Over the past decade, our understanding of coronary atherosclerosis has evolved from a paradigm dominated by coronary stenosis towards a deeper, more nuanced appreciation of coronary anatomy and physiology. Following the observation that most myocardial infarctions are caused by lesions deemed non-obstructive, there has been continued fervent search for imaging markers of plaque likely to cause adverse events.
Coronary computed tomography angiography (CCTA) has been established as a reliable noninvasive imaging modality for the evaluation of coronary artery disease in stable patients with chest pain (1). It has the advantage of being highly accurate in ruling out the presence of obstructive coronary disease due to its high negative predictive value (sensitivity in the range of 85–95% and specificity in the range of 83–97%) (2-4). Further, the detection of non-obstructive coronary artery disease on CCTA has been shown to have significant prognostic value, increasing all-cause mortality by 2-fold in the presence of any non-obstructive plaque (5). Additionally, as an anatomic imaging modality capable of directly visualizing atherosclerotic plaque, CCTA can identify imaging correlates of pathologically determined vulnerable plaque, such as thin-cap fibroatheroma and necrotic core. On the other hand, the amount of calcium in non-obstructive lesions has been shown not to be associated with future coronary events, as opposed to the amount of non-calcified plaque, highlighting the notion that the presence of calcified plaque likely confers stability (6).
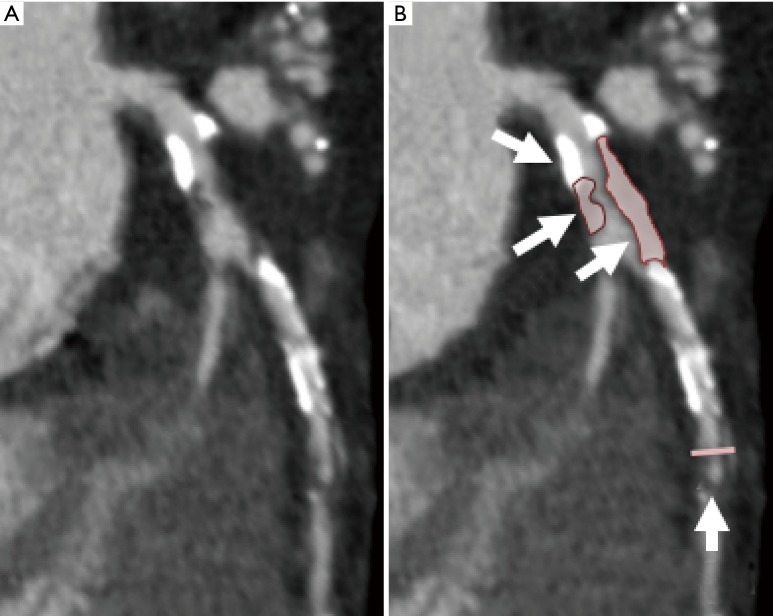
Studies using CCTA have demonstrated an association between 4 major plaque characteristics and future risk of acute coronary syndrome (ACS): positive remodeling, low attenuation plaque (<30 Hounsfield Units), spotty calcification and the napkin ring sign (7-10). The recent Coronary Artery Disease-RADS guidelines of the Society of Cardiovascular Computed Tomography (SCCT) support the reporting of the presence of these high-risk plaque (HRP) features (11). However, the extent to which these plaque characteristics improve risk stratification in stable patients undergoing evaluation for ischemic heart disease remains in question (Figure 1).
Figure 1.
Evaluation of atherosclerotic plaque on noninvasive imaging. (A) shows atherosclerotic plaque in the left anterior descending artery on coronary CT angiography, while (B) highlights some of the HRP features such as low attenuation plaque and positive remodeling. HRP, high-risk plaque.
