Abstract
The robust specification of organ development depends on coordinated cell-cell communication. This process requires signal integration among multiple pathways, relying on second messengers such as calcium ions. Calcium signaling encodes a significant portion of the cellular state by regulating transcription factors, enzymes, and cytoskeletal proteins. However, the relationships between the inputs specifying cell and organ development, calcium signaling dynamics, and final organ morphology are poorly understood. Here, we have designed a quantitative image-analysis pipeline for decoding organ-level calcium signaling. With this pipeline, we extracted spatiotemporal features of calcium signaling dynamics during the development of the Drosophila larval wing disc, a genetic model for organogenesis. We identified specific classes of wing phenotypes that resulted from calcium signaling pathway perturbations, including defects in gross morphology, vein differentiation, and overall size. We found four qualitative classes of calcium signaling activity. These classes can be ordered based on agonist stimulation strength Gαq-mediated signaling. In vivo calcium signaling dynamics depend on both receptor tyrosine kinase/phospholipase C γ and G protein-coupled receptor/phospholipase C β activities. We found that spatially patterned calcium dynamics correlate with known differential growth rates between anterior and posterior compartments. Integrated calcium signaling activity decreases with increasing tissue size, and it responds to morphogenetic perturbations that impact organ growth. Together, these findings define how calcium signaling dynamics integrate upstream inputs to mediate multiple response outputs in developing epithelial organs.
Introduction
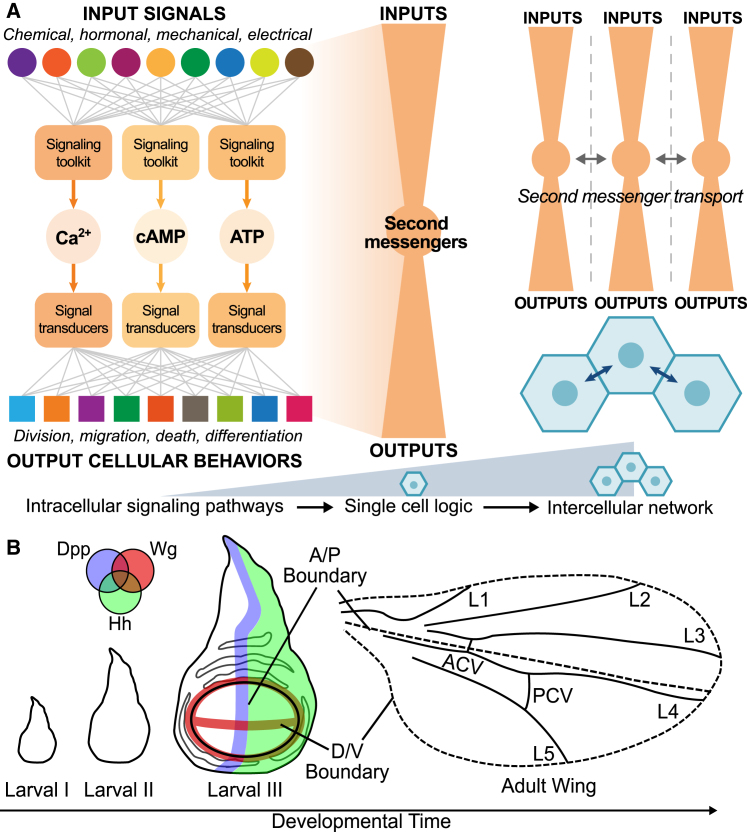
Organ development requires the coordination of many cells to form a structurally integrated tissue. Important properties of the final organ architecture include its shape, size, and spatial distribution of cell types. Notably, the information processing network required for development resembles a “bow-tie” network structure with many input signals that are funneled through a limited number of second messengers (1, 2, 3) (Fig. 1 A). Signal integration and pathway crosstalk result in many possible downstream outputs that are determined by effector proteins that regulate cellular processes, including cell division, migration, mechanical properties, death, and cell differentiation state (1). However, how these diverse input signals regulate the dynamics of second messengers is poorly understood. Further, how organ-level properties, such as size and shape, emerge from the integration of second messenger signaling remains to be fully elucidated.
Figure 1.
The wing disc as a model system of signal integration during organogenesis. (A) Second messengers are central nodes of a bow-tie structure that integrates various input signals to inform cellular behaviors. The information from a coupled intercellular network results in emergent tissue-scale phenomena. (B) Schematic describing the development of the Drosophila wing from the wing disc is shown. The wing disc grows rapidly during larval stages and develops into the adult wing. Spatial patterning of cell fates is determined by the combined actions of morphogenetic signals, including Hedgehog (Hh), BMP/Decapentaplegic (Dpp), and Wnt/Wingless (Wg). After larval growth, the wing disc undergoes morphogenesis to form the wing and the thorax. The larval pouch is approximately an oval. The pouch cells later form the wing blade. In the adult wing, there are L1–L5 veins, anterior crossvein (ACV), and posterior crossvein (PCV). This study focuses on Ca2+ signaling dynamics in the third instar wing disc pouch, after primary patterning of anterior/posterior and dorsal/ventral compartments has occurred. To see this figure in color, go online.
A key second messenger that serves as a central node in the bow-tie structure is the calcium ion (Ca2+) (4). Ca2+ signaling is a ubiquitous transducer of cellular information and plays key roles in regulating cell behaviors, such as cell division, growth, and death (5). Ca2+ dynamics regulate cellular properties and behavior during animal development, and perturbations to Ca2+ signaling often lead to disease (3, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16). Cells can encode complex signals into a Ca2+ signaling “signature,” which includes amplitude, frequency, and integrated intensity of Ca2+ oscillations (13, 17). Cells decode these signaling signatures by modulating the activities of downstream enzymes and transcription factors (Fig. 1 A).
Intercellular Ca2+ signaling is correlated with many developmental processes. For example, they have been found to regulate scale development in the butterfly (18). Ca2+ waves are indispensable to activate Drosophila egg development (19), and Ca2+ spikes are important for development of Drosophila and Xenopus embryos (8, 11, 16, 20, 21). Ca2+ signaling responds to Hedgehog (Hh) signaling in the frog neural cord (22), correlates with Decapentaplegic (Dpp) secretion in Drosophila imaginal discs (23), and is indispensable for human neural rosette development (24). Ca2+ dynamics also are essential for cell migration and tissue contractility in zebrafish, Japanese newt, and chick embryos (9, 20, 25). Recently, intercellular Ca2+ transients (ICTs) have been observed in the Drosophila wing disc, both in vivo and ex vivo (15, 26, 27, 28), and have been implicated as a first response to wounding (27) and robustness in regeneration (15), tissue homeostasis (26), and mechanotransduction (15, 29). Inhibition of Ca2+ significantly also rescues cancerous overgrowth of wings, thus showing its regulatory role in tissue growth (30). However, a quantitative characterization of Ca2+ dynamics in organ development is lacking, in part because of a lack of image-processing methods and a suitable model system to analyze the stochastic nature of the signals. Consequently, there is a need for a systems-level description of Ca2+ signaling dynamics to decode the role of Ca2+ signaling in organ development.
The Drosophila wing imaginal disc pouch is a premier model system to study how epithelial cells undergo specific morphogenetic steps to form the intricate structure of an adult wing (31, 32, 33, 34, 35, 36) (Fig. 1 B). The wing disc is a powerful model system because of the availability of tools to perturb gene expression in a specific region of a tissue (37). Multiple conserved regulatory modules for tissue development have been discovered in the wing disc. In the larval organ, morphogens divide the wing disc pouch into regions that define the differentiation state of cells and coordinate morphogenesis (Fig. 1 B). Morphogen signals that are important for wing disc development include Hh (reviewed in (38)) and Dpp (35, 39), which define the anterior/posterior axis. Wg (reviewed in (40)) patterns the dorsal/ventral axis. Widely available genetic tools and simple geometry make the Drosophila wing disc a powerful platform to decode Ca2+ signaling at the systems level.
Here, we have developed an image-processing pipeline to quantitatively investigate the relationships between Ca2+ signaling and organ size. We first genetically inhibited key components of the core Ca2+ signaling pathway, termed elsewhere as the “Ca2+ signaling toolkit” (5), to define the range of adult wing phenotypes. Next, we performed a dose-response experiment of fly extract (FEX) to order the specific classes of Ca2+ signaling based on the relative concentration of agonist-based stimulation. We use the term “Ca2+ signaling activity” to collectively refer to these four Ca2+ signaling classes. We investigated how these classes of Ca2+ signaling correlate with disc age and size, both in vivo and ex vivo. We established that FEX stimulates Ca2+ through Gαq/phospholipase C (PLC) β signaling through genetic perturbation experiments. Next, we developed advanced image-analysis tools to handle the large data sets to extract quantitative Ca2+ dynamics measurements. Using this image-analysis pipeline, we identified a negative power-law correlation between integrated Ca2+ signaling activity and wing disc pouch size. We examined how the genetic state of the tissue modulates Ca2+ signaling dynamics through genetic perturbation. Ca2+ signaling activity responds to perturbations that impact the morphogenic state of the tissue, resulting in deviations from the quantitative correlation curve between Ca2+ signaling activity and developmental progression. Together, these trends indicate that Ca2+ signaling provides a biochemical readout of organ size. The results suggest Ca2+ could be involved in modulating cell proliferation activity during larval growth. In sum, this study provides significant evidence that Ca2+ signaling contributes to intercellular consensus-building during organ development (41). This research paves the road of revealing the quantitative and mechanistic regulation of organ development by Ca2+ signaling in future studies.
