Abstract
Ovarian clear cell carcinoma (OCCC) is associated with a frequent loss in ARID1A function. ARID1A reportedly suppresses histone deacetylase (HDAC)6 in OCCC directly. Here, we evaluated the clinical significance of HDAC6 expression and its related factors in terms of ARID1A status. Immunohistochemical expression of HDAC6, hypoxia inducible factors-1α (HIF-1α), programmed death-1 ligand (PD-L1), CD44 (cancer stem cell marker), and ARID1A was analysed for 106 OCCC patients. High nuclear HDAC6 expression correlated with patient death (p = 0.038). In the multivariate analysis of overall survival, surgical status (complete or incomplete resection) (hazard ratio (HR) = 17.5; p = <0.001), HDAC6 nuclear expression (HR = 1.68; p = 0.034), and PD-L1 expression (HR = 1.95; p = 0.022) were the independent prognostic factors. HDAC6 upregulation and ARID1A loss did not necessarily occur simultaneously. High HDAC6 expression was associated with poor prognosis in OCCC with ARID1A loss; this was not observed without ARID1A loss. HDAC6 expression showed a significant positive correlation with HIF-1α, PD-L1, and CD44. In OCCC, HDAC6 involvement in prognosis depended on ARID1A status. HDAC6 also led to immuno- and hypoxia- tolerance and cancer stem cell phenotype. HDAC6 is a promising therapeutic target for OCCC with loss of ARID1A.
Introduction
Ovarian clear cell carcinoma (OCCC) ranks second as the leading cause of death from epithelial ovarian cancer (EOC)1 and is associated with the worst prognosis among the major subtypes of EOC when diagnosed at the advanced stages2,3. Typically, OCCC exhibits a low response rate to the platinum-based standard chemotherapy used to treat EOC. To date, we have proposed several therapeutic target substances and pathways for OCCC4,5. The most common somatic mutation identified in OCCC is that in ARID1A (46–57%)6,7, a factor that promotes SWI/SNF-mediated chromatin remodelling and displays one of the highest mutation rates among the epigenetic regulators in cancers8,9. Therapeutic strategies that harness this genetic characteristic are being explored10.
Histone deacetylases (HDACs) are chromatin-modifying enzymes involved in the regulation of many aspects of cell biology, including tissue differentiation, apoptosis, migration, mitosis, and angiogenesis via the deacetylation of histone or non-histone proteins11. Eighteen HDAC family members have been identified in humans11. The pan-HDAC inhibitor has been demonstrated to exhibit cytotoxic effects in various cancers, including EOC12. However, its activities of targeting multiple HDACs lead to various toxicities, which limits its application in the treatment of cancers13. More selective and effective HDAC inhibitors are therefore required in cancer therapy. In our previous study, HDAC6 and HDAC7 showed higher expression in OCCC than in other histological subtypes of EOC, and were expected to be poor prognostic factors14. Although HDAC7-selective inhibitors are yet to be well-developed, HDAC6-selective inhibitors are clinically used as antitumour agents.
HDAC6 increases deacetylated α-tubulin levels. This in turn enhances microtubule dynamics and leads to cancer cell growth (Fig. 1)15,16. HDAC6 is associated with several chemoresistant factors (Fig. 1) and upregulation of programmed death-1 ligand (PD-L1), which leads to cancer immune tolerance17. Hypoxia inducible factor-1α (HIF-1α) protein expression, transcriptional activity18, and tumour angiogenesis19 are induced by HDAC6, and the cancer stem cell phenotype is maintained by HDAC6 via CD4420. HDAC6-selective inhibitors are currently in clinical trials for multiple myeloma21,22. Recently, Bitler et al.23 identified that ARID1A directly suppresses HDAC6 in OCCC and provided evidence that HDAC6 may be a promising therapeutic target in ARID1A-mutated cancers. However, the significance of the association between HDAC6 and ARID1A has not been well documented in clinical samples. Herein, we investigated the significance of HDAC6 as a therapeutic target in OCCC and the usefulness of ARID1A as its biomarker using clinical samples.
