: Research support- Carl Zeiss Meditec, Centervue, Genentech, Heidelberg Engineering, Konan, National Eye Institute, Optos, Optovue, and Tomey; Consultant- Aerie Pharmaceuticals, Alcon, Allergan, Eyenovia, Novartis, Unity, Valeant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014; 311(18): 1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004; 363(9422): 1711–20. [DOI] [PubMed] [Google Scholar]
- 3.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016; 57(9): OCT451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21(4): 359–93. [DOI] [PubMed] [Google Scholar]
- 5.Grieshaber MC, Mozaffarieh M, Flammer J. What is the link between vascular dysregulation and glaucoma? Surv Ophthalmol 2007; 52 Suppl 2: S144–54. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri K Optical coherence tomography angiography and glaucoma: searching for the missing link. Expert Rev Med Devices 2016; 13(10): 879–80. [DOI] [PubMed] [Google Scholar]
- 7.Shoji T, Zangwill LM, Akagi T, et al. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. Am J Ophthalmol 2017; 182: 107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012; 20(4): 4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology 2017; 124(5): 709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016; 123(12): 2498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cennamo G, Montorio D, Velotti N, Sparnelli F, Reibaldi M, Cennamo G. Optical coherence tomography angiography in pre-perimetric open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2017; 255(9): 1787–93. [DOI] [PubMed] [Google Scholar]
- 12.Akil H, Huang AS, Francis BA, Sadda SR, Chopra V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One 2017; 12(2): e0170476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res 2013; 32: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol 2014; 132(3): 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol 1990; 300(1): 5–25. [DOI] [PubMed] [Google Scholar]
- 16.Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol 2017. [DOI] [PubMed] [Google Scholar]
- 17.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One 2017; 12(3): e0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan KH, Lam AKN, Leung CK. Optical Coherence Tomography Angiography Compared With Optical Coherence Tomography Macular Measurements for Detection of Glaucoma. JAMA Ophthalmol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triolo G, Rabiolo A, Shemonski ND, et al. Optical Coherence Tomography Angiography Macular and Peripapillary Vessel Perfusion Density in Healthy Subjects, Glaucoma Suspects, and Glaucoma Patients. Invest Ophthalmol Vis Sci 2017; 58(13): 5713–22. [DOI] [PubMed] [Google Scholar]
- 20.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 2017; 124(11): 1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS. Optical Coherence Tomography Angiography of the Superficial Microvasculature in the Macular and Peripapillary Areas in Glaucomatous and Healthy Eyes. Invest Ophthalmol Vis Sci 2017; 58(9): 3637–45. [DOI] [PubMed] [Google Scholar]
- 22.Penteado RC, Zangwill LM, Daga FB, et al. Optical Coherence Tomography Angiography Macular Vascular Density Measurements and the Central 10-2 Visual Field in Glaucoma. J Glaucoma 2018; 27(6): 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009; 127(9): 1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydogan T, Akcay BIS, Kardes E, Ergin A. Evaluation of spectral domain optical coherence tomography parameters in ocular hypertension, preperimetric, and early glaucoma. Indian J Ophthalmol 2017; 65(11): 1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cvenkel B, Sustar M, Perovsek D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc Ophthalmol 2017; 135(1): 17–28. [DOI] [PubMed] [Google Scholar]
- 26.Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2017; 125(4): 578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodapp E, Parish IIRK, Anderson DR Clinical Decisions in Glaucoma. St Louis, MO: Mosby; 1993: 52–61. [Google Scholar]
- 28.Zhang C, Tatham AJ, Abe RY, et al. Macular Ganglion Cell Inner Plexiform Layer Thickness in Glaucomatous Eyes with Localized Retinal Nerve Fiber Layer Defects. PLoS One 2016; 11(8): e0160549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci 2010; 51(7): 3531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci 2009; 50(4): 1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Tatham AJ, Weinreb RN, et al. Relationship between ganglion cell layer thickness and estimated retinal ganglion cell counts in the glaucomatous macula. Ophthalmology 2014; 121(12): 2371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YK, Jeoung JW, Park KH. Inferior Macular Damage in Glaucoma: Its Relationship to Retinal Nerve Fiber Layer Defect in Macular Vulnerability Zone. J Glaucoma 2017; 26(2): 126–32. [DOI] [PubMed] [Google Scholar]
- 33.Choi J, Kwon J, Shin JW, Lee J, Lee S, Kook MS. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One 2017; 12(9): e0184948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurysheva NI, Maslova EV, Trubilina AV, Ardzhevnishvili TD, Fomin AV. Macular blood flow in glaucoma. Vestn Oftalmol 2017; 133(2): 29–38. [DOI] [PubMed] [Google Scholar]
- 35.Alnawaiseh M, Lahme L, Muller V, Rosentreter A, Eter N. Correlation of flow density, as measured using optical coherence tomography angiography, with structural and functional parameters in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2018; 256(3): 589–97. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Yu J, Kong X, Sun X, Jiang C. Macular microvasculature alterations in patients with primary open-angle glaucoma: A cross-sectional study. Medicine (Baltimore) 2016; 95(33): e4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renard JP, Fenolland JR, El Chehab H, et al. [Analysis of macular ganglion cell complex (GCC) with spectral-domain optical coherence tomography (SD-OCT) in glaucoma]. J Fr Ophtalmol 2013; 36(4): 299–309. [DOI] [PubMed] [Google Scholar]
- 38.Pazos M, Dyrda AA, Biarnes M, et al. Diagnostic Accuracy of Spectralis SD OCT Automated Macular Layers Segmentation to Discriminate Normal from Early Glaucomatous Eyes. Ophthalmology 2017; 124(8): 1218–28. [DOI] [PubMed] [Google Scholar]
- 39.Spaide RF, Curcio CA. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol 2017; 135(3): 259–62. [DOI] [PubMed] [Google Scholar]
- 40.Hammel N, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol 2017; 178: 38–50. [DOI] [PubMed] [Google Scholar]
- 41.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Angle Closure and Primary Angle Closure Glaucoma. Am J Ophthalmol 2017; 177: 106–15. [DOI] [PubMed] [Google Scholar]
- 42.Feke GT, Bex PJ, Taylor CP, et al. Effect of brimonidine on retinal vascular autoregulation and short-term visual function in normal tension glaucoma. Am J Ophthalmol 2014; 158(1): 105–12 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siesky B, Harris A, Brizendine E, et al. Literature review and meta-analysis of topical carbonic anhydrase inhibitors and ocular blood flow. Surv Ophthalmol 2009; 54(1): 33–46. [DOI] [PubMed] [Google Scholar]
- 44.lafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016; 57(13): 5780–7. [DOI] [PubMed] [Google Scholar]
- 45.von Hanno T, Lade AC, Mathiesen EB, Peto T, Njolstad I, Bertelsen G. Macular thickness in healthy eyes of adults (N = 4508) and relation to sex, age and refraction: the Tromso Eye Study (2007-2008). Acta Ophthalmol 2017; 95(3): 262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 2017; 57: 46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