Materials and Methods
Fly strains and genetics
Phenotypic analysis in Fig. 2 was performed using the MS1096-Gal4 line (BL#8860). A nub > GCaMP6f reporter tester line was used to measure relative Ca2+ signals in the wing disc pouch (29, 42). Gene perturbations were generated by crossing the tester line to either RNA interference (RNAi)-based transgenic lines (UAS-Gene XRNAi) or gene overexpression lines (UAS-Gene X). When possible, multiple independent RNAi lines were tested for each gene investigated. As a second round of controls, the tester line was also crossed to UAS-mCherry to obtain ratiometric measurements (Fig. S6). Validation of Ca2+-based RNAi lines is reported in the Supporting Materials and Methods, Section 5 and in (29). Further details on genetic crosses and validation are detailed in the Supporting Materials and Methods.
Figure 2.
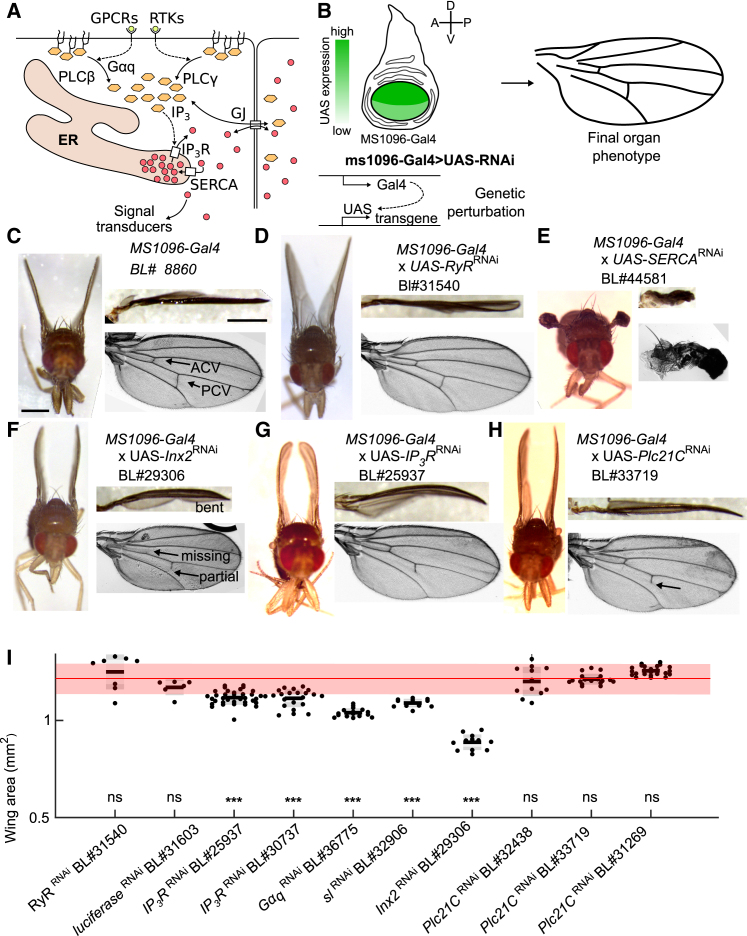
Core calcium signaling components modulate wing size, shape, and vein differentiation. (A) Schematic representing mechanism of the core IP3R-mediated Ca2+ signaling pathway is shown. ER, endoplasmic reticulum; Gαq, G protein α q subunit; GJ, gap junction; GPCR, G protein-coupled receptor; IP3, inositol trisphosphate; IP3R, IP3 receptor; PLC, phospholipase C, which includes multiple isoforms: PLCγ homolog (sl) and PLCβ homologs (Plc21C and norpA); RTK, receptor tyrosine kinase; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase. (B) Schematic of expression pattern of MS1096-Gal4, which is expressed more strongly in the dorsal compartment, is shown. The GAL4/UAS system was used to express RNAi constructs starting during the third instar larval stage. Adult wing phenotypes were used to provide a readout of final phenotype. (C–H) Micrographs of adult fly, orthogonal view of wing, and mounted view of wing for indicated crosses are shown. Scale bars represent 0.5 mm. (I) Total area of adult wings under various perturbations of Ca2+ signaling genes driven by MS1096-Gal4. The gray boxes indicate standard deviation. ns, not significant. ∗p < 0.05; ∗∗∗p < 0.001 by t-test after Bonferroni correction. The red band provides the average and SD of multiple control crosses. To see this figure in color, go online.
In vivo imaging setup
Wandering third instar larvae were collected for imaging and rinsed in deionized water. Larvae were dried and then adhered to a coverslip for imaging with Scotch tape covering the larvae. The larvae were attached with their spiracles facing toward the coverslip to align the wing discs toward the microscope. The larvae were imaged at 20× magnification for 20 min on an EVOS FL Auto microscope (Thermo Fisher Scientific, Waltham, MA). Images were taken every 15 s.
Organ culture media
Organ culture studies were performed as detailed in (29, 43) with modifications. We used 15% FEX for genetic perturbation studies performed ex vivo because it improved the signal/noise ratio for reliable quantification of Ca2+ dynamics, although there was more activity than in vivo measurements. ZB media +15% FEX contains 79.4% (v/v) ZB media (44), 0.6% (v/v) of 1 mg/mL insulin (Sigma-Aldrich, St. Louis, MO), 15% (v/v) ZB-based FEX, and 5% penicillin/streptomycin (Gibco, Carlsbad, CA). ZB media was developed as a chemically defined media to support Drosophila cell culture (44) and was used as the basal media for all experiments. The text of Supporting Materials and Methods provides details of FEX preparation.
Wing disc imaging setup
Wing discs were dissected from third instar larvae, cultured, and imaged as detailed in the Supporting Materials and Methods. All experiments were performed immediately after dissection to minimize the time in culture. Discs were imaged at three z-planes with a step size of 10 μm and 20× magnification and 10 s intervals for a total period of 1 h, with 200 ms exposure time and 50 mW 488 nm laser exposure at 44–70% laser intensity (Fig. S5). For some experiments, mCherry signal was imaged with a 560 nm laser (50% laser intensity).
Ex vivo data preprocessing
Microscopy resulted in four-dimensional time-lapse data (512 pixels by 512 pixels by three z-planes by 361 time points). The z-stack data was max-projected in FIJI (45) to yield z-projected time-lapse videos. Time points were selected so that discs were only analyzed during times in which disks did not move in the z-direction, with the shortest time lapse analyzed being 20 min.
Image segmentation and registration
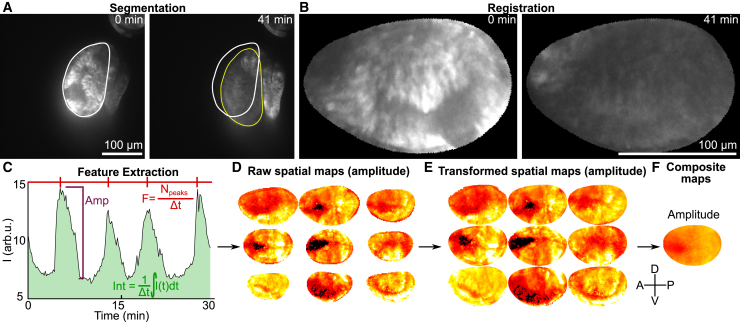
For the image frame at each time point, a region of interest (ROI) defining the pouch was obtained using a novel deep-learning-based segmentation algorithm that we developed and is described in (46). Each frame was segmented with a fully convolutional network (FCN) module, and the ROI boundaries were refined by a graph-search algorithm. The FCN module was trained on 800 images of wing discs expressing nub-GCaMP6f with various stages of Ca2+ signaling activity. B-spline-based registration was used to transform image frames onto a shape defined by the first time point. The pipeline ensures that coordinates within the pouch are consistent from frame to frame, allowing spatial analysis of Ca2+ signatures.
The FCN was used to segment only the pouch region, excluding the nub-expressing hinge region dorsal to the pouch. Note that the traditional image segmentation methods explored could not distinguish well between these two types of regions. For data that had both a GCaMP6f and RFP channel, Rigid Registration was used in FIJI (45), as the RFP channel does not exhibit oscillatory dynamics.
Identification of the axes
The posterior and D compartments were identified manually based on the characteristic shapes of the pouch (Figs. 1 B and S1). Pouches were flipped so that the A compartment was on the left and the D compartment was on the top. A custom MATLAB (The MathWorks, Natick, MA) graphical user interface was used to reduce error in manual pouch orientation (Fig. S2). The graphical user interface also helped to reduce error in the manual pouch orientation. Details are found in Supporting Materials and Methods and Fig. S2.
Feature extraction
Images of each wing disc pouch were divided into square ROIs of 2.8 μm. We obtained time-averaged Ca2+ signatures for each ROI and represented them as a spatial map (see Fig. 6 D). Each set of spatial maps was averaged to obtain a composite spatial map for each feature. The Ca2+ signatures include amplitude, frequency, and integrated intensity. The Ca2+ signature was extracted by taking average intensity (F(t)) from 4 × 4 pixel (2.8 × 2.8 μm) spatial bins arrayed with square packing across the segmented disc pouch (Fig. S1, A–C). Video durations ranged from 20 to 60 min. Further details are included in the Supporting Materials and Methods Text and Figs. S1 and S2.
Figure 6.