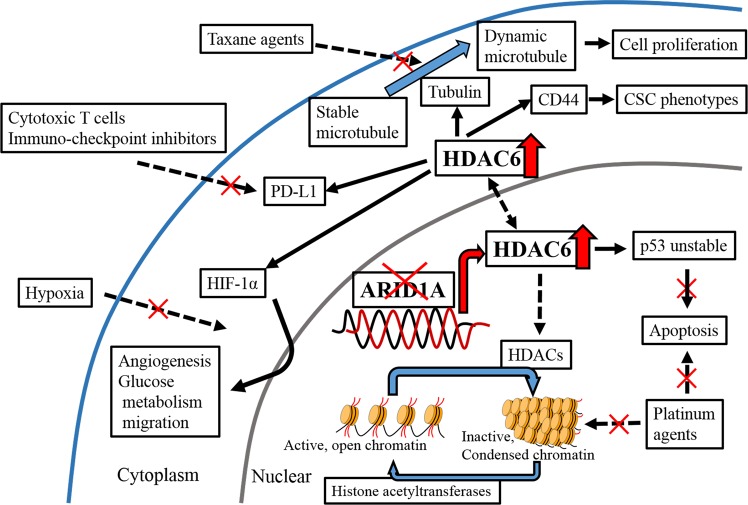
Figure 1.
Scheme of HDAC6 functions: When ARID1A loss occurs, suppression of HDAC6 is relieved. In the nucleus, HDAC6 destabilizes p53 by deacetylation, and suppresses apoptosis. As a member of the HDAC family, HDAC6 inactivates chromatin by deacetylation of the core histones. These effects of HDAC6 in the nucleus are responsible for the resistance to platinum agents. In the cytoplasm, HDAC6 leads to cell proliferation, angiogenesis, glucose metabolism, glucose transport, and CSC phenotypes via tubulin, HIF-1α, PD-L1, and CD44. In addition, HDAC6 result in tolerance to taxane agents, cytotoxic T cells, immuno-checkpoints inhibitors, and hypoxic stress.
Results
Clinicopathological characteristics and immunohistochemical (IHC) expressions
Table 1 lists the characteristics of the patients. The age of patients ranged from 32 to 80 years, with the average being 55.7 years. All patients included in the study were Japanese. Patients with OCCC were classified after surgery as International Federation of Obstetrics and Gynaecology (FIGO) stage I (n = 71), stage II (n = 16), stage III (n = 17), and stage IV (n = 2). A total of 90 (84.9%) patients underwent complete surgical resection, while 85 (80.2%) patients underwent adjuvant chemotherapy after surgery. Lymphadenectomy was performed in 62 patients (58.5%), 9.7% of which had lymph nodes metastasis. All adjuvant chemotherapies administered to patients contained platinum-agents, 84.7% of which were combined with taxane-agents. The median follow-up duration was 54.2 months for survivors (range, 10–121 months). Recurrence and death were observed in 32 (30.2%) and 23 (21.7%) patients, respectively.
Table 1.
Clinicopathological characteristics of OCCC patients (n = 106).
| Variable | N (%) |
|---|---|
| Age | |
| Median (range) | 55.7 (32–80) |
| >56 | 50 (47.2) |
| ≤56 | 56 (52.8) |
| FIGO stage | |
| I | 71 (67.0) |
| II | 16 (15.1) |
| III | 17 (16.0) |
| IV | 2 (1.9) |
| Surgical procedures | |
| TAH + BSO + OM | 93 (87.8) |
| BSO + OM | 1 (0.9) |
| USO + OM | 11 (10.4) |
| OM | 1 (0.9) |
| Lymphadenectomy | |
| Yes | 62 (58.5) |
| No | 44 (41.5) |
| Surgical status | |
| Complete resection | 90 (84.9) |
| Incomplete resection | 16 (15.1) |
| Adjuvant chemotherapy | |
| Yes | 85 (80.2) |
| Paclitaxel + Carboplatin | 60 |
| Docetaxel + Carboplatin | 12 |
| Irinotecan + Cisplatin | 12 |
| Gemcitabine + Carboplatin | 1 |
| No | 21 (19.8) |
| Recurrence | |
| Yes | 32 (30.2) |
| No | 74 (69.8) |
| Death | |
| Yes | 23 (21.7) |
| No | 83 (78.3) |
FIGO, the International Federation of Obstetrics and Gynaecology; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; OM, omentectomy; USO, unilateral salpingo-oophorectomy.
A positive expression of ARID1A was observed in 54 patients (50.9%), while a high expression of HDAC6 was observed in 59 (55.7%) and 20 (18.9%) (nucleus and cytoplasm, respectively) patients; high expression was observed in 60 (56.6%), 7 (6.6%), and 24 (22.6%), patients for HIF-1α, PD-L1, and CD44, respectively. The correlations between patient characteristics and IHC expressions are shown in Table 2. No correlation was found between all IHC expressions (HDAC6, HIF-1α, PD-L1, ARID1A, and CD44) and age, FIGO stage, and surgical status. A high expression of CD44 was correlated with recurrence (p = 0.018), while a high expression of HDAC6 (nucleus), HIF-1α, PD-L1, and CD44 correlated with death (p = 0.038, 0.047, 0.038, and 0.036, respectively). There was no significant correlation between ARID1A loss and any of the available clinicopathological parameters.
