Abstract
The cellular prion protein (PrPC) is a zinc-binding protein that contributes to the regulation of Zn2+ and other divalent species of the central nervous system. Zn2+ coordinates to the flexible, N-terminal repeat region of PrPC and drives a tertiary contact between this repeat region and a well-defined cleft of the C-terminal domain. The tertiary structure promoted by Zn2+ is thought to regulate inherent PrPC toxicity. Despite the emerging consensus regarding the interaction between Zn2+ and PrPC, there is little direct spectroscopic confirmation of the metal ion’s coordination details. Here, we address this conceptual gap by using Cd2+ as a surrogate for Zn2+. NMR finds that Cd2+ binds exclusively to the His imidazole side chains of the repeat segment, with a dissociation constant of ∼1.2 mM, and promotes an N-terminal-C-terminal cis interaction very similar to that observed with Zn2+. Analysis of 113Cd NMR spectra of PrPC, along with relevant control proteins and peptides, suggests that coordination of Cd2+ in the full-length protein is consistent with a three- or four-His geometry. Examination of the mutation E199K in mouse PrPC (E200K in humans), responsible for inherited Creutzfeldt-Jakob disease, finds that the mutation lowers metal ion affinity and weakens the cis interaction. These findings not only provide deeper insight into PrPC metal ion coordination but they also suggest new perspectives on the role of familial mutations in prion disease.
Introduction
Transmissible spongiform encephalopathies, also known as prion diseases, are a class of fatal neurodegenerative diseases for which there is no cure or treatment (1). Examples of prion diseases are Kuru and Creutzfeldt-Jakob disease (CJD) in humans, scrapie in sheep, chronic wasting disease in cervids, and mad cow disease (2). Prion diseases originate from genetic, sporadic, or infectious routes and involve misfolding of the predominately helical cellular prion protein (PrPC) to the β-sheet rich scrapie form (2). Similar to Alzheimer’s disease, sporadic disease accounts for the majority of prion cases in humans.
PrPC is expressed throughout the body but appears to be concentrated primarily at pre- and postsynaptic neuronal membranes (3, 4, 5). The precise physiological function of PrPC is largely unknown; however, the protein’s well-documented ability to coordinate Cu2+ and Zn2+ suggests a role in metal ion homeostasis (6, 7, 8, 9, 10). Mature human PrPC is a 208-amino-acid protein with two N-linked glycans and a glycophosphatidylinositol moiety that anchors the protein to the extracellular membrane surface. The protein has two distinct domains: the C-terminal domain (residues 126–230) composed of three α-helices, two short antiparallel β-strands, and a disulfide bond linking helices two and three and the N-terminal domain (residues 23–125), a flexible segment that coordinates both Cu2+ and Zn2+ in vivo (7, 10, 11, 12, 13).
Zn2+ is one of the most abundant trace metal in the brain with roles in diverse functions, including structural support in certain transcription factors, as catalytic elements in zinc metalloenzymes and as an abundant counterion in presynaptic glutamate vesicles (13, 14). Glutamate release results in a synaptic (Zn2+) spike, and recent findings suggest that Zn2+ binding to PrPC stimulates Zn2+ transport back into neurons through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, thereby restoring normal synaptic metal ion concentrations (14). Our lab demonstrated previously that Zn2+ binds to a PrPC N-terminal segment composed of the sequence (PHGGGWGQ)4 (residues 60–91 in human PrPC), termed the octarepeat (OR) domain, with a dissociation constant (Kd) of ∼200 μM (Fig. 1) (10). This OR segment is essential for Zn2+ binding and subsequent transport of the ion through AMPA receptors.
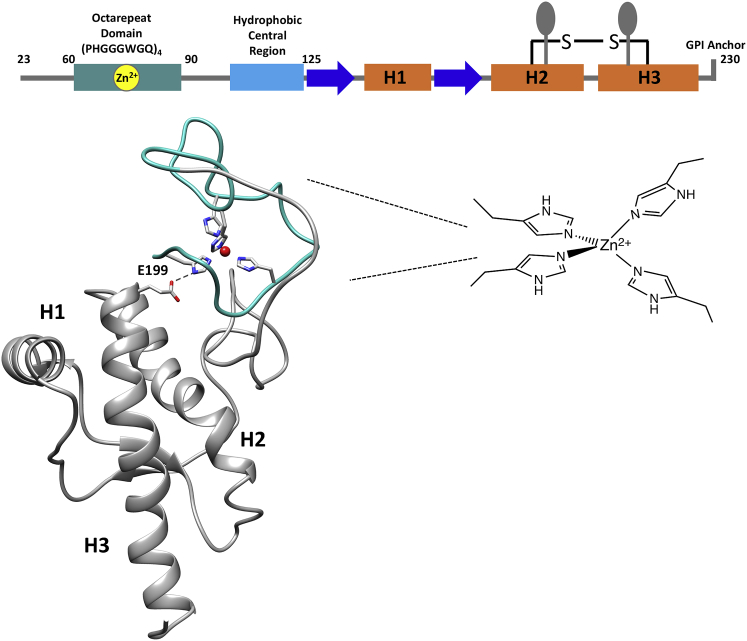
Figure 1.
Sequence and structural model of mouse PrPC-Zn2+ complex. (top) Linear sequence diagram of PrPC(23–230) (mouse sequence) with numbering indicating important N-terminal segments, along with secondary structure and posttranslational modifications of the folded C-terminal domain. Coloring shows the octarepeat (OR) domain (light green) that coordinates Zn2+, β sheets (blue), helices (orange), CR segment (light blue), glycans (ovals), disulfide bond linking helices two and three, and glycophosphatidylinositol anchor at residue 230. (bottom) Three-dimensional ribbon model of PrPC with Zn2+ (red sphere) coordinated to the OR domain His residues and docked against a C-terminal cleft formed by helices 1–3 (Spevacek et al. (15)). Also shown is residue E199 (mouse sequence) with its carboxylate group forming a hydrogen bond with the remote NH of a coordinating His residue.
Although once thought to be noninteracting, we recently demonstrated that PrPC’s N-terminal and C-terminal domains have an important interdomain interaction driven by the addition of physiological metal ions (6, 7, 15). Evidence for this cis interaction came from detailed double electron-electron resonance (DEER) EPR and 1H-15N heteronuclear single-quantum coherence (HSQC) NMR. Specifically, Spevacek et al. (15) demonstrated that the addition of Zn2+ drives the N-terminal OR to make a direct contact with a negatively charged patch on C-terminal surface composed of residues from helices two and three as well as the N-terminal end of the β1-α1 loop extending to the beginning of helix 1. Interestingly, a significant number of pathological mutations responsible for inherited prion disease reside on this surface, and those tested systematically weakened the observed cis interaction (15). Therefore, it was hypothesized that PrPC’s cis interaction plays a role in regulating the prion protein (PrP), with a decrease in this cis interaction promoting prion-mediated toxicity.
