Abstract
Chronic diseases are often comorbid and present a weighty burden for communities in the 21st century. The present investigation depicted patterns of multimorbidity in the general population and examined its association with the individual- and area-level factors in an urban sample of non-elderly adults of Brazil. Data were from the cross-sectional São Paulo Megacity Mental Health Survey, a stratified multistage area probability sampling investigation. Trained interviewers assessed mental morbidities and asked about physical conditions for 1,571 community-dwelling women and 1,142 men, aged between 18 and 64 years. Principal component analysis depicted patterns of physical-mental multimorbidity, by sex. Following, the patterns of multimorbidity were subjected to multilevel regression analysis, taking into account individual- and area-level variables. Three patterns of clustering were found for women: ‘irritable mood and headache’, ‘chronic diseases and pain’, and ‘substance use disorders’. Among men, the patterns were: ‘chronic pain and respiratory disease’, ‘psychiatric disorders’, and ‘chronic diseases’. Multilevel analyses showed associations between multimorbidity patterns and both individual- and area-level determinants. Our findings call for a reformulation of health-care systems worldwide, especially in low-resource countries. Replacing the single-disease framework by multi-disease patterns in health-care settings can improve the ability of general practitioners in the health-care of person-centred needs.
Introduction
Multimorbidity is a taxing concept to delimitate1, but it can be defined as a non-random association pattern between diseases2. Generally, multimorbidity is conceptualized as the co-occurrence of multiple chronic or long-term medical conditions. Multimorbidity includes both physical and mental illnesses and is distinguished from comorbidity due to the absence of an index disease or condition as is the case of the second3. The tendency of chronic conditions to cluster into distinct configurations represents the norm in older age people, resulting in higher disease persistence, functional disability, polypharmacy, health-care service use, and mortality3–5.
Although multimorbidity is a worldwide concern, most of beyond chance combination of medical and psychiatric conditions has been recognized in developed countries6. However, this phenomenon is not restricted to the elderly living in high-income countries (HICs). Multimorbidity affects more young people in low- and middle-income countries (LMICs) than in HICs7. Chronic non-communicable diseases represent a large share of disease burden in LMICs8, which start during peak economically active years of age. For example, the World Health Survey has estimated a mean prevalence of multimorbidity of 7.8% in 28 LMICs9. Moreover, in WHO’s Study on global AGEing and adult health (SAGE), over one-fifth of participants from six LMICs reported multiple morbidities10. All this burden of chronic multimorbidity contradicts the preceding belief that mortality-related burden still prevails in LMICs. The complex service needs of growing multimorbid populations in low-resource countries challenge policymakers to restructure health-care delivery.
In the general population, episodes of ill-health seem unevenly distributed among individuals, wherein some groups experience more diseases than others11. When pairs of conditions co-occur more frequently than expected, putative shared risk factors might be involved or one of the two processes operates as the risk factor for the other5. This morbidity-dependent mechanism might rely on a self-perpetuating and mutually reinforcing causality1. The reciprocal influence of general medical conditions and mental disorders is apparent when individuals reporting long-lasting psychiatric disorders also present more medical illnesses12. For example, generalized anxiety and dysthymia are the strongest predictors of medical multimorbidity. Similarly, hypertension and asthma are the strongest predictors for psychiatric multimorbidity. In this sense, the extent of multimorbidity has also been associated with age, sex, educational level, country’s income, and social inequality6,13,14. As a consequence, the resulting health-care needs of multimorbid people suggest a pressing reorganization of the health system in forthcoming decades15. Considering insufficient budget allocated to health in LMICs, reorienting health-care resources to identify and target adverse effects of morbidity burden is the utmost priority.
Over and beyond individual predictors, area-level or contextual factors may exert a joint effect16,17. Across all ages, individuals from deprived areas are more likely to present multimorbidity than those living in affluent areas13,14. Nevertheless, the majority of studies examining patterns of multimorbidity and area-level determinants were performed with clinical samples in developed countries, where social inequality is deemed to be lower than in LMICs6,17.
The numerous service needs of growing multimorbid populations challenge policymakers to rethink health-care delivery, but comprehensive information of health-care utilization by subjects with multimorbidity is scant, in terms of provision and expenditure16,18. Furthermore, considerable gaps still remain in the literature: Which are the individual- and area-level determinants of multimorbidity in the general population of LMICs? What is its impact on health-care systems?
It was estimated that at least 19 million adults present multimorbidity in Brazil19. A recent publication on global burden of diseases of Brazil has demonstrated that the morbidity profile has not changed substantially in the country since 1990: most of the leading causes of years lived with disability (YLDs) are non-communicable and chronic diseases (e.g., low back & neck pain, migraine, depressive disorders, anxiety disorders, etc.)20. In past decades, despite a gradual improvement, with the expansion of universal health coverage, structural problems still persist in health-care organisation and governance in the Brazilian health system. Moreover, limited funding and suboptimal resource allocation lead to persistent regional disparities in access to health-care services and health indicators21,22. For dwellers of an urbanized São Paulo metropolitan area, the 4th densely populated area in the globe, the availability of health-care services remains below standards of adequacy23–25. In the current study, we aim to investigate patterns of multimorbidity in the non-elderly general population of the metropolitan area of São Paulo26, located in a middle-income country. We also examine the association of multimorbidity with health-care utilization, as well as the determinants of individual- and area-level variables.
Methods
This is a multilevel analysis of the influence of individual- and area-level factors on patterns of physical–mental multimorbidity and health-care use in the general population of the metropolitan area of São Paulo.
Sampling
Cross-sectional data were drawn from the São Paulo Megacity Mental Health Survey26, the Brazilian branch of the World Mental Health Survey initiative27. A household representative sample of individuals aged 18 years or older living in the São Paulo metropolitan area was selected through stratified, multistage area probability sampling. This area comprises the city of São Paulo and its 38 surrounding municipalities, with an estimated population of 21 million inhabitants28. At the time of data collection, from May 2005 to May 2007, there were approximately 11 million inhabitants aged 18 years or older. After exclusion of 200 elderly with cognitive impairment, a total of 5,037 eligible respondents accepted to be interviewed in their households. The global response rate was 81.3%. A detailed description of the sampling procedure was presented elsewhere29.
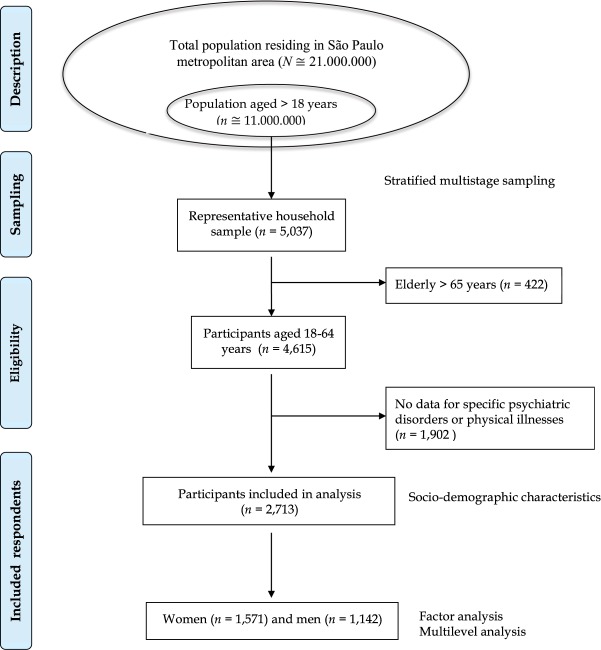
In the current analysis, to avoid the cumulative aging effect of multiple diseases, we excluded 422 participants aged over 65 years, resulting in a representative sample of 4,615 subjects aged between 18 and 64 years. Specific psychiatric disorders (e.g., post-traumatic stress, obsessive-compulsive, and premenstrual dysphoric disorders) were asked only for a representative, probability sub-sample. We excluded 1,902 individuals because they were not assessed for all morbidities. Therefore, data from 2,713 respondents whose answers contained complete dataset on psychiatric and physical morbidity were analysed (Fig. 1).