Table 2.
The correlations between patient characteristics and IHC expressions.
| Age (mean = 55.7) | FIGO stage | Residual tumour | Recurrence | Death | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <56 | ≥56 | p value | I + II | III + IV | p value | Yes | No | p value | Yes | No | p value | Yes | No | p value | ||
| ALL | 50 | 56 | 87 | 19 | 90 | 16 | 32 | 74 | 23 | 83 | ||||||
| ARID1A | Positive | 27 | 27 | 0.345 | 43 | 11 | 0.340 | 44 | 10 | 0.233 | 15 | 39 | 0.367 | 10 | 44 | 0.283 |
| Loss | 23 | 29 | 44 | 8 | 46 | 6 | 17 | 35 | 13 | 39 | ||||||
| HDAC6N | High | 26 | 33 | 0.301 | 49 | 10 | 0.482 | 49 | 10 | 0.376 | 20 | 39 | 0.237 | 17 | 42 | 0.038 |
| Low | 24 | 23 | 38 | 9 | 41 | 6 | 12 | 35 | 6 | 41 | ||||||
| HDAC6C | High | 8 | 12 | 0.322 | 16 | 4 | 0.504 | 17 | 3 | 0.647 | 8 | 12 | 0.212 | 6 | 14 | 0.237 |
| Low | 42 | 44 | 71 | 15 | 73 | 13 | 24 | 62 | 17 | 69 | ||||||
| HIF-1α | High | 29 | 31 | 0.469 | 49 | 11 | 0.554 | 50 | 10 | 0.408 | 22 | 38 | 0.073 | 17 | 43 | 0.047 |
| Low | 21 | 25 | 38 | 8 | 40 | 6 | 10 | 36 | 6 | 40 | ||||||
| PD-L1 | High | 3 | 4 | 0.564 | 5 | 2 | 0.367 | 5 | 2 | 0.285 | 4 | 3 | 0.121 | 4 | 3 | 0.038 |
| Low | 47 | 52 | 82 | 17 | 85 | 14 | 28 | 71 | 19 | 80 | ||||||
| CD44 | High | 12 | 12 | 0.466 | 18 | 6 | 0.229 | 18 | 6 | 0.114 | 12 | 12 | 0.018 | 9 | 15 | 0.036 |
| Low | 38 | 44 | 69 | 13 | 72 | 10 | 20 | 62 | 14 | 68 | ||||||
HDAC6N, histone deacetylase 6 nuclear expression; HDAC6C, HDAC6 cytoplasmic expression; HIF-1α, hypoxia inducible factor-1α; PD-L1, programmed death-1 ligand. p value < 0.05 is shown in bold.
Correlation with survival and IHC expressions
In the univariate analysis using the Cox proportional hazards model, high expression of PD-L1 and CD44, FIGO stage, and surgical status were found as the prognostic factors for progression free survival (PFS) and overall survival (OS) (Table 3). In the multivariate analysis, high expression of HIF-1α (hazard ratio (HR) = 1.75; 95% CI, 1.17 to 2.61, p = 0.006), PD-L1 (HR = 2.34; 95% CI, 1.28 to 4.30, p = 0.006), and CD44 (HR = 1.62; 95% CI, 1.10 to 2.38, p = 0.014), and surgical status (complete vs. incomplete resection) were demonstrated as the independent prognostic factors for PFS. High expression of HDAC6 (nuclear) (HR = 1.68; 95% CI, 1.04 to 2.70, p = 0.034) and PD-L1 (HR = 1.95; 95% CI, 1.10 to 3.45, p = 0.022), and surgical status were the independent prognostic factors for OS (Table 3).
Table 3.