In addition to Zn2+, Cu2+ also drives a cis interaction, as shown by Evans et al. (6). This was demonstrated using paramagnetic relaxation enhancement NMR and DEER EPR. Paramagnetic relaxation enhancement broadening of PrPC 1H-15N HSQC NMR crosspeaks with 1.0 equivalent of Cu2+ localized the cis interaction to the same C-terminal PrPC surface identified in the Zn2+ studies. The specific location of the Cu2+ ion was further refined using DEER-EPR-derived distance restraints, along with trilateration calculation (6). These structural data suggest that physiologically relevant metal ions are essential for stabilizing higher order structure in PrPC. Recent monoclonal antibody and electrophysiology experiments underscore the importance of this newly discovered cis interaction (16). These experiments show consistently that the PrPC C-terminal domain regulates the otherwise toxic N-terminal executive domain. Using mutagenesis and select PrPC constructs, we have provided strong evidence that this regulatory function requires the Zn2+/Cu2+-promoted cis interaction identified by our magnetic resonance experiments (6, 7, 15, 16, 17).
Cu2+ is a paramagnetic species, which enables EPR experiments for assessing details of the metal ion’s coordination environment. Using EPR with 1.0 equivalent of Cu2+, we showed that the copper ion is bound to the four imidazole side chains of the OR histidines (9). This coordination environment is preserved in both the OR domain alone when expressed as a polypeptide as well as in the full-length protein. This information was essential in developing a structural model of Cu2+-occupied PrPC.
Unlike Cu2+, there is no convenient magnetic resonance method for probing the coordination environment of Zn2+. However, given the essential role of Zn2+ in PrPC physiology, as evidenced by the ion’s ability to trigger divalent ion transport through the AMPA receptor (14), it is very important to evaluate the precise details of how this physiological ion coordinates within PrPC. We have shown previously with indirect methods such as mass spectrometry mapping applied to OR peptides that, much like Cu2+, Zn2+ binds to the OR through imidazole coordination (10). However, this has yet to be demonstrated in the full-length PrP. As such, it is unclear whether Zn2+ remains confined solely by the histidine residues of the OR segment or, alternatively, forms a direct bond to the C-terminal residue side chains.
To investigate the molecular details, we report here the application of 113Cd NMR spectroscopy to probe Zn2+ coordination in PrPC. Cd2+, like Zn2+, is a transition metal in group 12 of the periodic table and therefore forms a stable divalent ion with a diamagnetic d10 electron configuration (18). Being separated by just one period, the ionic radii of Cd2+ and Zn2+ are similar at 0.98 and 0.74 Å, respectively (18). As demonstrated in the context of other proteins, Cd2+ is an excellent Zn2+ surrogate recapitulating zinc’s coordination properties (19). For example, the zinc metalloenzymes carbonic anhydrase B and C were studied using 113Cd NMR spectroscopy (20, 21). The enzymes retained activity with Cd2+ at the catalytic center, and analysis of the 113Cd NMR spectra distinguished between competing coordination models by identifying an exchangeable water molecule at the active site (22).
Cd has two spin-1/2 isotopes: 111Cd and 113Cd. Of these, 113Cd is somewhat more sensitive and thus more desirable for NMR studies (23). 113Cd NMR offers several advantages for probing metal ion binding sites (22, 24, 25). 113Cd NMR signals are spread over a remarkably wide chemical shift range, ∼900 ppm, with specific resonances sensitive to coordination geometry and specific coordinating atoms (23). 113Cd chemical shifts are predictable with deshielding following the empirical relationship S > N > O (26, 27, 28). Consequently, 113Cd NMR provides a sensitive probe for assessing the metal ion coordination environment. Although 113Cd is a low-sensitivity nucleus, enrichment to 94.8% (natural abundance of 113Cd is 12.26%) allows for acquisition of high-quality spectra in ∼12 h using a broad-band, nitrogen-cooled cryoprobe on a 1H 500-MHz instrument (corresponding to a 113Cd resonance frequency of ∼111 MHz) (29). Finally, because of the uniqueness of 113Cd, spectra are devoid of background signals.
In this study, we use 1H-15N NSQC NMR to compare the interactions of Zn2+ and Cd2+ with PrPC. Titration studies and 2JNH couplings from HSQC experiments are used to assess the Cd2+-PrPC Kd (30, 31). Next, using 113Cd NMR, we compare binding in the isolated OR domain and in the full-length protein. Finally, we evaluate PrPC mutants relevant to prion toxicity and disease using both NMR and isothermal titration calorimetry (ITC). Together, these data advance the understanding of metal ion coordination in PrPC and show, specifically, that 1) Cd2+ is an excellent Zn2+ surrogate and useful tool for probing the features of metal ion coordination in PrPC, 2) Cd2+ binding in both the OR domain and full protein is dominated by His coordination and is spectroscopically equivalent, 3) a highly penetrant, familial, disease-associated mutant that alters C-terminal domain charge triggers a loss of metal ion binding affinity, and 4) basic residues of the C-terminal domain may stabilize the global PrPC fold through hydrogen bonding to the metal ion coordinating His residues of the OR segment.
Materials and Methods
15N-labeled protein expression
PrP and its variants were constructed using the template plasmid pJexpress 414 mouse PrP (DNA 2.0) containing full-length Mus musculus PrP (23–230). All constructs and mutations were confirmed by DNA sequencing. Protein expression was carried out in Escherichia coli BL21Star (DE3) (Invitrogen, Carlsbad, CA). For 1H-15N HSQC NMR experiments, 15N-labeled proteins were grown per the protocols reported by Evans et al. (6). N-terminal PrP (23–125) was produced by introducing a tobacco etch virus cleavage site to remove the C-terminal (126–230) domain (32).
Peptide synthesis
The linear peptide (referred to as the 4-Octa peptide or peptide) KKRPKPWGQPHGGGWGQPHGGSWGQPHGGSWGQPHGGGWGQ-NH2, corresponding to KKRPKP-PrP(56–90)-NH2, (molecular weight = 4291.66, ε = 28,450 cm−1M−1) was prepared by solid-phase peptide synthesis using standard fluorenylmethoxycarbonyl chemistry protocols on a Liberty 1 Microwave Peptide Synthesizer (CEM). 4-Octa peptide was cleaved from ChemMatrix Rink amide resin (Sigma Aldrich, St. Louis, MO) and purified by reverse-phase C18 high-performance liquid chromatography and lyophilized for long-term storage once it reached analytical purity.
NMR spectroscopy
PrPC and PrP-derived peptide samples for 1H-15N NMR experiments were prepared at 300 μM protein or peptide in a buffer containing 10 mM 2-(N-morpholino)ethanesulfonic acid (MES; Sigma), 10% D2O, at pH 6.0 or 7.0, depending on the specific experiment. Subsequent to the addition of 1.0 mM CdCl2, the pH was measured and adjusted, if necessary. 1H-15N HSQC spectra were recorded at 25°C on an 800-MHz spectrometer (Bruker, Billerica, MA) at the University of California, Santa Cruz NMR facility (Santa Cruz, CA). NMR spectra were analyzed with NMRPipe (33) and Sparky. Structural analysis was performed with Chimera (34). Protein assignments were achieved using previously determined values from Evans et al. (6).