Figure 1.
Flow diagram of the study sampling.
For multilevel analysis, characteristics of 69 areas of respondents’ residence were considered. The area-level of the model took into account the 31 administrative regions or “sub-prefeituras” of the city of São Paulo (average of 355,467 residents) and each one of the 38 municipalities located in this metropolitan area (average of 232,751 residents).
Assessment of socio-demographics
Information on sex, age, marital status, education, family income, and employment were collected. Sex was coded as male or female. Age was categorized as 18–34, 35–49, and 50–64-year-old (yo) brackets. Marital status was defined as married/cohabitating, previously married (widowed/separated/divorced) and never married. Years of schooling were coded as fundamental (≤4 years), basic (5–8 years), high school (9–11 years) and college (≥12 years). Socioeconomic status (SES) was expressed as the family income divided by the average income of the sample, resulting in four quartiles. Employment status was categorized as employed, student, homemaker, retired and unemployed.
Assessment of mental disorders
The World Mental Health version of the Composite International Diagnostic Interview (WMH-CIDI) was used. The WMH-CIDI is a fully structured instrument applied by lay evaluators30, which has acceptable validity and reliability31. The algorithm of this instrument allows generating the diagnosis of mental disorders according to DSM-IV and ICD-10.
Four classes of 12-month mental disorders (anxiety, mood, impulse-control, and substance use disorders [SUD]), plus premenstrual dysphoria (in women) and heavy drinking, were considered. Heavy drinking was defined as the consumption of more than 40 g/day of alcohol for men and more than 20 g/day for women32.
Anxiety disorders comprised panic, agoraphobia without panic, simple phobia, social phobia, generalized anxiety, adult separation anxiety, obsessive-compulsive, and post-traumatic stress disorders. Mood disorders included major depression, dysthymia, and bipolar disorder. Impulse-control disorders were intermittent explosive, oppositional-defiant, conduct, and attention-deficit/hyperactivity disorder. The substance use disorders (SUD) covered alcohol and drug abuse and dependence.
Assessment of physical illnesses
Chronic physical illnesses were assessed with a standard checklist of diseases. This checklist, used in all studies of the World Mental Health Initiative27, has produced more adequate illness prevalence than those estimates resultant from inventories with open-ended questions33,34. In earlier studies35,36, it has also demonstrated a moderate to good concordance with clinical records. Respondents reported whether a doctor or other health professional ever told them they had the condition in the previous year.
Ten prevalent medical conditions were considered in the analysis: cardiovascular diseases (heart attack, heart disease, and stroke), hypertension, diabetes mellitus, arthritis, chronic musculoskeletal pain, headache or migraine, digestive, respiratory (seasonal allergies, asthma, obstructive pulmonary disease, and emphysema), neurological diseases (Parkinson disease, epilepsy, and multiple sclerosis), and cancer.
Severity assessment
Respondents self-rated the impairment caused by each morbidity assessed during the worst month in the past year using the Sheehan Disability Scale37. The 10-point visual analogue scale assessed disability in the following domains: work role performance, household maintenance, social life, and intimate relationship. The scale scores ranged from none, 0; mild, 1–3; moderate, 4–6; to severe, 7–10. Respondents who had reported more than 1 disorder were assigned to the highest score for any single disorder.
Participant’s functional status was categorized as severe if the respondent has shown a high level of impairment on the Sheehan Disability Scale in at least one of the morbidities presented; were diagnosed with bipolar disorder, or substance dependence with physiologic signs; had attempted suicide in the past year38, in conjunction with one or more core DSM-IV psychiatric disorder.
Among those cases that were not categorized as severe, respondents were labelled ‘moderate’ if they had at least one disorder with a moderate level of impairment on any domain or substance dependence without physiological signs. The remaining respondents with any active disorder were categorized as ‘mild’. Accordingly, 12-month disease severity level of each respondent was classified as ‘no’, ‘mild’, ‘moderate’, or ‘severe’.
Use of service
The use of any medical service was asked through the question: “Do you have a physician who you usually consult when you need routine care?”. The availability of a regular physician for routine medical treatment in the past 12 months was analysed as absence (0) or presence (≥1) of treatment access.
Respondents were also asked whether they “ever saw any professional treatment for problems with their emotions, nerves, mental health, or use of substances” in the 12 months prior to the interview. In the present paper, the use of mental health-care refers to either a general medical or mental health sector for the treatment of any mental disorders. The general medical sector included primary care physicians, nurses, or other health-care professionals. The specialized mental health sector included psychiatrists and other professionals (psychologists, social workers, or counsellors in a mental health setting).
Statistical analysis
In the analytic approach, the variance estimation procedures with complex sample survey data were employed to account the stratified multistage area sampling design. To adjust for differences in the chance of selection and non-response within-households, weights were applied to adjust the prevalence rates. The differential sampling of WMH-CIDI was corrected, wherein a sub-sample of participants has answered to all questions about psychiatric and physical morbidities. Finally, a post-stratification weight was used to equate the sample distribution to the population distribution in the 2000 census28.
First, the weighted prevalence of each disease variable was calculated both for the total sample and by sex. Between-sex differences were assessed by chi-square test. Likewise, we determined the number of diseases in the same respondent, both for the total sample and by sex. As a substantial difference was apparent, we built a sex-specific histogram for the number of diseases by each age bracket. Also, selected prevalent conditions (over 5%) were analysed through a graphical matrix in accordance with family socioeconomic status (SES) quartiles. The high-SES quartile was compared to low-SES quartile in a bubble graph. Accordingly, this graph of conditional probabilities on the pairwise correlation between selected prevalent conditions further provides a visual appraisal of these relationships. The conditional relationship of multimorbidity was shown as the percentage of persons who presented both conditions.
Second, since all observed variables on psychiatric and physical morbidities were binary, a principal component analysis determined the covariance structure of the data and built the factor scores of each extracted component, separately by sex. Both Kaiser’s eigenvalue > 1.0 rule-of-thumb criteria39 and Cattell’s scree test40 established the number of components to extract, and both orthogonal and oblique rotations were applied for interpretation.
After the identification of models, we investigated the relationship between each multimorbidity pattern and risk factors, i.e., between latent and observed variables. Five multilevel regression models estimated the simultaneous influence of participants’ individual- and area-level characteristics on each sex-specific pattern of multimorbidity41. Individual-level information was included in the first model: socio-demographic variables (age bracket, marital status, and years of education), morbidity severity, and access to health-care facilities (medical treatment and mental health-care service).
