Abstract
The transition from childhood to adolescence is marked by increasingly sophisticated social cognitive abilities that are paralleled by significant functional maturation of the brain. However, the role of social and neurobiological development in facilitating age differences in prosocial behavior remains unclear. Using a cross-sectional sample of children and adolescents (n = 51; 8–16 years), we examined the age-related correlates of prosocial behavior. Youth made costly and non-costly prosocial decisions to anonymous peers during a functional magnetic resonance imaging scan. Among a subsample of youth who made prosocial decisions (n = 35), we found quadratic age differences in neural activation that peaked in early adolescence relative to childhood and older adolescence. In particular, early adolescents showed heightened recruitment of the posterior superior temporal sulcus (pSTS), temporal pole and inferior frontal gyrus (IFG) when engaging in costly prosocial behavior at the expense of gaining a reward, whereas they evoked heightened pSTS and dorsolateral prefrontal cortex/IFG activation when engaging in costly vs non-costly forms of prosocial behavior. Given that we did not find age differences in prosocial behavior, this suggests that early adolescents show unique patterns of brain activation to inform similar levels of prosocial behavior.
Keywords: prosocial behavior, pSTS, fMRI, childhood, adolescence
Introduction
Engagement in prosocial behavior, or voluntary actions intended to benefit others, is associated with a wide range of positive social and health outcomes during childhood and adolescence (e.g. Eisenberg et al., 2006). Mentalizing, or the ability to understand the mental states of oneself or others, is considered an important contributor to several types of prosocial behaviors, including giving and sharing (Eisenberg and Miller, 1987; McDonald and Messinger, 2011). Recent studies have begun to uncover the neurobiological mechanisms that promote prosocial behaviors across development (Telzer et al., 2011; van den Bos et al., 2011; Tusche et al., 2016). However, there is little convergence from prior literature on whether the development of prosocial behavior from childhood to adolescence increases linearly (e.g. increasing from childhood to adolescence) or non-linearly (e.g. peaking in early adolescence). A better understanding of the neural correlates of prosocial decision-making may help resolve discrepancies in the behavioral research, especially given that there is significant structural (Mills et al., 2014) and functional (Burnett and Blakemore, 2009; Burnett et al., 2011; Klapwijk et al., 2013) maturation within neural circuits that support social cognition (e.g. mentalizing) and prosocial decision-making during this developmental window. Thus, the current study combined behavioral and neuroimaging methods to better interrogate age-related differences in the behavioral and neural correlates of prosocial behavior from childhood to adolescence. Delineating the specific age-related patterns is important in identifying potential sensitive periods in promoting other-oriented considerations and behaviors.
The developmental psychology and cognitive neuroscience literature converge on two possible patterns of prosocial development from childhood to adolescence: (i) linear increases in prosocial behavior from childhood to adolescence and (ii) non-linear differences, marked by peaks in prosocial behavior in early adolescence. One line of research has shown that early adolescents exhibit more prosocial behavior than children (Eisenberg and Fabes, 1998; Fabes et al., 1999), suggesting prosocial behavior may increase linearly with age. Linear age patterns of prosocial development suggest prosocial tendencies may develop and continue to increase from childhood to adulthood, likely due to improvements in social-cognitive skills that facilitate other-oriented considerations. Yet, another line of work has found differences even within the adolescent years, with greater prosocial tendencies observed in early adolescents compared to late adolescents (Carlo et al., 2007; Güroğlu et al., 2014b; Meuwese et al., 2015; van Hoorn et al., 2016). These latter results provide initial evidence of non-linear (i.e. quadratic) age-related patterns in prosocial development, such that early adolescents engage more frequently in prosocial behaviors than children and mid-to-late adolescents. Non-linear age patterns of prosocial development suggest that early adolescence is a particularly sensitive window for prosocial development because of unique developmental processes that have yet to converge at earlier or later ages. Taken together, these data highlight the need to more clearly delineate between linear and non-linear age differences to better understand prosocial development from childhood to adolescence.
Emerging research has delineated the neural correlates involved in prosocial decision-making in youth. A network of the brain involved in mentalizing, comprising of the posterior superior temporal sulcus (pSTS), temporoparietal junction (TPJ), dorsomedial and medial prefrontal cortices (dmPFC, mPFC) and temporal pole, is correlated with youths’ prosocial decisions (see Do et al., 2017 for a review; van Hoorn et al., 2016), as well as perspective taking and empathy abilities (Masten et al., 2010; Kral et al., 2017). A recent study reported age-related differences in the recruitment of the mentalizing network during prosocial decision-making (van Hoorn et al., 2016), such that early adolescents show greater dmPFC and pSTS activation when making prosocial decisions in the presence of anonymous peers compared to late adolescents (van Hoorn et al., 2016). These results support non-linear developmental patterns in the brain that peak during early adolescence, which is perhaps reflective of the ongoing maturation of social cognitive skills during this developmental window (Dumontheil et al., 2010; van den Bos et al., 2010; Overgaauw et al., 2014). Furthermore, prior work has shown that significant changes in perspective-taking abilities mediate the relation between age and prosocial behavior in children and adolescents (Güroğlu et al., 2014a). Therefore, neural reactivity in regions that support social cognition may critically underlie other-focused, prosocial behavior, which changes across childhood and adolescence.
Current study
Although prior research suggests there are developmental differences in the neural correlates of prosocial decision-making, these studies have focused exclusively on either adolescents (Telzer et al., 2013, 2014; van Hoorn et al., 2016; Will et al., 2018) or adults (Telzer et al., 2015; van der Meulen et al., 2016) and have not included child participants nor a wider age range to test for age-related associations across childhood and adolescence. In the current study, we used a cross-sectional design to examine the behavioral and neural correlates of prosocial decision-making in youth from ages 8–16 years. While there are several types of prosocial behaviors, including sharing or group cooperation (Eisenberg et al., 2006; Padilla-Walker and Carlo, 2007), we operationalized prosocial behavior as the act of giving rewards to another individual. During functional magnetic resonance imaging (fMRI), participants made two types of prosocial decisions on an experimental task: (i) give points to anonymous peers at a cost to themselves (costly prosocial behavior) or (ii) give points to anonymous peers at no cost to themselves (non-costly prosocial behavior). The first goal was to delineate the neural correlates involved in engaging in costly prosocial behavior compared to receiving rewards for oneself (i.e. keeping points for oneself) or engaging in non-costly types of prosocial behavior. The second goal was to test for both linear and quadratic age associations with prosocial behavior at the behavioral and neural level, independent of the frequency of prosocial behavior. The third goal was to examine whether neural activation to prosocial behavior predicts individual differences in the frequency of prosocial behavior, independent of age.