The Cd2+ Kd was determined from 1H-15N-HSQC spectra using nonlinear least-squares fitting to the following equation (35):
where [P]t and [L]t are the total protein and ligand (Cd2+) concentrations, respectively, and , determined from the fitting procedure, is the difference in chemical shift between the free and fully bound protein.
For 113Cd NMR experiments, all samples were prepared in buffer containing 10 mM MES buffer (Sigma), 10% D2O at pH 6.0 and 25°C, with either 300 μM protein, EDTA or 1.8 mM imidazole, and 1.0 mM 113CdCl2 (Cambridge Isotopes; 95% isotopically labeled). 113Cd NMR acquisition was performed on the San Francisco State University Bruker AVANCE NEO 500 MHz (11.7 T) spectrometer, fitted with a nitrogen-cooled cryoprobe. The spectrometer was tuned to 110.9 MHz. All samples were externally referenced to 0.1 M Cd(ClO4)2 (aqueous [aq]).
Analysis leading to the conclusion of fast exchange in the 113Cd spectra utilized the following relations:
where is the difference in 113Cd chemical shift between free Cd2+ and the fully bound metal ion, and νo is the spectrometer frequency. kon is estimated to be diffusion controlled and ∼109 M−1s−1. With a protein concentration of 300 μM, kex >> Δω thereby satisfying fast exchange consistent with a single 113Cd NMR line.
ITC
ITC experiments were conducted using a MicroCal VPITC calorimeter. Zinc chloride titrations were performed by adding 2.0 mM zinc chloride into each PrPC construct (18–45 μM) at pH 7.4 in 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS). Cadmium chloride titrations were performed by titrating 20 mM cadmium chloride into each PrPC construct (45–60 μM) at pH 6.0 in 10 mM MES. Each construct was dialyzed overnight before running the experiments in either 50 mM MOPS pH 7.4 (Zn2+ titrations) or 10 mM MES pH 6.0 (Cd2+ titrations). Metal ions used in the titrations were prepared by diluting a 1.0 M stock in water to either 2.0 mM for zinc chloride or 20 mM for cadmium chloride using the MOPS or MES dialysis buffer, respectively. Because of the large heats of dilution with cadmium chloride, the integrated ITC data were background corrected. Data analysis was performed using the Origin calorimetry software package. Experiments were replicated two to three times for each construct, and the reported error is the difference between the highest and lowest Kd measured for each set of measurements.
Results
Cadmium induces a cis interaction between PrPC N-terminal and C-terminal domains
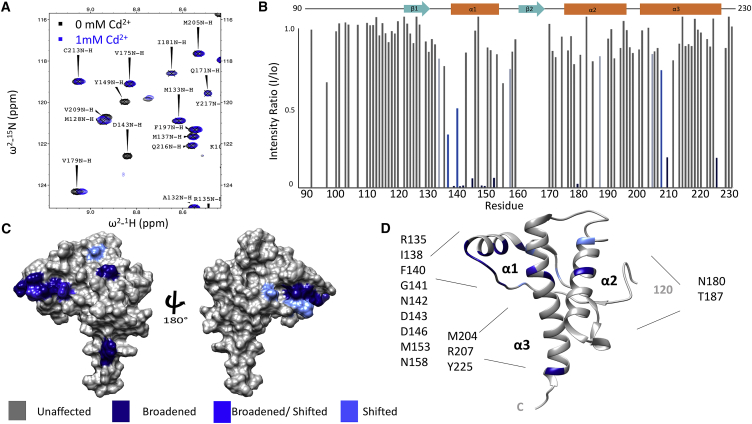
Previous 1H-15N HSQC NMR experiments performed on uniformly 15N-labeled PrPC with one to three equivalents of Zn2+ identified a collection of C-terminal residues that exhibited both linewidth broadening and/or changes in chemical shift of select crosspeaks. The affected residues, when mapped to the three-dimensional structure of the C-terminal domain of PrPC, identified a shallow cavity localized primarily to a surface patch composed of adjacent sides of helices 2 and 3 (α2 and α3), along with the residues corresponding to the β1-α1 loop. When combined with EPR DEER experiments and suitable controls, these NMR data demonstrated that the Zn2+-OR segment docks to a well-defined, negatively charged patch on the PrPC C-terminal domain (Fig. 1) (15). Divalent Zn is diamagnetic; we therefore attributed changes in the line shapes and chemical shifts of the affected C-terminal residues to intermediate exchange molecular dynamics at the interface between the interacting N-terminal and C-terminal domains. To confirm the use of Cd2+ as a viable Zn2+ surrogate, we performed parallel 1H-15N HSQC NMR experiments at 25°C on 15N-labeled wild-type PrPC(23–230) (300 μM) in the presence of 1.0 mM Cd2+ (added as CdCl2) at pH 6.0 and pH 7.0.
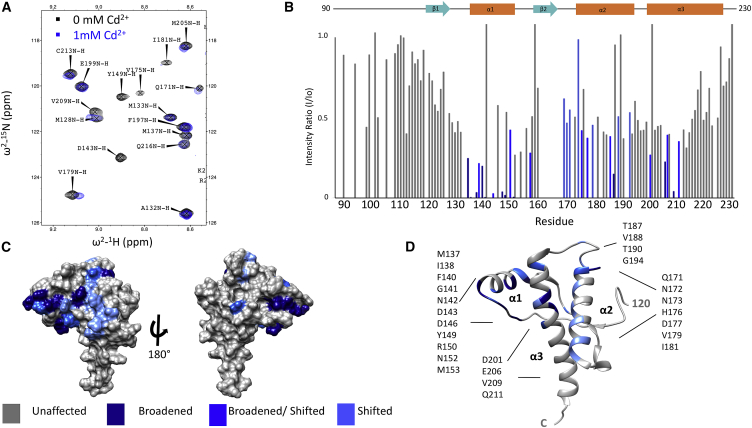
Fig. 2 A shows a segment of the 800-MHz 1H-15N HSQC NMR spectrum. As with Zn2+, we find that select crosspeaks exhibit changes in linewidth and chemical shift (15). To evaluate these spectral changes, values for intensity ratio (I/Io) were calculated for all assigned C-terminal resonances. These ratios are obtained by dividing the crosspeak intensity in the presence of Cd2+ by the intensity of the crosspeak before addition of Cd2+. The average and SD of the I/Io values were calculated, and standard Z-score analysis (6) was performed to determine a threshold for residues broadened more than 1.0 SD from the mean by the presence of Cd2+. In addition, crosspeaks exhibiting changes in chemical shift greater than 0.1 ppm (measured by [(Δδ1H)2 + (Δδ15N/9)2]1/2) were deemed to be significantly affected by the addition of Cd2+. The results are summarized in Fig. 2 B, which shows I/Io versus residue position for the C-terminal domain. Affected residue crosspeaks exceeding statistical significance are noted by dark blue (I/Io exceeding one SD the mean), medium blue (I/Io greater than 0.5 SD with a chemical shift change greater than 0.1 ppm), and in light blue (0.1 ppm or greater change in chemical shift) (6, 35). When plotted onto surface (Fig. 2 C) or ribbon (Fig. 2 D) diagrams, the data identify a patch of affected residues similar to those observed by the addition of Zn2+. Specifically, the Cd2+ caused either broadening, shifting or broadening and shifting of proximal residues on α2, α3, and the β1-α1 loop. The 27 affected residues are all proximal to each other and, of these, 12 are equivalent to those affected by Zn2+ (Fig. S1). Spectra acquired at pH 6.0 and 7.0 gave similar results; however, for experiments that follow, we standardized on the lower pH value because it was found to give narrower 113Cd line shapes (see below, Fig. 5). Noting that Zn2+ does not bind to PrPC(90–230), these results show that like Zn2+, Cd2+ drives a well-defined cis interaction between the PrPC Cd2+-occupied N-terminal domain and the shallow cavity of the C-terminal domain.