Thereafter, four area-level models were sequentially incorporated to the equation for examining the influence of following area-level administrative information: education, median income, socio-economic inequality (Gini coefficient), and violence (homicide rate per 100.000 inhabitants). Area-level median income and education (measured by the proportion of individuals that completed basic education) were categorized as low, medium and high by tertiles to allow for possible non-linearity, using the results from the 2010 Census28.
The intraclass correlation coefficient (ICC) was estimated for each random intercept model to determine the proportion of total variance explained by area-level variance while accounting for the non-independence of individual observations within groups. In other words, ICC expresses the correlation between individuals belonging to the same group. For interpretation, a non-zero ICC implies that the observations are not independent. The lower the ICC between two observations within the same cluster, the lower the variability is between the clusters and the higher the variability is within the clusters. If all the responses from observations in the same cluster are exactly the same, the ICC equals 1. If all the observations are independent of one another, the ICC equals zero.
Descriptive and principal component analyses were performed using the SPSS/PASW 17 software (www-01.ibm.com) and the multilevel models were estimated with R 3.2.2 software (www.cran.r-project.org). The level of significance was set at p < 0.05 for two-tailed tests.
Ethics approval and informed consent
The Research and Ethics Committee of the University of São Paulo Medical School approved the procedures for recruitment, obtaining informed consent, and protecting human subjects during field works. Respondents were interviewed after signing an informed written consent and being assured of confidentiality.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 and, also, the authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides.
Results
Socio-demographic characteristics of the sample
Table 1 depicts socio-demographic characteristics of the 2,713 community-dwelling respondents aged 18–64 years old (yo) in the São Paulo metropolitan area. Women represented 52.4% of the sample, around half of the respondents (48.1%) were aged 18–34 yo, 35.5% were 35–49 yo and 16.4% were 50–64 yo. The majority (61.2%) had average schooling of 5–11 years and 60.9% was married or cohabiting. Family income, or the proxy for socio-economic status (SES), was evenly distributed across groups: low (22.6%), middle-low (28.5%), medium-high (22.7%), and high (26.2%). The majority (68.9%) was employed at the time of the interview.
Table 1.
Descriptive characteristics of the participants of the São Paulo Megacity Mental Health Survey (N = 2,713, aged 18–64 years).
| Variable | n | % |
|---|---|---|
| Sex | ||
| Men | 1,142 | 47.63 |
| Women | 1,571 | 52.37 |
| Age, years | ||
| 18–34 yo | 1,091 | 48.11 |
| 35–49 yo | 1,028 | 35.45 |
| 50–64 yo | 594 | 16.44 |
| Education (years of schooling) | ||
| Low (0–4) | 644 | 19.34 |
| Low-average (5–8) | 700 | 22.82 |
| High-average (9–11) | 952 | 38.35 |
| High (≥12) | 417 | 19.49 |
| Marital status | ||
| Married/cohabiting | 1,734 | 60.87 |
| Previous married | 492 | 13.60 |
| Never married | 487 | 25.53 |
| Family income | ||
| Low | 694 | 22.58 |
| Low-average | 759 | 28.47 |
| High-average | 615 | 22.72 |
| High | 645 | 26.23 |
| Employment status | ||
| Employed | 1,706 | 68.94 |
| Student | 30 | 1.71 |
| Homemaker | 453 | 12.98 |
| Retired | 140 | 3.94 |
| Unemployed | 384 | 12.43 |
% Weighted proportion.
Prevalence of 12-month morbidities
In the total sample, anxiety (20.7%) and mood disorders (12.5%) were the most prevalent psychiatric disorders in the past year (Table 2). For the female sub-sample, anxiety and mood disorders were also prevalent, respectively in 27.2% and 17.5% of women. Premenstrual dysphoria was reported by more than half of women (51.2%). In the male sub-sample, the most prevalent disorders were heavy drinking (16.6%) and anxiety disorders (13.6%). Significant sex differences were observed for all classes of psychiatric disorders, except impulse-control disorders.
Table 2.
Prevalence of 12-month mental disorders and physical illnesses in the general population of the São Paulo metropolitan area, total sample (N = 2,713, aged 18–64 years) and by sex.
| Total (n = 2,713) | Women (n = 1,571) | Men (n = 1,142) | χ2 | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | SE | n | % | SE | n | % | SE | |||
| Psychiatric disorders | |||||||||||
| Anxiety disorder | 795 | 20.7 | 0.9 | 553 | 27.2 | 1.6 | 242 | 13.6 | 1.1 | 38.8 | < 0.0001 |
| Mood disorder | 548 | 12.5 | 0.9 | 410 | 17.5 | 1.5 | 138 | 7.1 | 0.8 | 42.6 | < 0.0001 |
| Heavy drinking | 278 | 10.3 | 0.8 | 88 | 4.7 | 0.6 | 190 | 16.6 | 1.6 | 68.9 | < 0.0001 |
| Impulse-control disorder | 189 | 4.8 | 0.5 | 103 | 4.6 | 0.6 | 86 | 5.0 | 0.7 | 0.3 | 0.59 |
| Substance use disorder | 161 | 4.1 | 0.4 | 37 | 1.8 | 0.3 | 124 | 6.7 | 0.8 | 46.6 | < 0.0001 |
| Premenstrual dysphoriac | — | — | — | 843 | 51.2 | 2.3 | — | — | — | NA | — |
| Physical illnesses | |||||||||||
| Musculoskeletal pain | 1,083 | 32.8 | 1.5 | 700 | 37.4 | 2.2 | 383 | 27.7 | 1.7 | 15.8 | 0.0002 |
| Head/migraine | 984 | 30.7 | 1.5 | 736 | 40.9 | 2.2 | 248 | 19.5 | 2.1 | 44.5 | < 0.0001 |
| Respiratory disease | 705 | 24.7 | 1.3 | 496 | 30.1 | 1.8 | 209 | 18.7 | 2.0 | 14.0 | 0.0005 |
| Hypertension | 579 | 17.1 | 1.1 | 359 | 18.0 | 1.4 | 220 | 16.2 | 1.7 | 0.6 | 0.45 |
| Arthritis | 244 | 6.5 | 0.7 | 195 | 9.3 | 1.1 | 49 | 3.5 | 0.8 | 17.5 | 0.0001 |
| Cardiovascular disease | 196 | 4.3 | 0.4 | 124 | 4.7 | 0.6 | 72 | 3.7 | 0.6 | 1.1 | 0.30 |
| Diabetes | 147 | 3.9 | 0.5 | 88 | 3.7 | 0.6 | 59 | 4.0 | 0.8 | 0.1 | 0.79 |
| Digestive disease | 118 | 2.2 | 0.3 | 78 | 2.6 | 0.4 | 40 | 1.7 | 0.3 | 2.8 | 0.10 |
| Neurological disease | 66 | 1.6 | 0.3 | 37 | 1.6 | 0.3 | 29 | 1.5 | 0.4 | 0.1 | 0.78 |
| Cancer | 19 | 0.5 | 0.1 | 14 | 0.6 | 0.2 | 5 | 0.4 | 0.2 | 0.4 | 0.51 |
n: subsample of respondents aged between 18–64 years, considering those who have answered to all mental disorders and general medical conditions.
