Abstract Abstract
A new, critically endangered species of softshell turtle, Pelodiscusvariegatussp. n. is described from north-central Vietnam and Hainan Island, China, distinguished by a unique set of genetic and morphological traits from all other congeners (P.axenaria, P.maackii, P.parviformis, P.sinensis, and unnamed genetic lineages). Morphologically, P.variegatus is characterized, among others, by its strong ventral ornamentation in all age classes.
Keywords: China, genetics, morphology, softshell turtles, Vietnam
Introduction
“Chinese softshell turtles” were considered for decades to represent the morphologically highly variable and geographically widespread species Pelodiscussinensis (Wiegmann, 1834), distributed from the Russian Far East through the Korean Peninsula, eastern and central China to Vietnam (e.g., Pope 1935; Wermuth and Mertens 1961, 1977; Pritchard 1979; Meylan 1987; Ernst and Barbour 1989). Another species, P.maackii (Brandt, 1857), from the northernmost part of the distributional range, was resurrected from the synonymy of P.sinensis by Chkhikvadze (1987) employing osteological features. On the basis of morphological characters, two additional species from central China were described in the 1990s, P.axenaria (Zhou, Zhang & Fang, 1991) and P.parviformis Tang, 1997. However, the validity of the latter three species was repeatedly questioned or rejected (Ernst et al. 2000; TTWG 2007). Using three mitochondrial DNA (mtDNA) fragments and one nuclear locus, Fritz et al. (2010) confirmed that Pelodiscus represents a species complex. These authors tentatively recognized P.axenaria and P.maackii and highlighted that the assignment of scientific names was difficult because the identity of the oldest available name, Trionyxsinensis Wiegmann, 1834, remained unclear. Stuckas and Fritz (2011) designated a lectotype for this species and succeeded in sequencing approximately 1500 base pairs of mtDNA of this 180-year-old type specimen. This allowed the conclusive recognition of four genetically distinct species, P.axenaria, P.maackii, P.parviformis, and P.sinensis. Yang et al. (2011) arrived at the same judgment with respect to P.parviformis after evaluating molecular and morphological data but emphasized the near-impossibility of distinguishing P.axenaria from P.parviformis by external characters alone. Finally, based on comprehensive sampling, Gong et al. (2018) examined the diversity of Pelodiscus using mtDNA and five nuclear loci. These authors showed that the diversity of Pelodiscus is still underestimated and provided evidence that two genetically and morphologically distinct Pelodiscus species occur syntopically in northern Vietnam. One of these species was found widely distributed and occurring also in China, whereas the other seemed to be confined to northern and central Vietnam. Mitochondrial DNA of the two species was only moderately divergent, albeit representing distinct lineages. In contrast, the studied nuclear loci revealed discrete gene pools, suggestive of ancient mitochondrial capture. In the present study, we describe this as yet unnamed taxon with introgressed mitochondria.
Taxonomy
Pelodiscus variegatus sp. n.
http://zoobank.org/95F21749-6C4A-439F-BBC0-A44B6F82F1AA
Figs 1 , 2 , 3 , 4 , Tables 1 , 2 , 3 , 4
Figure 1.
Dorsal and ventral aspects of the holotype of Pelodiscusvariegatus sp. n. (IEBR 4480, adult female, 134.2 mm PL). Photographs Balázs Buzás.
Figure 2.
Two paratypes of Pelodiscusvariegatus sp. n. in life. A, C MTD 44045, female, 75.2 mm PLB, D MTD 42834, female, 86.6 mm PL. Photographs Thomas Ziegler.
Figure 3.
Plastral views of two freshly dead paratypes of Pelodiscusvariegatus sp. n. A HNHM 2018.112.1, female, 77.4 mm PLB AQT001-HTVN2018, female, 77.3 mm PL. Photographs An Vinh Ong.
Figure 4.
Variation in plastral ornamentation of Pelodiscusvariegatus sp. n. A HNHM 2018.111.1, adult male, 109.7 mm PLB ZFMK 44212, adult male, 114.4 mm PLC juvenile, ZMB 49776, 50.0 mm PLD hatchling, AMNH 30125, 37.2 mm PL. Not to scale. Photographs Balázs Buzás (A), Balázs Farkas (B), Frank Tillack (C), Lauren Vonnahme (D).
Table 1.
Selection of diagnostic sites of the 12S rRNA gene for Pelodiscus species (84 wild-caught individuals from Gong et al. 2018). Positions refer to the 400-bp-long reference alignment in the Supporting Information. G, H, and I are genetic lineages that represent putatively distinct taxa (Gong et al. 2018).
| n | 13 | 14 | 20 | 34 | 96 | 222 | 223 | 224 | 225 | 234 | 327 | 330 | 384 | 385 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.variegatus sp. n. | 4 | T | T | T | T | C | T | T | T | T | A | C | T | G | C |
| P. axenaria | 5 | . | . | A | C | T | C | . | C | A | C | T | C | C | T |
| G | 1 | . | . | . | . | T | . | . | . | . | . | . | . | . | . |
| H | 1 | . | . | . | . | T | . | . | . | . | . | . | . | . | . |
| I | 6 | . | . | . | C | T | C | . | C | A | C | . | . | C | . |
| P. maackii | 10 | . | . | C | . | T | . | G | . | . | . | . | . | . | . |
| P. parviformis | 8 | C | C | C | C | T | . | G | . | . | . | . | . | . | . |
| P. sinensis | 49 | . | . | . | . | T | . | . | . | . | . | . | . | A/. | . |
Table 2.