Figure 2.
Cd2+ promotes an interdomain interaction wild-type PrP at pH 6.0. (A) A selected region of the 1H-15N HSQC of wild-type PrP in the absence of metal (black) and in the presence of 1.0 mM of Cd2+ (blue). (B) A bar graph of I/Io for residues 90–23 of PrPC in the presence of Cd2+. (C and D) Surface and ribbon plots, respectively, of C-terminal residues affected by the presence of Cd2+ (coordinates from Protein Data Bank (PDB): 1XYX). Affected residues are noted specifically on the ribbon diagram.
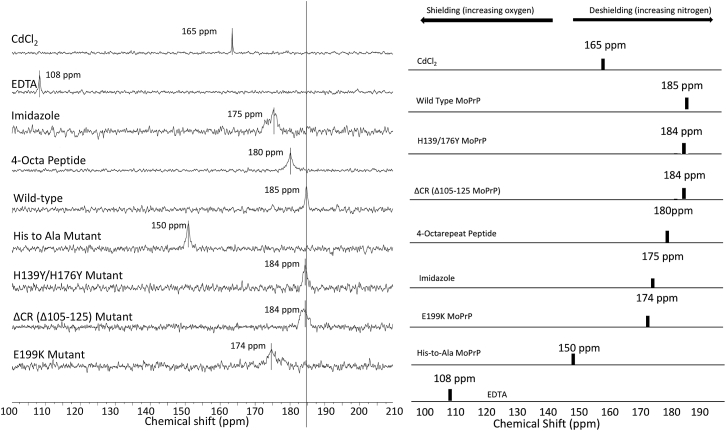
Figure 5.
113Cd NMR spectra of controls, octarepeat (OR) segment, full-length PrPC, and relevant mutants. (left) 113Cd NMR spectra with chemical shifts referenced to 0.1 M Cd(ClO4)2 (aq). Spectra for the OR peptide (4-Octa peptide) and all proteins were acquired with 300 μM peptide/protein and 1.0 mM 113Cd2+. The vertical line is drawn as a reference against wild-type protein. (right) A stick diagram of the 113Cd NMR spectra ordered by chemical shift from high to low (with reference CdCl2 at the top). As noted at the top, nitrogen coordination decreases chemical shielding, leading to higher chemical shift values, whereas oxygen coordination shifts in the opposite direction.
Cd2+ coordinates to imidazole groups of OR histidines
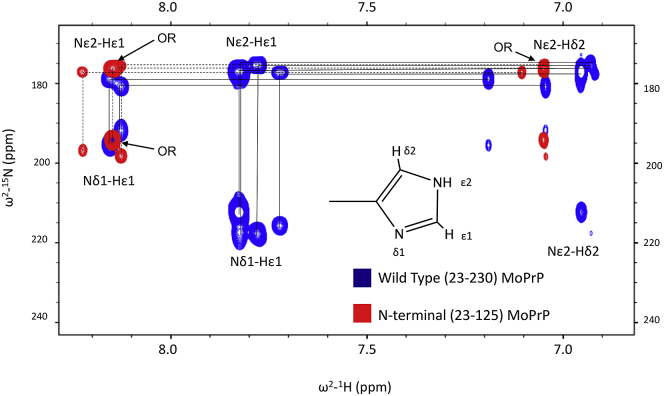
We previously used chemical mapping to show that Zn2+ coordinates specifically to His imidazole residues in the PrPC OR segment with a Kd of ∼200 μM (10). To examine Cd2+ coordination details and measure its binding affinity, we applied 1H-15N HSQC with pulse sequence modifications to highlight 2JNH scalar couplings (30). With this pulse sequence, His side chains give characteristic patterns depending on the protonation state of the imidazole ring. Spectra of full-length PrPC and PrP (23–125), both in the absence of Cd2+, are compared in Fig. 3. PrPC contains 9 His residues, 6 of which are in the flexible region 23–125. Examination of Fig. 3 shows that Hε1 crosspeaks with 1H chemical shifts above 8.1 ppm are readily assigned to this N-terminal segment. In addition, prominence of the specific crosspeak patterns of Nδ1-Hε1, Nε2-Hε1, and Nε2-Hδ2 for each imidazole is consistent with protonation of the ε2 nitrogen of the imidazole ring. Three separate patterns are observed for PrP (23–125) with approximate volume ratios of 4:1:1. We therefore assigned the four OR His residues to more intense set crosspeaks.
Figure 3.
2JNH-HSQC of 15N-labeled N-terminal PrP and full-length PrPC. 1H-15N-HSQC spectra with pulse sequence modified to highlight 2JNH couplings showing the imidazole region of the spectrum for mouse PrP(23–125) (red, correlated crosspeaks connected by dashed lines) and full-length PrP (blue, peaks connected by solid lines). The pattern of connections from Nδ1-Hε1 → Nε2-Hε1 → Nε2-Hδ2 is characteristic of the protonation at Nε2. Crosspeaks from the octarepeat (OR) His residues are labeled “OR.”