%: weighted prevalence.
cPremenstrual dysphoria refers to women.
SE: standard error; NA: not applicable.
For physical illnesses, musculoskeletal pain (32.8%), headache/migraine (30.7%), respiratory disease (24.7%), and hypertension (17.1%) were the most prevalent conditions in the total sample (Table 2). Women more likely reported painful conditions (musculoskeletal pain, headache/migraine, and arthritis) and respiratory diseases than men.
Number and type of multimorbidity
Multimorbidity was common for people younger than 65 years and the prevalence increased with age. Almost half of the respondents reported more than one condition during the past year, being 20.7% reported two and the remaining 27.8%, three to 10 co-occurring conditions (Table 3). Women significantly complained of more diseases than men, as well as more multimorbidity. Figure 2 shows the number of comorbid conditions by sex and age bracket. A salient dose-response gradient between age and number of morbidities was observed for both sexes: the higher the age bracket, the greater the proportion of concomitant conditions. In general, women reported more comorbid conditions than men for all age brackets.
Table 3.
Total number and proportion of morbidities in the general population of the São Paulo metropolitan area, total sample (N = 2,713) and by sex.
| Number of conditions | Total | Women† | Men | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0 | 456 | 24.43 | 191 | 19.19 | 265 | 30.19 | <0.0001 |
| 1 | 616 | 27.10 | 308 | 24.44 | 308 | 30.02 | 0.0620 |
| 2 | 577 | 20.68 | 334 | 21.66 | 243 | 19.60 | 0.3800 |
| 3 | 440 | 12.90 | 292 | 14.74 | 148 | 10.88 | 0.0284 |
| 4 | 294 | 7.92 | 200 | 10.14 | 94 | 5.48 | 0.0015 |
| 5 | 184 | 4.09 | 134 | 5.79 | 50 | 2.21 | 0.0003 |
| 6 | 75 | 1.68 | 56 | 2.43 | 19 | 0.86 | 0.0052 |
| 7 | 43 | 0.75 | 36 | 1.12 | 7 | 0.34 | 0.0201 |
| 8 | 21 | 0.33 | 16 | 0.40 | 5 | 0.27 | 0.4938 |
| 9 | 6 | 0.11 | 3 | 0.07 | 3 | 0.15 | 0.4534 |
| 10 | 1 | 0.01 | 1 | 0.02 | — | — | NA |
| Total | 2,713 | 100.00 | 1,571 | 100.00 | 1,142 | 100.00 | |
†Includes premenstrual dysphoric disorder.
Figure 2.
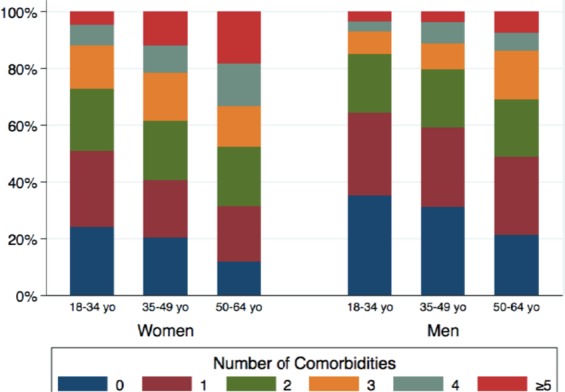
Number of comorbid conditions by sex and age bracket.
Regarding the co-occurrence of different conditions, the most common pairwise relationship was between musculoskeletal pain and arthritis, anxiety and mood disorders, and mood disorders and migraine. When respondents from the higher and the lower SES were compared, substantial influence of the socio-economic dissimilarity was demonstrated (Fig. 3). For example, when high-SES respondents with anxiety were compared with those low-SES ones, the proportion of arthritis was twice higher among the former. Conversely, the multimorbidity of heavy drinking and anxiety was doubled among respondents from low SES.
Figure 3.
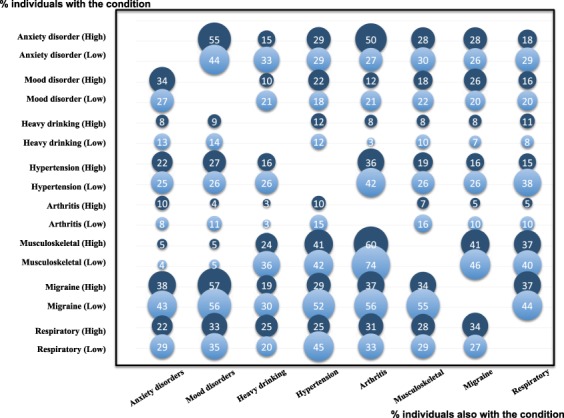
Conditional pairwise proportion of selected multimorbidities among people with common and frequent morbidities from the most affluent (dark blue) and the most deprived (light blue) socioeconomic quartiles.
Patterns of multimorbidity
For both women and men, the model selected by the Kaiser’s criterion was the one with 3 factors and explained 31.2% and 30.4% of the total variance, respectively (Table 4). Nevertheless, the morbidity composition and factor loading in each factor varied by sex.
Table 4.
Pattern matrix of 12-month multimorbidity of psychiatric disorders and general medical conditions in the general population of the São Paulo metropolitan area (N = 2,713, aged 18–64 years), by sex.
| Variable | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor 1 Irritable mood & headache | Factor 2 Chronic diseases & chronic pain | Factor 3 Substance use disorders | h 2 | Factor 1 Chronic pain & respiratory disease | Factor 2 Psychiatric disorders | Factor 3 Chronic diseases | h 2 | |
| Premenstrual dysphoria | 0.66 | 0.16 | 0.06 | 0.43 | ||||
| Mood disorder | 0.64 | 0.02 | 0.05 | 0.41 | 0.24 | 0.57 | 0.20 | 0.46 |
| Anxiety disorder | 0.61 | −0.04 | 0.08 | 0.38 | 0.14 | 0.47 | 0.29 | 0.34 |
| Impulse-control disorder | 0.47 | 0.12 | 0.23 | 0.26 | −0.09 | 0.66 | 0.06 | 0.44 |
| Headache/migraine | 0.43 | −0.14 | −0.09 | 0.24 | 0.68 | 0.03 | −0.06 | 0.46 |
| Respiratory disease | 0.26 | −0.03 | −0.09 | 0.09 | 0.38 | 0.00 | 0.01 | 0.14 |
| Hypertension | −0.08 | −0.72 | 0.10 | 0.49 | 0.01 | −0.02 | 0.69 | 0.50 |
| Cardiovascular disease | 0.02 | −0.60 | 0.07 | 0.35 | 0.04 | 0.02 | 0.55 | 0.31 |
| Diabetes mellitus | −0.15 | −0.59 | 0.11 | 0.34 | −0.26 | 0.01 | 0.68 | 0.48 |
| Arthritis | 0.05 | −0.54 | −0.11 | 0.34 | 0.35 | −0.17 | 0.22 | 0.21 |
| Musculoskeletal pain | 0.34 | −0.37 | −0.16 | 0.35 | 0.63 | 0.08 | 0.09 | 0.43 |
| Digestive disease | 0.21 | −0.25 | −0.10 | 0.14 | 0.34 | −0.02 | 0.02 | 0.12 |
| Heavy drinking | 0.14 | −0.12 | 0.76 | 0.57 | −0.11 | 0.39 | −0.18 | 0.20 |
| Substance use disorder | 0.21 | −0.07 | 0.69 | 0.49 | −0.06 | 0.61 | −0.10 | 0.38 |
| Neurological disease | 0.18 | −0.03 | −0.21 | 0.09 | 0.19 | 0.18 | −0.01 | 0.07 |
| Cancer | 0.05 | −0.01 | −0.20 | 0.04 | 0.12 | −0.01 | −0.07 | 0.02 |
| % of variance explained | 13.7 | 10.1 | 7.4 | 13.1 | 9.6 | 7.7 | ||
| Between-factor correlation | ||||||||
| Factor 1 | 1.00 | 1.00 | ||||||
| Factor 2 | −0.17 | 1.00 | 0.08 | 1.00 | ||||
| Factor 3 | −0.09 | 0.16 | 1.00 | 0.13 | −0.01 | 1.00 | ||
h2: communality.