Selection of diagnostic sites of the cyt b gene for Pelodiscus species (94 wild-caught individuals from Gong et al. 2018). Positions refer to the 1168-bp-long reference alignment in the Supporting Information. G, H, and I are genetic lineages that represent putatively distinct taxa (Gong et al. 2018).
| n | 130 | 132 | 147 | 148 | 195 | 204 | 285 | 288 | 315 | 477 | 520 | 730 | 741 | 1081 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.variegatus sp. n. | 4 | A | A | C | C | G | T | T | C | C | T | T | A | T | T |
| P. axenaria | 10 | C | C | T | T | A | C | . | T | . | . | C | G | C | C |
| G | 1 | C | C | . | . | . | C | C | . | T | . | C | G | C | C |
| H | 1 | C | C | . | . | . | C | C | . | T | . | C | G | C | C |
| I | 6 | C | C | . | T | A | C | . | T | . | . | C | G | C | C |
| P. maackii | 11 | C | C | . | A | C | C | . | . | . | C | G | C | C | |
| P. parviformis | 10 | C | C | . | . | A | C | C | . | T | C | C | G | C | C |
| P. sinensis | 51 | C | . | . | . | . | C | C | . | T | . | C* | G* | C | C |
*Among 51 sequences of P.sinensis, only the holotype had the same character state as P.variegatus.
Table 3.
Selection of diagnostic sites of the mtDNA fragment comprising the partial ND4 gene and adjacent DNA coding for tRNAs for Pelodiscus species (91 wild-caught individuals from Gong et al. 2018). Positions refer to the 838-bp-long reference alignment in the Supporting Information. G, H, and I are genetic lineages that represent putatively distinct taxa (Gong et al. 2018).
| n | 10 | 18 | 31 | 64 | 94 | 148 | 151 | 211 | 262 | 263 | 301 | 305 | 508 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.variegatus sp. n. | 4 | C | T | C | A | G | A | G | G | C | C | C | T | A |
| P. axenaria | 10 | . | . | T | G | A | C | A | A | T | T | T | C | G |
| G | 1 | . | . | . | . | A | . | . | A | . | . | . | . | G |
| H | 1 | . | . | . | . | A | . | . | A | . | . | . | . | G |
| I | 6 | T | C | T | . | A | C | A | A | T | . | . | A | G |
| P. maackii | 10 | . | . | . | . | A | . | . | A | . | . | . | . | G |
| P. parviformis | 9 | . | . | . | . | A | G | A | A | . | . | . | . | G** |
| P. sinensis | 50 | . | . | . | . | A | G* | . | A/. | . | . | . | . | G** |
*Among 50 P.sinensis, only one individual had the same character state as P.variegatus.
**Among nine P.parviformis and 50 P.sinensis, two and one individuals, respectively, had the same character state as P.variegatus.
Table 4.
Diagnostic morphological features of adults of Pelodiscus species.
| P. axenaria | P. maackii | P. parviformis | P. sinensis | P.variegatus sp. n. | |
|---|---|---|---|---|---|
| Maximum carapace length (in cm) | 20 | more than 35 | 16 | 23 | 23 |
| Prevalent carapace color | yellowish brown | olive brown to dark brown | yellowish brown | olive green to olive gray | yellowish brown |
| Carapace pattern | blurred dark mottling with indistinct stellate spots and ill-defined half oval blotches around perimeter of leathery margin | fine dark-edged yellowish to orange spots, background sometimes dark-mottled | dark marbling with indistinct stellate spots and ill-defined half oval blotches around perimeter of leathery margin | none or small, irregular black blotches and vermiculations or small, faint stellate spots | complex dark marbling, large, irregularly disposed black stellate spots and half oval blotches around perimeter of leathery margin |
| Prevalent plastron color | yellowish white | white to straw yellow | yellowish white | snow white to pinkish white | pinkish white to pale reddish orange |
| Plastral pattern | a single dark gray central figure enclosed by hypo- and xiphiplastra, underside of leathery margin of carapace unmarked | no pattern, underside of leathery margin of carapace unmarked | no pattern, underside of leathery margin of carapace unmarked | no pattern or relatively small, faint round to oval dark markings, underside of leathery margin of carapace unmarked | distinct, large dark blotches, underside of leathery margin of carapace pigmented |
| Head and neck pattern | numerous fine dark brown to black markings, pre- and postocular stripes thin and discontinuous | fine dark-edged yellowish to orange spots, pre- and postocular stripes thick, edged in yellowish white | numerous fine dark brown to black markings, pre- and postocular stripes thin and discontinuous | a few scattered dark and light markings, pre- and postocular stripes of medium thickness, sometimes accentuated with white | numerous fine dark brown to black markings edged in yellowish white, pre- and postocular stripes thick |
| Throat pattern | minuscule, indistinct yellowish white spots | large light, dark-edged markings | minuscule, barely discernible black spots | small whitish spots or large light, dark-edged markings | large light, dark-edged markings |
| Carapace tuberculation | dorsal tubercles in longitudinal series more or less discrete, central tubercle in front of marginal ridge of carapace small | tubercles restricted to leathery margin, central tubercle in front of marginal ridge of carapace distinct | dorsal tubercles in longitudinal series more or less discrete, central tubercle in front of marginal ridge of carapace small | dorsal tubercles in longitudinal series more or less discrete, central tubercle in front of marginal ridge of carapace small | dorsal tubercles more or less fused with one another in longitudinal series, central tubercle in front of marginal ridge of carapace indistinct |
| Medial keel | high | low, carapace flat or longitudinally depressed in middle | high | low, carapace evenly arched or longitudinally depressed in middle | high |
Holotype.