Methods
Participants
Fifty-six healthy children and adolescents were recruited from community advertisements. All participants were right-handed and free of MRI contraindications (e.g. braces). Five participants were excluded [n = 1 due to excessive motion (>2 mm in any direction), n = 3 due to technical errors, n = 1 did not complete scan]. The final sample included 51 children and adolescents between ages 8–16 years (M = 13.55, s.d. = 2.72; 28 females). Parent reports of children’s ethnicity or race were comprised of 76.50% European American, 3.90% African American, 3.90% Asian American, 3.90% Hispanic or Latino and 11.80% Multi-ethnic. Annual family income was categorized into five bins: 10.7%, <$14 999–$29 999; 27.7%, $30 000–$59 999; 10.7%, $60 000–$89 999; 31.9%, $90 000–$119 999; and 19.2%, $120 000–$150 000+. Parent reports of maximum educational attainment (maternal | paternal) were comprised of 3.9% | 6.4% less than high school diploma, 6.1% | 12.8% high school diploma, 20.4% | 6.4% some college, 14.3% | 12.8% associate’s degree, 32.7% | 27.7% bachelor’s degree, 0% | 2.1% some graduate school, 18.4% | 27.7% master’s degree (M.A., M.S.) and 4.1% | 4.3% professional degree (e.g. M.D., Ph.D.). As described below, a subsample was included in the fMRI analyses of prosocial behavior, which included 35 children and adolescents between 8–16 years (M = 13.71, s.d. = 2.65; 20 females). There were no significant differences between the included and excluded participants for age, gender, ethnicity, annual family income or parental education. Parents and youth provided written consent and assent, respectively, in accordance with the university’s Institutional Review Board guidelines.
Costly giving fMRI task
We used a costly giving task from previous work on prosocial decision-making in adolescents (Telzer et al., 2013, 2014, 2015) that was modified to be more child-appropriate. Participants were informed that they would be playing a game in which their decisions would impact peers who previously participated in the study. In reality, the peers featured in the task were confederates. Participants saw pictures of the previous participants, whose pictures were from several databases (e.g. National Institute of Mental Health Child Emotional Faces Picture Set) (Egger et al., 2011) that were equally distributed by age (range: 7–17 years), gender and race (White, Black and Asian). On each trial, participants viewed two pictures of themselves and/or other peers and made decisions to ‘keep’ the points shown above one picture or ‘share’ (i.e. give) the points shown above the other picture by pressing their right or left index finger, respectively (Figure 1). To control for motor-related neural activation and heuristic responding, the location of the pictures and response options were counterbalanced on the left or right of the screen across trials. The point values associated with each decision (i.e. Keep or Share) ranged from 1–10 to create variability in responses and decrease heuristic responding. Participants were instructed that the total points kept for themselves or given to other peers during the task could be exchanged for non-monetary prizes (e.g. candy) in real life. Notably, participants were informed that the other players would not know what their decisions were. No information was provided on how the peers would receive points that were given to them during the task or if/how they would receive points that were ostensibly given to them from previous participants. Participants were not told that future participants would play the game, so reciprocity or anticipation of future earnings was not expected. Participants were not shown a running total of their own or peers’ earned points.
Fig. 1.
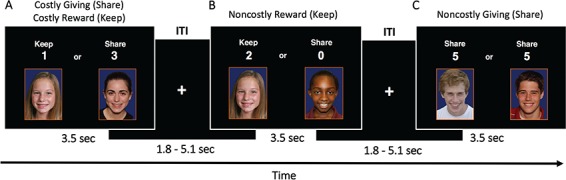
Schematic representation of Costly Giving fMRI task. There were four trial types of interest based on the type of decision made on the Costly Giving fMRI task: Costly Giving, Costly Reward, Non-costly Reward and Non-costly Giving. The choice yoked to each trial type is indicated in parentheses. On Costly Giving trials, participants chose to forgo personal gains to give points to an unknown peer. On Costly Reward trials, participants chose to keep points for oneself at the expense of another peer gaining greater rewards. On Non-costly Reward trials, participants chose to keep points for oneself at no cost or benefit to another peer. On Non-costly Giving trials, participants chose between one of two unknown peers to give points to at no cost to their own gains (i.e. no option to keep points for oneself). ITI = inter-trial interval.
Four trials of interest were defined based on the type of decisions that participants made on the task: Costly Giving, Costly Reward, Non-costly Giving and Non-costly Reward. On Costly Giving trials, participants gave a higher amount of points to another peer at the expense of gaining fewer points for themselves (Figure 1A). On Costly Reward trials, participants kept a lower amount of points for themselves at the expense of another person gaining more points (Figure 1A). On Non-costly Reward trials, participants kept a higher amount of points for themselves over giving zero points to a peer, thereby measuring the receipt of a personal reward when there was no benefit or cost to others (Figure 1B). On Non-costly Giving trials, participants chose between one of two peers to give points to (i.e. there was no option to keep points for themselves) (Figure 1C). Across two functional runs, participants completed 60 Costly trials (which for analyses are divided into Costly Giving and Costly Reward based on their decisions), 30 Non-costly Giving trials and 30 Non-costly Reward trials, which were presented in a randomized order. Following previous research (Telzer et al., 2011), more costly trials were administered since responses on non-costly trials are highly consistent (i.e. participants often choose to give on Non-costly Giving trials and keep on Non-costly Reward trials) relative to costly trials, which show greater response variability and are further divided into two trials types based on their decision. The stimulus event (i.e. faces and decisions) was presented for 3.5 s followed by a jittered inter-trial interval (ITI), which displayed a fixation cross for a range of 1.8–5.1 s.
Our primary interest was examining the unique psychological and neurobiological processes associated with Costly Giving decisions, which involve a conflict between gaining a personal reward and giving a reward to another individual and thus reflect prosocial behavior motivated by more other-focused concerns. Specifically, we conducted three comparisons to delineate how Costly Giving is unique from the receipt of personal rewards or engagement in non-costly types of prosocial behavior: (i) Costly Giving > Costly Reward, (ii) Costly Giving > Non-costly Reward and (iii) Costly Giving >Non-costly Giving. First, we examined differences between Costly Giving and Costly Reward decisions, as Costly Giving decisions tend to be more difficult and altruistic than Costly Reward decisions, which instead prioritize personal gains at the expense of potential gains for others. Second, we contrasted how Costly Giving decisions differ from gaining a personal reward at no cost to others’ outcomes (i.e. Non-costly Reward), which is consistent with prior work examining differences between eudaimonic, prosocial choices and hedonic, personally rewarding choices (Moll et al., 2006; Telzer, Masten et al., 2010; Telzer et al., 2011; Telzer et al., 2014). Finally, we examined differences between costly and non-costly prosocial decisions in order to isolate psychological processes related to prosocial behavior with and without an autonomous, costly component, respectively. Compared to Costly Giving decisions, Non-costly Giving decisions involve involuntarily giving to others without the option to keep rewards for oneself, thereby eliciting prosocial behavior that is neither autonomous nor self-sacrificing because outcomes do not impact one’s own gains.
