Abstract
Background
There is a paucity of data regarding the racial and sex disparities in the outcomes of multi-vessel percutaneous coronary interventions (MVPCI).
Methods
The National Inpatient Sample (NIS) was examined for the years 2010 to 2014 to incorporate adult MVPCI-related hospitalizations using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes. We excluded patients with the missing race or gender data from the final scrutiny. Discharge weights were used to obtain the national estimations. The principal outcomes were MVPCI-related racial and gender disparities in terms of the in-hospital mortality and complications, and diagnostic and therapeutic healthcare resource utilization. Secondary outcomes were the length of hospital stay (LOS) and hospitalization charges. We used the Chi-square test and t-test/ANOVA test to equate dichotomous and continuous variables respectively. A two-tailed P of <0.05 was considered clinically significant.
Results
An estimated 769,502 MVPCI-related hospitalizations were recorded from 2010 to 2014 after excluding patients with the missing data (70,954; 8.4%). Black male and female were the youngest (62±13, 64±14 years). The highest non-elective admissions (M: 72.8%, F: 71.2%) were reported among Hispanics. Non-whites showed a higher proportion of comorbidities with lower resource utilization than whites. Hispanic males (OR 1.23) showed the highest odds of the in-hospital mortality whereas among females, Asians (OR 1.51), blacks (OR 1.35), followed by Hispanics (OR 1.22) revealed higher odds of in-hospital mortality. Odds of cardiac complications were highest amongst Asians (M: OR 1.19, F: OR 1.40). Black (6±8 days) and Hispanic (7±9 days) showed the highest length of stay among males and females respectively. Total hospitalization charges were highest among Asians. There was a greater increase in the all-cause mortality in non-whites from 2010 to 2014.
Conclusions
This study determines the existence of racial disparities in resource utilization and outcomes in MVPCI. There is an instant need for interventions designed to govern these healthcare discrepancies.
Keywords: Multivessel coronary artery disease, multi-vessel percutaneous coronary interventions (MVPCI), racial/ethnic disparities, sex/gender disparities, all-cause mortality
Introduction
Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are performed in more than a million patients annually in the United States (US) (1). Among the patients undergoing coronary angiography, the multivessel disease is found to be present in up to 50% of the patients (2). However, among the patients with higher surgical risk, PCI is preferred for revascularization. Complete coronary revascularization shows the better outcomes of mortality and reinfarction in patients with acute myocardial infarction (AMI), but if not done in a timely and appropriate manner can lead to detrimental effects. Although a lot of development has been accomplished in the field of PCI, it still fails to serve the population equally with increasing evidence suggesting decreased long-term post-PCI survival among blacks compared to whites (3). The past four decades have witnessed a decline in the coronary heart disease (CHD)-associated mortality rate in the US but this decline is an accompanied by the racial disparity. Between 2000 and 2008, the rate of mortality associated with acute CHD declined steeply for whites as compared to blacks (4). Similarly, the rate of hospitalizations related to AMI decreased between the years 2002 and 2007 more for whites than for blacks (4). Studies have reported racial and ethnicity-related health disparities in the US with non-whites susceptible to worse outcomes than whites (5). Existing data suggest a higher risk of cardiovascular disease susceptibility, related death, and deprivation from beneficial interventions like coronary angiography and PCI among blacks, Asians, and Hispanics (5,6). These groups also show a racial difference in the outcome of the procedures such as coronary artery bypass graft (CABG) and PCI. However, the data in regards to the outcome disparities after PCI remain inconsistent (7). Multi-vessel percutaneous coronary interventions (MVPCI) can be challenging at times even for experienced operators and to the best of our knowledge, no study has evaluated the racial or sex disparities in high-risk MVPCI outcomes. As affirmed by Kurian and Cardarelli, better understanding and consciousness of the variations of cardiovascular disease outcomes by race and ethnicity may help clinicians to develop socially sensitive interventions, corrective action plans, and administrations engaged towards issues in each of this population (8). The main objective of this study was to look at the racial disparities in the resource utilization and outcomes of MVPCI using the largest inpatient US database, the Nationwide Inpatient Sample (NIS).
Methods
Study population and variables
We reviewed the 2010 to 2014 NIS databases to identify all MVPCI-related hospitalizations. The NIS is a database developed as part of the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ) (www.hcup-us.ahrq.gov/nisoverview.jsp). It is formulated to estimate a stratified 20% sample of community hospitals serving the US population, excluding rehabilitation and long-term acute care hospitals. The NIS database essentially has all-payers information. The sampling framework includes over 95% of discharges from US hospitals according to defined strata based on the owner type, a number of beds, location, the teaching status, and the region. The patients <18 years old and those with missing documentation on race/ethnicity or sex were excluded from the final study analysis. The study was exempt from an IRB approval since the NIS is a publically available dataset and contains only deidentified information.
Among all the MVPCI-related hospitalizations, we studied the demographics, type (elective/non-elective) and day (Weekday/Weekend) of admission, hospital characteristics including hospital bed size (small, medium or large), location/teaching status (rural, urban non-teaching or urban teaching), region, median household income quartiles and payer status, relevant comorbidities, resource utilization (coronary angiography catheterization, intravascular ultrasound, fractional flow reserve, bare-metal stent, drug-eluting stent, intra-aortic balloon pump, ventricular assist device, coronary atherectomy) for the diagnostic and therapeutics and the outcomes in terms of in-hospital mortality and post-procedural complications (cardiovascular, postoperative bleeding, postoperative infections, postoperative stroke and postoperative acute kidney injury).