Institute of Ecology and Biological Resources, Hanoi: IEBR 4480, adult female preserved in alcohol, Thai Thinh village, Kinh Mon District, Hai Duong Province, Vietnam, leg. Cuong The Pham, 20 June 2009 (Fig. 1).
Paratypes
(all preserved in alcohol). American Museum of Natural History, New York: AMNH 30125, hatchling, Nodoa (= Nada, Danzhou), Hainan Province, China, leg. Clifford H Pope, December 1922–July 1923; Hungarian Natural History Museum, Budapest: HNHM 2018.111.1, adult male, same data as for the holotype; HNHM 2018.112.1, female, Song Rac Lake, Cam Xuyen District, Cam Lac Commune, Ha Tinh Province, Vietnam (18.1665N, 106.0957E), leg. An Vinh Ong, Quang Xuan Hoang and Trung Van Vo, 20 October 2018; Museum of Zoology, Senckenberg Dresden: MTD 42534, adult female, through local trade (Nha Trang), Khanh Hoa Province, Vietnam, leg. Edgar Lehr, February 2000; MTD 42834, female, through local trade (from lowland forest northwest of Ky Thuong village; Ziegler 2002), Ha Tinh Province, Vietnam, leg. Thomas Ziegler, 12 August 1997 (field number TZ 584); MTD 44045, female, through local trade (allegedly captured in a sandy stream in Phong Nha–Ke Bang Reserve; Ziegler et al. 2004), Quang Binh Province, Vietnam, leg. Thomas Ziegler, August/September 2001 (field number TZ V8); Natural History Museum Vienna: NMW 30221:1–6, six hatchlings, Phuc Son, Tan Yen District, Bac Giang Province, Vietnam, leg. Hans Fruhstorfer, 1903; Zoological Collection, Vinh University, Vinh, Nghe An Province: AQT001-HTVN2018, female, Song Rac Lake, Cam Xuyen District, Cam Lac Commune, Ha Tinh Province, Vietnam (18.1665N, 106.0957E), leg. An Vinh Ong, Quang Xuan Hoang and Trung Van Vo, 20 October 2018; Zoological Research Museum Alexander Koenig, Bonn: ZFMK 101820, female, through local trade (surroundings of the area named “Chin Xai” by local people; Ziegler 2002), Ha Tinh Province, Vietnam, leg. Thomas Ziegler, 23 August 1997 (field number TZ 679).
Additional specimens
(all preserved in alcohol). American Museum of Natural History, New York: AMNH 28345, adult female, Nodoa (= Nada, Danzhou), Hainan Province, China, leg. Clifford H Pope, December 1922–July 1923; Field Museum, Chicago: FMNH 6626 and 6627, females, Nodoa (= Nada), Hainan Province, China, leg. Clifford H Pope, 1923; Museum of Vertebrate Zoology, Berkeley: MVZ 23946, male, Kachek (= Jiaji, Qionghai), Hainan Province, China, 20 m a.s.l. (23.36667N, 116.65E), leg. J Linsley Gressitt, 8 August 1935; Natural History Museum Vienna: NMW 30219:1, female, River of Mount Wuchi (= Wanquan He, Wuzhi Shan), Hainan Province, China, don. Franz Steindachner, 1906; NMW 30232:3, adult male, Kau-kong River (to be identified with Gaogong He according to Zhao and Adler 1993), Hainan Province, China, don. Franz Steindachner, 1906; Naturalis Leiden: RMNH 4752 and 4753, juveniles, “Annam” (= possibly Phuc Son, Tan Yen District, Bac Giang Province, Vietnam, the declared origin of all of Fruhstorfer’s specimens in other collections), leg. Hans Fruhstorfer, 1903; Zoological Research Museum Alexander Koenig, Bonn: ZFMK 44212 and 44213, adult males, near Hanoi, Vietnam, leg. Ivan Rehák, March 1984; ZFMK 44214, adult female, near Hanoi, Vietnam, leg. Ivan Rehák, March 1984; ZFMK 59199 and 59200, females, Red River (Song Hong), Hanoi, Vietnam, leg. Václav Laňka, June 1985; Natural History Museum Berlin: ZMB 29614, 49775 and 49776, juveniles, Phuc Son, Tan Yen District, Bac Giang Province, Vietnam, leg. Hans Fruhstorfer.