fMRI data acquisition and analysis
Imaging data were collected with a 3 Tesla Siemens Trio MRI scanner. The task included T2*-weighted echoplanar images [EPI; slice thickness = 3 mm, 38 slices, repetition time (TR) = 2 s]; [echo time (TE) = 25 ms, matrix = 92 × 92, field of view (FOV) = 230 mm, voxel size = 2.5 × 2.5 × 3 mm3]. A T1*-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) scan (TR = 2.3 s, TE = 2.1 ms, FOV = 256, matrix = 192 × 192, sagittal plane, slice thickness = 1 mm, 160 slices) and a T2-weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4 s, TE = 63 ms, FOV = 230, matrix = 256 × 256, sagittal plane, slice thickness = 1 mm, 187 slices) were acquired for registration purposes. EPI and MBW scans were acquired at an oblique axial orientation in order to maximize brain coverage and reduce signal dropout.
Neuroimaging data were pre-processed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Pre-processing included spatial realignment to correct for head motion and co-registration of all images to the high-resolution MPRAGE structural scan, which was then segmented into gray matter, white matter and cerebrospinal fluid. The normalization transformation matrices were applied to the functional and MBW structural images to transform them into standard stereotactic space as defined by the Montreal Neurological Institute (MNI) and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8 mm full-width-at-half maximum Gaussian kernel to increase the signal-to-noise ratio. Each trial was convolved with a canonical hemodynamic response function and filtered in the temporal domain using a non-linear high-pass filter (128 s cutoff) to remove low-frequency drift across the time series. A restricted maximum likelihood algorithm with an autoregressive model order of 1 was used to estimate serial autocorrelations.
Individual level, fixed-effects analyses were conducted using the general linear model (GLM) in SPM8. The task was modeled as an event-related design, with each trial’s onset defined as the presentation of the face stimuli and duration defined as the reaction time of the decision. The events of interest included (i) giving at the expense of gaining a reward (Costly Giving), (ii) gaining a reward at the expense of giving to another individual (Costly Reward), (iii) gaining a reward at no cost to another individual’s gains (Non-costly Reward) and (iv) giving at no expense to one’s gains (Non-costly Giving). All events were modeled with a parametric regressor [i.e. parametric modulation (PM)] to control for the magnitude of the points that could be given/kept across trials, which may reflect decisions motivated by a desire to maximize the total points awarded to whoever stood to gain the most (participant or peer confederate; i.e. utility maximization) instead of truly prosocial considerations. Thus, neural responses observed in the event of interest (e.g. Costly Giving) controlled for neural responses that tracked linear increases in utility maximization. The PM was the trial-level difference between the amount of points that could be given vs kept on Costly Giving and Costly Reward trials (range: 1–5 point difference), the amount of points that were given to another peer on Non-costly Giving trials (as points that could be given were equal between the two decisions) and the amount of points that were kept for oneself on Non-costly Reward trials (as points that could be given were always zero). Point values were mean centered within each event type for each individual. Decisions to give points when there was no benefit to a peer on Non-costly Reward trials were modeled separately as covariates of no interest and were excluded from further analyses. The rest periods and jittered ITIs were not explicitly modeled and therefore served as an implicit baseline. Six motion parameters were modeled as regressors of no interest. Using the parameter estimates from the GLM, linear contrast images comparing each of the conditions of interest were calculated for each individual.
Individual subject contrasts were then submitted to random-effects, group-level analyses in GLMFlex, which corrects for variance–covariance inequality, partitions error terms, removes outliers and sudden activation changes in the brain and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). To correct for multiple comparisons, we conducted a Monte Carlo simulation using the updated version (April 2016) of 3dFWHMx and 3dClustSim programs from the AFNI software package (Ward, 2000). We submitted the group residuals generated from GLMFlex’s random-effects, group-level analysis to the 3dFWHMx program to calculate the spatial group smoothness, assuming an auto-correlation function (−acf). This approach is notably similar to cluster correction methods used in SPM and FMRIB Software Library (FSL) and thus deviates from Analysis of Functional Neuroimaging (AFNI’s) default method of estimating the group smoothness using the residuals from first-level analyses. We entered the estimated group smoothness parameters into the 3dClustSim program, which conducted 10,000 Monte Carlo simulations on each contrast’s group-level brain mask using a voxel-wise threshold of P < 0.001, corresponding to P < 0.05, Family-Wise Error corrected, to determine the minimum cluster size for the whole brain (range: 20–23 contiguous voxels).
To investigate the neural correlates of prosocial behavior, group-level analyses were constrained to participants who had sufficient rates of prosocial decisions on the Costly Giving condition (n = 35). To ensure we had adequate statistical power in the individual subject GLM, 16 participants were excluded from fMRI analyses due to low rates of costly giving decisions (n = 8 never made a costly giving decision and n = 8 made <4 costly giving decisions in a single run of the task). Given that there were no demographic differences between the two sets of participants, we used this conservative exclusion criterion to ensure that there were enough trials per condition to appropriately examine the neural correlates of prosocial decision-making among youth who actually engaged in these behaviors. Subject-specific data on the frequency of prosocial decisions in the full (n = 51) and reduced (n = 35) samples are reported in Supplementary Figure 1.
At the group level, the first aim was to contrast neural responses to costly prosocial behavior relative to other types of reward- or prosocial-related decisions, controlling for utility maximization. Whole-brain t-tests were conducted to examine the main effects of Costly Giving decisions against the main effects of (i) gaining a personal reward over engaging in prosocial behavior (Costly Giving > Costly Reward), (ii) gaining a personal reward at no cost or benefit to another individual (Costly Giving > Non-costly Reward) and (iii) engaging in involuntary non-costly prosocial behavior (Costly Giving > Non-costly Giving). The second aim was to delineate age-related associations in neural activation during prosocial decision-making, controlling for differences in utility maximization and the frequency of prosocial behavior. We conducted whole-brain regression analyses correlating linear and quadratic age regressors with neural activation during the Costly Giving > Costly Reward, Costly Giving > Non-costly Reward and Costly Giving > Non-costly Giving contrasts, controlling for utility maximization and frequency of Costly Giving. The linear age predictor was a linear age variable used to detect differences with increasing age, and the quadratic age predictor was calculated by squaring age in order to detect non-linear age differences. All regression analyses using the quadratic age predictor controlled for the linear age predictor. Finally, the third aim was to investigate whether neural activation during costly prosocial decision-making covaried with the frequency of costly prosocial behavior, above and beyond differences in utility maximization and age. We conducted whole-brain regression analyses correlating the frequency of Costly Giving with neural activation during Costly Giving > Costly Reward decisions, controlling for utility maximization and linear and quadratic age. Given the participants’ highly consistent responses on non-costly trials (‘Give’ on Non-costly Giving trials: M = 100%, s.d. = 0%; ‘Keep’ on Non-costly Reward trials: M = 88.54%, s.d. = 21.05%), associations between the frequency of costly giving and the Costly Giving > Non-costly Giving and Costly Giving > Non-costly Reward contrasts were not expected to be of interest and thus were not analyzed. All reported fMRI results can be found on Neurovault: https://neurovault.org/collections/ZUSAPCBB/.