Outcomes
The principal outcomes were racial and gender disparities in terms of the in-hospital mortality, procedural complications, and healthcare resources utilization. Secondary outcomes were the length of hospital stay (LOS) and hospital charges. The NIS database dispenses up to 25 diagnoses for each discharge report and up to 15 procedural codes. MVPCI-related hospitalizations from January 1, 2010, to December 31, 2014, covering the 44 states, were evaluated using International Classification of Diseases, 9th revision, clinical modification (ICD-9-CM) procedure codes 00.41, 00.42, and 00.43. Prior research validates the implementation of the corresponding procedure codes to identify MVPCI, related outcomes, complications and healthcare resource utilization (2). Relevant comorbidities defined by the AHRQ definitions were extracted from the datasets (http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp). All demographic variables, comorbidities, and hospital characteristics are available on the HCUP website (https://www.hcup-us.ahrq.gov/nisoverview.jsp), and these variables were incorporated in a two-step hierarchical logistic regression model and depicted as a forest plot.
Statistical analysis
As a part of the self-weighing design, discharge weights (DISCWT) provided in the database were applied in the analysis to get the national estimates (https://www.hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course.jsp). We performed a bivariate analysis to assess the sex–race differences in the patients’ characteristics and outcomes. We applied the Chi-square test to compare dichotomous and categorical variables and the ANOVA test for continuous variables. A two-way hierarchical multivariate regression model was constructed to analyze the racial and sex disparities in terms of in-hospital mortality and procedural outcomes among patients undergoing MVPCI, adjusting for patient’s age, demographics, hospital characteristics and related comorbidities using complex sample function to reflect weights and strata provided in data. We incorporated 11 dummy variables (white female, black male, black female, Hispanic male, Hispanic female, Asian male, Asian female, native American male, native American female, other male and other female) in the model keeping the white male as a reference. The identical regression model for in-hospital mortality and complications was constructed. A two-sided significance level of 0.05 was considered as the clinically significant difference. All analyses were performed using IBM SPSS Statistics 22.0 (IBM Corp, Armonk, New York) software. Patient-data confidentiality was entertained through the data use agreement. However, the study was exempted from the institutional review board approval, as the NIS is an anonymous deidentified publicly available database.
Results
After excluding <18 years old patients, an estimated 840,455 (weighted N) MVPCI-related hospitalizations were identified in the US from 2010 to 2014. Total 70,954 (8.4%) patients with missing gender and/or race information were excluded from the study. A total of 769,502 (weighted N) patients were included in the final analysis. We further stratified the MVPCI cohort into twelve subcategories as per the race and gender as shown in Table 1.
Table 1. Baseline characteristics of study population undergoing MVPCI (n=769,502).
| Variables | White, % | Black, % | Hispanic, % | Asian, % | Native American, % | Other, % | P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| Number | 366,406 | 208,069 | 44,162 | 41,276 | 38,309 | 23,286 | 10,922 | 5,557 | 2,979 | 2,167 | 17,760 | 8,608 | ||||||
| Age in years | ||||||||||||||||||
| Mean age (years) | 66±12 | 70±13 | 62±13 | 64±14 | 63±13 | 66±15 | 64±13 | 69±14 | 64±12 | 66±12 | 64±13 | 68±14 | <0.001 | |||||
| 18–44 | 3.4 | 3.5 | 7.6 | 8.1 | 6.0 | 5.3 | 6.8 | 4.4 | 5.2 | 4.5 | 5.6 | 3.9 | <0.001 | |||||
| 45–64 | 39.5 | 28.2 | 50.6 | 41.0 | 45.9 | 34.0 | 43.9 | 26.2 | 46.3 | 39.7 | 45.2 | 31.0 | <0.001 | |||||
| 65–84 | 50.9 | 55.7 | 38.5 | 43.8 | 43.3 | 51.0 | 44.9 | 58.7 | 45.5 | 51.0 | 44.0 | 54.5 | <0.001 | |||||
| ≥85 | 6.0 | 12.2 | 2.8 | 6.6 | 4.0 | 8.3 | 4.0 | 10.0 | 2.8 | 4.6 | 4.7 | 9.6 | <0.001 | |||||
| Elective versus non-elective admission | ||||||||||||||||||
| Non-elective | 63.6 | 61.3 | 71.9 | 70.1 | 72.8 | 71.2 | 68.0 | 69.6 | 62.1 | 55.9 | 67.8 | 65.6 | <0.001 | |||||
| Elective | 36.4 | 38.7 | 28.1 | 29.9 | 27.2 | 28.8 | 32.0 | 30.4 | 37.9 | 44.1 | 32.2 | 34.4 | <0.001 | |||||