Pipeline for spatiotemporal quantification of calcium signatures. (A) Automatic segmentation of pouch region with deep-learning segmentation algorithm was developed for this study. Outlines represent the pouch at the initial time point (white) and the final time point (yellow). (B) Registration of raw images onto canonical pouch shapes is shown. (C) Extraction of amplitudes (Amp), frequency (F), and integrated intensity (Int) from Ca2+ traces for individual region of interest (ROI) was performed. (D) The Ca2+ signature is computed for each spatial position within each pouch to generate a spatial map. (E) Each spatial map is transformed onto a canonical coordinate system to align the anterior-posterior and dorsal-ventral axes. (F) Each set of transformed spatial maps is averaged at each position to generate a composite spatial map. The cross indicates the orientation of the wing disc. Scale bars, 100 μm. To see this figure in color, go online.
Qualitative analysis
The in vivo data were analyzed blindly and qualitatively by dividing each video into 135-s-clip segments and scoring them in random order. Ca2+ signaling activity in each segment was manually classified into one of the four previously mentioned categories. One sample may, in this way, be classified in multiple bins for the full video. Each clip was classified as having either no activity exceeding basal levels, cellular spikes, ICTs, intercellular Ca2+ waves (ICWs), fluttering, or being unanalyzable because of larval motion. If a time-lapse segment includes two or more categories, the most active category was annotated. This was done with a custom MATLAB script that divided each video into clips. One random clip was looped at a time until all the data had been classified. Afterwards, the fraction of time spent in each class was reported for each condition. Additional procedural details are included in the Supporting Materials and Methods.
Statistical analysis and visualization
The median value of each summary statistic for each ROI in each disc was compared across genetic conditions, pouch sizes, and between compartments (Fig. 6). ROIs within two spatial bins of the edge were excluded from the median to avoid edge effects. A two-tailed, unpaired, Student’s t-test was performed in comparisons across conditions. The visualization of the manifold identified in Fig. 8 is a multivariate kernel density estimate with a cutoff value selected to display a three-dimensional region that encompasses a uniform density of measurements. As discussed in the Supporting Materials and Methods, this was done to clarify the manifold along which developing wing discs lie in the “morphological cell signaling” space.
Figure 8.
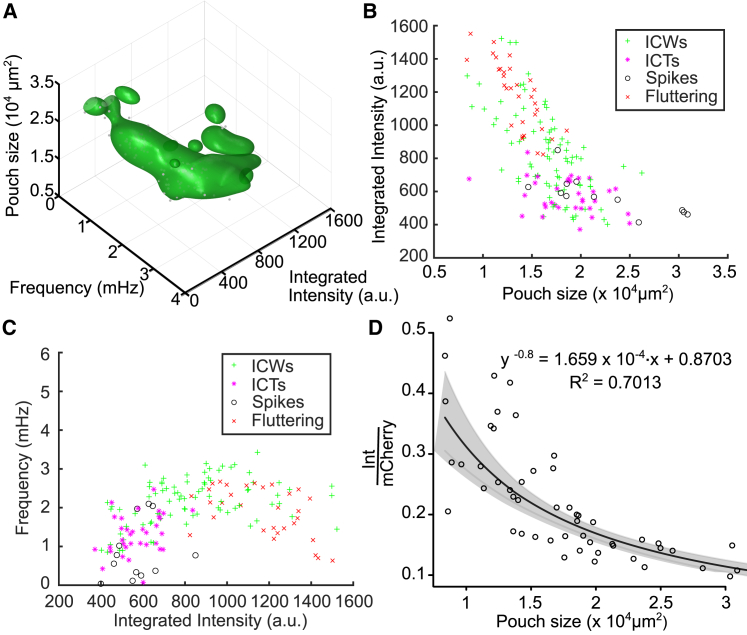
Transitions of agonist-stimulated calcium signaling as a function of pouch size. (A) Larval wing discs exhibit transitions in FEX (Gαq) agonist-stimulated Ca2+ signaling dynamics during development, forming a spiral in a three-dimensional plot of “morphological cell signaling space.” (B) Integrated intensity decreases with increasing pouch size. Colors represent dominant Ca2+ dynamics observed in the particular sample. (C) A parametric plot of a data set from (A) shows frequencies of Ca2+ oscillations increasing with increasing integrated intensity and then decreasing at higher amplitudes. Note that low-amplitude fluttering does not contribute to the measured frequency signal. Each wing disc was imaged for 1 h and with a 10 s interval. Each video was divided into 31 time segments. As a second level of analysis, dominant Ca2+ activity was scored blindly in each segment, respectively (colors, see legend). Ca2+ dynamics that were observed for the largest total time for each separate wing disc sample are represented as color dots in (B and C). (D) A regression curve fit to ratiometric average integrated intensity of Ca2+ signaling in wing pouches as a function of pouch size is shown (n = 53). To see this figure in color, go online.
Results
The core calcium signaling pathway regulates final wing size, shape, and patterning robustness
The regulation of cytosolic Ca2+ levels occurs by regulating fluxes of Ca2+ between Ca2+ stores, such as the endoplasmic reticulum, and the cytosol (Fig. 2 A). We knocked down genes known to regulate Ca2+ signaling dynamics and characterized final wing morphologies as an initial step to systematically identify phenotypic outputs. We used MS1096-Gal4 to express RNAi. MS1096-Gal4 is expressed in the wing pouch, the precursor of the wing blade, and has stronger expression in the dorsal than the ventral compartment (Fig. 2 B; (47, 48)). The parental MS1096-Gal4 line was crossed to control lines such as UAS-RyRRNAi (where RYR is not expected to have significant expression (49)) or other UAS-RNAi constructs that do not target endogenous messenger RNA (such as lucRNAi). These did not exhibit significant morphological or size defects (Fig. 2, C and D).
Based on the wealth of literature on wing development, adult wing phenotypes provide important functional information into the downstream outputs of calcium signaling. We found that there are four major categories of wing defects in adult wings when perturbing Ca2+ signaling genes: 1) crumpling and blistering (Fig. 2 E), 2) wing bending (Fig. 2, F–H), 3) loss of crossveins and other vein defects (Fig. S11), and 4) reduction in wing size. MS1096 > SERCARNAi led to a severe shriveled wing (Fig. 2 E) (15, 26). Knocking down gap junctions through MS1096 > Inx2RNAi (RNAi phenotype for line is described in Pézier et al. (50); see Table S1) led to significantly smaller wings with defects, including partial loss, total loss, and deviation in the posterior crossvein (PCV) and anterior crossvein (Fig. 2, F and I). We observed that partial loss of the PCV was always the loss of the posterior side of the PCV and never the anterior side (n = 33). MS1096 > IP3RRNAi exhibited similar wing defects (Fig. 2, G and I). The loss of crossveins is consistent with Ca2+ signaling playing a role in the secretion of growth and patterning molecules, such as Dpp (23). Earlier reports have also implicated Ca2+ signaling genes (including Stim and Orai) in vein patterning (26, 51).
Notably, wing size was also significantly reduced for RNAi knockdowns to many of the core Ca2+ signaling components. MS1096 > Inx2RNAi (Fig. 2 I), MS1096 > IP3RRNAi (Fig. 2 G), and MS1096 > GαqRNAi (data not shown) wings are all bent and show a significant reduction of wing size. If a certain gene regulates growth, the asymmetric perturbation by MS1096-Gal4 could lead to a differential reduction of dorsal and ventral wing area. This differential reduction of area could lead to bending of the final wing, which is formed by apposition of the dorsal and ventral compartments during pupal morphogenesis (Fig. 2, C–G). Together, these results demonstrate that Ca2+ signaling plays important roles in wing morphogenesis, vein patterning, and growth control.
Three homologs of the PLC family are present in Drosophila. Plc21C (PLCβ1) and norpA (PLCβ4) are stimulated by G protein-coupled receptors (GPCRs), and sl (PLCγ) is stimulated by receptor tyrosine kinases (RTKs), such as insulin receptor and epidermal growth factor receptor (52). We observed that MS1096 > Plc21CRNAi wings had a similar bent wing phenotype and defects in crossvein patterning compared to the MS1096 > IP3RRNAi and MS1096 > Inx2RNAi wings, but did not have the same wing size phenotypes (Figs. 2, H and I and S9). MS1096 > norpA also did not cause significant size phenotype (data not shown). An explanation for the weaker defect of MS1096 > Plc21CRNAi compared to knockdown of IP3R or Gαq could be that it is caused by incomplete knockdown for the given RNAi lines tested or redundancy between PLCβ homologs. Another possibility is that the bent wing phenotype arises from defects in the mechanical regulation of cells during morphogenesis and is a separate phenotype from growth regulation. Previously, sl has been shown to modulate wing size (53), which is in agreement with our results (Fig. 2 I).
Taken together, these results show that IP3R-mediated Ca2+ signaling contributes to multiple aspects of wing morphogenesis. Here, we have established that Ca2+ plays an important role in wing size control. These observations propelled us to investigate further the interplay and correlation between Ca2+ signaling dynamics and larval wing disc size, which is the stage of development governing overall organ growth as reviewed in (34, 54).