Results
Behavioral results
Descriptive statistics, including correlations between all study variables, are reported in Supplementary Table 1. Given participants’ highly consistent responses on non-costly trials, behavioral analyses of prosocial behavior were constrained to Costly Giving and Costly Reward trials (60 total); mean-level differences in the frequency and reaction time (RT) of the other trial types are reported in the Supplementary Materials. A series of generalized linear mixed models were estimated in SAS 9.4 to investigate the relation between RT, age and gender on youths’ propensity to give in the full sample (n = 51). Trials (Level 1; mean centered within participants) were nested within participants (Level 2; mean centered across participants), with the outcome variable defined as the binary decision to give vs keep on each trial (1 = give, 0 = keep). Likelihood ratio tests were conducted to determine whether the addition of trial- and person-level variables significantly improved the model fit (Raudenbush, 2004). All models included a random intercept and controlled for the main effect of gender (1 = female, 0 = male) and RT × gender interaction. For interpretation, unstandardized model estimates have been converted from logits to odds ratio (OR).
In Model 1, we tested the within- and between-person effect of RT on youths’ propensity to give on Costly Giving and Costly Reward trials. The RT of each trial’s decision (Level 1) significantly predicted youths’ propensity to give (b = 1.181, SE = 0.103, 95% confidence intervals (CI) [0.979, 1.382]; OR = 3.257, 95% CI [2.662, 3.984]), such that slower RT on a costly trial was associated with a greater likelihood of giving. The mean RT (MRT) of each participant (Level 2) also significantly predicted youths’ propensity to give (b = 2.400, SE = 0.798, 95% CI [0.796, 4.003]; OR = 11.023, 95% CI [2.217, 54.796]), such that an average participant with a slower average RT on costly trials showed a greater likelihood of giving. A log likelihood ratio test confirmed that the inclusion of the within- and between-person effects of RT significantly improved model fit from the null model (i.e. random intercept with no predictors), χ2(2) = 151.02, P < 0.000.
Next, we added the main and interaction effects of linear age (Model 2) and quadratic age (Model 3) to the generalized linear mixed model to examine whether age explained additional variance in the propensity to give above RT differences, controlling for gender. The linear and quadratic terms of age did not significantly predict the propensity to give, nor did their respective interactions with trial-level RT. There were also no main or interaction effects of gender on the propensity to give. A log likelihood ratio test indicated that the inclusion of age (linear, quadratic) and gender did not improve model fit, thus Model 1 was retained.
fMRI results
Neural activation to Costly Giving > Costly Reward decisions
Main effects. No regions showed significant differences to the Costly Giving > Costly Reward or the inverse contrast.
Age effects. There was no significant linear age effect on neural activation to Costly Giving > Costly Reward decisions or the inverse contrast (Table 2). There was a negative correlation between the quadratic regressor of age and several regions implicated in social processing, including the bilateral pSTS, left temporal pole, right inferior frontal gyrus (IFG) and right cuneus (Table 2). For descriptive purposes, we extracted parameter estimates of signal intensity from the regions that showed a quadratic age effect and plotted the patterns of activation against age. Neural activation in the pSTS, temporal pole, IFG and cuneus show an inverted U-shaped curve (Figure 2), such that early adolescents, compared to children and mid-adolescents, showed relatively heightened activation in these brain regions when making costly prosocial decisions vs making costly reward decisions.
Table 2.
Linear and quadratic effects of age on the neural correlates of costly prosocial behavior
| Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Age (Linear) | ||||||
| Costly Giving > Costly Reward a | ||||||
| None | ||||||
| Costly Giving > Non-costly Reward | ||||||
| None | ||||||
| Costly Giving > Non-costly Giving | ||||||
| R dmPFC | 9 | 21 | 56 | 34 | −5.75 | 192 |
| Age 2 (Quadratic) | ||||||
| Costly Giving > Costly Reward a | ||||||
| R pSTS | 21 | 54 | −37 | 4 | −4.97 | 223 |
| L temporal pole | 22 | −54 | 5 | −8 | −4.97 | 58 |
| R cuneus | 18 | 3 | −64 | 10 | −4.22 | 27 |
| L pSTS | 39 | −63 | −49 | 10 | −4.51 | 23 |
| R IFG | 44 | 57 | 20 | 16 | −3.81 | 21 |
| Costly Giving > Non-costly Reward | ||||||
| None | ||||||
| Costly Giving > Non-costly Giving | ||||||
| R pSTS | 21/22 | 63 | −28 | 4 | −4.35 | 51 |
| L dlPFC/IFG | 46 | −36 | 35 | 13 | −4.33 | 42 |
| L pSTS | 21/22 | −51 | −37 | 10 | −4.75 | 32 |
L and R refer to left and right hemispheres; BA refers to Brodmann area of peak voxel; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster, such that positive values indicate a positive association between age and neural activation to Condition A > Condition B and negative values indicate a negative association between age and neural activation to Condition A > Condition B; and x, y and z refer to MNI coordinates. fMRI results are reported at P < 0.001, with a corrected cluster size ranging from 20–23 contiguous voxels. aGiven n = 1 made <4 costly reward decisions in a single run of the task, group-level comparisons with Costly Reward trials were conducted in n = 34 from the subsample of youth who had sufficient rates of prosocial behavior on the task (n = 35).
Fig. 2.