| Median household income national quartile for patient ZIP code# | ||||||||||||||||||
| 0–25th | 24.7 | 27.0 | 52.0 | 55.7 | 39.9 | 43.2 | 12.0 | 12.4 | 49.0 | 57.7 | 24.5 | 32.7 | <0.001 | |||||
| 26–50th | 27.8 | 28.7 | 21.3 | 21.0 | 25.3 | 24.4 | 17.1 | 20.4 | 25.5 | 23.5 | 25.3 | 23.5 | <0.001 | |||||
| 51–75th | 25.4 | 24.7 | 16.1 | 14.7 | 22.5 | 20.8 | 28.3 | 27.1 | 16.1 | 14.8 | 24.4 | 22.0 | <0.001 | |||||
| 76–100th | 22.2 | 19.6 | 10.6 | 8.5 | 12.3 | 11.7 | 42.6 | 40.1 | 9.4 | 4.1 | 25.8 | 21.8 | <0.001 | |||||
| Control/ownership of hospital | ||||||||||||||||||
| Government, nonfederal | 8.6 | 8.3 | 13.7 | 12.1 | 13.2 | 12.0 | 8.9 | 8.8 | 19.7 | 26.8 | 9.0 | 8.7 | <0.001 | |||||
| Private, non-profit | 75.8 | 75.6 | 69.9 | 70.9 | 57.5 | 56.8 | 76.4 | 75.6 | 53.1 | 47.8 | 73.2 | 71.9 | <0.001 | |||||
| Private, invest-own | 15.6 | 16.2 | 16.4 | 17.0 | 29.3 | 31.2 | 14.7 | 15.6 | 27.2 | 25.4 | 17.8 | 19.4 | <0.001 | |||||
| Location/teaching status of the hospital | ||||||||||||||||||
| Rural | 6.5 | 7.1 | 4.0 | 3.4 | 3.0 | 3.4 | 1.1 | 1.0 | 22.4 | 29.4 | 3.4 | 4.5 | <0.001 | |||||
| Urban, non-teaching | 38.7 | 39.7 | 28.3 | 28.9 | 39.7 | 41.1 | 34.4 | 37.5 | 35.7 | 28.2 | 34.1 | 35.5 | <0.001 | |||||
| Urban, teaching | 54.8 | 53.2 | 67.7 | 67.7 | 57.2 | 55.5 | 64.5 | 61.5 | 41.9 | 42.4 | 62.5 | 59.9 | <0.001 | |||||
| Region of hospital | ||||||||||||||||||
| Northeast | 18.5 | 17.7 | 16.9 | 17.1 | 15.5 | 14.9 | 24.2 | 18.5 | 6.8 | 4.9 | 37.2 | 36.1 | <0.001 | |||||
| Midwest | 25.7 | 26.1 | 20.9 | 22.0 | 6.6 | 6.0 | 8.8 | 8.5 | 19.0 | 18.5 | 16.4 | 16.8 | <0.001 | |||||
| South | 37.9 | 39.4 | 53.4 | 53.0 | 42.5 | 43.7 | 15.2 | 14.2 | 43.9 | 47.0 | 30.4 | 31.4 | <0.001 | |||||
| West | 17.8 | 16.8 | 8.8 | 8.0 | 35.4 | 35.4 | 51.8 | 58.8 | 30.4 | 29.6 | 16.1 | 15.6 | <0.001 | |||||
| Primary expected payer | ||||||||||||||||||
| Medicare | 58.8 | 71.2 | 56.7 | 66.6 | 52.0 | 61.2 | 42.2 | 62.9 | 54.4 | 64.0 | 46.5 | 63.3 | <0.001 | |||||
| Medicaid | 4.8 | 5.6 | 12.1 | 13.2 | 13.0 | 16.6 | 16.0 | 15.6 | 10.3 | 11.1 | 12.8 | 14.3 | <0.001 | |||||
| Private including HMO | 29.2 | 18.6 | 21.4 | 14.9 | 23.2 | 14.3 | 34.2 | 16.5 | 22.5 | 15.5 | 30.6 | 16.5 | <0.001 | |||||
| Self-pay | 4.1 | 2.8 | 5.6 | 3.3 | 7.0 | 4.7 | 4.3 | 3.2 | 3.3 | 3.1 | 6.8 | 4.3 | <0.001 | |||||
| No charge | 0.4 | 0.3 | 0.5 | 0.4 | 1.3 | 0.7 | 0.5 | 0.1 | 0.0 | 0.0 | 0.3 | 0.5 | <0.001 | |||||
| Other | 2.7 | 1.4 | 3.7 | 1.6 | 3.6 | 2.6 | 2.9 | 1.8 | 9.4 | 6.2 | 2.9 | 1.1 | <0.001 | |||||
| Length of stay (days) | 4±6 | 5±6 | 6±8 | 7±9 | 6±8 | 7±9 | 5±8 | 6±10 | 5±6 | 5±5 | 4±6 | 6±9 | <0.001 | |||||
| ≤3 days | 64.3 | 55.6 | 47.0 | 41.6 | 52.5 | 45.9 | 64.4 | 51.4 | 61.2 | 58.9 | 64.4 | 52.0 | <0.001 | |||||
| ≥13 days | 6.5 | 8.4 | 13.1 | 15.5 | 10.7 | 13.2 | 7.4 | 10.7 | 7.4 | 8.1 | 7.4 | 12.4 | <0.001 | |||||
| Total hospital charges | $95,741 | $97,388 | $106,725 | $106,618 | $128,096 | $130,956 | $128,842 | $139,186 | $ 97,679 | $ 82,917 | $102,641 | $111,564 | <0.001 | |||||
| All-cause in-hospital mortality | 2.0 | 2.6 | 1.8 | 2.5 | 2.5 | 3.0 | 2.2 | 3.8 | 1.2 | 1.6 | 1.6 | 3.4 | <0.001 | |||||
P value <0.05 indicates clinical significance. #, the quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. Derived from https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp. MVPCI, multi-vessel percutaneous coronary interventions; HMO, health maintenance organization.
Baseline characteristics (Table 1)
Among all the races, black males and females were the youngest (62±13, 64±14 years). The Hispanic males and females undergoing MVPCI were found to have the highest non-elective admission (72.8%, 71.2%; P<0.001). Hispanic males (2.5%), and Asian females (3.8%) showed the highest in-hospital mortality. Black males (52.0%) and native-American females (57.7%) were in the lowest median household income group. As expected, MVPCI was predominantly performed at the urban teaching hospitals for all ethnicities, predominantly in black male (M) and female (F), 67.7%, 67.7%; P<0.001. Black (M, 53.4%; F, 53.0%; P<0.001) and Hispanic (M, 42.5%; F, 43.7%; P<0.001) ethnicities frequently visited the Southern hospitals, whereas, Asian (M, 51.8%; F, 58.8%; P<0.001) and white (M, 25.7%; F, 26.1%; P<0.001) patients visited hospitals in the West and the Midwest region. Black (6±8 days) and Hispanic (7±9 days) patients showed the highest mean LOS among the male and the female gender respectively. Surprisingly, total hospitalization charges were highest among the Asians for both sexes (M: $128,842 and F: $139,186, P<0.001).