Varying agonist concentration results in four ordinal classes of calcium signaling activity ex vivo
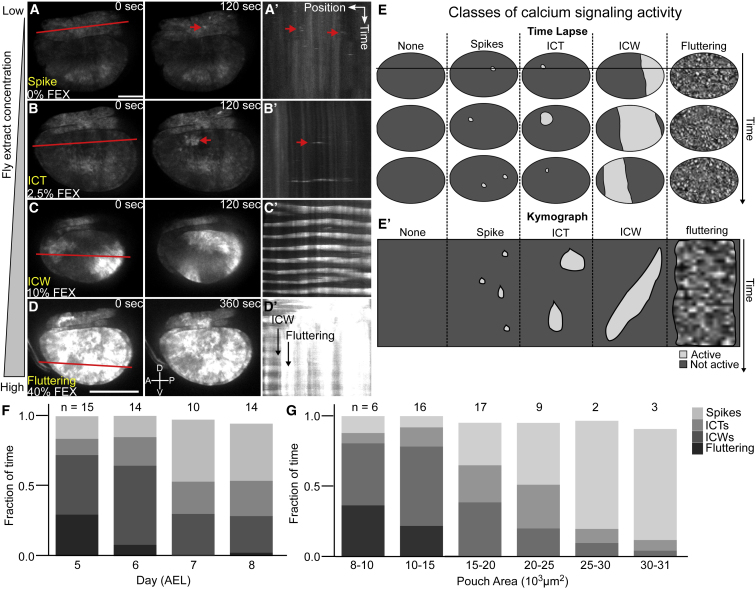
Previously, FEX has been reported to stimulate Ca2+ activity by our group and others (15, 26, 28, 29). We first tested whether the extent of Ca2+ signaling exhibits a concentration dependence on FEX. To do so, we performed ex vivo imaging of Ca2+ signaling activity in the wing disc pouch using a genetically encoded Ca2+ sensor (nub > GCaMP6f). Wing discs were cultured with variable concentrations of FEX. We observed a consistent progression of four classes of Ca2+ signaling activity as the concentration of FEX increased (Fig. 3, A–E): 1) local Ca2+ spikes that occur in single cells (0% v/v FEX, Fig. 3 A and A′; Video S14); 2) stochastic, short-distance ICTs (2.5% v/v FEX, Fig. 3, B and B′; Video S15); 3) oscillatory long-range ICTs that we term ICWs (5–20% v/v FEX, Fig. 3, C and C′; Videos S16, S17, and S18); and 4) elevated cytoplasmic Ca2+ exhibiting rapid, low-amplitude oscillations that we term “fluttering” (40% v/v FEX, Fig. 3, Dand D′; Video S19). Because the observed Ca2+ activity classes depend on the dose of FEX, we established the Ca2+ signaling activity to be ordinal, with spikes being the least active and fluttering being the most active. These results suggest that transitions in Ca2+ activity occur as the overall level of IP3R-based signaling increases.
Figure 3.
Increased stimulation of calcium signaling results in a progression of signaling classes. Wing discs were cultured in chemically defined ZB media with varying concentrations of fly extract (FEX): (A) 0%, (B) 2.5%, (C) 10%, and (D) 40% v/v FEX. These conditions stimulate four classes of Ca2+ signaling activity: spikes, ICTs, ICWs, and “fluttering” Ca2+ oscillations (Videos S14, S15, S16, S17, S18, and S19). (A–D) Montages from ex vivo time-lapse videos are shown. (A′–D′) Kymographs of the corresponding time-lapse videos along the indicated red lines are shown. Note: the kymograph slice in A was selected in the hinge region to clearly indicate examples of spikes. (E) Schematic of four classes of Ca2+ signaling activity is shown. The horizontal line represents the x-y position of the kymograph. (E′) Schematic representation of kymographs taken from (E) is shown. (F and G) Average fraction of time in each class of Ca2+ signaling activity for 15% FEX varies by (F) pouch age and (G) pouch size. Wing disks are oriented with anterior to the left and dorsal compartment to the top. AEL indicates days after egg laying. Position and timescale bars, 25 μm and 15 min. For (F and G), p < 0.001 for ordinal logistic regression model versus constant model. n indicates the sample size. To see this figure in color, go online.
nub-Gal4>UAS-GCaMP6f, in ZB media + 0% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 2.5% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 5% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 10% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 20% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 40% FEX.
Next, we quantified the percentage of each class of Ca2+ signaling activity in wing discs of different ages and sizes throughout development while keeping the FEX concentration constant at 15%. The highest activity observed within each time segment established the annotated signaling class. For example, if ICTs and ICWs occur in the same time within the pouch, we characterized that fraction of time as ICW activity. We found that the occurrence of the highest Ca2+ signaling activities (ICWs and fluttering) decreased from 72% at 5 days after egg laying (AEL) to 29% at 8 days AEL (Fig. 3 F). Also, the occurrence of the highest Ca2+ signaling activity deceased from 80% in small pouches (<8 × 103 μm2) to 5% in large pouches (>30 × 103 μm2, Fig. 3 G). Importantly, our observations demonstrate that ex vivo Ca2+ signaling activity in 15% FEX correlates strongly with both the age of the larvae and the size of the wing disc pouch.
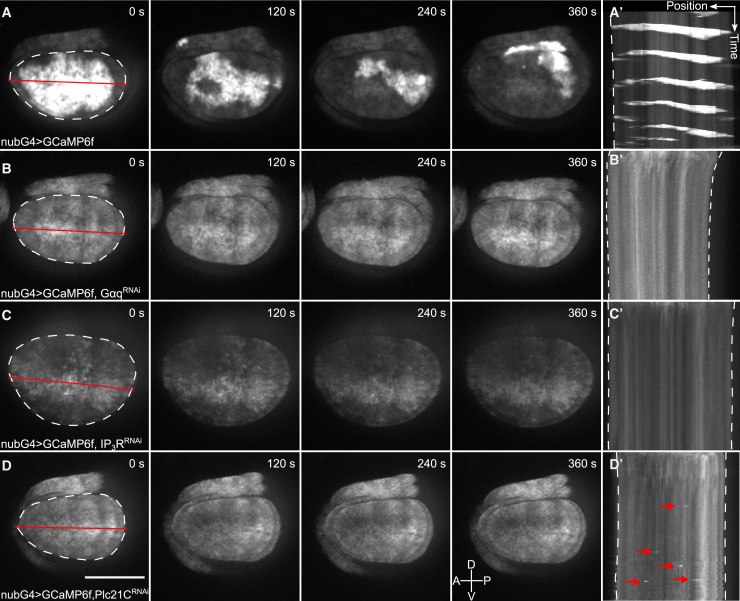
To investigate the mechanism of ICW formation, we systematically inhibited Ca2+ signaling genes using RNAi when stimulated with 15% v/v FEX. Importantly, we found significantly fewer ICWs when Plc21C, IP3R, or Gαq were knocked down (Fig. 4, A–D; Videos S11, S12, and S13). Knockdown of Plc21C still resulted in localized cell-level spike activity but not ICWs. This suggests PLC is either partially knocked down, or there is redundancy between PLC isoforms (Fig. 4 D). Overall, these results demonstrate that FEX-induced ICWs require GPCR/Gαq-based signaling. Thus, our ex vivo results provide additional insights into the mechanism of intercellular Ca2+ signaling propagation in the wing disc.
Figure 4.
FEX-induced calcium waves require GPCR/Gαq signaling. (A–D) Montages from ex vivo time-lapse videos and (A′–D′) kymographs of the corresponding time-lapse videos are shown. The genotypes of the wing discs are indicated on the bottom left. We observed ICWs in (A) and no Ca2+ signaling activity in (B–D). The cross indicates the orientation of the wing disc (Videos S10, S11, S12, and S13). The red arrows indicate single-cell spikes. Position and timescale bars, 25 μm and 15 min. White dotted line represents the pouch boundary. Red line represents the x-y position of the kymograph. The RNAi lines are as follows: GαqRNAi, BL#36775; IP3RRNAi, BL#25937; and Plc21CRNAi, BL#31719. To see this figure in color, go online.
nub-G4>UAS-GCaMP6f, UAS-GαqRNAi, ex vivo, BL# 36775.
nub-G4>UAS-GCaMP6f, UAS-IP3RRNAi, ex vivo, BL# 25937.
nub-G4>UAS-GCaMP6f, UAS-Plc21CRNAi, ex vivo, BL# 31269.
Calcium signaling dynamics are regulated during wing disc development
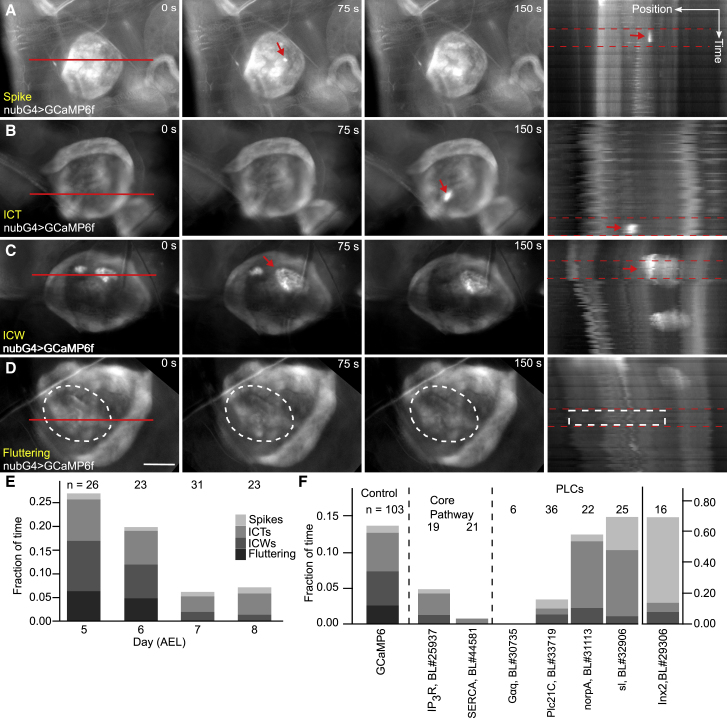
In vivo Ca2+ transients were noted in previous reports. However, a systematic characterization has not been performed because of the technical challenges of in vivo imaging. To address this knowledge gap, we tested whether in vivo Ca2+ signaling activity is a regulated phenomenon. We gently immobilized the larvae with tape on a coverslip to enable in vivo imaging for durations of 20 min. We developed a semi-automated pipeline to blindly classify Ca2+ signaling activity in video segments, matching the qualitative ex vivo analysis. Interestingly, we identified and quantified the same four classes of Ca2+ signaling activity that are observed ex vivo (Fig. 3, E and E′): 1) local Ca2+ spikes (Fig. 5 A; Video S1), 2) ICTs (Fig. 5 B; Video S2), 3) ICWs (Fig. 5 C; Video S3), and 4) fluttering (Fig. 5 D; Video S4).