Diagnosis.
In the 12S rRNA gene, Pelodiscusvariegatus differs from all other species and genetic lineages of Pelodiscus by the presence of cytosine (C) instead of thymine (T) at position 96 of the reference alignment (Suppl. material 1). In the cyt b gene, P.variegatus differs from all other species and genetic lineages of Pelodiscus by the presence of adenine (A) instead of cytosine (C) in position 130 and by the presence of thymine (T) instead of cytosine (C) in positions 204, 741, and 1081 of the reference alignment (Suppl. material 2). In the mtDNA fragment corresponding to the partial ND4 gene plus adjacent DNA coding for tRNAs, P.variegatus differs from all other species and genetic lineages of Pelodiscus by the presence of adenine (A) instead of guanine (G) in position 94 of the reference alignment (Suppl. material 3). These and further species-specific differences are shown in Tables 1–3.
In addition to the genetic distinctiveness of P.variegatus, the strong ventral ornamentation clearly sets apart adult individuals from P.maackii, which has a uniform yellowish white or straw yellow plastron devoid of any markings in adults; from P.sinensis, which may retain faint remnants of its juvenile pattern on its snow white to reddish white plastron but the round to oval spots are usually isolated and proportionally much smaller; from P.axenaria, which has a yellowish white plastron with just a single large black central figure enclosed by the hypo- and xiphiplastra throughout its life (Zhou et al. 1991); and from P.parviformis, which has an unmarked yellowish white plastron at all ages. According to the specimens investigated and data available to us, P.variegatus also reaches a much smaller maximum size (23 cm CL; AMNH 28345) than P.maackii (at least 35 cm CL; Brandt 1857) but grows bigger than P.parviformis (16 cm CL; NMW 30232:6); P.sinensis (23 cm CL; ZMB 9784) and P.axenaria (20 cm CL; Gong et al. 2018) attain dimensions resembling P.variegatus. The diagnostic morphological features of adults of these five Pelodiscus species are summarized in Table 4.
Description of the holotype.
Carapace length (CL) 171.0 mm, carapace width (CW) 148.0 mm, plastron length (PL) 134.2 mm, head width (HW) 32.2 mm, eye diameter 9.8 mm, interorbital distance 5.4 mm, snout length (SL) 13.3 mm. Carapace oval, slightly domed but with a medial keel, widest at level of the posterior buttress spurs of the hypoplastra. Marginal ridge low, central tubercle indistinct. Dorsal surface roughened by longitudinal ridging and smaller protuberances spread over the leathery margin. The yellowish gray carapace is adorned with an extremely complex greenish black pattern consisting of reticulations and stellate spots enclosed by incomplete rings of the same color, finely dotted with siskin green on either side of the vertebral keel, with those above the pelvis being more pronounced but with additional ones towards the perimeter of the bony disk. The ridging of the carapace is enhanced by the siskin green color of the vertebral keel and the longitudinal rows of tubercles.
Ventral surfaces yellowish white with distinct greenish gray blotches extending onto the plastron. Two dark patches below the neck along the anterior carapace margin, one oval mark between the epiplastra, one on both sides behind the armpits and continuing towards but not reaching the hyoplastra, as well as on the hyo- and hypoplastra and the xiphiplastra, the latter meeting along the midline but not contacting those covering the hyo- and hypoplastra. Additional blotches at the insertion of the hindlimbs and some vague, bruise-like markings on the bridge and the underside of the leathery margin.
Head, extended to posterior level of eyes, terminating in flexible snout. Jaws closed, each covered by fleshy lips except anteriorly where the horny beaks are exposed. Top of head with fine, greenish black specks and streaks. Pre-, sub- and postocular stripes thick (approx. 2 mm wide), locally interrupted and yellowish black in color with thin siskin green outlines. Chin with a contrasting yellowish white pattern on yellowish gray ground, which gradually fades towards the throat and gets almost indiscernible at the base of the neck.
Fore- and hindfeet well-webbed, having five digits each, with claws on the first three digits only. Each forelimb with four antebranchial scales, three of them free-edged. These are wide, the upper one stretching across nearly the whole width of the forelimb (approx. 16 mm long) and the lower two overlapping each other (approx. 20 mm together). Each hindlimb with two horny scales, one smooth on the posterodorsal surface while the other, which is free-edged, is located on the posteroventral surface.
Tail short, barely extending beyond the rear margin of the carapace.
Undersurface of soft parts of body yellowish white embellished with large yellowish gray markings, encroaching on soles and palms, and on either side of the tail.
Variations.