The racial distribution of comorbidities
Table 2 describes the comparison of comorbidities in the patients undergoing MVPCI. Among the cardiovascular comorbidities, black males and females had a significantly higher proportion of congestive heart failure (6.5%, 7.5%), hypertension (82.5%, 83.6%), atherosclerosis (34.3%, 40.6%), cardiomyopathy (7.6%, 5.7%), peripheral vascular disease (34.1%, 37.7%), and thromboembolism (10.4%, 12.3%) as compared to whites. Blacks also had a higher prevalence of uncomplicated diabetes (30.6%, 36.3%), diabetes with complications (16.7%, 18.6%), renal failure (38.5%, 40.6%) and drug abuse (5.4%, 2.3%) among males and females respectively as shown in Table 2. Asian females had a considerably higher prevalence of coagulopathy (6.6%), cerebrovascular disease (4.8%), and previous history of CABG (10.5%) (all P were <0.001).
Table 2. Distribution of comorbidities in patients undergoing multivessel percutaneous coronary interventions.
| Comorbidity | White, % | Black, % | Hispanic, % | Asian, % | Native American, % | Other, % | P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| Alcohol abuse | 3.5 | 1.0 | 4.2 | 1.0 | 3.6 | 0.3 | 1.0 | 0.2 | 4.8 | 0.2 | 2.8 | 1.1 | <0.001 | |||||
| Congestive heart failure | 3.4 | 4.3 | 6.5 | 7.7 | 4.6 | 5.8 | 3.3 | 4.8 | 3.5 | 4.9 | 2.5 | 4.6 | <0.001 | |||||
| Chronic pulmonary disease | 20.2 | 26.0 | 15.7 | 19.5 | 11.6 | 14.9 | 9.7 | 11.6 | 20.3 | 18.6 | 14.3 | 19.1 | <0.001 | |||||
| Coagulopathy | 4.1 | 3.8 | 4.6 | 4.3 | 4.4 | 4.5 | 4.2 | 6.6 | 3.6 | 4.4 | 4.1 | 4.2 | <0.001 | |||||
| Diabetes, uncomplicated | 27.9 | 29.0 | 30.6 | 36.3 | 35.9 | 40.2 | 37.9 | 40.0 | 36.3 | 45.3 | 33.1 | 37.7 | <0.001 | |||||
| Diabetes with complications | 8.7 | 9.4 | 16.7 | 18.6 | 19.0 | 23.0 | 11.6 | 15.6 | 17.5 | 18.5 | 8.8 | 13.1 | <0.001 | |||||
| Drug abuse | 1.4 | 0.9 | 5.4 | 2.3 | 1.9 | 0.8 | 0.7 | 0.7 | 2.2 | 0.2 | 1.7 | 1.0 | <0.001 | |||||
| Smoking | 46.9 | 39.2 | 42.7 | 32.8 | 35.8 | 20.1 | 34.5 | 13.9 | 41.7 | 32.2 | 39.7 | 28.9 | <0.001 | |||||
| Hypertension | 74.7 | 76.9 | 82.5 | 83.6 | 78.9 | 80.4 | 74.5 | 80.6 | 79.6 | 81.2 | 76.4 | 78.7 | <0.001 | |||||
| Liver disease | 1.5 | 1.2 | 2.6 | 1.5 | 2.3 | 1.7 | 1.7 | 1.9 | 1.3 | 1.2 | 1.1 | 1.1 | <0.001 | |||||
| Obesity | 12.7 | 14.9 | 10.7 | 18.3 | 11.5 | 16.5 | 5.3 | 7.1 | 12.8 | 15.7 | 10.4 | 15.0 | <0.001 | |||||
| Peripheral vascular disorders | 28.7 | 34.4 | 34.1 | 37.7 | 30.9 | 36.9 | 18.0 | 26.6 | 37.7 | 42.4 | 22.5 | 31.0 | <0.001 | |||||
| Pulmonary circulation disorders | 0.7 | 1.3 | 1.3 | 2.1 | 0.8 | 1.3 | 0.5 | 1.5 | 0.3 | 1.1 | 0.5 | 0.9 | <0.001 | |||||
| Renal failure | 17.5 | 18.4 | 38.5 | 40.6 | 28.4 | 32.3 | 23.1 | 32.1 | 25.3 | 28.7 | 18.6 | 24.8 | <0.001 | |||||
| Valvular heart disease | 1.4 | 2.3 | 1.6 | 2.3 | 1.4 | 1.9 | 0.9 | 1.1 | 1.4 | 1.1 | 0.8 | 1.8 | <0.001 | |||||
| Dyslipidemia | 66.1 | 61.5 | 54.9 | 52.2 | 63.1 | 58.6 | 70.6 | 63.1 | 61.5 | 56.5 | 65.4 | 60.0 | <0.001 | |||||
| Atherosclerosis | 30.7 | 38.3 | 34.3 | 40.6 | 29.5 | 37.6 | 17.9 | 27.0 | 37.6 | 43.4 | 23.6 | 33.2 | <0.001 | |||||
| Cardiomyopathy | 4.5 | 3.9 | 7.6 | 5.7 | 5.0 | 5.2 | 5.7 | 4.6 | 3.9 | 5.1 | 3.5 | 4.5 | <0.001 | |||||
| Cerebrovascular Diseases | 2.0 | 3.0 | 2.4 | 2.9 | 2.2 | 2.5 | 1.9 | 4.8 | 1.0 | 2.5 | 2.4 | 2.5 | <0.001 | |||||
| Thromboembolism | 5.9 | 8.2 | 10.4 | 12.3 | 5.2 | 8.0 | 3.1 | 7.1 | 4.6 | 6.7 | 4.2 | 7.6 | <0.001 | |||||
| Previous history of MI | 15.3 | 11.6 | 12.8 | 10.8 | 13.1 | 10.3 | 13.6 | 11.4 | 12.4 | 10.5 | 13.2 | 9.6 | <0.001 | |||||
| Family history of CAD | 8.8 | 7.0 | 4.5 | 3.7 | 6.1 | 4.4 | 7.6 | 4.1 | 6.8 | 6.7 | 8.6 | 6.9 | <0.001 | |||||
| Previous PCI | 18.7 | 14.7 | 14.0 | 12.4 | 15.1 | 13.2 | 17.5 | 13.9 | 16.0 | 11.8 | 18.5 | 14.6 | <0.001 | |||||
| Previous CABG | 13.4 | 8.9 | 8.2 | 7.2 | 10.8 | 9.8 | 10.5 | 10.5 | 14.0 | 10.1 | 10.2 | 8.6 | <0.001 | |||||
MI, myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Procedural outcomes and in-hospital mortality trend (Table 3 and Figure 1)
Table 3. In-hospital mortality trends after multivessel-percutaneous coronary interventions.