Univariable and multivariable analyses using the Cox proportional hazards model of overall survival for OCCC patients.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | CI | p value | |
| Age | 1.01 | 0.45–2.30 | 0.978 | |||
| ARID1A | 1.51 | 0.66–3.45 | 0.329 | |||
| HDAC6N | 1.56 | 0.98–2.48 | 0.063 | 1.68 | 1.04–2.70 | 0.034 |
| HDAC6C | 1.30 | 0.81–2.07 | 0.273 | |||
| HIF-1α | 1.56 | 0.98–2.49 | 0.061 | |||
| PD-L1 | 2.08 | 1.21–3.58 | 0.009 | 1.95 | 1.10–3.45 | 0.022 |
| CD44 | 1.63 | 1.07–2.49 | 0.022 | |||
| FIGO stage | 6.68 | 2.94–15.2 | <0.001 | |||
| Residual tumour | 15.2 | 6.49–35–7 | <0.001 | 17.2 | 6.90–43.5 | <0.001 |
HR, hazard ratio; CI, confidence interval; HDAC6N, histone deacetylase 6 nuclear expression; HDAC6C, HDAC6 cytoplasmic expression; HIF-1α, hypoxia inducible factor-1α; PD-L1, programmed death-1 ligand. p value < 0.05 is shown in bold.
Dependency of HDAC6 and HIF-1α expression on ARID1A
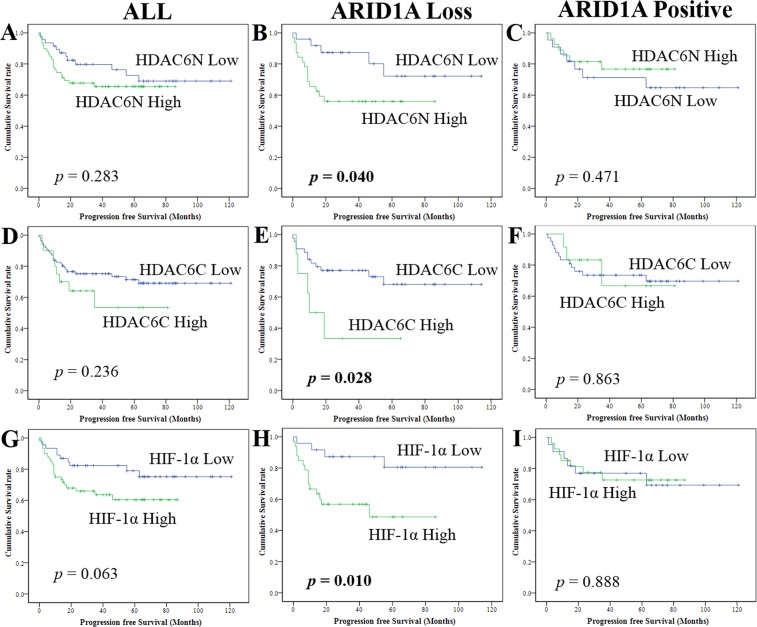
A subgroup analysis was performed in the presence or absence of ARID1A loss (Fig. 2). A high expression of HDAC6 (nucleus, p = 0.040; cytoplasm, p = 0.028) had an adverse effect on the PFS in patients with ARID1A loss; however, a high expression of HDAC6 had no adverse effect on the PFS in all patients (nucleus, p = 0.283; cytoplasm, p = 0.236) and in those without ARID1A loss (nucleus, p = 0.417; cytoplasm, p = 0.863) (Fig. 2A–F). A high expression of HIF-1α (p = 0.010) had an adverse effect on the PFS of patients with ARID1A loss; however, this was not observed in all patients (p = 0.063) and in patients without ARID1A loss (p = 0.888) (Fig. 2G–I).
Figure 2.
Kaplan-Meier survival analysis: Nuclear HDAC6 in (A) all cases, (B) with, and (C) without ARID1A loss. Cytoplasmic HDAC6 in (D) all cases, (E) with, and (F) without ARID1A loss. HIF-1α in (G) all cases, (H) with, and (I) without ARID1A loss. p values, log rank test.
Correlation among IHC expressions
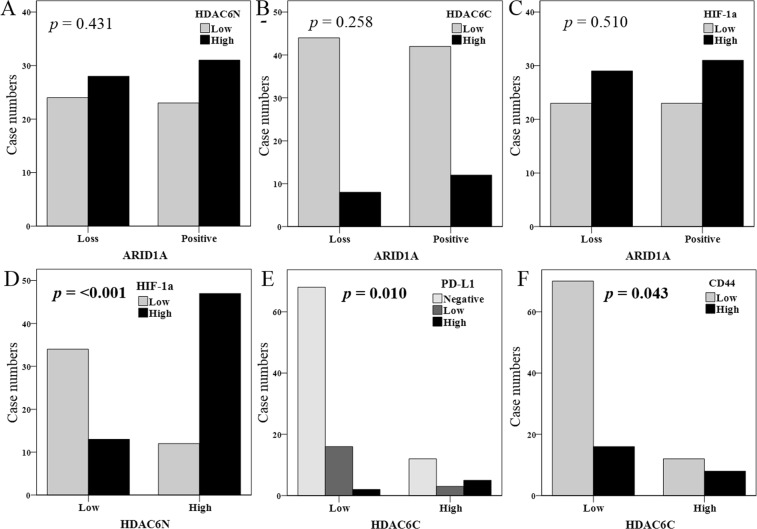
ARID1A loss also showed a significantly positive correlation with the high expression of PD-L1 (Table 4); however, this was not observed with the high expression of HDAC6 (nucleus, p = 0.431, Fig. 3A; cytoplasm, p = 0.258, Fig. 3B) and HIF-1α (p = 0.510, Fig. 3C). The nuclear high expression of HDAC6 also showed a significantly positive correlation with HIF-1α (p = <0.001, Fig. 3D). The cytoplasmic high expression of HDAC6 showed a significantly positive correlation with PD-L1 (p = 0.010, Fig. 3E) and CD44 (p = 0.043, Fig. 3F).