Among women, the first component was labelled as “irritable mood and headache” and encompassed premenstrual dysphoric, mood, anxiety, impulse-control disorders, and headache/migraine. The second component of “chronic diseases and pain” included hypertension, cardiovascular illnesses, arthritis, diabetes, and musculoskeletal pain. The third component, “substance use”, included heavy drinking and substance use disorders (SUD). Respiratory, digestive, neurological illnesses and cancer presented low communality (<0.15) and did not contribute to the model (factor loading < 0.3).
For men, the first component, “chronic pain and respiratory disease”, included headache/migraine, musculoskeletal pain, arthritis, respiratory, and digestive illnesses. The second component was named “psychiatric disorders” and included impulse-control, mood, anxiety disorders, SUD, and heavy drinking. Finally, the third component described a dimension of “chronic diseases” that contained hypertension, cardiovascular illnesses, and diabetes. Neurological illness and cancer did not contribute to the model.
Multilevel analysis
Multilevel analysis, or hierarchical models, is an analytical approach that permits the examination of simultaneous effects of individual-, and group-level variables on outcome variables, allowing depicting the isolated and combined influence of variables of each level on the outcome variable41. Therefore, ten sex-specific linear models examined the influence of individual- and contextual-level variables, separately for women and men. In general, the intraclass correlation (ICC) varied greatly across models (Supplementary Table S1). Table 5 shows the model of each sex-specific pattern of multimorbidity that showed the lowest ICC. Individual-level variables like age, education, Sheehan category of disease severity, and use of health-care services were associated with increased likelihood of presenting multimorbid patterns. Jointly, the area-level variables explained 24% and 32% of the variance, respectively in women with “irritable mood” and men with “psychiatric disorders”. The remaining models explained less than 20% of the variance, and no influence of area-level variables was shown among men with “chronic diseases” (ICC = 0).
Table 5.
Multilevel analysis of patterns of multimorbidity in the general population of the São Paulo metropolitan area, by sex.
| Women | Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irritable mood & headache | Chronic diseases & pain | Substance use disorders | Chronic pain & respiratory disease | Psychiatric disorders | Chronic diseases | |||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | |
| Age bracket | ||||||||||||
| 18–34 yo† | ||||||||||||
| 35–49 yo | 0.04 | (−0.02; 0.10) | −0.31* | (−0.40; −0.23) | −0.11* | (−0.21; −0.02) | 0.04 | (−0.06; 0.15) | −0.18* | (−0.25; −0.11) | 0.30* | (0.20; 0.41) |
| 50–64 yo | −0.07 | (−0.15; 0.00) | −0.99* | (−1.10; −0.89) | −0.30* | (−0.42; −0.18) | 0.08 | (−0.05; 0.21) | −0.30* | (−0.38; −0.22) | 0.83* | (0.71; 0.95) |
| Marital status | ||||||||||||
| Single† | ||||||||||||
| Married/Cohabiting | 0.02 | (−0.05; 0.10) | −0.03 | (−0.13; 0.07) | −0.05 | (−0.17; 0.06) | 0.19* | (0.07; 0.31) | 0.00 | (−0.08; 0.07) | 0.05 | (−0.06; 0.16) |
| Separated/Widowed/Divorced | 0.00 | (−0.09; 0.09) | 0.06 | (−0.06; 0.18) | 0.06 | (−0.07; 0.20) | −0.04 | (−0.22; 0.14) | 0.02 | (−0.09; 0.13) | 0.10 | (−0.07; 0.26) |
| Educational level | ||||||||||||
| 1† | ||||||||||||
| 2 | 0.04 | (−0.03; 0.11) | 0.21* | (0.11; 0.31) | −0.07 | (−0.18; 0.05) | −0.08 | (−0.21; 0.04) | 0.02 | (−0.06; 0.10) | −0.05 | (−0.17; 0.06) |
| 3 | 0.01 | (−0.06; 0.09) | 0.30* | (0.20; 0.40) | 0.01 | (−0.10; 0.13) | −0.23* | (−0.35; −0.10) | 0.01 | (−0.07; 0.09) | −0.09 | (−0.20; 0.03) |
| 4 | −0.02 | (−0.11; 0.07) | 0.50* | (0.38; 0.63) | 0.02 | (−0.12; 0.16) | −0.16* | (−0.31; −0.01) | 0.00 | (−0.10; 0.09) | −0.13 | (−0.27; 0.00) |
| Severity | ||||||||||||
| 1 | 1.82* | (1.73; 1.90) | −0.28* | (−0.39; −0.16) | 0.42* | (0.29; 0.55) | 0.66* | (0.49; 0.83) | 2.37* | (2.26; 2.48) | 0.36* | (0.20; 0.52) |
| 2 | 1.45* | (1.37; 1.54) | −0.13* | (−0.24; −0.01) | 0.04 | (−0.09; 0.16) | 0.59* | (0.41; 0.77) | 1.67* | (1.56; 1.79) | 0.75* | (0.58; 0.92) |
| 3 | 1.23* | (1.14; 1.32) | −0.09 | (−0.21; 0.04) | 0.23* | (0.09; 0.37) | −0.01 | (−0.17; 0.16) | 1.57* | (1.47; 1.67) | 0.30* | (0.15; 0.45) |
| 4† | ||||||||||||
| Medical treatment | ||||||||||||
| No† | ||||||||||||
| Yes | 0.36* | (0.28; 0.44) | −0.21* | (−0.32; −0.10) | −0.22* | (−0.34; −0.09) | 0.25* | (0.06; 0.43) | 0.08 | (−0.04; 0.20) | 0.33* | (0.16; 0.50) |
| Mental health-care | ||||||||||||
| No† | ||||||||||||
| Yes | −0.13* | (−0.21; −0.05) | 0.30* | (0.19; 0.41) | 0.12 | (−0.01; 0.25) | −0.28* | (−0.38; −0.18) | 0.01 | (−0.05; 0.07) | −0.27* | (−0.36; −0.17) |
| Between individual ICC | 0.42 | 0.59 | 0.17 | 0.14 | 0.44 | 0.00 | ||||||
| Area-level education | ||||||||||||
| Low† | ||||||||||||
| Medium | ||||||||||||
| High | ||||||||||||
| Area-level income | ||||||||||||
| Low† | ||||||||||||
| Medium | −0.05 | (−0.12; 0.02) | ||||||||||
| High | 0.05 | (−0.03; 0.12) | ||||||||||
| Gini coefficient | ||||||||||||
| Low† | ||||||||||||
| Medium | 0.07* | (0.00; 0.14) | ||||||||||
| High | 0.01 | (−0.06; 0.08) | ||||||||||
| Area Violence | ||||||||||||
| Low† | ||||||||||||
| Medium | 0.03 | (−0.06; 0.12) | 0.18* | (0.06; 0.30) | ||||||||
| High | −0.12* | (−0.21; −0.03) | 0.09 | (−0.03; 0.22) | ||||||||
| Between Area ICC | 0.24 | 0.18 | 0.17 | 0.09 | 0.32 | 0.00 | ||||||
yo: year-old, ICC: Intra-class Correlation.