| Year | White, % | Black, % | Hispanic, % | Asian, % | Native American, % | Others, % | P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| 2010 | 1.7 | 2.3 | 1.4 | 2.4 | 2.5 | 2.7 | 2.4 | 3.2 | 0.6 | 0.7 | 1.3 | 1.2 | <0.001 | |||||
| 2011 | 1.7 | 2.4 | 1.5 | 2.8 | 1.6 | 2.2 | 0.6 | 2.9 | 1.2 | 0.0 | 2.4 | 3.5 | <0.001 | |||||
| 2012 | 1.9 | 2.6 | 2.2 | 2.4 | 3.0 | 2.7 | 2.8 | 4.0 | 2.2 | 1.0 | 0.8 | 3.5 | <0.001 | |||||
| 2013 | 2.1 | 2.8 | 1.7 | 2.1 | 2.3 | 2.6 | 2.7 | 4.2 | 0.0 | 4.1 | 2.2 | 4.6 | <0.001 | |||||
| 2014 | 2.4 | 2.9 | 2.1 | 2.8 | 3.2 | 5.1 | 2.4 | 4.8 | 2.0 | 3.3 | 1.5 | 3.6 | <0.001 | |||||
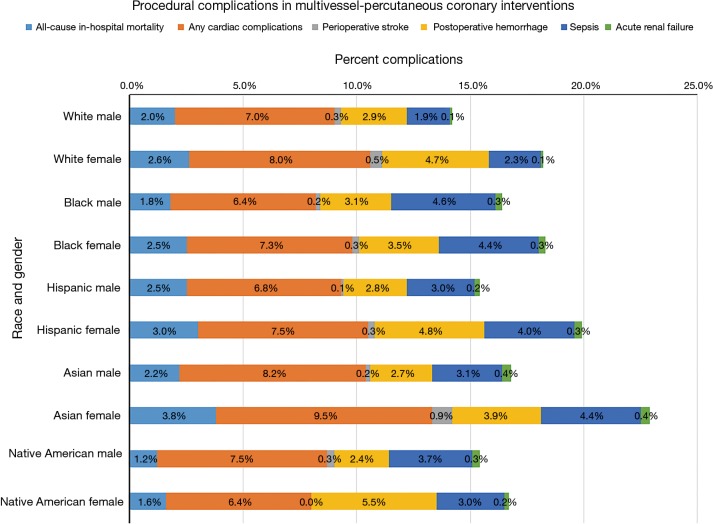
Figure 1.
Procedural complications in multivessel percutaneous coronary interventions by race and sex.
The all-cause in-hospital mortality among the Hispanics (M and F, 2.5% and 3.0%) and Asians (M and F, 2.2% and 3.8%) were higher in both the genders as compared to whites (P<0.001). Sepsis and acute renal failure were found to be higher among the non-whites as compared to whites (P<0.001). The all-cause cardiac complication rate was higher among the Asians (M and F, 8.2% and 9.5%) and native American males (7.5%) (P<0.001). Native American females had the highest rate of stent thrombosis followed by Asian females (P<0.001).
There was an increase in the all-cause mortality in all races between 2010 and 2014. However, as compared to whites (2010: M, 1.7% and F, 2.3%; 2014: M, 2.4% and F, 2.9%), there was a greater increase in non-whites; Hispanics (2010: M, 2.5% and F, 2.7%; 2014: M, 3.2% and F, 5.1%), Asians (2010: M, 2.4% and F, 3.2%; 2014: M, 2.4% and F, 4.8% and native-American females (2010: 0.7%, 2014: 3.3%) (P<0.001).
Multivariate analysis
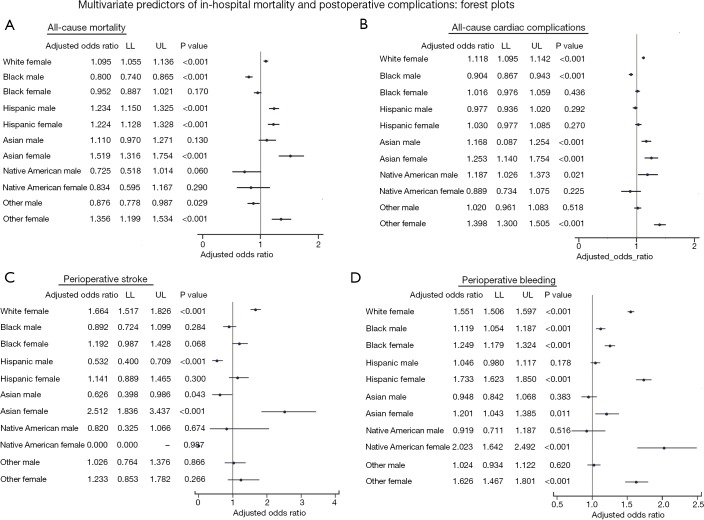
Figure 2 depicts the multivariate analysis of racial disparities in terms of adjusted odds for the all-cause-hospital mortality, any cardiac complications, perioperative stroke, and postoperative bleeding among patients who underwent MVPCI, keeping White males as a referent category.