Figure 5.
Overall calcium activity decreases throughout development in vivo and is regulated by multiple PLCs. (A–D) Montages from in vivo time-lapse videos of nub > GCaMP6f larval wing discs are shown. A diverse range of Ca2+ dynamics are observed. The red lines represent the x-y position of the kymograph and are 200 μm long. The red arrows indicate the Ca2+ events. The white circle indicates the area of fluttering Ca2+ oscillation. Because of its nature, the supporting videos demonstrate the fluttering oscillation much more clearly (Videos S1, S2, S3, and S4). (A′–D′) The kymograph of the corresponding time-lapse videos is shown. The kymographs between red dotted lines correspond to the montage on the left. Scale bars, 50 μm. Position and timescale bars, 50 μm and 5 min. (E) The duration of each category of Ca2+ oscillation as a fraction of total time is shown. The total Ca2+ activation time (the height of the column) decreases as wing disks grow older. p < 10−15 by ordinal regression. (F) In vivo Ca2+ signaling transients are regulated by multiple PLC isoforms, either through Gαq/PLCβ (Plc21C), which greatly inhibits large waves, or RTK/PLCγ, which also modulates Ca2+ signaling levels. Videos S5, S6, S7, S8, S9, and S10 provide representative examples for each genetic perturbation. For Inx2, the y-axis is on the right, showing upregulation of small cellular spikes. Labels represent crosses of UAS-RNAi lines to parental nub > GCaMP6f line. AEL indicates days after egg laying. To see this figure in color, go online.
nub-Gal4>UAS-GCaMP6f, in vivo, spike.
nub-Gal4>UAS-GCaMP6f, in vivo, ICT.
nub-Gal4>UAS-GCaMP6f, in vivo, ICW.
nub-Gal4>UAS-GCaMP6f, in vivo, fluttering.
Next, we quantified the percentage of four classes in wing discs of different ages and sizes throughout development (n = 103 videos). We found that observable Ca2+ signaling activity decreases from 27% to 6% of the time from 5 to 8 days AEL (Fig. 5 E). Ca2+ signaling activity also shifts from fluttering and ICW classes at day 5 to spikes and ICTs at day 8 (Fig. 5 E, p < 10−15 by ordinal regression). Note, the GCaMP6f test line shows slower growth compared to nontransgenic flies, resulting in delayed larval development.
To verify the mechanisms regulating in vivo Ca2+ activity, we performed similar perturbations with UAS-RNAi lines (Fig. 5 F; Tables S6 and S7; Videos S5, S6, S7, S8, S9, and S10). We observed that genetically knocking down SERCA and IP3R greatly reduced Ca2+ signaling activity. Perturbation of GPCR-stimulated signaling through Plc21CRNAi inhibited ICWs. Perturbation of RTK signaling through sl/PLCγ also reduced the observed proportion of higher classes of Ca2+ signaling activity but increased the proportion of time with observed cellular spikes (Fig. 5 F; Tables S6 and S7). The disruption of gap junctions through Inx2RNAi led to a significant increase in the number of spikes throughout the tissue, but Ca2+ signals did not travel from cell to cell across the tissue. This suggests that gap-junction communication synchronizes Ca2+ activity across the tissue and leads to the reduced frequency of initial stimulatory events. The increase in cellular Ca2+ spike activity with gap junctions inhibited may suggest that it is easier to stimulate smaller systems. Inx2RNAi-expressing wings are significantly smaller, suggesting the possibility that part of the growth phenotype is due to reduced coordination in Ca2+ signaling synchronization between cells.
nub-G4>UAS-GCaMP6f, UAS-IP3RRNAi, in vivo, BL# 25937.
nub-G4>UAS-GCaMP6f, UAS-SERCARNAi, in vivo, BL# 44581.
nub-G4>UAS-GCaMP6f, UAS-Inx2RNAi, in vivo, BL# 29306.
nub-G4>UAS-GCaMP6f, UAS-Plc21CRNAi, in vivo, BL# 31269.
nub-G4>UAS-GCaMP6f, UAS-norpARNAi, in vivo, BL# 31113.
nub-G4>UAS-GCaMP6f, UAS-slRNAi, in vivo, BL# 32906.
To summarize, the multiple classes of observed Ca2+ signaling activity are a recurring phenomenon during in vivo development (29). Importantly, we found that Ca2+ signaling activity in wing discs correlate with the larval age. This correlation suggests that Ca2+ signaling activity decreases as wing discs approach their final size (Fig. 5 E).
Calcium signaling activity is spatiotemporally patterned by morphogen signaling
It is challenging to perform quantitative spatial analysis of Ca2+ signaling in vivo because of larval motion, optical interference from the cuticle, and limited imaging duration. To overcome this challenge, we developed a quantitative pipeline to analyze ex vivo-imaged wing discs, which enabled more detailed spatiotemporal analysis (Fig. 6; Fig. S1–S4). In these experiments, we stimulated Ca2+ signaling with 15% FEX and recorded and analyzed Ca2+ signaling responses under a range of genetic perturbations. We then inferred how the “organ state” influences the calcium responses.
As suspended micro-organs, wing discs often exhibit movement, even in ex vivo cultures. Image registration is a problem with dynamic systems because the most distinct landmarks in the image are constantly changing as the signal moves along the tissue. To solve this problem, we built a pipeline that detects the borders of the pouch using a deep neural network approach that transforms the underlying images onto the initial mask (Figs. 6, A and B and S4; (46)). Ca2+ signatures were extracted from signals that are taken from a square grid across the tissue (Fig. 6, C and D). The resulting spatial maps were transformed onto a canonical domain represented by the average positions of the pouch boundaries and axes using a previously described method (Fig. 6 E; (55)). Finally, we report the median of the spatial maps (Fig. 6 F).
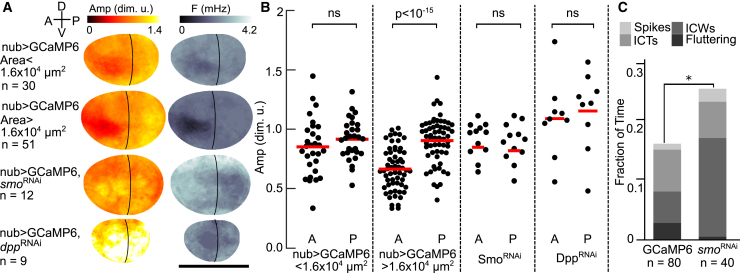
The spatial analysis revealed that a time-averaged patterning of amplitude emerges as the wing disc grows bigger. Across all wing discs, the average amplitude is 33% higher in the posterior compartment than in the anterior compartment (Fig. 7 A). In smaller discs (area < 1.6 × 104 μm2), the difference of amplitude between the anterior compartment and the posterior compartment is not significant (Fig. 7 B). In larger discs (area > 1.6 × 104 μm2), the patterning of amplitude emerges between the anterior and the posterior compartments, in which the amplitude is lower in the anterior compartment and higher in the posterior compartment, and the difference is significant (Fig. 7 B).
Figure 7.
Calcium signaling is spatially patterned in the developing wing disc. (A) Composite spatial maps of median summary statistics of Ca2+ features are shown. Wing disc pouches expressing nub > GCaMP6f were grouped by pouch size. These wing disc pouches followed one of two patterns: small pouches (<1.6 × 104μm2) with uniformly high amplitude in both compartments and larger pouches (>1.6 × 104μm2) with a lower median amplitude in the anterior compartment. Inhibition of Hh and Dpp signaling led to an increase in frequency (F) and uniformly high amplitude. The black curve represents the approximate anterior-posterior boundary. Orientations of the wing discs are indicated in upper left corner. (B) The amplitude measurement of anterior and posterior compartments in control discs (small and large) and smoRNAi and dppRNAi discs are shown. In nub > GCaMP6f discs, the difference of amplitude between the two compartments is significant in large disks. The difference is not significant in smoRNAi (composite of three independent RNAi lines, Fig. S9) and dppRNAi discs, and the median amplitude appears uniform near the anterior dorsal-ventral boundary region. The p values were obtained by paired t-tests. (C) Inhibition of Hh signaling by smoRNAi increases overall Ca2+ signaling activity in wing discs in vivo. Sample size represents the number of analyzed videos. Scale bars, 200 μm. Total Ca2+ activity by proportions test as described in Tables S6 and S7. To see this figure in color, go online.