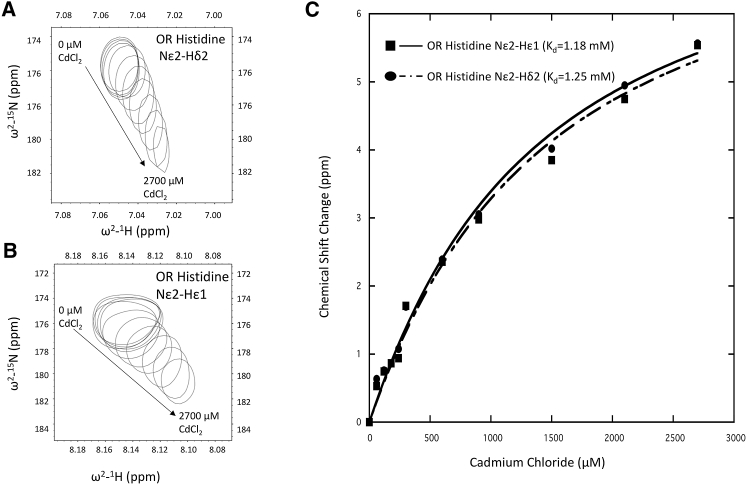
Next, we added increasing concentrations of Cd2+ to a solution of 300 μM PrP (23–125) and observed a progressive chemical shift changes of the Nε2-Hε1 and Nε2-Hδ2 crosspeaks, assigned to the OR His residues (Fig. 4). The peaks exhibited only slight broadening with increasing [Cd2+], consistent with fast exchange. These data suggest that Cd2+ coordinates preferentially to the ε2 nitrogen, perhaps displacing the exchangeable proton. Plotting the Nε2 15N chemical shifts derived from the Nε2-Hε1 and Nε2-Hδ2 crosspeaks gave saturable binding curves, which we fit independently to a standard model for equilibrium fast exchange (35). Both curves gave similar results with an approximate Kd of 1.2 mM (Table 1). We also attempted titration with full-length PrPC; however, the relevant 2JNH crosspeaks exhibited significant broadening, likely because of intermediate exchange, and were therefore not amenable to fast-exchange binding analysis. Taken together, these NMR experiments demonstrate that Cd2+, like Zn2+, binds to the OR His residues but with a significant reduction in affinity.
Figure 4.
Determination of Kd from 2JNH HSQC chemical shifts versus Cd2+. (A) Nε2-Hδ2 and (B) Nε2-Hε1 2JNH HSQC crosspeaks of N15-labeled PrP (23–125) shift as a function of added cadmium chloride. (C) A plot of 15N chemical shift change versus CdCl2. Data were fitted to the Kd expression derived for fast chemical exchange (Materials and Methods) to determine binding constant for Cd2+ to the OR domain (Table 1). Resulting Kd values derived from the Nε2-Hδ2 and Nε2-Hε1 crosspeaks are ∼1.25 and 1.18 mM, respectively.
Table 1.
Kd for OR Histidine ε Nitrogen
| Kd (μM) | Error (μM) | |
|---|---|---|
| OR histidine Nε2-Hε1 | 1187 | ±200 |
| OR histidine Nε2-Hδ2 | 1253 | ±245 |
The top represents OR histidine Nε2-Hε1, and the bottom represents OR histidine Nε2-Hδ2.
113Cd NMR of wild-type PrPC and relevant mutants
With the goal of assessing the Cd2+ coordination environment, we carried out direct NMR measurements on 113Cd combined with PrPC and various relevant constructs. With an 11.7 T magnetic field (500 MHz for 1H), the 113Cd resonance frequency is 110.9 MHz (29). All spectra were referenced to the chemical shift of 0.10 M Cd (ClO4)2 (aq), which places the chemical shift of aquo 113Cd at 0 ppm (29). Given the low gyromagnetic ratio of 113Cd, it was essential to adjust sample conditions to give the best possible spectra. Consequently, we prepared peptides and proteins to concentrations of 300 μM, which is near the PrPC solubility limit, along with 1.0 mM 113CdCl2. Under these conditions, a spectrometer fitted with a cryoprobe was capable of acquiring resolvable spectra in approximately 12 h.
Resulting spectra are shown in Fig. 5. As shown in the insert, nitrogen coordination leads to deshielding of the 113Cd center, with concomitant resonances of higher chemical shift values. Oxygen coordination produces resonance signals of lower chemical shifts (23). The differences between nitrogen and oxygen coordination are reflected in the comparison of imidazole (175 ppm) and EDTA (108 ppm).
All peptide and protein samples gave only a single resonance line consistent with fast exchange between the coordinated species and free Cd2+ in buffered solution, as seen with the 1H-15N HSQC above (Fig. 2). A peptide containing the isolated 4-Octa peptide, OR segment (KKRPKP-PrP (56–90)-NH2) produces a single line with a 113Cd chemical shift close to that of imidazole, consistent with our findings above that Cd2+ coordinates primarily through His side chains. Full-length PrPC is further deshielded relative to the isolated OR by ∼5 ppm. Consequently, either the cis interaction described above further stabilizes OR-Cd2+ coordination or the Cd2+ coordination shell is additionally enhanced by C-terminal His residues. To test for this latter case, we prepared PrPC(H139Y, H176Y), which replaces two C-terminal His residues at the Cd2+-OR docking interface, specifically, H139 on the β1-α1 loop and H176 on α2. The resulting 113Cd spectrum is equivalent to that of wild-type PrPC, suggesting that either Cd2+ remains fully confined by coordination to the OR His residues or that one or two of the C-terminal His residues replace OR His residues thereby maintaining a four-His coordination environment.
To directly test the involvement of Cd2+ binding to the flexible N-terminal PrPC domain, we prepared a mutant in which all OR His residues, His95, and His110 were mutated to Ala. This His-to-Ala mutant shows a strong shift toward a more shielded 113Cd signal of ∼35 ppm, close to that of CdCl2 in aq solution. We therefore conclude that, indeed, the N-terminal segment provides the primary coordination environment for Cd2+ with a three- or four-His coordination shell, as previously found for Zn2+.
The fast-exchange conditions observed for 113Cd provide an opportunity to estimate the chemical shift of the OR -113Cd2+ species. Under fast exchange, the observed signal is a weighted average of the free and fully PrPC-bound 113Cd chemical shifts. Using a Kd = 1.2 mM determined for the isolated 4-Octa OR segment (peptide concentration = 300 μM and [Cd2+] = 1.0 Mm), we calculate that the concentration of the bound OR-Cd2+ species is 126 μM or 12.6% of the total 113Cd2+ in solution. Given the free 113Cd2+ signal of 165 ppm (from CdCl2) and exchanged average signal of 180 ppm, the fully bound species is determined to be approximately 300 ppm. This value aligns well with published values of 310–320 ppm for superoxide dismutase, which coordinates through a histidine-rich environment (36, 37, 38).
Next, we evaluated two important mutants than link directly to prion disease. ΔCR PrPC is a designed deletion mutant in which residues 105–125, corresponding to the central region, are eliminated. Loss of this 21-residue segment between the OR and globular C-terminal domain drives severe cerebellar degeneration and neonatal lethality in laboratory mice (39). Electrophysiological experiments performed on cells transfected with the ΔCR PrPC gene show spontaneous cationic currents associated with the early stages of prion disease (40, 41). In cell culture, these currents are inhibited by the addition of Cu2+, which binds with high affinity to the OR (16). Similar to Zn2+, Cu2+ drives a cis interaction, and previous work from our lab showed a loss of this copper-promoted interaction in ΔCR PrPC. Fig. 5 shows that ΔCR PrPC gives a 113Cd spectrum approximately equivalent to that of wild-type PrPC. Consequently, there is no loss of Cd2+ coordination in ΔCR PrPC; however, we cannot rule out a potential loss of the metal-ion-promoted cis interaction.