Figure 2.
Odds of procedural complications by multivariate analysis: forest plot. (A) Hispanic males (aOR 1.23) showed the highest odds of in-hospital mortality, whereas among females, Asian (aOR 1.51), black (aOR 1.35), followed by Hispanics (aOR 1.22) revealed higher odds of in-hospital mortality; (B) the odds of any cardiac complications in males were higher amongst native Americans and Asians (aOR 1.19, 1.17) whereas, in females, Asian females (aOR 1.40, 1.25) revealed higher odds; (C) the risk of perioperative stroke was highest amongst the Asian and white females (aOR 2.51 and 1.66); (D) black males (aOR 1.11), and native American (aOR 2.02) and Hispanic (aOR 1.73) females had greater odds of postoperative bleeding complications.
Hispanic males (aOR 1.23) showed the highest odds of in-hospital mortality, whereas among females, Asian (aOR 1.51), black (aOR 1.35), followed by Hispanics (aOR 1.22) revealed higher odds of in-hospital mortality. The odds of any cardiac complications in males were higher amongst native Americans and Asians (aOR 1.19, 1.17) whereas, in females, Asian females (aOR 1.40, 1.25) revealed higher odds. The risk of perioperative stroke was highest amongst the Asian and white females (aOR 2.51 and 1.66). Black males (aOR 1.11) and native American and Hispanic females (aOR 2.02, 1.73) had greater odds of postoperative bleeding complications. All P were <0.001.
Resource utilization disparities
Table 4 illustrates the race and gender differences in the resource utilization in MVPCI. The resource utilization among black was lower in terms of coronary angiography, left heart catheterization, drug-eluting stent, intra-vascular ultrasound, coronary atherectomy as compared to whites. Intra-aortic balloon pump and ventricular assist device usage were highest among Asians and lowest in blacks. Coronary atherectomy, which is often used for calcified coronary lesions, was revealed to be used less in blacks. The utilization of fractional flow reserve (FFR) for stenotic lesion risk assessment was lower among blacks and Hispanics. All P were <0.001.
Table 4. Racial disparities in resource utilization during multivessel percutaneous coronary interventions.
| Resource utilization | White, % | Black, % | Hispanic, % | Asian, % | Native American, % | Other, % | P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| Coronary angiography | 48.4 | 38.3 | 35.8 | 27.9 | 49.2 | 38.9 | 65.9 | 51.7 | 45.7 | 30.5 | 57.4 | 44.7 | <0.001 | |||||
| Left heart catheterization | 48.9 | 39.3 | 37.4 | 29.4 | 50.0 | 40.2 | 64.2 | 49.3 | 46.5 | 33.3 | 56.1 | 43.6 | <0.001 | |||||
| Bare-metal stent | 11.0 | 9.0 | 9.5 | 6.5 | 10.7 | 7.6 | 9.1 | 7.0 | 8.8 | 7.6 | 12.0 | 10.1 | <0.001 | |||||
| Drug-eluting stent | 47.9 | 36.7 | 33.5 | 27.0 | 45.3 | 36.4 | 66.5 | 49.7 | 43.6 | 30.8 | 57.4 | 43.5 | <0.001 | |||||
| Intra-aortic balloon pump | 2.5 | 2.0 | 1.8 | 1.1 | 3.0 | 2.3 | 5.0 | 3.5 | 4.3 | 0.9 | 3.7 | 3.2 | <0.001 | |||||
| Ventricular assist device | 0.7 | 0.4 | 0.8 | 0.4 | 0.8 | 0.5 | 1.0 | 0.6 | 0.9 | 0.0 | 1.5 | 0.8 | <0.001 | |||||
| Intra-vascular ultrasound | 5.1 | 4.0 | 3.2 | 2.8 | 5.6 | 4.4 | 6.6 | 4.3 | 3.8 | 3.4 | 7.8 | 8.2 | <0.001 | |||||
| Coronary atherectomy | 1.4 | 1.1 | .9 | .6 | 1.7 | 1.2 | 2.8 | 2.1 | 1.6 | 0.2 | 1.6 | 1.5 | <0.001 | |||||
| Fractional flow reserve | 1.5 | 1.2 | 1.1 | 0.8 | 1.3 | 1.1 | 1.9 | 1.5 | 0.8 | 0.9 | 1.8 | 0.9 | <0.001 | |||||
Discussion
To our knowledge, this is the first study evaluating racial and gender disparities in MVPCI outcomes and resource utilization. This study presents the data of the patients who underwent PCI over a period of five years from 2010 through 2014 from the largest US inpatient database. We found that significant racial and sex disparities existed in terms of procedural outcomes and resource utilization in patients who underwent MVPCI. Black males formed the youngest patient cohort while black females had the most prolonged LOS. Blacks had the highest frequency of comorbidities with surprisingly lowest resource utilization as compared to whites. Asian females undergoing MVPCI had the highest all-cause in-hospital mortality and post-procedural cardiac complications. Unfortunately, the trend in the in-hospital mortality in patients undergoing MVPCI was found to be increased significantly over the study period with the largest increment among Hispanic females.