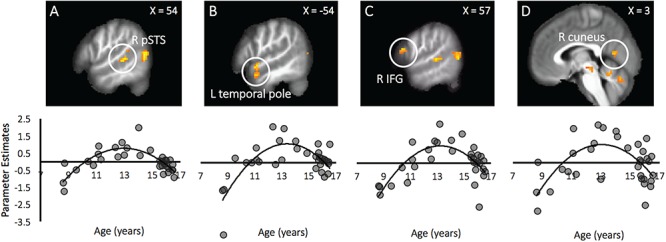
Age differences in neural responses to costly giving vs costly reward decisions. There were quadratic age differences in the brain regions supporting decisions to forgo personal rewards to be prosocial to another peer from childhood to adolescence (Costly Giving > Costly Reward). Compared to children and mid-adolescents, early adolescents exhibited relatively greater activation in the (A) pSTS, (B) temporal pole, (C) IFG and (D) cuneus during costly prosocial behavior.
Neural activation to Costly Giving > Non-costly Reward decisions
Main effects. No regions showed differences in activation during Costly Giving relative to Non-costly Reward decisions or vice versa.
Age effects. There were no linear or quadratic age differences on the neural correlates of costly giving relative to non-costly reward decisions (Table 2).
Neural activation to Costly Giving > Non-costly Giving decisions
Main effects. Youth exhibited greater activation in the inferior temporal gyrus and precuneus during costly prosocial behavior relative to non-costly prosocial behavior (Table 1). No regions showed differences during non-costly relative to costly prosocial behavior.
Table 1.
Summary of whole-brain activation during costly prosocial behavior
| Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Costly Giving > Costly Reward a | ||||||
| None | ||||||
| Costly Giving > Non-costly Reward | ||||||
| None | ||||||
| Costly Giving > Non-costly Giving | ||||||
| L inferior temporal gyrus | 37 | −51 | −58 | −23 | −4.08 | 32 |
| L precuneus | 0 | −58 | 22 | −4.25 | 26 |
L and R refer to left and right hemispheres; BA refers to Brodmann area of peak voxel; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster, such that positive values indicate greater activation to Condition A > Condition B and negative values indicate less activation to Condition A > Condition B (or greater activation to Condition B > Condition A); and x, y and z refer to MNI coordinates. fMRI results are reported at P < 0.001, with a corrected cluster size ranging from 20–23 contiguous voxels. aGiven n = 1 made <4 costly reward decisions in a single run of the task, group-level comparisons with Costly Reward trials were conducted in n = 34 from the subsample of youth who had sufficient rates of prosocial behavior on the task.
Age effects. Linear age was negatively associated with activation in the dmPFC during Costly Giving > Non-costly Giving decisions (Table 2), such that there were age-related decreases in the dmPFC from childhood to adolescence during costly giving vs non-costly giving. Quadratic age was negatively correlated with activation in the bilateral pSTS and left IFG/dorsolateral prefrontal cortex (dlPFC; Table 2). For descriptive purposes, we extracted parameter estimates of signal intensity from significant brain regions during Costly Giving > Non-costly Giving and plotted the patterns of activation against age (Figure 3). Early adolescents exhibited relatively heightened activation in the pSTS and IFG/dlPFC when engaging in costly prosocial behavior compared to non-costly prosocial behavior.
Fig. 3.
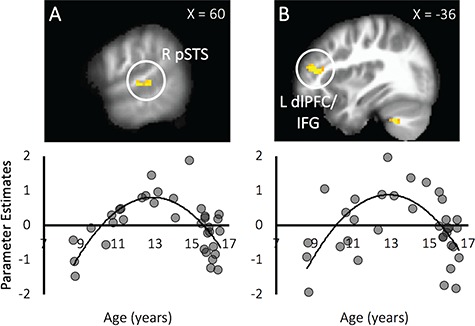
Age differences in neural responses to costly vs non-costly giving decisions. There were quadratic age associations in neural reactivity to costly vs non-costly prosocial behavior that peaked during early adolescence, such that early adolescents recruited relatively greater activation in the (A) pSTS and (B) dlPFC/IFG than children and mid-adolescents.
Associations between neural activation to prosocial decisions and frequency of prosocial behavior
Finally, we tested whether neural activation during costly prosocial decisions was associated with individual differences in the frequency of costly giving, independent of age or utility maximization. Greater frequency of costly giving was negatively correlated with dorsal anterior cingulate cortex (dACC) activation (xyz = 0, 35, 19, t = −5.036, k = 45; P < 0.001: 21 contiguous voxels) during Costly Giving > Costly Reward decisions, controlling for linear and quadratic age. For descriptive purposes, we extracted parameter estimates of signal intensity from the dACC in response to Costly Giving > Costly Reward and plotted the patterns of activation against the frequency of costly giving. Controlling for age, youth who exhibited less dACC activation when choosing to forgo gaining personal rewards to give rewards to another individual engaged in more costly prosocial behavior on the task (Figure 4).
Fig. 4.
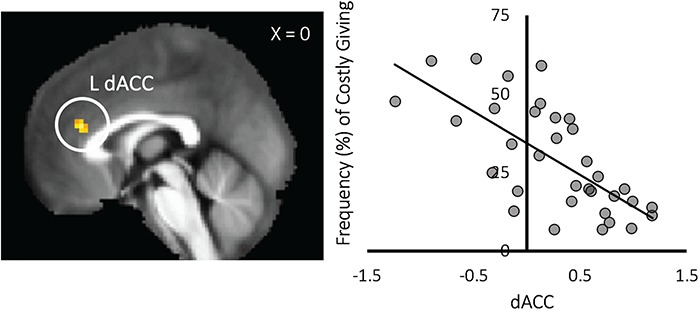
Relation between frequency of costly giving and dACC activation to costly giving vs costly reward decisions. Controlling for age, participants who exhibited less activation in the dACC engaged in higher rates of costly prosocial behavior.
Discussion
The present study addressed three aims: (i) examine neurobiological differences when youth engaged in costly and non-costly forms of prosocial behaviors, (ii) elucidate the age-related correlates of prosocial decision-making from childhood to adolescence and (iii) examine associations between neural responses and the frequency of prosocial behavior, independent of age. Although there were largely no differences in neural reactivity to costly giving relative to the other decision types at the main effect level, we found quadratic age differences in the neural correlates of costly prosocial behavior, with relative peaks in activation during early adolescence compared to childhood and mid-adolescence. Not only did early adolescents exhibit greater activation in social-cognitive regions (pSTS, temporal pole, IFG) when engaging in costly prosocial behaviors over the receipt of costly rewards, but they also exhibited greater activation in social-cognitive (pSTS) and regulatory (IFG/dlPFC) regions when making costly relative to non-costly prosocial decisions. To our knowledge, this is the first study to investigate age-related differences in the neural correlates of prosocial decision-making across a wide developmental period spanning childhood and adolescence, providing converging evidence for quadratic age-related differences in the brain. Moreover, regardless of age, youth who exhibited less dACC reactivity during costly prosocial behaviors engaged in higher rates of costly giving, suggesting that brain regions involved in affective processing or conflict monitoring may be critically involved in youths’ overall propensity to give.