Table 4.
Spearman’s correlations among IHC expressions.
| HDAC6N | HDAC6C | HIF-1α | PD-L1 | ARID1A | CD44 | ||
|---|---|---|---|---|---|---|---|
| HDAC6N | Correlation coefficient | 1 | 0.236 | 0.521 | 0.008 | 0.036 | −0.107 |
| p value | 0.015 | <0.001 | 0.936 | 0.715 | 0.275 | ||
| HDAC6C | Correlation coefficient | 1 | −0.064 | 0.357 | 0.087 | 0.200 | |
| p value | 0.513 | <0.001 | 0.373 | 0.040 | |||
| HIF-1α | Correlation coefficient | 1 | −0.074 | 0.017 | −0.118 | ||
| p value | 0.452 | 0.866 | 0.23 | ||||
| PD-L1 | Correlation coefficient | 1 | −0.271 | 0.219 | |||
| p value | 0.005 | 0.024 | |||||
| ARID1A | Correlation coefficient | 1 | −0.004 | ||||
| p value | 0.965 | ||||||
| CD44 | Correlation coefficient | 1 | |||||
| p value |
HDAC6N, histone deacetylase 6 nuclear expression; HDAC6C, HDAC6 cytoplasmic expression; HIF-1α, hypoxia inducible factor-1α; PD-L1, programmed death-1 ligand. p value < 0.05 is shown in bold.
Figure 3.
Correlations among IHC expressions, using the Chi-square test.
Discussion
In the present study, OCCC patients with high nuclear expression of HDAC6 had a poor prognosis regardless of FIGO stage and surgical status, the latter of which is a well-known important prognostic factor in EOC. These results suggest that HDAC6 is one of the refractory factors to the standard treatments in OCCC. The standard chemotherapy for EOC is a combination of platinum and taxane agents; however, OCCC patients are resistant to this combination. The deacetylation of alpha-tubulin, induced by HDAC6, decreases the effect of taxane agents as a microtubule-stabilizing agent24. When HDAC6 is inhibited, taxane resistance is reversed in EOC cell lines24,25. HDAC6 upregulation leads to tumour cisplatin resistance, and depletion of HDAC6 enhances cisplatin-induced DNA damage and apoptosis26. HDAC6-selective inhibitors exhibit an anti-tumour effect in breast cancer27,28, gastric cancer19, multiple myeloma21,22, and lymphoma29. Therefore, we suggest that HDAC6 is a potentially key therapeutic target for OCCC. Notably, HDAC6-selective inhibitors are well-tolerated and show minimal toxicity in clinical trials21,22. HDAC6-selective inhibitors may therefore improve the efficacy and adverse effects such as kidney failure30 and peripheral neuropathy31 that often accompany the standard chemotherapy for EOC.