In the February issue of JAMA Cardiology, Ferencik and colleagues elaborate on their investigation of HRP within the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial. In this prespecified secondary analysis of the PROMISE trial, the authors sought to determine the association between HRP and major adverse cardiovascular events (MACE, a conglomerate of death, myocardial infarction or unstable angina) using a nested observational study design of patients who were randomized to undergo CCTA as part of an evaluation for ischemic heart disease (12). The analysis included 4,415 individuals with stable, symptomatic chest pain who underwent CCTA imaging and were followed for a median of 25 months. HRP features were qualitatively defined and determined by a core laboratory. For the purposes of the study they included: positive remodeling, low attenuation plaque and napkin-ring sign. It should be mentioned that one particular recognized HRP feature not included in this analysis was spotty calcification. The overall incidence of MACE over the follow up period was relatively low at 3%, despite the fact that the mean number of risk factors per patient was 2.36 (SD 1.08) and that a large proportion of the cohort were considered at high long term risk when assessed with either the Framingham risk score (40.7% were assessed to have a risk of >20%) or atherosclerotic cardiovascular disease (ASCVD) risk score (66.8% were considered to have a 10 year risk of ≥7.5%). Overall, the presence of HRP was associated with a higher risk of MACE [6.4% vs. 2.4%, unadjusted hazard ratio (HR), 2.73; 95% CI: 1.89–3.93], which remained significant after adjustment for ASCVD risk score and significant stenosis (defined as ≥70% diameter stenosis in any coronary artery or ≥50% stenosis in the left main artery). More comprehensive adjustment for clinical variables or CCTA variables was not provided and thus the possibility of residual confounding in the association between HRP and MACE remains a consideration. In a series of subgroup analyses, the authors highlight the greater magnitude of association between HRP and MACE among women compared with men [adjusted HR (aHR) 2.41 vs. 1.40, respectively] and patients under the median age for the cohort (aHR 2.33 vs. 1.36, respectively), highlighting the potential use of HRP as a risk stratification tool in such subgroups of individuals with lower atherosclerotic burden and lower occurrence of obstructive coronary artery disease. While this may be true, no formal test of interaction was provided in the study to support the assertion that sex or age modify the effect of HRP on MACE.
Despite net reclassification improvement, the addition of HRP to a model that included significant stenosis and the ASCVD score did not improve discrimination [area under the curve (AUC) of 0.69 without HRP vs. AUC of 0.71 with HRP, P=0.12]. In terms of test characteristics, the poor positive predictive value of HRP stands out as a factor that may limit its clinical application. This is in part due to the low rate of MACE and short follow up duration. It is important to keep in mind that the PROMISE trial was not designed to evaluate the natural progression of coronary artery disease, and downstream change in medical therapy after CCTA performance could have affected the occurrence of MACE and its relationship to HRP.
The major strengths of the aforementioned study include the relatively large cohort of patients in addition to the prespecified nature of the analysis and core lab assessment of CCTA for significant stenosis and qualitative measures of HRP features. Additional features of the study population (mean age of 60.5, equal distribution of males/females albeit with a predominance of non-Hispanic white individuals) enhance generalizability. The performance of sensitivity analyses for both ≥50% and ≥70% stenosis also increases the applicability of the findings to clinical practice. Finally, MACE was constructed to include hard outcomes: death, myocardial infarction or unstable angina. Limitations worth highlighting include the relatively low event rate (MACE event rate of 3%) and modest interobserver agreement amongst readers for HRP on CCTA (κ=0.56). The latter highlights the need for improved automated methods of measuring HRP and standardization in its reporting. An additional limitation of the study was use of the ASCVD risk score to control for confounding. As iterated in the accompanying editorial by Gibbons (13), the ASCVD risk score was intended for use in asymptomatic populations and adjustment for factors such as symptom typicality, dyspnea, and comorbid disease would seem germane to this type of analysis.
The findings by Ferencik et al. are in keeping with prior investigations in patients presenting to the emergency department with chest pain. Compared to outpatients with stable chest pain, findings from the Rule Out Myocardial Infarction using Computer Assisted Tomography-II (ROMICAT-II) trial found that the presence of HRP (in this instance, HRP was defined as presence of at least one of the 4 major plaque characteristics associated with HRP) was significantly associated with the occurrence of ACS in 472 patients who presented with acute chest pain and underwent CCTA for diagnostic evaluation [odds ratio (OR) 8.9; 95% CI: 1.8–43.3] (14). The incidence of ACS was 7.8%, and similar to the analysis of the PROMISE trial, the incidence of HRP was positively correlated with increasing degree of diameter stenosis. Additionally, HRP had incremental prognostic value beyond clinical features and diameter stenosis (AUC of 0.776 for baseline clinical characteristics, AUC of 0.935 for combined clinical characteristics and diameter stenosis ≥50%, and AUC of 0.959 for the addition of HRP to clinical characteristics and diameter stenosis). More recently, Chang and colleagues performed a nested case-control study in which 234 patients with incident ACS and undergoing CCTA were propensity matched to 234 patients without ACS (15). The presence of HRP (defined as the presence of at least 2 features of spotty calcification, low attenuation plaque or positive remodeling) was associated with ACS on a per-patient (HR 1.59; 95% CI: 1.22–2.08) and a per-lesion (HR 1.95; 95% CI: 1.32–2.90) basis.