†Reference category.
*p < 0.05.
Regarding contextual variables, in women, socio-economic inequality had a small effect on “irritable mood and headache” (β = 0.07), and residing in violent location was inversely associated with “chronic diseases and pain” (β = −0.12). For men, violence was associated with the pattern of “chronic pain and respiratory diseases” (β = 0.18). Furthermore, women with “chronic diseases and pain” and “substance use” presented little influence of area-level variables. While men with “chronic diseases” presented no influence of area-level variables, contextual influence in the occurrence of the pattern of “psychiatric disorders” among men was observed.
Regarding the use of health-care services, medical treatment was associated with “irritable mood and headache” in women (β = 0.36), and mental health-care was associated with “chronic diseases and pain” (β = 0.30). Among men, “chronic diseases” and “chronic pain and respiratory diseases” were associated with medical treatments (β = 0.33 and 0.25, respectively), and inversely associated with mental health-care (β = −0.27 and −0.28, respectively).
Discussion
The presence of multiple conditions affecting the same individual is an important problem in the Brazilian population, even in younger brackets, and around one-fourth of adults aged 18–64 years-old presents a pattern of multimorbidity encompassing physical, mental illnesses or both. Additionally, men and women present distinct profiles of morbidity clustering, indicating the necessity of gender-focused preventive and intervention programs. To our knowledge, this is the first study, using a multilevel approach, which addresses the interrelationship of multiple conditions investigating physical but also mental disorders among younger adults of a general population. The strengths of the present work include the assessment of individuals of a developing country, with the combination of diagnosed psychiatric and physical illness in the classification of multimorbidity, and the examination of area-level determinants of disease clustering. The key findings of the present study have to be highlighted in the domain of public health.
In the present study, the prevalence of multimorbidity was twice higher than previously reported in Brazil14. An earlier study also suggested that multimorbidity likely affects older people, women, and those with low educational level14. In addition to these findings, we have shown that there was a salient sex difference, aging effect, and socioeconomic influence on the patterns of multimorbidity. Women and men presented different patterns of physical-mental multimorbidity42. While women were more affected by an irritable pattern of mood psychopathology and pain, men suffered more painful and respiratory diseases. However, the patterns of multimorbidity for both sexes could explain around 30% of the data variance and some low-prevalence diseases did not fit into any factor.
In comparison with previous findings, it rendered clear that the burden of multiple co-occurring chronic diseases is not high just in older age groups13,43, but a substantial proportion of economically-active younger adults in Brazil were also affected by multimorbidity. In the same direction, the Scottish study by Barnett and colleagues13 has indicated that the onset of multimorbidity might occur 10–15 years earlier in individuals living in deprived areas. Because chronic diseases generally start during peak economically-active years in LMICs7, multimorbidity clusters have disabling penalties and deeply affect the productivity of working populations, demanding prevention, early identification, and timely treatment.
Regarding the patterns of multimorbidity, despite heterogeneous methodology (number of selected diseases, diagnostic tool, and statistical procedures), three dimensions of multimorbidity were regularly identified6: cardiovascular and metabolic diseases; mental health problems; and musculoskeletal diseases. Previously, data from the Brazilian National Health Survey 201314 had also reported an analogous pattern of multimorbidity. Our results were in line with these key findings: ‘irritable mood and headache’ was the most important pattern among women, while ‘chronic pain and respiratory diseases’ among men. Noteworthy, the individual- and area-level characteristics affect these clustering patterns.
The singular case of São Paulo, located in an upper-middle-income country in late epidemiological transition and with high rates of inequality, may anticipate the policy planning and gradual transformation of low-income countries toward an optimal functioning of patient-centred health systems. In LMICs, the inverse care law is persistently confirmed by the fact that highly deprived areas also present less availability of health-care services44. Moreover, the current Brazilian health-care system, which is highly focused in specialized clinics21,22, should be readapted and strengthened to accommodate the multimorbidity epidemic in a person-centred holistic approach. Therefore, the combined recognition of morbidities that frequently co-occur may reduce the need of multiple visits to overloaded professionals in LMICs, facilitating comprehensive management of clustered health-care needs in communities.
The example of São Paulo indicated that multimorbidity is dependent on contextual characteristics of residence, but the impact of improved living conditions on well-being and health indicators remains to be demonstrated. Lessons that have been learned from different LMICs should be used to reduce the vicious circle of adverse disadvantages16,45–49, with the integration and scaling-up of the mental health component in primary care, due to its close relationship with the physical component50–52. Forecasting the worldwide growth of the ageing population, policymakers will have to face multimorbidity as a global challenge in the near future. The current unmet needs of the general population23–25 already indicate that it is necessary to deliver earlier preventive and treatment programs by the health-care systems, especially in LMICs. Hence, preventive efforts should start well before older age, focusing those people living in areas of high socio-economic inequality45.
A further consequence of these findings refers to the education of the teams that will face the tasks of prevention and treatment of at-risk populations. The requirement of a multiple-disease approach needs to be strengthened in the medical training in order to fulfil the requirements indispensable to deal with this uncovering epidemiologic profile13. The educational emphasis of health-care professionals should incorporate longitudinal clerkship that helps students to acquire a broad understanding of the emerging challenges of long-term multimorbidity conditions presented by aging adults53. For example, skills for handling multiple diseases should be developed through the gradual integration of general clinical reasoning within the patients’ undivided physical-mental needs54,55.
Limitations
Despite the several strengths of this study, our findings should be interpreted bearing in mind some limitations. Because the present study aimed to investigate common morbidities among the general population aged 18–64-year-old in their household, those treatment-seeking, institutionalized and homeless people were not included in the sample. Also, non-affective psychosis and other severe mental disorders were not included in the analysis. Likewise, the association between suicidality and multimorbidity was not considered as a potential outcome of this sample56.
Moreover, although the cross-sectional nature of the results presented herein has shown the size, patterns, and determinants of multimorbidity burden, the underlying causal relationships between co-occurring chronic diseases and patients’ disabilities need to be demonstrated in a more solid basis. The joint evolutionary course of multiple disorders might be captured with refined probabilistic models57.