The present study also showed the coexistence of an upregulation in HDAC6 and ARID1A loss, leading to a shorter survival for OCCC patients than for patients having either one of the two factors; these activities do not necessarily happen simultaneously. Bitler et al.23 showed that an HDAC6-selective inhibitor (ACY-1215, ricolinostat) suppressed the proliferation of ARID1A-mutated OCCC cell lines and improved the survival of mice bearing ARID1A-mutated OCCC compared to that of mice bearing ARID1A-wild type OCCC. Fukumoto et al.32 also reported that the pan-HDAC inhibitor improved the survival of mice bearing ARID1A-mutated OCCC, while Gupta et al.33 demonstrated that HDAC inhibitors responded to ARID1A-mutated urothelial cancers when compared to that in ARID1A-wild type urothelial cancers, in two clinical trials. These observations therefore indicate that ARID1A status is an important biomarker in the treatment of HDAC. A high PD-L1 expression demonstrated a positive correlation with high HDAC6 cytoplasmic expression and ARID1A loss. When HDAC6 is inhibited, immunotherapy response is enhanced with PD-L1 blockage34–36. Shen et al.37 showed that treatment with an anti-PD-L1 antibody reduced tumour burden and prolonged survival of ARID1A-mutated mice; this was not observed in the ARID1A-wild type EOCs. HIF-1α and CD44 also showed a positive correlation with HDAC6. Therefore, HDAC6 may serve as a therapeutic target for OCCC with ARID1A loss associated with PD-L1, HIF-1α, and CD44 expression. Given that a loss in ARID1A is frequent in cancers9, the present findings may have implications beyond the established OCCC.
Our study had several limitations. The sample size used in this study was small, and the survival analysis was only performed with a few events. However, when considering the low incidence of OCCC, the present study included a relatively large number of patients. Before drawing a conclusion based on the results of this study, further confirmation is warranted via a multi-ethnic population study and on a larger scale. Secondly, the present study consisted solely of semi-quantitated IHC analysis and lacked both quantitative protein analysis and molecular correlations; the molecular correlations in OCCC between ARID1A and HDAC6 have already been reported23. The novelty of the present study is its verification of the findings in clinical samples. However, further studies are required to quantitatively analyse HDAC6 protein and mRNA expression.
In conclusion, the involvement of HDAC6 in OCCC prognosis was demonstrated to depend on the ARID1A status. HDAC6 was observed to function as a promising therapeutic target for OCCC with ARID1A loss, in close association with immuno-modulation, response to hypoxia, and cancer stem cell phenotype. HDAC6-selective inhibitors are expected to have safe and synergistic effects when combined with the current standard chemotherapy for EOC.
Methods
Patients and samples
Patient electronic medical charts from the Saitama Medical University International Medical Centre from 2007 to 2016 were reviewed under the approval of the institutional review board (IRB number, 16–257). All methods were performed in accordance with the 1975 Declaration of Helsinki. All tumour specimens in the pathological analysis were obtained with informed consent (or a formal waiver of consent) with an approval from the Ethics Committee of our hospital. We recruited 106 patients with OCCC, whose tumours were surgically resected and pathologically confirmed. The clinicopathological characteristics of these cases, such as age, recurrence/PFS, death/OS, FIGO stage, surgical status (complete resection or incomplete resection), and treatment methods were reviewed.
IHC staining
IHC expression of HDAC6, ARID1A, HIF-1α, PD-L1, and CD44 was analysed using tissue microarray (KIN-2, AZUMAYA, Tokyo, Japan). Tissue microarray was generated from 2 cylindrical cores that were 3.0 mm in diameter in each block; these were punched out of paraffin-embedded tissue blocks corresponding to the representative histological findings, and then inserted into a recipient block. A total of 106 tissue blocks were cut into 4-μm serial sections, and were each run through an automated system by Dako Autostainer Link 48 (Agilent technologies, CA, USA) as per the manufacturer’s protocol. The following primary antibodies were used: polyclonal rabbit anti-HDAC6 (dilution, 1:500; ab1440, Abcam, Cambridge, UK), polyclonal rabbit anti-HIF-1α (dilution, 1:100; NB100–479; Novus Biologicals, CO, USA), monoclonal rabbit anti-PD-L1 (dilution, 1:100; 28-8 pharmDx; Dako North America, CA, USA), monoclonal rabbit anti-ARID1A (dilution, 1:1000; ERP13501-73; Abcam, Cambridge, UK), and monoclonal mouse anti-CD44 (dilution, 1:200; 156-3C11; Thermo Fisher Scientific, MA, USA). For all antibodies, the Target Retrieval Solution (pH 9.0, HDAC6 and CD44; pH 6.0, ARID1A, HIF-1α, and PD-L1) was applied for antigen retrieval at 98 °C for 20 min. Sections were incubated with the primary antibodies at 25 °C for 60 min, followed by incubation with a secondary antibody (EnVision FLEX/HRP, Agilent technologies, CA, USA) at 25 °C for 30 min. The chromogen reaction was performed with diaminobenzidine plus the H2O2 substrate at 25 °C for 10 min.