The discovery of anterior-posterior patterning of amplitudes suggests that upstream morphogen pathways may affect Ca2+ dynamics. To test this, we perturbed the Hh signaling pathways, which direct the development of the anterior-posterior compartment boundary (56). Smoothened (Smo), a GPCR (57), is a primary transducer of Hh signaling (58). We also perturbed Dpp signaling, which is downstream of Hh signaling and also directs anterior-posterior patterning and growth (35, 39, 59). We knocked down smo and dpp using RNAi expression in the wing disc pouches of wandering larvae to test if the spatial asymmetry in average amplitudes is downstream of Hh signaling (nub > GCaMP6f/ > smoRNAi; Video S21) or Dpp signaling (nub > GCaMP6f/ > dppRNAi; Video S22). We found that inhibition of either Hh or Dpp signaling was sufficient to abolish anterior-posterior spatial patterning of ICW amplitudes and leads to a higher frequency in the entire pouch (Fig. 7, A and B). These results were confirmed by evaluating multiple independent RNAi lines against smo, which are reported individually in Fig. S9, and combined together in Fig. 7, A and B. Further, the results of increased activity of nub > GCaMP6f/ > smoRNAi were replicated in vivo (Fig. 7 C). Hh suppression resulted in roughly doubling the amount of time when fluttering or ICWs were observed in the pouch (Fig. 7 C). Additionally, Hh suppression led to a decrease in the average pouch size for wandering larvae, which are near the end of development. These results demonstrate that spatial patterning of the amplitude of Ca2+ oscillations is downstream of Hh and Dpp signaling, which are two morphogen pathways involved in regulating patterning and tissue growth. It provides support for a model in which agonist-stimulated Ca2+ signaling activity provides a readout of morphogenetic states of the organ during development.
nub-G4>UAS-GCaMP6f, UAS-SmoRNAi, ex vivo, BL# 43134.
nub-G4>UAS-GCaMP6f, UAS-DppRNAi, ex vivo, BL# 25782.
Calcium signaling activity decreases with wing disc pouch size in a power-law relationship
To determine how stimulated Ca2+ signaling dynamics change as wing disc sizes increase, we created a high-dimensional summary map relating organ size to the features of Ca2+ signaling (Fig. 8 A; Videos S20, S21, S22, S23, and S24). We plotted the integrated intensity and frequency of Ca2+ signaling activity of all wing disc imaged against their pouch sizes (with wing disc pouch size as a proxy for overall organ size). The result demonstrates several interesting correlations between features of Ca2+ signaling activity and wing disc sizes, which form a manifold in high-dimensional morphological-cell signaling space (Fig. 8, A–C). Integrated intensity was found to decrease with pouch size (Fig. 8 B). Additionally, we found that frequency increases with integrated intensity and then decreases again (Fig. 8 C). Further, we analyzed the full data set for qualitative characteristics of calcium signaling, similar to the in vivo experiments. We found that there is a progression from fluttering disc to oscillatory waves to smaller-scale stochastic spiking activity (Fig. 8 B). Overall, these findings further confirm stimulated Ca2+ signaling responses decline with increasing organ size until the end of larval development.
nub-G4>UAS-GCaMP6f, UAS-Inx2RNAi, ex vivo, BL# 29306.
nub-G4>UAS-GCaMP6f, UAS-PtenRNAi, ex vivo, BL# 25841.
nub-G4>UAS-GCaMP6f, UAS-mCherry, ex vivo, BL# 35787.
The full data set plotted in Fig. 8, A–C includes a subset of discs that also expressed a red fluorescent protein under the same Gal4 driver (expressing nub > GCaMP6f/ > mCherry; Video S24). Similar results between nub > GCaMP6f and nub > GCaMP6f/ >mCherry suggest Gal4 dilution is not a significant concern (Fig. S6 E). For discs coexpressing mCherry, we could normalize GCaMP6f fluorescence to mCherry fluorescence (Fig. S6 C). This allowed us to obtain ratiometric estimates of Ca2+ signaling that better account for sensor expression. For the data with mCherry expression, we observed a power-scaling relationship in which average ratiometric integrated intensity (normalized to mCherry expression) scales with pouch area (R2 = 0.70, Fig. 8 D; Figs. S7 and S8). This relationship utilizes a Box-Cox power-scaling transformation (60) to maximize the log-likelihood estimation of the power exponent (Fig. S6 A). This power-scaling relationship served as a better fit compared to linear and exponential models because of a more normal distribution of residuals and the least variance between model residual values (Fig. S7).
Calcium signaling responds to perturbations to morphogen and growth factor signaling
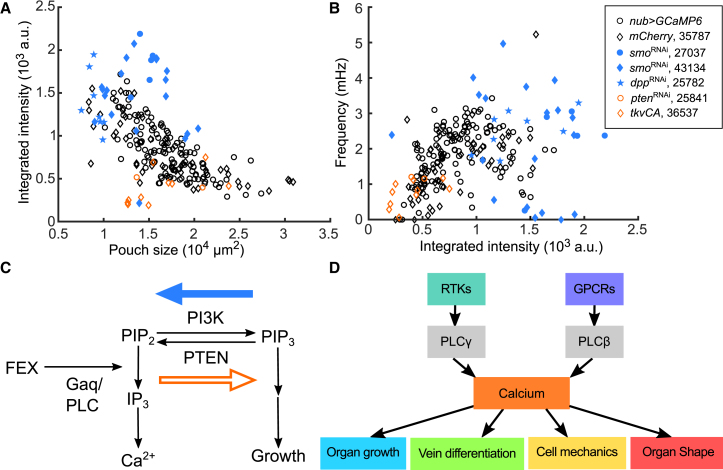
Finally, we investigated the effects of multiple regulators of organ growth to further clarify the connections between organ growth and stimulated Ca2+ responses. We ectopically activated the Dpp pathway through the uniform expression of the constitutively active form of the Dpp receptor, tkvCA (39, 61, 62). Additionally, we ectopically stimulated growth through the inhibition of the tumor suppressor phosphatase and tensin homolog (Pten), which results in high levels of insulin/AKT signaling without impacting wing patterning (63, 64; Video S23). According to previous reports, both conditions lead to increases in cell division and growth (35, 59, 65). We found that these growth-inducing perturbations resulted in a decrease in Ca2+ signaling activity relative to control discs when exogenously stimulated (Fig. 9, A and B, orange). Inhibiting Hh and Dpp signaling with smoRNAi and dppRNAi expression, respectively, lead to reduced growth (Fig. S8) and shows an increased integrated intensity, amplitude, and frequency of Ca2+ oscillations in wing disc pouches (Fig. 9, A and B, blue solid points of various shapes).
Figure 9.
Integrated model of calcium signaling transduction in the Drosophila wing disc. (A) Genetic perturbations impacting morphogen and growth pathways result in deviations in agonist (FEX)-stimulated Ca2+ signaling responses. Data points of different colors and shapes represent wing discs with various genotypes that are indicated in the legend. Blue-colored, solid data points result in patterning defects and reduced wing growth. Higher Ca2+ signaling responses are produced for a given stimulus (15% FEX in all cases). Orange-colored, nonsolid data points result in increased growth. Reduced Ca2+ signaling responses are observed for the same stimulus. (B) The parametric plot of the same data set shows increased frequency in Ca2+ transients for the growth-suppression perturbations and a decreased frequency for growth-enhancement perturbations. Bloomington stock numbers for UAS-RNAi transgenic lines are included. All the perturbations shown here were driven by the nub > GCaMP6f tester line. (C) An inferred model is shown consistent with observations in (A) and (B). (D) Schematic summarizing key findings from this work and the literature is shown. Both RTK/PLCγ− and GPCR/PLCβ-based signaling contributes to Ca2+ activity in vivo and ex vivo (inputs). Perturbations to core Ca2+ signaling pathway result in a range of developmental phenotypes (outputs), including wing size, vein differentiation, cell mechanics, and overall tissue shape. To see this figure in color, go online.
In sum, our results provide multiple lines of evidence that reveal general trends when wing disc growth is perturbed: morphogenetic perturbations that reduce growth lead to abnormally higher levels of integrated Ca2+ signaling activity above the manifold, relating size to integrated Ca2+ activity. In contrast, perturbations that promote growth lead to reduced levels of total (integrated) Ca2+ signaling.
Discussion
Here, we have performed a systems-level analysis of Ca2+ signaling in a model system of organ growth and morphogenesis. To do so, we have established an innovative “Ca2+ decoding” image-processing pipeline. This work advances the analysis of Ca2+ signaling dynamics in organ systems. It demonstrates how a significant portion of cellular information processing occurs through the coupling of signaling dynamics during organ development. This pipeline also can be used to develop spatiotemporal maps of larval wing growth and patterning for other signal integrators such as cAMP (66) and for readouts of central growth pathways such as Hippo signaling (67).
This work has established multiple inputs and outputs for the calcium bow-tie network during wing development (Fig. 9 D). We also identified four classes of spontaneous Ca2+ signaling activity during in vivo development in the wing disc: 1) cellular Ca2+ spikes, 2) ICTs, 3) ICWs, and 4) elevated Ca2+ fluttering. We found that increasing Gαq-mediated signaling with increasing concentrations of FEX leads to a natural progression from low (class 1 and 2) to higher levels of Ca2+ signaling responses (classes 3 and 4). These four signaling classes occur both ex vivo and in vivo. Importantly, we found that multiple classes of Ca2+ activity occur and are a regulated phenomenon in vivo. These findings contradict previous suggestions that ICWs may be an ex vivo artifact (26, 68). Future work is needed to specify the full set of specific RTKs, GPCRs, and morphogens that modulate Ca2+ dynamics in vivo.