Beyond prognosis, further inquiry is needed to understand the implications of medical therapy on HRP features. Most recently, Vaidya and colleagues evaluated the effect of low-dose colchicine therapy, in addition to optimal medical therapy, on low attenuation plaque volume (LAPV) in 80 patients post ACS, and demonstrated that colchicine therapy was significantly associated with greater reduction in LAPV (P=0.039), even after multivariate adjustment (16). However, endpoints did not include clinical outcomes. Further, invasive and noninvasive imaging has been extensively used to assess the effect of medical therapy on quantitative and qualitative plaque features (17-19). For instance, intravascular ultrasound evaluation showed that statin therapy promoted coronary atheroma calcification independent of plaque regression (17). In another study of 140 patients with acute myocardial infarction who underwent serial CCTA, early aggressive statin therapy was associated with a 23% increase in total dense calcium volume as compared with standard dose statin (19). Moreover, a recent study of 1,255 patients undergoing serial CCTA imaging found that statin therapy was associated with slower progression of atherosclerotic plaque volume, with an increase in plaque calcification and a 35% reduction in the development of HRP (20). Thus, the work by Ferencik and colleagues adds to the growing literature supporting the association between HRP and major cardiac events, and extends the field to a large stable symptomatic population. Atherosclerotic plaque characterization using CCTA will continue to be an active area of investigation as we seek to rigorously determine the utility of HRP features above and beyond clinical and imaging characteristics in clinical practice.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by Executive Editor-in-Chief Jianxing He (Director of the Thoracic Surgery Department, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: Dr. Min serves on the scientific advisory board of Arineta, on the speaker’s bureau of GE Healthcare, and owns equity in Cleerly. The other authors have no conflicts of interest to declare.
References
- 1.Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;55:2663-99. 10.1016/j.jacc.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724-32. 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 3.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. 10.1056/NEJMoa0806576 [DOI] [PubMed] [Google Scholar]
- 4.Schuijf JD, Pundziute G, Jukema JW, et al. Diagnostic accuracy of 64-slice multislice computed tomography in the noninvasive evaluation of significant coronary artery disease. Am J Cardiol 2006;98:145-8. 10.1016/j.amjcard.2006.01.092 [DOI] [PubMed] [Google Scholar]
- 5.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58:510-9. 10.1016/j.jacc.2010.11.078 [DOI] [PubMed] [Google Scholar]
- 6.Kristensen TS, Kofoed KF, Kühl JT, et al. Prognostic implications of nonobstructive coronary plaques in patients with non-ST-segment elevation myocardial infarction: a multidetector computed tomography study. J Am Coll Cardiol 2011;58:502-9. 10.1016/j.jacc.2011.01.058 [DOI] [PubMed] [Google Scholar]
- 7.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 8.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26. 10.1016/j.jacc.2007.03.044 [DOI] [PubMed] [Google Scholar]
- 9.Motoyama S, Sarai M, Narula J, et al. Coronary CT angiography and high-risk plaque morphology. Cardiovasc Interv Ther 2013;28:1-8. 10.1007/s12928-012-0140-1 [DOI] [PubMed] [Google Scholar]
- 10.Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging 2013;6:448-57. 10.1016/j.jcmg.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 11.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269-81. 10.1016/j.jcct.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018;3:144-52. 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons RJ. High-Risk Coronary Atherosclerotic Plaque Assessment by Coronary Computed Tomography Angiography-Should We Use It? JAMA Cardiol 2018;3:153-4. 10.1001/jamacardio.2017.5016 [DOI] [PubMed] [Google Scholar]
- 14.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684-92. 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HJ, Lin F, Lee SE, et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol 2018;71:2511-22. 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidya K, Arnott C, Martínez GJ, et al. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging 2018;11:305-16. 10.1016/j.jcmg.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 17.Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273-82. 10.1016/j.jacc.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 18.Räber L, Taniwaki M, Zaugg S, et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur Heart J 2015;36:490-500. 10.1093/eurheartj/ehu373 [DOI] [PubMed] [Google Scholar]
- 19.Auscher S, Heinsen L, Nieman K, et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: Assessment with serial coronary CT angiography. Atherosclerosis 2015;241:579-87. 10.1016/j.atherosclerosis.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Chang HJ, Sung JM, et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM (Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging) Study. JACC Cardiovasc Imaging 2018. [Epub ahead of print].