Sonel et al. from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines?) study from 400 US hospitals reported blacks to be younger as compared to whites (median age; 61 vs. 70 years) admitted for unstable angina (9). Similar findings were reported from the GWTG-CAD (Get With the Guidelines-Coronary Artery Disease) program by Cavender et al. (median age; 48 vs. 51 years) and by Slater et al. from the Dynamic registry (median age; 48 vs. 51 years) for patients undergoing PCI (3,10). These studies support our current findings with blacks undergoing MVPCI were younger as compared to whites. Genetic predisposition, higher predisposition to the cardiovascular comorbidities (3,8) and higher frequency of drug abuse (11,12) could be the potential reasons for the advanced disease at a younger age in blacks. Shaw et al. used the National Cardiovascular Data Registry to evaluate patients undergoing angiographic evaluation and found higher in-hospital mortality for Hispanic and white women presenting with ACS (13). Blacks and Hispanics comprise the largest percentage of emergency admission MVPCI with prolonged hospital stay as compared to whites. The higher burden of comorbidities could be a contributory factor towards increased emergency MVPCI leading to the extended LOS and increased hospitalization cost among non-whites compared to whites (2). Although extended LOS was not found in Asians, higher proportions of procedural complications and resource utilization could be the conceivable reasons for the inflated cost among Asians.
Data from the CRUSADE initiative, CathPCI registry, Cooperative Cardiovascular project, and Dynamic Registry unanimously showed the higher burden of comorbidities like CHF, hypertension, diabetes, renal failure and previous stroke in black, Hispanics, and Asians as compared to whites. However, despite the higher burden of cardiovascular comorbidities among the black consistent with the previous studies (3,9,14,15), the incidence of several post-procedural complications was strikingly higher among Asians, followed by blacks and Hispanics (13).
Shaw et al. reported highest post-procedural mortality rates in Asian (2.17%) undergoing coronary angiography after acute chest pain, which is consistent with our results with Asians (M 2.2% and F 3.8%) encountering the highest in-hospital mortality (13,16). The trends in mortality increased over time among all ethnic groups with the highest increment among Hispanics, Females dominated males in terms of in-hospital mortality. Arora et al. reported poor outcomes among the females undergoing MVPCI with post-procedural mortality odds of 1.63 (2). However, Singh et al. suggested no mortality difference among males and females in a cohort of 18,885 patients who underwent PCI (17). Similarly, higher post-procedural complications and mortality among the Asians could be explained by the fact that they were the ones with the higher utilization of invasive procedures (14).
Kumar et al. reported the lower utilization of drug-eluting stents among blacks (71.9% vs. 74.8%) as compared to whites (14). A study by Rodriguez et al. reported a lower rate of PCI or CABG among the blacks vs. whites (47.7% vs. 58.0%) (18). Another study by Slater et al. also reported a lower rate of prior PCI (27.9% vs. 29.9%) and CABG (11.4% vs. 17.6%) among blacks than whites (3). These studies back our findings of blacks as the least resource utilizers and Asians as the highest as compared to whites (15). The major reason for this disparity has been suggested to be the underuse of procedures among minorities, the over-use among whites, physician bias, and the disparities in the clinical presentation of the disease among the various races (9,16,19-21). Khambatta et al. showed in their review that one study noted that the percentage of black receiving the highest efficacy antiplatelet, Prasugrel, was less (10% vs. 14.5%) than white (22). In another study reviewing CABG procedures in New York, black patients were more likely to be referred to the cardiovascular surgeons with the higher risk-adjusted mortality rates than white patient population. A study from the National Registry of the Myocardial Infarction (NRMI-2) reported that black patients were less likely (aOR 0.76, 95% CI: 0.71–0.80) to receive thrombolytic therapy and coronary angiography (aOR 0.85, 95% CI: 0.77 to 0.95) than their white counterparts (23).
We also noted the underutilization of intravascular ultrasound (IVUS) for the detection of the severity of calcifications and fractional flow reserve (FFR) for stenotic lesions assessment among blacks despite the higher incidence of atherosclerosis. As reported by Sedlis et al., the reluctance to undergo an invasive procedure and a higher refusal rate (two times higher odds) for the procedure by African Americans as compared to Caucasians offer possible explanations for the underutilization of resources (15,24). Longer waiting hours by Asians from the onset of symptoms to initiation of the treatment have also been reported to poorly influence the outcomes (25). Patients’ race influencing the physician’s referral for cardiac procedures has also been reported as one of the influential factors. Sedlis and colleagues reported the higher odds (OR =1.70) of referral for surgery for Caucasian than African Americans (24,26,27). Lower household income among blacks and the type of hospital facility have been found to play key roles in the nature of the care being provided (9).
The major strength of our study lies in the fact that we present our findings from the largest inpatient database in the US, thus providing a better generalizability of the results for the entire population as done previously to assess the healthcare disparities in cardiovascular interventions (28). As the database has been used in the past to look at procedural outcomes, the validation is high. However, there are a few limitations of this study, which needs to be reported. The NIS database uses ICD-9-CM coding, which involves administrative coding, which can be associated with errors by under- or overreporting of the disease. With ICD-9-CM coding, we cannot provide further details about the coronary vessels being operated upon. Since the NIS does not provide information on the follow-up so we could not assess the long-term outcomes following MVPCI.
Conclusions
This retrospective study provides useful insights into a number of racial and gender disparities regarding the in-hospital mortality, post-procedural outcomes, and resource utilization in patients undergoing MVPCI using the largest nationally representative database from the US. Although blacks presented at a relatively younger age and with a high frequency of cardiovascular comorbidities, they were found to use the least number of cardiovascular procedures and resources as compared to any other race. Asians were the highest resource utilizers with the highest all-cause-hospital mortality. There is a need for further study to look at the factors governing these distinctions, which could fill the gaps witnessed in this study.
Acknowledgements
None.