We demonstrated a negative correlation between the stimulated Ca2+ signaling responses and the wing disc age and size for third instar larvae. Overall, these observations provide evidence for Ca2+ signaling as a readout for overall organ size in the developing wing and a regulator of cellular processes during larval wing development. Through linear regression analysis, we demonstrated a negative power-law correlation between larval age/pouch size and integrated Ca2+ signaling activity. These findings suggest that Ca2+ signaling decreases during the latter stages of larval wing disc growth. The maximal log-likelihood estimation of the power exponent occurred when the estimate had a value of −0.8 ± 0.5. This is consistent with many allometric scaling relationships observed in biological systems wherein quarter-power scaling frequently occurs (69, 70). For example, quarter-power scaling has been observed in the organism metabolic rate, lifespan, growth rate, heart rate, and the concentrations of metabolic enzymes (69). A −0.75-scaling relationship is consistent, near the maximal log-likelihood estimation, and within the 95% confidence interval of the optimal exponent power (Fig. S6 A). This, in turn, may indicate that the underlying metabolic trajectory of organ growth influences the level of agonist-stimulated calcium signaling activity.
Further, we observed anterior-posterior patterning of Ca2+ signaling activity amplitudes in the wing disc. The amplitude is higher in the posterior than in the anterior compartment. As these compartments have been shown to grow at different rates, this result is consistent with the correlation between Ca2+ signaling activity and the growth state of each compartment (71). There are several possible explanations for why there is an absence of amplitude patterning between anterior and posterior compartments for larger discs in Hh (smoRNAi) or Dpp (dppRNAi) signaling-perturbed discs. First, Hh and Dpp signaling may be directly responsible for patterning the anterior-posterior amplitude difference, perhaps through regulation of cAMP levels (72, 73). Second, this may be because the sizes of anterior and posterior compartments are similar under those conditions. Identifying the cause of this phenomenon may yield insight into additional patterning roles for Ca2+ signaling in wing development, including the pupal stages when vein differentiation occurs. Recently, Ca2+ signaling has been connected to proper Hh signaling in zebrafish embryo (74). Our work suggests that Ca2+ signaling may generally be involved in modulating morphogenesis mediated by Hh signaling and other morphogen pathways.
Future work is needed to identify specific mechanisms connecting signal transduction inputs to phenotypic outputs. In a recent article, cellular Ca2+ spikes were found to correlate with secretion of Dpp, a key regulator of wing disc size and tissue patterning (23). We speculate that local cellular spike activity might be connected to the positive regulation of organ growth. SmoRNAi and dppRNAi leads to smaller wing discs and higher integrated Ca2+ intensity when Ca2+ signaling is stimulated by agonists. The data points from growth-reducing perturbation (smoRNAi and dppRNAi, blue solid data points) lie above the negative correlation curve of the control wing discs (black circle or diamond data points, Fig. 9, A and B). In contrast, genetic perturbations leading to more growth (tkvCA and PtenRNAi, orange data points, Fig. 9, A and B) result in reduced Ca2+ signaling responses when stimulated.
These results imply a common underlying regulatory mechanism. As a launching point for future work, we speculate by proposing a simple model that explains the results reported here (Fig. 9 C). First, our experiments demonstrate that FEX stimulates Gαq/PLCβ activity, which results in IP3 generation and IP3-regulated Ca2+ release. Sufficient IP3 production may lead to phosphatidylinositol bisphosphate (PIP2) substrate depletion. In other systems, PIP2 is often rate limiting for Ca2+ signaling (75, 76). PIP2 is also required for phosphatidylinositol trisphosphate generation, which then stimulates cell growth through PI3K/AKT signaling (77). It follows that reduced PI3K signaling resulting from decreased growth stimulation (indirectly through inhibition of Hh or Dpp signaling in our experiments) will lead to higher PIP2 substrate availability and a stronger Ca2+ response. Conversely, decreased PIP2 availability through the inhibition of PTEN (which converts phosphatidylinositol trisphosphate to PIP2) (63, 65) or through constitutively active Dpp signaling (78) would lead to attenuated Ca2+ signaling responses when stimulated by FEX (Fig. 9 C).
This interpretation of the data provides a generalizable and testable hypothesis for future work: if PIP2 levels are more abundant (reduced PI3K signaling and growth activity), more IP3 can be generated, resulting in more Ca2+ signaling for a given agonist response. If PIP2 substrate levels are limiting (as results when PTEN is inhibited or more growth is stimulated), less IP3-stimulated Ca2+ signaling can occur. This hypothetical model would predict that sufficient overexpression of Gαq could lead to reduced organ growth by depleting PIP2 substrate availability for growth stimulation. Future work may identify such relationships across biological systems because all of these molecular components are present in most eukaryotic cells. We term this hypothetical model the “Ca2+ shunt” hypothesis of growth control.
Ca2+ signaling likely modulates other aspects of growth control during larval development. Ca2+ may integrate signals about the availability of nutrients or about mechanical constraints on the tissue. Several known effectors of size control pathways, such as kibra, a regulator of Hippo signaling (79, 80, 81), have Ca2+ signaling binding domains as annotated by InterPro (82).
Additionally, this work motivates new questions regarding how gap-junction communication, and by extension, membrane voltage, influences the overall control of organ size (4). A decrease in cell-cell gap-junction permeability occurs over the course of wing development (83). As gap junctions become less permeable, Ca2+ and IP3 diffuse a shorter distance before being reabsorbed into the endoplasmic reticulum or decaying, respectively. This would explain the transition from ICWs to ICTs and spikes as well as why amplitude is spatially patterned in large discs as development proceeds. Other studies have also implicated gap-junction communication in organ size control (84). For example, Inx2RNAi suppresses growth in the developing eye disc (85). Connexin43 mutants that disrupt gap-junction communication lead to short fin in zebrafish (86). Gap-junction communication also regulates cell differentiation as Inx2-mediated Ca2+ flux is essential for border cell specification in Drosophila (87). Our results suggest that part of the role of gap-junction communication in regulating size and influencing tissue patterning is through the regulation of Ca2+ transients across the tissue. Taken together, it is therefore likely that the role of Ca2+ signaling in wing growth is conserved in other organs.
Our phenotypic analysis provides additional evidence that the Ca2+ signaling module contributes to modulating wing morphogenesis during pupal development and vein cell differentiation. It should be noted that the crossvein defects suggest that these veins are particularly sensitive to levels of morphogen signaling, including Dpp (88). In particular, Dpp signaling has been linked to Ca2+ signaling in the developing wing (23). Perturbing Ca2+ signaling may also be enhancing the crossvein defects that can occur in the MS1096-Gal4 line, which impacts Beadex gene function (89). Future work will need to investigate the mechanisms leading to wing shape and vein differentiation defects, which are specified during pupal development.
Computational modeling is essential for future efforts to decode the regulation and function of Ca2+ signaling (3). Understanding the specific roles of Ca2+ signaling in organ development will require computational models that couple multiple signals of Ca2+ signaling across multiple spatiotemporal scales (90, 91, 92, 93). For example, computational models are particularly useful at the systems level to understand mechanisms for the coupled transport of Ca2+ and wound healing (6). Regarding this study, our findings that the integrated Ca2+ intensity decreases with development is consistent with a model from the neocortex being applied to our wing disc, in which Ca2+ signaling dynamics are weakly coupled with cell-cycle progression and can influence cell-cycle synchrony with neighbors (94, 95). In sum, this effort demonstrates key roles of Ca2+ signaling as a signal integrator in epithelial growth and morphogenesis.
Author Contributions
J.J.Z., Q.W., P.A.B., and C.N. designed and conceived the study. Q.W., D.K.S., F.J.H., C.N., M.K.L, and N.A.-W. performed experiments. P.A.B. developed methods for feature extraction, statistical analysis, and manual annotation. P.A.B. and F.J.H. implemented qualitative analysis pipeline, performed statistical analysis, and performed manual classification of in vivo data. J.C., P.L., and D.Z.C. developed automated methods for image segmentation and registration. Q.W., P.A.B., F.J.H., and J.J.Z. analyzed data and wrote the manuscript. J.J.Z supervised the study.
Acknowledgments
We thank Mark Alber, Christopher Paolucci, Jeffrey Kantor, Gregory Reeves, Alyssa Lesko, Erin Howe, and Yogesh Goyal for helpful feedback, Brandon Greenawalt (Notre Dame Center for Social Research) for helpful conversations regarding statistical methodology, Simon Restrepo for sharing early unpublished observations, Jahmel Jordon and Kara Snyder for technical support, and members of the Zartman Lab for critiques.
The work in this manuscript was supported in part by National Institutes of Health grant R35GM124935, National Science Foundation awards CBET-1403887, CBET-1553826, CNS-1629914, CCF-1217906, and CCF-1617735, Harper Cancer Research Institute Research like a Champion awards (Q.W.), Walther Cancer Foundation Interdisciplinary Interface Training Project (P.A.B.), and the Notre Dame Advanced Diagnostics & Therapeutics Berry Fellowship (C.N.). The authors gratefully acknowledge the Notre Dame Integrated Imaging Facility.
Editor: Stanislav Shvartsman.
Footnotes
Pavel A. Brodskiy and Qinfeng Wu contributed equally to this work.