Interpretation of IHC results
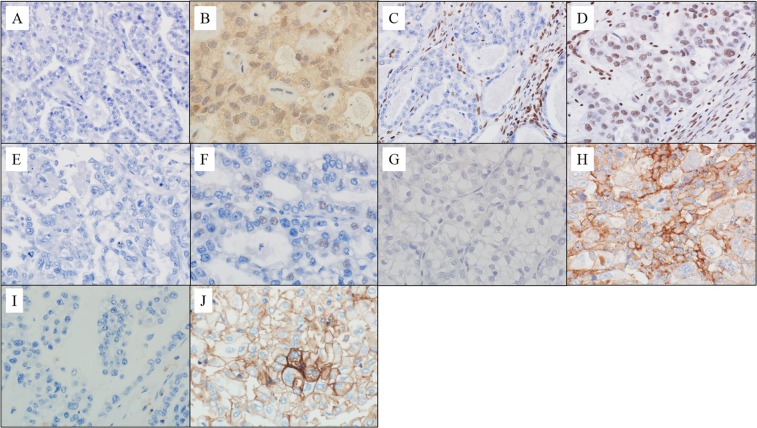
IHC evaluation was performed by one pathologist (Masanori Yasuda) and one physician (Mitsutake Yano) with subspecialties in gynaecological oncology; both of them were blinded to the clinicopathological parameters (Fig. 4). The following four-tiered scoring scheme was used: negative (0%), weak (1–50%), moderate (51–80%), and marked (81–100%). To optimize the PFS and OS differences, the raw data were binarised for statistical analysis as follows: in HDAC6, HIF-1α, PD-L1, and CD44. The moderate and marked expression were grouped as high-level, whereas the completely negative and weak expression were grouped as low level. For ARID1A, the completely negative expression was categorised as the loss-group, whereas the weak, moderate, and marked expressions were categorised as positive groups. This categorisation was based on the evidence that the complete absence of ARID1A expression is significantly correlated with its mutation status38.
Figure 4.
IHC expressions. HDAC6 ((A) low; (B) high), ARID1A ((C) loss; (D) positive), HIF-1α ((E) low; (F) high), PD-L1 ((G) low; (H) high), and CD44 ((I) low; (J) high).
Statistical analysis
IHC expressions and the clinicopathological parameters were assessed using the Pearson chi-square test or the Fisher exact test. Spearman’s rank correlation was used to determine whether there was a positive or a negative correlation between the factors. Univariable survival analysis was performed by generating Kaplan-Meier curves, and differences between the groups were assessed using the log rank statistic. Univariable and multivariable survival analyses were performed using the Cox proportional hazards model. All analyses were performed using SPSS v24.0 (SPSS Inc, IL, USA). P values < 0.05 were considered significant.
Acknowledgements
We thank Kouichi Kamada, Yusuke Hosonuma, Satoshi Kanno, Nobuyuki Suzuki, and Yasuo Kamakura, Department of Pathology, Saitama Medical University International Medical Center, for their great technical support. We would like to thank Editage (www.editage.jp) for English language editing. Hidaka Research Projects in the Saitama Medical University (Grant numbers: 29-D-1-1) and Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (Research Project Numbers: 15K08355 and 18K06997).
Author Contributions
M.Y. contributed to the conception, design, acquisition, analysis and interpretation of data, and drafting of the manuscript. M.Y. contributed to the conception, design, critical revision of the manuscript for the inclusion of important intellectual content, and supervised the writing. K.H. contributed to the acquisition of data and supervision. M.M. contributed to the acquisition of data. H.N. contributed to the critical revision of the manuscript to ensure that important intellectual content was present. M.M., M.M., T.K., and N.O. contributed to the conception and design of the manuscript to ensure important intellectual content was present.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitsutake Yano, Email: yano1210@oita-u.ac.jp.
Masanori Yasuda, Email: m_yasuda@saitama-med.ac.jp.