Ethical Statement: The study was exempt from an IRB approval since the NIS is a publically available dataset and contains only deidentified information.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Epstein AM, Weissman JS, Schneider EC, et al. Race and gender disparities in rates of cardiac revascularization: do they reflect appropriate use of procedures or problems in quality of care? Med Care 2003;41:1240-55. 10.1097/01.MLR.0000093423.38746.8C [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Panaich SS, Patel NJ, et al. Multivessel Percutaneous Coronary Interventions in the United States: Insights From the Nationwide Inpatient Sample. Angiology 2016;67:326-35. 10.1177/0003319715593853 [DOI] [PubMed] [Google Scholar]
- 3.Slater J, Selzer F, Dorbala S, et al. Ethnic differences in the presentation, treatment strategy, and outcomes of percutaneous coronary intervention (a report from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2003;92:773-8. 10.1016/S0002-9149(03)00881-6 [DOI] [PubMed] [Google Scholar]
- 4.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768-74. 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham G. Population-based approaches to understanding disparities in cardiovascular disease risk in the United States. Int J Gen Med 2014;7:393-400. 10.2147/IJGM.S65528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobi JA, Parikh SV, McGuire DK, et al. Racial disparity in clinical outcomes following primary percutaneous coronary intervention for ST elevation myocardial infarction: influence of process of care. J Interv Cardiol 2007;20:182-7. 10.1111/j.1540-8183.2007.00263.x [DOI] [PubMed] [Google Scholar]
- 7.Rumsfeld JS, Epstein AJ. Racial Disparities in Cardiovascular Procedure Outcomes: Turn Down the Volume. J Am Coll Cardiol 2006;47:425-6. 10.1016/j.jacc.2005.10.025 [DOI] [PubMed] [Google Scholar]
- 8.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238-45. 10.2174/1573403X11666141122220003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 2005;111:1225-32. 10.1161/01.CIR.0000157732.03358.64 [DOI] [PubMed] [Google Scholar]
- 10.Cavender MA, Rassi AN, Fonarow GC, et al. Relationship of race/ethnicity with door-to-balloon time and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: findings from Get With the Guidelines-Coronary Artery Disease. Clin Cardiol 2013;36:749-56. 10.1002/clc.22213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai R, Patel U, Rupareliya C, et al. Impact of Cocaine Use on Acute Ischemic Stroke Patients: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017;9:e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai R, Patel U, Sharma S, et al. Recreational Marijuana Use and Acute Myocardial Infarction: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017;9:e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787-801. 10.1161/CIRCULATIONAHA.107.726562 [DOI] [PubMed] [Google Scholar]
- 14.Kumar RS, Douglas PS, Peterson ED, et al. Effect of race and ethnicity on outcomes with drug-eluting and bare metal stents: results in 423 965 patients in the linked National Cardiovascular Data Registry and centers for Medicare & Medicaid services payer databases. Circulation 2013;127:1395-403. 10.1161/CIRCULATIONAHA.113.001437 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Rathore SS, Radford MJ, et al. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med 2001;344:1443-9. 10.1056/NEJM200105103441906 [DOI] [PubMed] [Google Scholar]
- 16.Gaglia MA, Jr, Steinberg DH, Pinto Slottow TL, et al. Racial disparities in outcomes following percutaneous coronary intervention with drug-eluting stents. Am J Cardiol 2009;103:653-8. 10.1016/j.amjcard.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Rihal CS, Gersh BJ, et al. Mortality Differences Between Men and Women After Percutaneous Coronary Interventions: 12-Year, Single-Center Experience. J Am Coll Cardiol 2008;51:2313-20. 10.1016/j.jacc.2008.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez F, Foody JM, Wang Y, et al. Young Hispanic Women Experience Higher In-Hospital Mortality Following an Acute Myocardial Infarction. J Am Heart Assoc 2015;4:e002089. 10.1161/JAHA.115.002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation 2003;108:286-91. 10.1161/01.CIR.0000079164.95019.5A [DOI] [PubMed] [Google Scholar]
- 20.Groeneveld PW, Kruse GB, Chen Z, et al. Variation in cardiac procedure use and racial disparity among Veterans Affairs Hospitals. Am Heart J 2007;153:320-7. 10.1016/j.ahj.2006.10.032 [DOI] [PubMed] [Google Scholar]
- 21.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007;298:1525-32. 10.1001/jama.298.13.1525 [DOI] [PubMed] [Google Scholar]
- 22.Khambatta S, Seth M, Rosman HS, et al. The association between patient race, treatment, and outcomes of patients undergoing contemporary percutaneous coronary intervention: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J 2013;165:893-901.e2. 10.1016/j.ahj.2013.02.030 [DOI] [PubMed] [Google Scholar]
- 23.Taylor HA, Jr, Canto JG, Sanderson B, et al. Management and outcomes for black patients with acute myocardial infarction in the reperfusion era. National Registry of Myocardial Infarction 2 Investigators. Am J Cardiol 1998;82:1019-23. 10.1016/S0002-9149(98)00547-5 [DOI] [PubMed] [Google Scholar]
- 24.Sedlis SP, Fisher VJ, Tice D, et al. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. J Clin Epidemiol 1997;50:899-901. 10.1016/S0895-4356(97)00089-9 [DOI] [PubMed] [Google Scholar]
- 25.King KM, Khan NA, Quan H. Ethnic variation in acute myocardial infarction presentation and access to care. Am J Cardiol 2009;103:1368-73. 10.1016/j.amjcard.2009.01.344 [DOI] [PubMed] [Google Scholar]
- 26.Capers Q, Sharalaya Z. Racial Disparities in Cardiovascular Care: A Review of Culprits and Potential Solutions. J Racial Ethn Health Disparities 2014;1:171-80. 10.1007/s40615-014-0021-7 [DOI] [Google Scholar]
- 27.Bertoni AG, Goonan KL, Bonds DE, et al. Racial and ethnic disparities in cardiac catheterization for acute myocardial infarction in the United States, 1995--2001. J Natl Med Assoc 2005;97:317-23. [PMC free article] [PubMed] [Google Scholar]
- 28.Desai R, Mirza O, Sachdeva R, et al. Sex and racial disparities in fractional flow reserve-guided percutaneous coronary intervention utilization: a 5-year national experience. Ann Transl Med 2018;6:198. 10.21037/atm.2018.03.15 [DOI] [PMC free article] [PubMed] [Google Scholar]