There is considerable, in part also age-dependent, variation in pattern intensity among Pelodiscusvariegatus. For instance, our male paratype (HNHM 2018.111.1; 109.7 mm PL; Fig. 4A) has somewhat smaller but much more conspicuous stellate spots on its carapace and very large dark (greenish black) markings on its undersurfaces. Those on the hyo-, hypo- and xiphiplastra are fused into a single mushroom-shaped figure, while the “bruises” on the bridge and the ventral surface of the leathery margin also manifest themselves as true blotches.
In one of our female paratypes (MTD 44045; 75.2 mm PL; Fig. 2A, C) the dark blotches on either side of the tail are connected with those at the insertion of the hindlimbs, the leathery margin and the bridge, whereas the ones in front of the entoplastron are fused with those in the armpits, extend towards the central patch between the epiplastra and actually reach the marks on the anterior edge of the plastron, at the base of the neck.
In a battered, presumably very old male examined by us (ZFMK 44212; 116.4 mm PL; Fig. 4B) comparable in size to our male paratype, the ventral ornamentation has lost definition but is still perceptible.
Hatchlings (Fig. 4C, D) have similar markings to adults but the overall effect is even more striking. The parts colored yellowish white in preservative are orpiment to reddish orange in life (Farkas and McCormack 2010) and fade remarkably slowly as age advances (Figs 2–3). In some individuals the light blotches framed with black on the sides of the neck intermingle to form wide bands on a yellowish gray (in life yellowish brown) ground (Fig. 2A). The subocular stripes are occasionally reduced to short streaks and vary between two and three in number. Although the dark longitudinal striation on the nape of juveniles dissolves in adults, a central spot typically remains discernible just in front of the marginal ridge.
Our modest sample size does not allow us to draw definite conclusions about ontogenetic variation and it is presently unknown at what CL sexual maturity is reached in this species. Individuals are sexually clearly dimorphic, with males having much longer and thicker tails, at a PL of 98.8 mm (ZFMK 44213) but a slight variation in TL can be noticed even among hatchlings (NMW 30221:1–6). Anyhow, smaller (younger) specimens appear to possess proportionally wider heads and rounder shells than larger (older) ones. PL/HW 3.50–4.44, mean 3.925; CL/CW 1.02–1.23, mean 1.149; HW/SL 1.98–2.60, mean 2.213, CL/PL 1.18–1.43, mean 1.283.
Distribution.
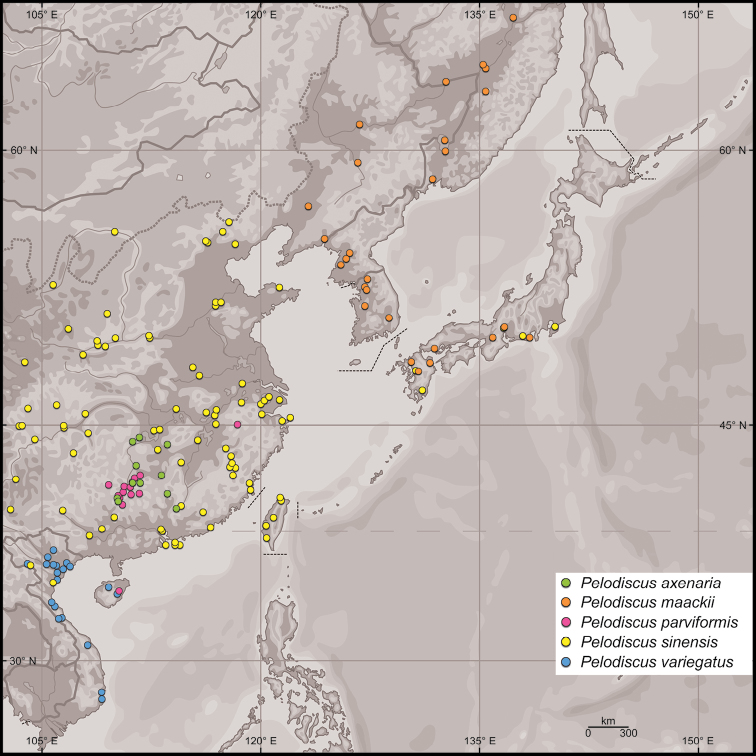
The exact range is unknown but includes lowland areas in the provinces Bac Giang, Ha Tinh (Fig. 5), Hai Duong (own data), “Hai Hung” (a former administrative unit encompassing present-day Hai Duong and Hung Yen; Rudolphi and Weser 1998), Ninh Binh (Rudolphi and Weser 1998; Nguyen et al. 2009; Farkas and McCormack 2010), Phu Tho (Nguyen et al. 2009), Quang Binh (own data), Quang Nam (Farkas and McCormack 2010), Tuyen Quang (Rudolphi and Weser 1998) and Yen Bai (Nguyen et al. 2009) of Vietnam as well as the lower reaches of the Gaogong and Wanquan rivers in Hainan Province, China (own data; Fig. 6). In northeastern Vietnam the distribution area of Pelodiscusvariegatus overlaps with that of Paleasteindachneri (Bac Giang Province, NMW 23395, NMW 23480:2, Siebenrock 1906; Ha Tinh Province, ZFMK 81539, ZFMK 81540, Ziegler 2002; Quang Binh Province, Ziegler et al. 2004) and further south (Gong et al. 2018) with that of Pelodiscussinensis, believed to have been introduced (Nguyen et al. 2009). On Hainan, Pelodiscusvariegatus seems to be sympatric with Pelodiscusparviformis (vouchers NMW 30232:1–2, NMW 30232:4–8; see Remarks) and Paleasteindachneri (NMW 20373; Siebenrock 1906). In Vietnam, most records fall within the “Northeast Lowlands Subregion” of Bain and Hurley (2011; encompassing the “Red River System” of Kottelat 1989). The zoogeographical affinities of Hainan lie also with this area (as well as mainland southwestern China), while the southern portion of the purported range forms part of the “Central–South Vietnam Lowlands Subregion” of Bain and Hurley (2011) or the “Annam River System” of Kottelat (1989).