Conclusion
Multimorbidity is the greatest global challenge of the 21st century. It affects not just older patients in HICs, but also the general population in LMICs. International health systems face a sizable proportion of people younger than 65 years with multimorbidity, whose management must be personalized. The government of LMICs should reorganize and coordinate health delivery to integrate the whole needs of people with multimorbidity, through training of health professionals and expansion of primary care. The clinician should be sensitive to the patient’s sex because distinctive patterns of illnesses affect women and men. The recovery of the economic burden of multimorbidity, as lost economic productivity due to multiple illnesses and premature deaths, begins with the recognition of key factors involved in its onset. Also, stakeholders must tailor structural financing for improving the living conditions of the disadvantaged people residing in deprived areas.
Future research priorities include uniform definition and measurement of multimorbidity to allow generating information with internal and external validity. The overlooked relationship between multimorbidity and suicidality should be explored in further studies56. Because increasing provision of treatment would not suffice to diminish the prevalence of common physical-mental morbidities in the general population58,59, balanced management of comorbid conditions in primary care would play a vital role in the framework of health systems, by shifting from a single-disease approach to a multimorbid approach.
Supplementary information
Acknowledgements
The State of São Paulo Research Foundation funded the São Paulo Megacity Mental Health Survey, SPMHS (FAPESP Grant 03/00204-3, http://www.fapesp.br/materia/176/projeto-tematico/projeto-tematico.htm). Instrument development was supported by the Foundation for Science and Technology of Vitoria, Espirito Santo, Brazil (Fundo de Apoio à Ciência e Tecnologia do Município de Vitória – FACITEC 002/2003). The São Paulo Megacity Mental Health Survey is carried out in conjunction with the World Health Organization World Mental Health (WMH) Survey Initiative. The authors thank the WMH staff for assistance with instrumentation, fieldwork, and data analysis. The main coordination center activities, at Harvard University, were supported by the United States National Institutes of Mental Health (R01-MH070884), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service (R13-MH066849, R01-MH069864, and R01-DA016558), the Fogarty International Center (FIRCA R03-TW006481), the Pan American Health Organization, the Eli Lilly and Company Foundation, Ortho-McNeil Pharmaceutical, Inc., Glaxo Smith Kline, Bristol-Myers Squibb, and Shire. National Council for Scientific and Technological Development supports Dr. L. H. Andrade (CNPq Grant 307623/2013-0). The authors declare herein that the founders of the survey had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The authors also declare that the commercial funders of the Harvard coordination center had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Y.-P.W., L.H.A., A.D.P.C.F. conceived the experiment, Y.-P.W., L.H.A., M.C.V. collected the data, Y.-P.W., B.P.N., B.M.C., G.L.S., C.F.N., L.H.A., A.D.P.C.F. performed statistical analyses, Y.-P.W., B.P.N., M.C.V., I.M.B., L.H.A., A.D.P.C.F. wrote the first version and interpreted the results. All authors reviewed and approved the manuscript.
Data Availability
The full dataset supporting the findings of the present observational study can be obtained upon request to the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura H. Andrade and Alexandre D. P. Chiavegatto Filho contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39326-8.
References
- 1.Lappenschaar M, Hommersom A, Lucas PJ. Probabilistic causal models of multimorbidity concepts. AMIA Ann Symp Proc. 2012;2012:475–484. [PMC free article] [PubMed] [Google Scholar]
- 2.van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–375. doi: 10.1016/S0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 3.Almirall J, Fortin M. The coexistence of terms to describe the presence of multiple concurrent diseases. J Comorb. 2013;3:4–9. doi: 10.15256/joc.2013.3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/S0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 5.Schäfer I, et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5:e15941. doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. 2013;369:1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet390, 1260-1344 (2017). [DOI] [PMC free article] [PubMed]
- 9.Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. doi: 10.1186/s12889-015-2008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arokiasamy P, et al. BMC Med. 2015. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? p. 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schellevis, F.G. Comorbidity of chronic diseases in general practice. [PhD thesis]. Nijmegen, Nijmegen University (1993). [DOI] [PubMed]
- 12.Andrade LH, Benseñor IM, Viana MC, Andreoni S, Wang YP. Clustering of psychiatric and somatic illnesses in the general population: multimorbidity and socioeconomic correlates. Braz J Med Biol Res. 2010;43:483–491. doi: 10.1590/S0100-879X2010007500024. [DOI] [PubMed] [Google Scholar]
- 13.Barnett K, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 14.Rzewuska M, et al. Epidemiology of multimorbidity within the Brazilian adult general population: Evidence from the 2013 National Health Survey (PNS 2013) PLoS One. 2017;12:e0171813. doi: 10.1371/journal.pone.0171813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S. Multimorbidity–older adults need health care that can count past one. Lancet. 2015;385:587–589. doi: 10.1016/S0140-6736(14)61596-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee JT, Hamid F, Pati S, Atun R, Millett C. Impact of Noncommunicable Disease Multimorbidity on Healthcare Utilisation and Out-Of-Pocket Expenditures in Middle-Income Countries: Cross Sectional Analysis. PLoS One. 2015;10:e0127199. doi: 10.1371/journal.pone.0127199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palladino R, Tayu Lee J, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing. 2016;45:431–435. doi: 10.1093/ageing/afw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes BP, et al. Contextual and individual inequalities of multimorbidity in Brazilian adults: a cross-sectional national-based study. BMJ Open. 2017;7:e015885. doi: 10.1136/bmjopen-2017-015885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD 2016 Brazil Collaborators. Burden of disease in Brazil, 1990-2016: a systematic subnational analysis for the Global Burden of Disease Study 2016. Lancet392(10149), 760–775 (2018). [DOI] [PMC free article] [PubMed]
- 21.Amaral CE, et al. Systematic review of pathways to mental health care in Brazil: narrative synthesis of quantitative and qualitative studies. Int J Mental Health Systems. 2018;12:65. doi: 10.1186/s13033-018-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massuda A, Hone T, Leles FAG, de Castro MC, Atun R. The Brazilian health system at crossroads: progress, crisis and resilience. BMJ Glob Health. 2018;3(4):e000829. doi: 10.1136/bmjgh-2018-000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiavegatto Filho AD, Kawachi I, Wang YP, Viana MC, Andrade LH. Does income inequality get under the skin? A multilevel analysis of depression, anxiety and mental disorders in Sao Paulo, Brazil. J Epidemiol Community Health. 2013;67:966–972. doi: 10.1136/jech-2013-202626. [DOI] [PubMed] [Google Scholar]
- 24.Campanha AM, et al. Pharmacoepidemiol Drug Saf. 2015. Use of psychotropic medications in São Paulo Metropolitan Area, Brazil: pattern of healthcare provision to general population; pp. 1207–1214. [DOI] [PubMed] [Google Scholar]
- 25.Wang YP, et al. Patterns and predictors of health service use among people with mental disorders in São Paulo metropolitan area, Brazil. Epidemiol Psychiatr Sci. 2017;26:89–101. doi: 10.1017/S2045796016000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade LH, et al. Mental disorders in megacities: findings from the São Paulo megacity mental health survey, Brazil. PLoS One. 2012;7:e31879. doi: 10.1371/journal.pone.0031879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler RC, et al. The WHO World Mental Health (WMH) Surveys. Psychiatrie (Stuttg) 2009;6(1):5–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Instituto Brasileiro de Geografia e Estatística (IBGE). Sistema IBGE de Recuperação Automática – SIDRA. Contagem da população 2007 tabelas. [acessed on May 4, 2018]. Available at: http://www.sidra.ibge.gov.br/bda/tabela/protabl.asp?c=793&z=cd&o=17&i=P (Table 793). (no data).