Finally, we examined murine PrPC(E199K), a mutation that corresponds to familial E200K in humans (42, 43). Families that carry this fully penetrant E200K mutation develop midlife CJD (44). Spevacek et al. demonstrated that the E199K mutation exhibits a weakened cis interaction, relative to wild-type, upon the addition of Zn2+ (15). They postulated that because E199 is located on the N-terminal end of helix 3 contributing to a large concentration of negatively charged C-terminal residues, the mutation to a Lys confers toxicity by reducing this localized negative charge thereby weakening electrostatic contributions to the cis interaction (15). The 113Cd spectrum of PrPC(E199K) shows a significant shift relative to that obtained from wild-type PrPC, with a chemical shift reflecting a partial loss of nitrogen coordination. Complementing the findings of Spevacek et al. (15), these data suggest partial release of the metal ion.
We next performed ITC measurements on both wild-type PrPC and the E199K mutant to test whether the difference in 113Cd chemical shift arises from a change in metal ion affinity. Kd values were determined for Cd2+ and Zn2+ binding to both proteins, as well as Zn2+ binding to the N-terminal peptide segment (PrP(23–125)). Kd values are reported in Table 2 (ITC curves are in Fig. S2). Wild-type PrPC gives a Kd of ∼3.0 mM, in reasonable agreement with 1.2 mM obtained from NMR using the fast exchange approximation (above). Comparing wild-type and mutant PrPC, we find that there is a very slight increase in Kd for Cd2+ binding to PrPC(E199K) relative to wild-type PrPC; however, the difference is within experimental error. By contrast, the Kd difference for Zn2+ shows a factor of two increase for PrPC(E199K) compared to wild-type PrPC, thus reflecting a lower affinity for the mutant. In addition, Kd for PrPC(E199K) is approximately equivalent to that obtained from N-terminal PrP (23–125). Together, these data demonstrate that the C-terminal domain in wild-type PrPC enhances the OR domain’s affinity for Zn2+ and that this enhancement is lost in PrPC(E199K).
Table 2.
Kd for Zn2+ and Cd2+ Determined by ITC
| Zn2+Kd (μM)a | Cd2+Kd (mM)a | |
|---|---|---|
| Wild-type PrPC | 16.9 ± 1.2 | 3.0 ± 1.8 |
| PrPC(E199K) | 33.6 ± 0.3 | 3.3 ± 0.8 |
| N-terminal PrP(23–125) | 38.0 ± 0.1 | N/A |
N/A, not acquired.
Note different units for Zn2+ (μM) and Cd2+ (mM).
To further explore the consequence of the E199K mutation, we performed 15N-1H HSQC NMR in the presence of 1.0 mM CdCl2. Fig. 6 shows that the E199K mutation significantly weakens the observed cis interaction relative to wild-type, similar to our previous observations with Zn2+. The majority of the remaining broadened residues are localized to helix 1; line shape broadening of crosspeaks from residues at the respective N-termini of helices 2 and 3 is much less pronounced. Collectively, with the ITC measurements above, these data suggest that residues E199 in the wild-type protein promotes the cis interaction and enhances metal ion binding affinity.
Figure 6.
Cd2+-promoted interdomain interaction PrPC(E199K) is significantly weakened relative to the wild-type. The PrPC mutation E200K (E199K in mouse) correlates with the human prion CJD. (A) A selected region of the 1H-15N HSQC of wild-type PrP in the absence of metal (black) and in the presence of 1.0 mM of Cd2+ (blue). (B) A bar graph of I/Io for residues 90–23 of PrPC in the presence of Cd2+. (C and D) Surface and ribbon plots, respectively, of C-terminal residues affected by the presence of Cd2+ (coordinates from PDB: 1XYX). Affected residues are noted specifically on the ribbon diagram.
Discussion
PrPC is a Zn2+ binding protein, and emerging evidence suggests that uptake of this essential metal ion of the central nervous system drives an intramolecular cis interaction that is critical for regulating PrPC function and arresting inherent toxicity. Previous to this current study, we used chemical mapping performed on an OR peptide to suggest that Zn2+ coordinates exclusively to OR His side-chain imidazole groups (10). Here, we used Cd2+ as a Zn2+ surrogate to carefully investigate the metal ion coordination features and the resulting cis interaction. As with Zn2+, 1H-15N HSQC NMR experiments show that the binding of Cd2+ to the N-terminal OR segment of PrPC leads to broadening of select C-terminal residues located to a shallow, negatively charged cleft formed primarily by three α-helices. Next, we used a modified 1H-15N HSQC NMR sequence that selects for 2JNH scalar couplings to assess the Cd2+-PrPC Kd, in turn establishing optimal conditions for 113Cd NMR studies (23, 30, 31). Finally, 113Cd NMR studies allowed us to compare Cd2+ complexation features among the isolated OR, the full-length PrPC protein, as well as important mutants.
Given the remarkable chemical shift sensitivity of 113Cd, the agreement between the isolated OR and the full-length protein provides strong evidence that the OR alone is responsible for direct Cd2+ coordination (23). Consistent with this proposal, elimination of N-terminal His residues leads to a significant change in 113Cd chemical shift. Moreover, analysis of the fast-exchange signal finds a calculated chemical shift of the Cd2+-PrPC species to be consistent with coordination by three- or four-His nitrogen atom configuration (35, 45). Together, these findings with Cd2+ as a surrogate support our previous proposal that PrPC takes up Zn2+ with three- or four-His coordination in the repeat domain.
After our chemical mapping studies (10), several extended x-ray absorption fine-structure (EXAFS) and related x-ray studies investigated Zn2+ coordination to PrPC (46, 47). Stellato et al. probed the competition between Zn2+ and Cu2+ coordination and showed that at low Cu2+ occupancy, Zn2+ partially displaces His side chains from the copper centers (48). In a separate study, this group found that Zn2+ does not fully coordinate to all OR His residues but instead may facilitate OR peptide clustering (47). Given that this work was performed exclusively on OR peptides, it is not clear whether this type of metal ion-assisted cross-linking applies to full-length PrPC. Pushie et al. (46) combined density functional theory and EXAFS to carefully examine Zn2+ occupation of the OR. Interestingly, their calculations found little energy difference between Zn2+ coordinated to three imidazole groups and a single water versus Zn2+ coordinated to four imidazoles. This finding was supported by analysis of the EXAFS data, which were well fit with a model of only three imidazole groups (46). Our findings here suggest three- or four-His coordination as determined by extrapolation from the fast-exchange signal and comparison of the estimated chemical shift to that of known His-rich Cd2+ complexes. As such, our result, although an approximation, is consistent with the findings of Pushie et al. (46).