Figure 5.
Habitat of Pelodiscusvariegatus sp. n.: Song Rac Lake, Cam Xuyen District, Ha Tinh Province, Vietnam. Photograph An Vinh Ong.
Figure 6.
Currently known presence points of Pelodiscus species based on our own data as well as distribution maps published by the TTWG (2017) and Gong et al. (2018). Earlier records of P.sinensis from Hainan Island are referable to P.parviformis or P.variegatus sp. n. (see Remarks).
Etymology.
The specific epithet variegatus (spotted) is a Latin adjective in masculine gender alluding to the highly contrasting markings, especially the large plastral blotches, of the new species.
Remarks.
In addition to the characters used here for diagnosing P.variegatus, Gong et al. (2018) described some further genetic differences to other Pelodiscus species.
Fritz et al. (2010) suggested that the taxon now named Pelodiscusvariegatus resembles P.parviformis, prompting the TTWG (2011, 2012, 2014, 2017) to identify the Pelodiscus records from Vietnam with the latter species. However, as explained in Gong et al. (2018), this is no longer tenable in the face of the genetic distinctness of the two species.
Traditionally, Chinese softshell turtles from Hainan were identified as P.sinensis (e.g., Pope 1935; Ernst and Barbour 1989; Ernst et al. 2000; TTWG 2011, 2012, 2014, 2017). However, the few old (early 20th century) museum specimens serving as record sources represent either P.variegatus (AMNH 28345, AMNH 30125, FMNH 6626, FMNH 6627, MVZ 23946, NMW 30219:1, NMW 30232:3) or P.parviformis (NMW 30232:1–2, NMW 30232:4–8). Thus, the native occurrence of P.sinensis sensu stricto on Hainan seems questionable, even though this species is now most likely bred there in local farms. We cannot exclude that also some of the presence points of P.sinensis from southwestern mainland China mapped by the TTWG (2017) refer to P.parviformis or P.variegatus (and in part perhaps to P.axenaria).
Conservation implications.
While Pelodiscussinensis is listed as “Vulnerable (VU)” by the IUCN Red List of Threatened Species (Asian Turtle Trade Working Group 2000), the conservation status of P.axenaria, P.maackii, P.parviformis, and now P.variegatus, remains unassessed, in spite of their proven genetic distinctness (Fritz et al. 2010; Yang et al. 2011; Gong et al. 2018). Given their restricted distributional ranges and the intense exploitation to which they are subjected, all these species would certainly classify for a higher category rating. In this vein, the most recent red list of Chinese vertebrates compiled by Jiang et al. (2016) proposed the conservation status of P.axenaria, P.parviformis and P.sinensis be upgraded to “Endangered (EN)” and indicated P.maackii to be “Data Deficient (DD).” Rhodin et al. (2018) suggested for P.parviformis “Critically Endangered (CR)” and for P.sinensis “Endangered (EN),” whereas P.axenaria and P.maackii were identified as “Data Deficient (DD).” Consequently, also P.variegatus, which was included in P.parviformis by Rhodin et al. (2018), should be classified as “Critically Endangered (CR).”
Supplementary Material
Acknowledgments
Cuijuan Niu (Beijing), Marinus Hoogmoed (Belém, formerly Leiden), Carol Spencer, Mikaila Baskin (both Berkeley), Rainer Günther, Frank Tillack (both Berlin), Wolfgang Böhme, Morris Flecks (both Bonn), Judit Vörös, Viktória Szőke, Gábor Csorba (all Budapest), Alan Resetar, Joshua Mata (both Chicago), Markus Auer (Dresden), Gerold Schipper (Frankfurt am Main), Balázs Buzás (Fujairah), Truong Quang Nguyen (Hanoi), David Kizirian, David Dickey, Lauren Vonnahme (all New York), Georg Gassner, Richard Gemel, Franz Tiedemann (all Vienna), and Quang Xuan Hoang (Vinh) are acknowledged for access to collection material, providing photographs, as well as information and/or for various courtesies over the years. Anders Rhodin (Lunenburg) is thanked for his valuable comments on the first draft of this paper. Fieldwork in Vietnam was supported by the Volkswagen Foundation.