- 29.Viana MC, et al. Sao Paulo Megacity Mental Health Survey - a population-based epidemiological study of psychiatric morbidity in the Sao Paulo metropolitan area: aims, design and field implementation. Braz J Psychiatr. 2009;31:375–386. doi: 10.1590/S1516-44462009000400016. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Ustun TB. Int J Methods Psychiatr Res. 2004. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) pp. 93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haro JM, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res. 2006;15:167–180. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehm J, Taylor B, Patra J. Volume of alcohol consumption, patterns of drinking and burden of disease in the European region 2002. Addiction. 2006;101:1086–1095. doi: 10.1111/j.1360-0443.2006.01491.x. [DOI] [PubMed] [Google Scholar]
- 33.Schoenborn, C. A., Adams, P. F. & Schiller, J. S. Summary health statistics for the US population; National Health Interview Survey, 2000. Washington DC, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Vital Health Stat10(214) (2003).
- 34.Burkhauser RV, Daly MC, Houtenville AJ, Nargis N. Self-reported work-limitation data: What they can and cannot tell us. Demography. 2002;39(3):541–555. doi: 10.1353/dem.2002.0025. [DOI] [PubMed] [Google Scholar]
- 35.Andrade LH, et al. Days out-of-role due to common physical and mental health problems: results from the Sao Paulo Megacity Mental Health Survey, Brazil. Clinics (Sao Paulo) 2013;68(11):1392–1399. doi: 10.6061/clinics/2013(11)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight M, Stewart-Brown S, Fletcher L. Estimating health needs: the impact of a checklist of conditions and quality of life measurement on health information derived from community surveys. J Public Health Med. 2001;23(3):179–186. doi: 10.1093/pubmed/23.3.179. [DOI] [PubMed] [Google Scholar]
- 37.Sheehan TJ. Creating a psychosocial measurement model from stressful life events. Social Sci Med. 1996;43:265–271. doi: 10.1016/0277-9536(95)00377-0. [DOI] [PubMed] [Google Scholar]
- 38.Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20:141–151. doi: 10.1177/001316446002000116. [DOI] [Google Scholar]
- 40.Cattell, R.B. The scientific use of factor analysis in behavioral and life sciences. New York, Plenum (1978).
- 41.Diez Roux AV, Aiello AE. Multilevel analysis of infectious diseases. J Infect Dis. 2005;191(Suppl 1):S25–33. doi: 10.1086/425288. [DOI] [PubMed] [Google Scholar]
- 42.Gamma A, Angst J. Concurrent psychiatric comorbidity and multimorbidity in a community study: gender differences and quality of life. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II43–46. doi: 10.1007/BF03035126. [DOI] [PubMed] [Google Scholar]
- 43.Kowal P, Arokiasamy P, Afshar S, Pati S, Snodgrass JJ. Multimorbidity: health care that counts “past one” for 1·2 billion older adults. Lancet. 2015;385:2252–2253. doi: 10.1016/S0140-6736(15)61062-5. [DOI] [PubMed] [Google Scholar]
- 44.Mercer SW, Guthrie B, Furler J, Watt GC, Hart JT. Multimorbidity and the inverse care law in primary care. BMJ. 2012;344:e4152. doi: 10.1136/bmj.e4152. [DOI] [PubMed] [Google Scholar]
- 45.Schiøtz ML, Stockmarr A, Høst D, Glümer C, Frølich A. Social disparities in the prevalence of multimorbidity - A register-based population study. BMC Public Health. 2017;17:422. doi: 10.1186/s12889-017-4314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pati S, et al. Non communicable disease multimorbidity and associated health care utilization and expenditures in India: cross-sectional study. BMC Health Services Research. 2014;14:451. doi: 10.1186/1472-6963-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pati S, Swain S, Metsemakers J, Knottnerus JA, van den Akker M. Pattern and severity of multimorbidity among patients attending primary care settings in Odisha, India. PLoS One. 2017;12:e0183966. doi: 10.1371/journal.pone.0183966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vancampfort D, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low- and middle-income countries. Int J Behav Nutr Phys Act. 2017;14:6. doi: 10.1186/s12966-017-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen, I., Rathod, S., Kathree, T., Selohilwe, O. & Bhana, A. Risk correlates for physical-mental multimorbidities in South Africa: a cross-sectional study. Epidemiol Psychiatr Sci 1–9. [Epub ahead of print]. (2017). [DOI] [PMC free article] [PubMed]
- 50.Gureje O, et al. Integrating mental health into primary care in Nigeria: report of a demonstration project using the mental health gap action programme intervention guide. BMC Health Serv Res. 2015;15:242. doi: 10.1186/s12913-015-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spagnolo J, et al. Building system capacity for the integration of mental health at the level of primary care in Tunisia: a study protocol in global mental health. BMC Health Serv Res. 2017;17:38. doi: 10.1186/s12913-017-1992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diez-Canseco F, et al. Integration of a Technology-Based Mental Health Screening Program Into Routine Practices of Primary Health Care Services in Peru (The Allillanchu Project): Development and Implementation. J Med Internet Res. 2018;20:e100. doi: 10.2196/jmir.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yardley S, Cottrell E, Rees E, Protheroe J. Modelling successful primary care for multimorbidity: a realist synthesis of successes and failures in concurrent learning and healthcare delivery. BMC Fam Pract. 2015;16:23. doi: 10.1186/s12875-015-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muth C, et al. The Ariadne principles: how to handle multimorbidity in primary care consultations. BMC Med. 2014;12:223. doi: 10.1186/s12916-014-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis C, Wallace E, Kyne L, Cullen W, Smith SM. Training doctors to manage patients with multimorbidity: a systematic review. J Comorb. 2016;6:85–94. doi: 10.15256/joc.2016.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kavalidou K, Smith DJ, O’Connor RC. The role of physical and mental health multimorbidity in suicidal ideation. J Affect Disord. 2017;209:80–85. doi: 10.1016/j.jad.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 57.Lappenschaar M, et al. Multilevel temporal Bayesian networks can model longitudinal change in multimorbidity. J Clin Epidemiol. 2013;66:1405–1416. doi: 10.1016/j.jclinepi.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Jorm AF, Patten SB, Brugha TS, Mojtabai R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry. 2017;16:90–99. doi: 10.1002/wps.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mulder R, Rucklidge J, Wilkinson S. Why has increased provision of psychiatric treatment not reduced the prevalence of mental disorder? Aust N Z J Psychiatry. 2017;51:1176–1177. doi: 10.1177/0004867417727356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset supporting the findings of the present observational study can be obtained upon request to the authors.