Age-related correlates of prosocial behavior
Although there were no age-related differences in behavior, we found quadratic age associations in the neural correlates underlying costly prosocial decisions, which peaked in early adolescence relative to childhood and mid-adolescence. The frequency and MRT of costly prosocial decisions did not differ by age in the current study, which is consistent with prior experimental research on costly vs non-costly prosocial behavior in youth (Gummerum et al., 2008; Güroğlu et al., 2009; Güroğlu et al., 2014a). Despite similar rates of costly prosocial behavior across age, early adolescents make prosocial decisions differently than children and late adolescents, as evidenced by greater recruitment of the pSTS, temporal pole and IFG when engaging in costly prosocial behavior. In other words, when engaging in the same rate of prosocial behavior as their younger and older peers (and controlling for utility maximization), early adolescents demonstrate uniquely heightened activation in brain regions involved in social-cognitive and regulatory processing.
Developmental changes in social relationships and social cognition that are thought to converge during early adolescence may contribute to non-linear patterns of sociocognitive brain responses during prosocial decision-making. Relative to childhood and mid-adolescence, early adolescence is characterized by a significant shift in the importance and amount of time spent with family members to peers, which is associated with greater sensitivity to peer evaluation and increasingly advanced social-cognitive abilities (Steinberg and Morris, 2001; Brown, 2004; Somerville et al., 2013; Blakemore and Mills, 2014). Prosocial behaviors and other-oriented social skills (e.g. mentalizing) are instrumental for establishing positive peer relationships and maintaining peer acceptance from an early age (Lejuez et al., 2003; Fink et al., 2014; Slaughter et al., 2015; Will et al., 2018). Heightened recruitment of regions involved in social-cognitive and regulatory processing to prosocial behavior could provide a mechanism by which positive, prosocial behaviors are impacted by implicit peer influences (Somerville et al., 2018). Specifically, developmental shifts in the desire to establish or maintain peer acceptance may be related to increases in neural responses during prosocial decision-making from childhood to early adolescence but declines after this transition, when youth establish more independence from peers (and family) and become more autonomous decision-makers.
Costly Giving > Costly Reward decisions. At the neural level, we found quadratic age differences in the pSTS, temporal pole, IFG and cuneus during costly prosocial behavior relative to gaining a personal reward (Costly Giving > Costly Reward), which peaked in early adolescence relative to childhood and mid-adolescence. Decisions to engage in costly prosocial behavior are often complex and difficult because they require individuals to weigh other-oriented concerns against more selfish inclinations. Although we did not have a priori expectations about the role of the cuneus, previous research has implicated brain regions associated with social cognition, including the pSTS, temporal pole and IFG, in prosocial decision-making (van Hoorn et al., 2016; Will et al., 2018). In particular, the pSTS and temporal pole are involved in mentalizing about others’ intentions, emotions and beliefs (Saxe et al., 2004; Blakemore et al., 2007; Pfeifer et al., 2009; Blakemore and Mills, 2014) and empathic processing (Lamm and Singer, 2010; Morelli et al., 2014). Relative to other regions in the social brain network, the pSTS uniquely serves as a central hub for social information processing, which includes social perception, action observation and theory of mind (Yang et al., 2015) and has been associated with greater self-reported altruism in adults (Tankersley et al., 2007). Thus, the pSTS may be crucially activated when integrating the multiple social cognitive processes (e.g. mentalizing) inherent in costly prosocial behavior, a process that might be exaggerated during early adolescence due to developmental peaks in self-conscious emotions and sensitivity to peer evaluation (Somerville et al., 2013).
In addition to the pSTS and temporal pole, the IFG showed early adolescent peaks during costly prosocial relative to costly reward decisions. The IFG is important for both the representation of others’ actions (Iacoboni, 2009) and cognitive control (Aron et al., 2004). Our results suggest that the extent to which the IFG is activated when making costly prosocial decisions differentially motivates youth toward prosocial actions, with early adolescents more likely to recruit this region during prosocial decisions compared to children and mid-adolescents. Indeed, IFG reactivity to emotional faces explains the link between empathic concern in late childhood and subsequent prosocial behavior in early adolescence (Flournoy et al., 2016), underscoring the importance of engaging the IFG early in life in order to sustain or increase prosocial behaviors across development. Taken together, these results build on prior research showing regions implicated in social cognitive processing differentially support costly prosocial engagement from childhood to adolescence.
Costly Giving > Non-costly Giving decisions. Similar quadratic age-related differences were found in the pSTS and IFG/dlPFC during costly prosocial behavior compared to non-costly prosocial behavior, with peaks in early adolescence relative to childhood and mid-adolescence. Prosocial behaviors involving a personal cost (e.g. loss of potential gains) evoked greater activation in brain regions that support social-affective processing (pSTS) and cognitive regulation (IFG/dlPFC) compared to engaging in non-costly prosocial behaviors that have little effect on one’s self-interests. Given the need to voluntarily sacrifice one’s self-interests to help another individual, costly prosocial behaviors may require that early adolescents—more so than children or mid-adolescents—regulate their self-interests and consider others’ interests to a greater extent than when engaging in non-costly prosocial behaviors (e.g. sharing public goods). There was only one linear age effect, such that dmPFC showed age-related decreases during costly giving vs non-costly giving from childhood to adolescence. Given all but one analysis showed quadratic age associations, these findings collectively suggest that social-affective and regulatory brain systems are important for the deliberation of more autonomous and costly forms of prosocial behavior, with exaggerated effects during early adolescence compared to childhood and mid-adolescence.
dACC activation predicts frequency of giving
Independent of age, the frequency of costly giving behavior was negatively correlated with dACC activation during Costly Giving > Costly Reward decisions among youth who made costly prosocial decisions. Youth who engaged in higher rates of costly giving behavior exhibited less dACC activation when forgoing personal gains in order to be prosocial to another individual. Previous research has implicated the dACC in conflict monitoring (Carter et al., 1998) and the social pain that results from rejection or empathy for negative emotions (Morelli et al., 2014; Rotge et al., 2015). Thus, neural regions associated with conflict monitoring or unpleasant social emotions can moderate youths’ proclivity toward prosocial engagement. Perhaps having less conflicting emotions about the discrepancy between self-other rewards facilitates engagement in costly prosocial behavior, though future research should test this hypothesis.