References
- 1.Kobel M, et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 2010;29:203–211. doi: 10.1097/PGP.0b013e3181c042b6. [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Mackay HJ, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2010;20:945–952. doi: 10.1111/IGC.0b013e3181dd0110. [DOI] [PubMed] [Google Scholar]
- 4.Miyazawa M, et al. Therapeutic strategy targeting the mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma. Pathol. Int. 2009;59:19–27. doi: 10.1111/j.1440-1827.2008.02320.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirasawa T, et al. Alterations of hypoxia-induced factor signaling pathway due to mammalian target of rapamycin (mTOR) suppression in ovarian clear cell adenocarcinoma: in vivo and in vitro explorations for clinical trial. Int. J. Gynecol. Cancer. 2013;23:1210–1218. doi: 10.1097/IGC.0b013e31829d2d51. [DOI] [PubMed] [Google Scholar]
- 6.Jones S, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiegand KC, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadoch C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013;45:592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson CT, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 2016;13(7):13837. doi: 10.1038/ncomms13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 12.Sonnemann J, et al. Comparative evaluation of the treatment efficacy of suberoylanilide hydroxamic acid (SAHA) and paclitaxel in ovarian cancer cell lines and primary ovarian cancer cells from patients. BMC Cancer. 2006;6:183. doi: 10.1186/1471-2407-6-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modesitt SC, Sill M, Hoffman JS, Bender DP. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2008;109:182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Yano M, et al. Association of histone deacetylase expression with histology and prognosis of ovarian cancer. Oncol. Lett. 2018;15:3524–3531. doi: 10.3892/ol.2018.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyake Y, et al. Structural insights into HDAC6 tubulin deacetylation and its selective inhibition. Nat. Chem. Biol. 2016;12:748–754. doi: 10.1038/nchembio.2140. [DOI] [PubMed] [Google Scholar]
- 16.Hubbert C, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 17.Lienlaf M, et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol. Oncol. 2016;10:735–750. doi: 10.1016/j.molonc.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian DZ, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 19.Dong J, et al. A novel HDAC6 inhibitor exerts an anti-cancer effect by triggering cell cycle arrest and apoptosis in gastric cancer. Eur. J. Pharmacol. 2018;828:67–79. doi: 10.1016/j.ejphar.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Wang KH, et al. Increasing CD44+/CD24(−) tumor stem cells, and upregulation of COX-2 and HDAC6, as major functions of HER2 in breast tumorigenesis. Mol. Cancer. 2010;9:288. doi: 10.1186/1476-4598-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee AJ, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol. 2016;17:1569–1578. doi: 10.1016/S1470-2045(16)30375-8. [DOI] [PubMed] [Google Scholar]
- 22.Santo L, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119:2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitler BG, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat. Cell Biol. 2017;19:962–973. doi: 10.1038/ncb3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelucci A, et al. Suberoylanilide hydroxamic acid partly reverses resistance to paclitaxel in human ovarian cancer cell lines. Gynecol. Oncol. 2010;119:557–563. doi: 10.1016/j.ygyno.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Marcus AI, et al. Farnesyltransferase inhibitors reverse taxane resistance. Cancer Res. 2006;66:8838–8846. doi: 10.1158/0008-5472.CAN-06-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, et al. Depletion of HDAC6 enhances cisplatin-induced DNA damage and apoptosis in non-small cell lung cancer cells. PloS One. 2012;7:e44265. doi: 10.1371/journal.pone.0044265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma K, et al. Association of estrogen receptor alpha and histone deacetylase 6 causes rapid deacetylation of tubulin in breast cancer cells. Cancer Res. 2009;69:2935–2940. doi: 10.1158/0008-5472.CAN-08-3458. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, et al. HDAC6 inhibitor WT161 downregulates growth factor receptors in breast cancer. Oncotarget. 2017;8:80109–80123. doi: 10.18632/oncotarget.19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amengual JE, et al. Dual Targeting of Protein Degradation Pathways with the Selective HDAC6 Inhibitor ACY-1215 and Bortezomib Is Synergistic in Lymphoma. Clin. Cancer Res. 2015;21:4663–4675. doi: 10.1158/1078-0432.CCR-14-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang J, et al. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin. Sci. (Lond.) 2018;132:339–359. doi: 10.1042/CS20171417. [DOI] [PubMed] [Google Scholar]
- 31.Krukowski K, et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158:1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukumoto T, et al. Repurposing Pan-HDAC Inhibitors for ARID1A-Mutated Ovarian Cancer. Cell Rep. 2018;22:3393–3400. doi: 10.1016/j.celrep.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta, S. et al. Histone deacetylase inhibition has targeted clinical benefit in ARID1A mutated advanced urothelial carcinoma. Mol. Cancer Ther (2018). [DOI] [PMC free article] [PubMed]
- 34.Bae, J. et al. Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors. Leukemia (2018). [DOI] [PMC free article] [PubMed]
- 35.Ray A, et al. Combination of a novel HDAC6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma. Leukemia. 2018;32:843–846. doi: 10.1038/leu.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods DM, et al. HDAC Inhibition Upregulates PD-1 Ligands in Melanoma and Augments Immunotherapy with PD-1 Blockade. Cancer Immunol. Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen, J. et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med (2018). [DOI] [PMC free article] [PubMed]
- 38.Guan B, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am. J. Surg. Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]