A surprising finding of our study is the significant change in chemical shift associated with the E199K mutation. In humans, the parallel E200K mutation potently confers familial CJD (44, 49). Residue 200 is located near the N-terminal end of α3, so it is therefore reasonable to hypothesize that this mutation destabilizes the protein thereby promoting aggregation (6). However, biophysical studies with NMR and circular dichroism find that PrPC(E200K) maintains the same fold and stability as the wild-type protein (15, 50, 51). Residue E200 contributes to a highly conserved, negatively charged electrostatic patch on the PrPC C-terminal domain (15). In our previous investigations with PrPC-Zn2+ binding, we noted that all C-terminal disease promoting mutations involving acidic or basic residues results in a reduction of the patch’s negative charge character. Using 1H-15N HSQC NMR, we directly tested the influence of the Glu→Lys mutation and found a significant loss of the Zn2+-promoted cis interaction, especially in the vicinity of α3 (15). This finding led to the proposal of a new paradigm for understanding the E200K and related mutations in which alteration of the protein’s electrostatics results in a weakening of the regulatory cis interaction. Our findings here take this concept further by suggesting a loss of metal ion affinity. Testing this directly with ITC indeed shows that the affinity for Zn2+ is reduced by the Glu→Lys mutation, consistent with the change in 113Cd chemical shift found for PrPC(E199K) possibly from a loss of nitrogen coordination.
Integrating the observations above suggests a new scheme for understanding the role of residue E200 in stabilizing the PrPC cis interaction. The 113Cd NMR spectra comparing wild-type PrPC with the OR peptide reflects little change in the metal ion coordination environment. However, mutation to Lys causes a loss of the observed PrPC cis interaction promoted by either Zn2+ or Cd2+. These findings may be explained by proposing that E200 contributes to the second coordination sphere of the metal ion. Previous work by Spevacek et al. (15) used molecular dynamics simulations with distance restraints from DEER EPR to localize the OR-Zn2+ domain relative to the interfacial binding surface of the C-terminal domain. Inspection of the coordinates from these simulations finds that the side-chain carboxylate of E200 is within hydrogen bonding distance of an imidazole ring NH opposite to the nitrogen that coordinates Zn2+, as shown in Fig. 1. In this paradigm, the mutation E200K does not destabilize the C-terminal domain; instead mutation of the acidic glutamate results in the loss of a Glu-His hydrogen bond that is essential for regulating the otherwise toxic PrPC N-terminal executive domain.
In summary, 113Cd NMR has provided new and important insights into metal ion coordination in the PrP. Our studies continue to support the concept of an interdomain cis interaction promoted by coordination of Zn2+ to the OR; mutations that weaken this interaction correlate with inherited prion disease. This concept may prove useful in the continued study of PrPC function and treatment of prion diseases.
Author Contributions
K.A.M. and G.L.M. designed the experiments. K.A.M., G.P.R., R.B.L., and H.-W.L. performed the experiments. K.A.M. and G.L.M. wrote the manuscript. K.A.M., G.P.R., and G.L.M. edited the manuscript.
Acknowledgments
The authors extend their sincere gratitude to professor Ian M. Armitage, University of Minnesota, for helpful advice on the use of 113Cd as an NMR probe of metal ion coordination centers and to Kevin Schilling, University of California, Santa Cruz, who generously contributed protein for 113Cd NMR studies. We further thank Dr. Mark Swanson, PhD, San Francisco State University NMR Facility Manager, for help with acquisition of 113Cd spectra.
This work was funded by National Institutes of Health instrumentation grant S10OD018455, which supported acquisition of the University of California, Santa Cruz 800 MHz NMR spectrometer, and National Institutes of Health research grant R01GM065790 (awarded to G.L.M.). The San Francisco State University NMR instrument was supported by National Science Foundation Major Research Instrumentation grant DBI1625721.
Editor: Wendy Shaw.
Footnotes
Two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(19)30021-9.
Supporting Material
References
- 1.Prusiner S. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner S.B. Prion diseases and the BSE crisis. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 3.Herms J., Tings T., Kretzschmar H. Evidence of presynaptic location and function of the prion protein. J. Neurosci. 1999;19:8866–8875. doi: 10.1523/JNEUROSCI.19-20-08866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele A.D., Lindquist S., Aguzzi A. The prion protein knockout mouse: a phenotype under challenge. Prion. 2007;1:83–93. doi: 10.4161/pri.1.2.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millhauser G.L. Copper and the prion protein: methods, structures, function, and disease. Annu. Rev. Phys. Chem. 2007;58:299–320. doi: 10.1146/annurev.physchem.58.032806.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans E.G., Pushie M.J., Millhauser G.L. Interaction between prion protein’s copper-bound octarepeat domain and a charged C-terminal pocket suggests a mechanism for N-terminal regulation. Structure. 2016;24:1057–1067. doi: 10.1016/j.str.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans E.G.B., Millhauser G.L. Copper- and zinc-promoted interdomain structure in the prion protein: a mechanism for autoinhibition of the neurotoxic N-terminus. Prog. Mol. Biol. Transl. Sci. 2017;150:35–56. doi: 10.1016/bs.pmbts.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Aronoff-Spencer E., Burns C.S., Millhauser G.L. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry. 2000;39:13760–13771. doi: 10.1021/bi001472t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chattopadhyay M., Walter E.D., Millhauser G.L. The octarepeat domain of the prion protein binds Cu(II) with three distinct coordination modes at pH 7.4. J. Am. Chem. Soc. 2005;127:12647–12656. doi: 10.1021/ja053254z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter E.D., Stevens D.J., Millhauser G.L. The prion protein is a combined zinc and copper binding protein: Zn2+ alters the distribution of Cu2+ coordination modes. J. Am. Chem. Soc. 2007;129:15440–15441. doi: 10.1021/ja077146j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donne D.G., Viles J.H., Dyson H.J. Structure of the recombinant full-length hamster prion protein PrP(29-231): the N terminus is highly flexible. Proc. Natl. Acad. Sci. USA. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown L.R., Harris D.A. Copper and zinc cause delivery of the prion protein from the plasma membrane to a subset of early endosomes and the Golgi. J. Neurochem. 2003;87:353–363. doi: 10.1046/j.1471-4159.2003.01996.x. [DOI] [PubMed] [Google Scholar]
- 13.Watt N.T., Griffiths H.H., Hooper N.M. Neuronal zinc regulation and the prion protein. Prion. 2013;7:203–208. doi: 10.4161/pri.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt N.T., Taylor D.R., Hooper N.M. Prion protein facilitates uptake of zinc into neuronal cells. Nat. Commun. 2012;3:1134. doi: 10.1038/ncomms2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spevacek A.R., Evans E.G., Millhauser G.L. Zinc drives a tertiary fold in the prion protein with familial disease mutation sites at the interface. Structure. 2013;21:236–246. doi: 10.1016/j.str.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B., McDonald A.J., Harris D.A. The N-terminus of the prion protein is a toxic effector regulated by the C-terminus. eLife. 2017;6:e23473. doi: 10.7554/eLife.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald A.J., Wu B., Harris D.A. An inter-domain regulatory mechanism controls toxic activities of PrPC. Prion. 2017;11:388–397. doi: 10.1080/19336896.2017.1384894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton F.A., Wilkinson G. Wiley; New York: 1988. Advanced Inorganic Chemistry: A Comprehensive Text. [Google Scholar]
- 19.Otvos J.D., Armitage I.M., Coleman J.E. 31P NMR of alkaline phosphatase. Dependence of phosphate binding stoichiometry on metal ion content. J. Biol. Chem. 1979;254:4707–4713. [PubMed] [Google Scholar]
- 20.Armitage I.M., Pajer R.T., Coleman J.E. Cadmium-113 Fourier transform nuclear magnetic resonance of cadmium(II) carbonic anhydrases and cadmium(II) alkaline phosphatase. J. Am. Chem. Soc. 1976;98:5710–5712. doi: 10.1021/ja00434a058. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson N.B., Tibell L.A., Sudmeier J.L. Cadmium-113 NMR of carbonic anhydrases: effect of pH, bicarbonate, and cyanide. Proc. Natl. Acad. Sci. USA. 1980;77:3269–3272. doi: 10.1073/pnas.77.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage I.M., Schoot Uiterkamp A.J., Coleman J.E. 113Cd NMR as a probe of the active sites of metalloenzymes. J. Magn. Reson. 1978;29:375–392. [Google Scholar]
- 23.Armitage I.M., Drakenberg T., Reilly B. Use of (113)Cd NMR to probe the native metal binding sites in metalloproteins: an overview. Met. Ions Life Sci. 2013;11:117–144. doi: 10.1007/978-94-007-5179-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armitage I.M., Otvos J.D., Boulanger Y. Structure elucidation of the metal-binding sites in metallothionein by 113Cd NMR. Biophys. Biochem. 1982;13:2974–2980. [PubMed] [Google Scholar]
- 25.Armitage I.M., Boulanger Y. Cadmium-113 NMR. NMR New. Access. Nucl. 1983;2:337–365. [Google Scholar]
- 26.Maciel, G. E., and M. Borzo. High resolution l13Cd nuclear magnetic resonance by pulse Fourier transform. J. Chem. Soc., Chem. Commun. 19:394a.