Citation
Farkas B, Ziegler T, Pham CT, Ong AV, Fritz U (2019) A new species of Pelodiscus from northeastern Indochina (Testudines, Trionychidae). ZooKeys 824: 71–86. https://doi.org/10.3897/zookeys.824.31376
Supplementary materials
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of 397 bp of the 12S rRNA gene of different Pelodiscus species and lineages
Data type: molecular data
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of the cyt b gene of different Pelodiscus species and lineages (1168 bp)
Data type: molecular data
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of the partial ND4 gene and adjacent DNA coding for tRNAs (838 bp) of different Pelodiscus species and lineages
Data type: molecular data
References
- Asian Turtle Trade Working Group (2000) Pelodiscussinensis The IUCN Red List of Threatened Species 2000: e.T39620A97401140. https://www.iucnredlist.org/species/39620/97401140
- Bain RH, Hurley MM. (2011) A biogeographic synthesis of the amphibians and reptiles of Indochina. Bulletin of the American Museum of Natural History 360: 1–138. http://digitallibrary.amnh.org/handle/2246/6141 [Google Scholar]
- Brandt JF. (1857) Observationes quaedam ad generis Trionychum species duas novas spectantes auctore J.F. Brandt. Bulletin de la Classe physico-mathématique de l’Académie impériale des Sciences de Saint Pétersbourg 16: 110–111. [Google Scholar]
- Chkhikvadze VM. (1987) On systematic position of the USSR Far East soft-shelled turtle. Bulletin of the Academy of Sciences of the Georgian Soviet Socialist Republic 128: 609–611. [In Russian with Georgian and English summary] [Google Scholar]
- Ernst CH, Barbour RW. (1989) Turtles of the World. Smithsonian Institution Press, Washington, DC, xxii + 313 pp.
- Ernst CH, Altenburg RGM, Barbour RW. (2000) Turtles of the world. Version 1.2, CD-ROM. ETI BioInformatics, Amsterdam.
- Farkas B, McCormack T. (2010) Le Trionyx de Chine Pelodiscussinensis (Wiegmann, 1834) (Ang: Chinese softshell turtle). Chéloniens 18: 56–63. [Google Scholar]
- Fritz U, Gong S, Auer M, Kuchling G, Schneeweiss N, Hundsdörfer AK. (2010) The world’s economically most important chelonians represent a diverse species complex (Testudines: Trionychidae: Pelodiscus). Organisms, Diversity & Evolution 10: 227–242. 10.1007/s13127-010-0007-1 [DOI] [Google Scholar]
- Gong S, Vamberger M, Auer M, Praschag P, Fritz U. (2018) Millennium-old farm breeding of Chinese softshell turtles (Pelodiscus spp.) results in massive erosion of biodiversity. Science of Nature 105: 34. 10.1007/s00114-018-1558-9 [DOI] [PubMed]
- Jiang Zh, Jiang J, Wang Y, Zhang E, Zhang Y, Li L, Xie F, Cai B, Cao L, Zheng G, Dong L, Zhang Zh, Ding P, Luo Zh, Ding Ch, Ma Zh, Tang S, Cao W, Li Ch, Hu H, Ma Y, Wu Y, Wang Y, Zhou K, Liu Sh, Chen Y, Li J, Feng Z, Wang Y, Wang B, Li Ch, Song X, Cai L, Zhang Ch, Zeng Y, Meng Zh, Fang H, Ping X. (2016) Red list of China’s vertebrates. Shengwu Duoyangxing (Biodiversity Science) 24: 500–551. [Google Scholar]
- Kottelat M. (1989) Zoogeography of the fishes from Indochinese inland waters with an annotated check-list. Bulletin Zoölogisch Museum, Universiteit van Amsterdam 12: 1–53. [Google Scholar]
- Meylan PA. (1987) The phylogenetic relationships of soft-shelled turtles (family Trionychidae). Bulletin of the American Museum of Natural History 186: 1–101. http://digitallibrary.amnh.org/handle/2246/972 [Google Scholar]
- Nguyen VS, Ho TC, Nguyen QT. (2009) Herpetofauna of Vietnam. Edition Chimaira, Frankfurt am Main, 768 pp. [Google Scholar]
- Pope CH. (1935) The Reptiles of China – Natural History of Central Asia 10. American Museum of Natural History, New York, lii + 604 pp.