Limitations
A few limitations should be noted. First, we used a cross-sectional design in order to examine the behavioral and neural development of prosocial behavior from childhood through adolescence. While testing for non-linear age associations in cross-sectional data can start to address inconsistencies in prosocial development found in prior research (Do et al., 2016), longitudinal designs are particularly advantageous for investigating more complex growth trajectories (beyond linear and quadratic age associations) or diverging developmental trends based on gender (van der Graaff et al., 2014) or pubertal status (Braams et al., 2015) that may better characterize youths’ prosocial behavior. While the current results suggest that we should potentially capitalize on early adolescents’ heightened recruitment of social-cognitive regions to promote other-focused behaviors, an alternative possibility is that childhood and mid-adolescence are instead the sensitive periods for encouraging prosocial behavior because less recruitment of social-cognitive regions is needed to engage in the same rates of prosocial behavior. Longitudinal methods can provide a more comprehensive understanding of these age-related differences (see Kraemer et al., 2000), as well as explore how within-person individual differences can influence prosocial decision-making over time. Future longitudinal studies that utilize at least three time points should unpack the specific role of age-related changes in brain development that promotes prosocial behavior.
The subsample used in fMRI analyses of prosocial behavior was relatively small, thus limiting the generalizability of some of the current findings beyond youth who are inclined toward costly prosocial decision-making. While this approach allowed us to characterize age-related associations in the neural correlates of prosocial decision-making among youth who actually engaged in costly prosocial behaviors, we are limited in understanding prosocial development in youth who do not engage in the behavior. Youth likely engage in higher or more variable rates of prosocial behavior, which binary assessments of prosocial behavior are less sensitive at capturing than continuous measurement of prosocial behavior (e.g. van Hoorn et al., 2016). The experimental design may also be underpowered to detect true effects or retain true null effects due to our small sample size. Reported null results (e.g. between age and the frequency of prosocial decisions) may thus arise erroneously from a lack of statistical power. Future studies should address these study limitations by using continuous measurement of prosocial behavior and larger sample sizes, thereby increasing the statistical power necessary to probe age and possible gender differences in costly prosocial behavior among youth. Nonetheless, these results provide compelling evidence that ongoing neurobiological changes during childhood and adolescence differentially influence prosocial development.
Conclusion
The current study addresses an important gap in the literature on whether developmental differences in prosocial decision-making at the neural level follow linear or non-linear (e.g. quadratic) associations with age from childhood to mid-adolescence. Neural evidence supports quadratic age differences, such that early adolescents who engaged in prosocial decision-making show greater recruitment of social-cognitive and regulatory regions compared to children and mid-adolescents. Given that we did not find age differences in prosocial behavior, this suggests that early adolescents show unique patterns of brain activation to inform similar levels of prosocial behavior. Future research should examine how age-related differences in youths’ neural sensitivity to prosocial decision-making can foster other-oriented abilities and prosocial behaviors over time.
Funding
This research was supported by funds to E.H.T. from the National Institutes of Health (R01DA039923) and the Department of Psychology at the University of Illinois.
Supplementary Material
Acknowledgements
We would like to thank the research participants and the Biomedical Imaging Center staff at the University of Illinois.
References
- Aron A.R., Robbins T.W., Poldrack R.A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Ouden H., Choudhury S., Frith C. (2007). Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience, 2(2), 130–139. 10.1093/scan/nsm009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Braams B. R., Duijvenvoorde A. C. K., Peper J. S., Crone E. A. (2015). Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. Journal of Neuroscience, 35(18), 7226–38. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.B. (2004). Adolescents’ relationships with peers In: Lerner R., Steinberg L., editors. Handbook of Adolescent Psychology, 2nd edn, New York: Wiley, 363–394. [Google Scholar]
- Burnett S., Blakemore S.-J. (2009). Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience, 29(6), 1294–1301 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S., Sebastian C., Cohen Kadosh K., Blakemore S.-J. (2011). The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews, 35(8), 1654–1664 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo G., Crockett L.J., Randall B.A., Roesch S.C. (2007). A latent growth curve analysis of prosocial behavior among rural adolescents. Journal of Research on Adolescence, 17(2), 301–324 10.1111/j.1532-7795.2007.00524.x. [DOI] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280(5364), 747–749 10.1126/SCIENCE.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Do K.T., Guassi Moreira J.F., Telzer E.H. (2017). But is helping you worth the risk? Defining Prosocial Risk Taking in adolescence. Developmental Cognitive Neuroscience, 25, 260–71, 10.1016/j.dcn.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I.A., Blakemore S.-J. (2010). Online usage of theory of mind continues to develop in late adolescence. Developmental Science, 13(2), 331–338 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., et al. (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A. (1998). Prosocial development In: Damon W., Eisenberg N., editors. Handbook of Child Psychology: Vol. 3. Social, Emotional, and Personality development, 5th edn, New York, NY: Wiley, 701–778. [Google Scholar]
- Eisenberg N., Fabes R.A., Spinrad T.L. (2006) In: Eisenberg N., Damon W., Lerner R.M., editors. Handbook of Child Psychology: Vol. 3. Social, Emotional, and Personality Development, 6th edn, Hoboken, NJ, USA: John Wiley & Sons Inc. [Google Scholar]
- Eisenberg N., Miller P.A. (1987). The relation of empathy to prosocial and related behaviors. Psychological Bulletin, 101(1), 91–119. 10.1037/0033-2909.101.1.91. [DOI] [PubMed] [Google Scholar]
- Fabes R.A., Carlo G., Kupanoff K., Laible D. (1999). Early adolescence and prosocial/moral behavior I. Journal of Early Adolescence, 19(1), 5–16 10.1177/0272431699019001001. [DOI] [Google Scholar]
- Fink E., Begeer S., Hunt C., Rosnay M. (2014). False-belief understanding and social preference over the first 2 years of school: a longitudinal study. Child Development, 85(6), 2389–2403. 10.1111/cdev.12302 [DOI] [PubMed] [Google Scholar]