- 27.Kostelnik R.J., Bothner-By A.A. Cadmium-113 nuclear magnetic resonance studies of cadmium(II)ligand binding in aqueous solutions. I. The effect of diverse ligands on the cadmium-113 chemical shift. J. Magn. Reson. 1974;14:141–151. [Google Scholar]
- 28.Cardin A.D., Ellis P.D., Howard J.W. Cadmium-113 Fourier transform nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1975;97:1672–1679. [Google Scholar]
- 29.Lambert J.B., Riddell F.G. D. Reidel Publishing Company; Hingham, MA: 1982. The Multinuclear Approach to NMR Spectroscopy. [Google Scholar]
- 30.Pelton J.G., Torchia D.A., Roseman S. Tautomeric states of the active-site histidines of phosphorylated and unphosphorylated IIIGlc, a signal-transducing protein from Escherichia coli, using two-dimensional heteronuclear NMR techniques. Protein Sci. 1993;2:543–558. doi: 10.1002/pro.5560020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tettamanzi M.C., Keeler C., Hodsdon M.E. Analysis of site-specific histidine protonation in human prolactin. Biochemistry. 2008;47:8638–8647. doi: 10.1021/bi800444t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raran-Kurussi S., Cherry S., Waugh D.S. Removal of affinity tags with TEV protease. Methods Mol. Biol. 2017;1586:221–230. doi: 10.1007/978-1-4939-6887-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaglio F., Grzesiek S., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 34.Pettersen E.F., Goddard T.D., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 35.Williamson M.P. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 2013;73:1–16. doi: 10.1016/j.pnmrs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti P.S., Kennedy M.A., Bell T.W. Cadmium-113 NMR spectroscopy. Long bond interactions and chemical shielding in the cadmium complex of an unsaturated nitrogen analogue of 18-crown-6. J. Am. Chem. Soc. 1989;111:2063–2066. [Google Scholar]
- 37.Bailey D.B., Ellis P.D., Fee J.A. Cadmium-113 nuclear magnetic resonance studies of cadmium-substituted derivatives of bovine superoxide dismutase. Biochemistry. 1980;19:591–596. doi: 10.1021/bi00544a031. [DOI] [PubMed] [Google Scholar]
- 38.Kofod P., Bauer R., Bjerrum M.J. 113Cd-NMR investigation of a cadmium-substituted copper, zinc-containing superoxide dismutase from yeast. Eur. J. Biochem. 1991;8:607–611. doi: 10.1111/j.1432-1033.1991.tb16057.x. [DOI] [PubMed] [Google Scholar]
- 39.Li A., Christensen H.M., Harris D.A. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105-125. EMBO J. 2007;26:548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon I.H., Khatri N., Harris D.A. An N-terminal polybasic domain and cell surface localization are required for mutant prion protein toxicity. J. Biol. Chem. 2011;286:14724–14736. doi: 10.1074/jbc.M110.214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biasini E., Turnbaugh J.A., Harris D.A. The toxicity of a mutant prion protein is cell-autonomous, and can be suppressed by wild-type prion protein on adjacent cells. PLoS One. 2012;7:e33472. doi: 10.1371/journal.pone.0033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell J.E., Ironside J.W. Neuropathology of spongiform encephalopathies in humans. Br. Med. Bull. 1993;49:738–777. doi: 10.1093/oxfordjournals.bmb.a072645. [DOI] [PubMed] [Google Scholar]
- 43.Kong Q., Surewicz W.K., Montagna P. Inherited prion diseases. In: Prusiner S.B., editor. Prion Diseases and Biology. Cold Spring Harbor Laboratory Press; 2008. pp. 673–775. [Google Scholar]
- 44.Mead S. Prion disease genetics. Eur. J. Hum. Genet. 2006;14:273–281. doi: 10.1038/sj.ejhg.5201544. [DOI] [PubMed] [Google Scholar]
- 45.Borsari M. Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons, Ltd.; 2014. Cadmium: coordination chemistry; pp. 1–16. [Google Scholar]
- 46.Pushie M.J., Nienaber K.H., George G.N. Combined EXAFS and DFT structure calculations provide structural insights into the 1:1 multi-histidine complexes of Cu(II), Cu(I), and Zn(II) with the tandem octarepeats of the mammalian prion protein. Chemistry. 2014;20:9770–9783. doi: 10.1002/chem.201304201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stellato F., Minicozzi V., Morante S. Copper-zinc cross-modulation in prion protein binding. Eur. Biophys. J. 2014;43:631–642. doi: 10.1007/s00249-014-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stellato F., Spevacek A., Morante S. Zinc modulates copper coordination mode in prion protein octa-repeat subdomains. Eur. Biophys. J. 2011;40:1259–1270. doi: 10.1007/s00249-011-0713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minikel E.V., Vallabh S.M., MacArthur D.G. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 2016;8:322ra9. doi: 10.1126/scitranslmed.aad5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae S.H., Legname G., Dyson H.J. Prion proteins with pathogenic and protective mutations show similar structure and dynamics. Biochemistry. 2009;48:8120–8128. doi: 10.1021/bi900923b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liemann S., Glockshuber R. Influence of amino acid substitutions related to inherited human prion diseases on the thermodynamic stability of the cellular prion protein. Biochemistry. 1999;38:3258–3267. doi: 10.1021/bi982714g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.