- Pritchard PCH. (1979) Encyclopedia of turtles. T.F.H. Publications, Neptune, 895 pp. [Google Scholar]
- Rhodin AGJ, Stanford CB, van Dijk PP, Eisemberg C, Luiselli L, Mittermeier RA, Hudson R, Horne BD, Goode EV, Kuchling G, Walde A, Baard EHW, Berry KH, Bertolero A, Blanck TEG, Bour R, Buhlmann KA, Cayot LJ, Collett S, Currylow A, Das I, Diagne T, Ennen JR, Forero-Medina G, Frankel MG, Fritz U, García G, Gibbons JW, Gibbons PM, Gong S, Guntoro J, Hofmeyr MD, Iverson JB, Kiester AR, Lau M, Lawson DP, Lovich JE, Moll E, Páez VP, Palomo-Ramos R, Platt K, Platt SG, Pritchard PCH, Quinn HR, Rahman SC, Randrianjafizanaka ST, Schaffer J, Selman W, Shaffer HB, Sharma DSK, Shi H, Singh S, Spencer R, Stannard K, Sutcliffe S, Thomson S, Vogt RC. (2018) Global conservation status of turtles and tortoises (order Testudines). Chelonian Conservation and Biology 17: 135–161. 10.2744/CCB-1348.1 [DOI] [Google Scholar]
- Rudolphi M, Weser R. (1998) Die Weichschildkröten Nordvietnams unter besonderer Berücksichtigung der Nackendornen-Weichschildkröte, Paleasteindachneri (Siebenrock, 1906). Sauria (Berlin) 20(1): 3–14. [Google Scholar]
- Siebenrock F. (1906) Zur Kenntnis der Schildkrötenfauna der Insel Hainan. Zoologischer Anzeiger 30: 578–586. [Google Scholar]
- Stuckas H, Fritz U. (2011) Identity of Pelodiscussinensis revealed by DNA sequences of an approximately 180-year-old type specimen and a taxonomic reappraisal of Pelodiscus species (Testudines: Trionychidae). Journal of Zoological Systematics and Evolutionary Research 49: 335–339. 10.1111/j.1439-0469.2011.00632.x [DOI] [Google Scholar]
- TTWG [Turtle Taxonomy Working Group] (2007) An annotated list of modern turtle terminal taxa with comments on areas of taxonomic instability and recent change. Chelonian Research Monographs 4: 173–199. [Google Scholar]
- TTWG [Turtle Taxonomy Working Group] (2011) Turtles of the world, 2011 update: Annotated checklist of taxonomy, synonymy, distribution, and conservation status. Chelonian Research Monographs 5: 165–242. 10.3854/crm.5.000.checklist.v4.2011 [DOI] [Google Scholar]
- TTWG [Turtle Taxonomy Working Group] (2012) Turtles of the world, 2012 update: Annotated checklist of taxonomy, synonymy, distribution, and conservation status. Chelonian Research Monographs 5: 243–328. 10.3854/crm.5.000.checklist.v5.2012 [DOI] [Google Scholar]
- TTWG [Turtle Taxonomy Working Group] (2014) Turtles of the world: Annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status (7th edn). Chelonian Research Monographs 5: 329–479. 10.3854/crm.5.000.checklist.v7.2014 [DOI] [Google Scholar]
- TTWG [Turtle Taxonomy Working Group] (2017) Turtles of the world. Annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status (8th edn). Chelonian Research Monographs 7: 1–292. 10.3854/crm.7.checklist.atlas.v8.2017 [DOI] [Google Scholar]
- Wermuth H, Mertens R. (1961) Schildkröten, Krokodile, Brückenechsen. VEB Gustav Fischer Verlag, Jena, XXVI + (1) + 422 pp.
- Wermuth H, Mertens R. (1977) Liste der rezenten Amphibien und Reptilien. Testudines, Crocodylia, Rhynchocephalia. Das Tierreich 100: I–XXVII + 1–174.
- Yang P, Tang Y, Ding L, Guo X, Wang Y. (2011) Validity of Pelodiscusparviformis (Testudines: Trionychidae) inferred from molecular and morphological analysis. Asian Herpetological Research 2: 21–29. 10.3724/SP.J.1245.2011.00021 [DOI] [Google Scholar]
- Zhao E, Adler K. (1993) Herpetology of China. Society for the Study of Amphibians and Reptiles (Contributions to Herpetology 10), Oxford, Ohio, 521 pp. [Google Scholar]
- Zhou G, Zhang X, Fang Z. (1991) Bulletin of a new species Trionyx. Acta Scientiarum Naturalium Universitatis Normalis Hunanensis 14: 379–382. [In Chinese with English abstract] [Google Scholar]
- Ziegler T. (2002) Die Amphibien und Reptilien eines Tieflandfeuchtwald-Schutzgebietes in Vietnam. Natur & Tier Verlag, Münster, 342 pp. [Google Scholar]
- Ziegler T, Herrmann H-W, Vu NT, Le KQ, Nguyen TH, Cao XC, Luu MT, Dinh HT. (2004) The amphibians and reptiles of the Phong Nha–Ke Bang National Park, Quang Binh Province, Vietnam. Hamadryad 28: 19–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of 397 bp of the 12S rRNA gene of different Pelodiscus species and lineages
Data type: molecular data
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of the cyt b gene of different Pelodiscus species and lineages (1168 bp)
Data type: molecular data
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong, Uwe Fritz
Reference alignment of the partial ND4 gene and adjacent DNA coding for tRNAs (838 bp) of different Pelodiscus species and lineages
Data type: molecular data