- Flournoy J.C., Pfeifer J.H., Moore W.E., et al. (2016). Neural reactivity to emotional faces may mediate the relationship between childhood empathy and adolescent prosocial behavior. Child Development, 87(6), 1691–1702 10.1111/cdev.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummerum M., Keller M., Takezawa M., Mata J. (2008). To give or not to give: children’s and adolescents’ sharing and moral negotiations in economic decision situations. Child Development, 79(3), 562–576 10.1111/j.1467-8624.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- Güroğlu B., Bos W., Crone E. A. (2014a). Sharing and giving across adolescence: an experimental study examining the development of prosocial behavior. Frontiers in Psychology, 5(291), 1–13. 10.3389/fpsyg.2014.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B., Bos W., Crone E. A. (2009). Fairness considerations: increasing understanding of intentionality during adolescence. Journal of Experimental Child Psychology, 104(4), 398–409. 10.1016/j.jecp.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Güroğlu B., Will G.-J., Crone E.A. (2014b). Neural correlates of advantageous and disadvantageous inequity in sharing decisions. PLoS One, 9(9), e107996. 10.1371/journal.pone.0107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M. (2009). Imitation, empathy, and mirror neurons. Annual Review of Psychology, 60(1), 653–670 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Klapwijk E.T., Goddings A.L., Burnett Heyes S., Bird G., Viner R.M., Blakemore S.J. (2013). Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Hormones and Behavior, 64(2), 3140322. 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H. C., Yesavage J. A., Taylor J. L., Kupfer D. (2000). How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry, 157(2), 163–171. 10.1176/appi.ajp.157.2.163 10.1176/appi.ajp.157.2.163appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kral T.R.A., Solis E., Mumford J.A., et al. (2017). Neural correlates of empathic accuracy in adolescence. Social Cognitive and Affective Neuroscience, 12(11), 1701–10. 10.1093/scan/nsx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Singer T. (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214, 579–591 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W.M., Zvolensky M.J., Pedulla C.M. (2003). Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence, 26(4), 475–479 10.1016/S0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Pfeifer J.H., Dapretto M. (2010). Witnessing peer rejection during early adolescence: neural correlates of empathy for experiences of social exclusion. Social Neuroscience, 5(5–6), 496–507 10.1080/17470919.2010.490673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald N.M., Messinger D.S. (2011). The development of empathy: how, when, and why In: Acerbi A., Lombo J.A., Sanguineti J.J., editors. Free Will, Emotions, and Moral Actions: Philosophy and Neuroscience in Dialogue, Rome: IF-Press, 333–359. [Google Scholar]
- Meuwese R., Crone E. A., Rooij M., Güroğlu B. (2015). Development of equity preferences in boys and girls across adolescence. Child Development, 86(1), 145–158. 10.1111/cdev.12290 [DOI] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.-J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–131 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., Oliveira-Souza R., Grafman J. (2006). Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America, 103(42), 15623–15628. 10.1073/pnas.0604475103 10.1073/pnas.0604475103pnas.0604475103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Rameson L.T., Lieberman M.D. (2014). The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience, 9(1), 39–47 10.1093/scan/nss088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaauw S., Güroğlu B., Rieffe C., Crone E.A. (2014). Behavior and neural correlates of empathy in adolescents. Developmental Neuroscience, 36(3–4), 210–219 10.1159/000363318 10.1159/000363318000363318. [DOI] [PubMed] [Google Scholar]
- Padilla-Walker L.M., Carlo G. (2007). Personal values as a mediator between parent and peer expectations and adolescent behaviors. Journal of Family Psychology, 21(3), 538–541 10.1037/0893-3200.21.3.538. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. (2009). Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development, 80(4), 1016–1038 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S.W. (2004). HLM 6: Hierarchical Linear and Nonlinear Modeling, Lincolnwood, IL: Scientific Software International. [Google Scholar]
- Rotge J.-Y., Lemogne C., Hinfray S., et al. (2015). A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, 10, 19–27 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Carey S., Kanwisher N. (2004). Understanding other minds: linking developmental psychology and functional neuroimaging. Annual Review of Psychology, 55, 87–124 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Slaughter V., Imuta K., Peterson C.C., Henry J.D. (2015). Meta-analysis of theory of mind and peer popularity in the preschool and early school years. Child Development, 86(4), 1159–1174 10.1111/cdev.12372. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Haddara N., Sasse S.F., Skwara A.C., Moran J.M., Figner B. (2018). Dissecting ‘peer presence’ and ‘decisions’ to deepen understanding of peer influence on adolescent risky choice. Child Development., 10.1111/cdev.13081. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. (2001). Adolescent development. Annual Review of Psychology, 52(1), 83–110 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Tankersley D., Stowe C.J., Huettel S.A. (2007). Altruism is associated with an increased neural response to agency. Nature Neuroscience, 10(2), 150–151 10.1038/nn1833. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. (2013). Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience, 3, 45–52 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. (2014). Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences of the United States of America, 111(18), 6600–6605 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N., Qu Y. (2015). The ties that bind: group membership shapes the neural correlates of in-group favoritism. NeuroImage, 115, 42–51 10.1016/j.neuroimage.2015.04.035. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2010). Gaining while giving: an fMRI study of the rewards of family assistance among white and Latino youth. Social Neuroscience, 5(5–6), 508–518 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage, 58(1), 242–249 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A., Bockler A., Kanske P., Trautwein F.-M., Singer T. (2016). Decoding the charitable brain: empathy, perspective taking, and attention shifts differentially predict altruistic giving. Journal of Neuroscience, 36(17), 4719–4732 10.1523/JNEUROSCI.3392-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos W., Dijk E., Westenberg M., Rombouts S. A., Crone E. A. (2011). Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science, 22(1), 60–70. 10.1177/0956797610391102 10.1177/09567976103911020956797610391102 [DOI] [PubMed] [Google Scholar]
- Bos W., Westenberg M., Dijk E., Crone E. A. (2010). Development of trust and reciprocity in adolescence. Cognitive Development, 25(1), 90–102. 10.1016/j.cogdev.2009.07.004 [DOI] [Google Scholar]
- Graaff J., Branje S., De Wied M., Hawk S., Van Lier P., Meeus W. (2014). Perspective taking and empathic concern in adolescence: gender differences in developmental changes. Developmental Psychology, 50(3), 881–888. 10.1037/a0034325 [DOI] [PubMed] [Google Scholar]
- Meulen M., IJzendoorn M. H., Crone E. A., et al. (2016). Neural correlates of prosocial behavior: compensating social exclusion in a four-player cyberball game. PLoS One, 11(7), e0159045 10.1371/journal.pone.0159045 10.1371/journal.pone.0159045journal.pone.0159045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn J., Dijk E., Güroğlu B., Crone E. A. (2016). Neural correlates of prosocial peer influence on public goods game donations during adolescence. Social Cognitive and Affective Neuroscience, 11(6), 923–933. 10.1093/scan/nsw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. D. (2000). Simultaneous inference for fMRI data. Biophysics Research Institute. Medical College of Wisconsin Retrieved from https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf
- Will G.-J., Crone E.A., Van Lier P.A.C., Güroğlu B. (2018). Longitudinal links between childhood peer acceptance and the neural correlates of sharing. Developmental Science, 21, 1–0.e12489 10.1111/desc.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.Y.-J., Rosenblau G., Keifer C., Pelphrey K.A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience and Bio-behavioral Reviews, 51, 263–275 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