Supporting Materials and Methods, 11 figures, 7 tables, and 24 videos are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(19)30023-2.
Supporting Material
References
- 1.Dodd A.N., Kudla J., Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 2.Csete M., Doyle J. Bow ties, metabolism and disease. Trends Biotechnol. 2004;22:446–450. doi: 10.1016/j.tibtech.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Brodskiy P.A., Zartman J.J. Calcium as a signal integrator in developing epithelial tissues. Phys. Biol. 2018;15:051001. doi: 10.1088/1478-3975/aabb18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin M., Martyniuk C.J. The bioelectric code: an ancient computational medium for dynamic control of growth and form. Biosystems. 2018;164:76–93. doi: 10.1016/j.biosystems.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Antunes M., Pereira T., Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J. Cell Biol. 2013;202:365–379. doi: 10.1083/jcb.201211039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng H., Gerencser A.A., Jasper H. Signal integration by Ca2+ regulates intestinal stem-cell activity. Nature. 2015;528:212–217. doi: 10.1038/nature16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi K., Yamamoto T.S., Ueno N. Intracellular calcium signal at the leading edge regulates mesodermal sheet migration during Xenopus gastrulation. Sci. Rep. 2018;8:2433. doi: 10.1038/s41598-018-20747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam P.Y., Webb S.E., Miller A.L. Inhibition of stored Ca2+ release disrupts convergence-related cell movements in the lateral intermediate mesoderm resulting in abnormal positioning and morphology of the pronephric anlagen in intact zebrafish embryos. Dev. Growth Differ. 2009;51:429–442. doi: 10.1111/j.1440-169X.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- 10.Markova O., Lenne P.F. Calcium signaling in developing embryos: focus on the regulation of cell shape changes and collective movements. Semin. Cell Dev. Biol. 2012;23:298–307. doi: 10.1016/j.semcdb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Markova O., Sénatore S., Lenne P.F. Calcium spikes in epithelium: study on Drosophila early embryos. Sci. Rep. 2015;5:11379. doi: 10.1038/srep11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteith G.R., McAndrew D., Roberts-Thomson S.J. Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 13.Noren D.P., Chou W.H., Levchenko A. Endothelial cells decode VEGF-mediated Ca2+ signaling patterns to produce distinct functional responses. Sci. Signal. 2016;9:ra20. doi: 10.1126/scisignal.aad3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevarskaya N., Skryma R., Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 15.Restrepo S., Basler K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat. Commun. 2016;7:12450. doi: 10.1038/ncomms12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallingford J.B., Ewald A.J., Fraser S.E. Calcium signaling during convergent extension in Xenopus. Curr. Biol. 2001;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 17.Berridge M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 18.Ohno Y., Otaki J.M. Spontaneous long-range calcium waves in developing butterfly wings. BMC Dev. Biol. 2015;15:17. doi: 10.1186/s12861-015-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.York-Andersen A.H., Parton R.M., Weil T.T. A single and rapid calcium wave at egg activation in Drosophila. Biol. Open. 2015;4:553–560. doi: 10.1242/bio.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chernoff E.A., Hilfer S.R. Calcium dependence and contraction in somite formation. Tissue Cell. 1982;14:435–449. doi: 10.1016/0040-8166(82)90038-6. [DOI] [PubMed] [Google Scholar]
- 21.Kaneuchi T., Sartain C.V., Wolfner M.F. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belgacem Y.H., Borodinsky L.N. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc. Natl. Acad. Sci. USA. 2011;108:4482–4487. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahal G.R., Pradhan S.J., Bates E.A. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development. 2017;144:2771–2783. doi: 10.1242/dev.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hříbková H., Grabiec M., Sun Y.M. Calcium signaling mediates five types of cell morphological changes to form neural rosettes. J. Cell Sci. 2018;131:jcs206896. doi: 10.1242/jcs.206896. [DOI] [PubMed] [Google Scholar]
- 25.Takano K., Obata S., Asashima M. Development of Ca2+ signaling mechanisms and cell motility in presumptive ectodermal cells during amphibian gastrulation. Dev. Growth Differ. 2011;53:37–47. doi: 10.1111/j.1440-169X.2010.01220.x. [DOI] [PubMed] [Google Scholar]
- 26.Balaji R., Bielmeier C., Classen A.K. Calcium spikes, waves and oscillations in a large, patterned epithelial tissue. Sci. Rep. 2017;7:42786. doi: 10.1038/srep42786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narciso C., Wu Q., Zartman J. Patterning of wound-induced intercellular Ca(2+) flashes in a developing epithelium. Phys. Biol. 2015;12:056005. doi: 10.1088/1478-3975/12/5/056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q., Brodskiy P.A., Zartman J.J. In vivo relevance of intercellular calcium signaling in Drosophila wing development. bioRxiv. 2017 [Google Scholar]
- 29.Narciso C.E., Contento N.M., Zartman J.J. Release of applied mechanical loading stimulates intercellular calcium waves in Drosophila wing discs. Biophys. J. 2017;113:491–501. doi: 10.1016/j.bpj.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marada S., Stewart D.P., Ogden S.K. The unfolded protein response selectively targets active smoothened mutants. Mol. Cell. Biol. 2013;33:2375–2387. doi: 10.1128/MCB.01445-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchmann A., Alber M., Zartman J.J. Sizing it up: the mechanical feedback hypothesis of organ growth regulation. Semin. Cell Dev. Biol. 2014;35:73–81. doi: 10.1016/j.semcdb.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz de la Loza M.C., Thompson B.J. Forces shaping the Drosophila wing. Mech. Dev. 2017;144:23–32. doi: 10.1016/j.mod.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Hafen E., Stocker H. How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 2003;1:E86. doi: 10.1371/journal.pbio.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariharan I.K. Organ size control: lessons from Drosophila. Dev. Cell. 2015;34:255–265. doi: 10.1016/j.devcel.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Restrepo S., Zartman J.J., Basler K. Coordination of patterning and growth by the morphogen DPP. Curr. Biol. 2014;24:R245–R255. doi: 10.1016/j.cub.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 36.Narciso C., Zartman J. Reverse-engineering organogenesis through feedback loops between model systems. Curr. Opin. Biotechnol. 2018;52:1–8. doi: 10.1016/j.copbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy J.B. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 38.Basler K., Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- 39.Nellen D., Burke R., Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 40.Strigini M., Cohen S.M. Wingless gradient formation in the Drosophila wing. Curr. Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 41.Mathews J., Levin M. Gap junctional signaling in pattern regulation: physiological network connectivity instructs growth and form. Dev. Neurobiol. 2017;77:643–673. doi: 10.1002/dneu.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badura A., Sun X.R., Wang S.S. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics. 2014;1:025008. doi: 10.1117/1.NPh.1.2.025008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zartman J., Restrepo S., Basler K. A high-throughput template for optimizing Drosophila organ culture with response-surface methods. Development. 2013;140:667–674. doi: 10.1242/dev.088872. [DOI] [PubMed] [Google Scholar]
- 44.Burnette M., Brito-Robinson T., Zartman J. An inverse small molecule screen to design a chemically defined medium supporting long-term growth of Drosophila cell lines. Mol. Biosyst. 2014;10:2713–2723. doi: 10.1039/c4mb00155a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindelin J., Arganda-Carreras I., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang P., Chen J., Chen D.Z. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018) Institute of Electrical and Electronic Engineers; 2018. A new registration approach for dynamic analysis of calcium signals in organs; pp. 934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capdevila J., Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirth C.K., Truman J.W., Riddiford L.M. The ecdysone receptor controls the post-critical weight switch to nutrition-independent differentiation in Drosophila wing imaginal discs. Development. 2009;136:2345–2353. doi: 10.1242/dev.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graveley B.R., Brooks A.N., Celniker S.E. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pézier A.P., Jezzini S.H., Blagburn J.M. Shaking B mediates synaptic coupling between auditory sensory neurons and the giant fiber of Drosophila melanogaster. PLoS One. 2016;11:e0152211. doi: 10.1371/journal.pone.0152211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eid J.P., Arias A.M., Dziadek M. The Drosophila STIM1 orthologue, dSTIM, has roles in cell fate specification and tissue patterning. BMC Dev. Biol. 2008;8:104. doi: 10.1186/1471-213X-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukami K., Inanobe S., Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res. 2010;49:429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Murillo-Maldonado J.M., Zeineddine F.B., Riesgo-Escovar J.R. Insulin receptor-mediated signaling via phospholipase C-γ regulates growth and differentiation in Drosophila. PLoS One. 2011;6:e28067. doi: 10.1371/journal.pone.0028067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston L.A., Gallant P. Control of growth and organ size in Drosophila. BioEssays. 2002;24:54–64. doi: 10.1002/bies.10021. [DOI] [PubMed] [Google Scholar]
- 55.Schaffter T. Ecole Polytechnique Fédérale de Lausanne; 2014. From genes to organisms: Bioinformatics system models and software. PhD thesis. [Google Scholar]
- 56.Dahmann C., Oates A.C., Brand M. Boundary formation and maintenance in tissue development. Nat. Rev. Genet. 2011;12:43–55. doi: 10.1038/nrg2902. [DOI] [PubMed] [Google Scholar]
- 57.Ayers K.L., Thérond P.P. Evaluating smoothened as a G-protein-coupled receptor for hedgehog signalling. Trends Cell Biol. 2010;20:287–298. doi: 10.1016/j.tcb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Alcedo J., Ayzenzon M., Hooper J.E. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–232. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 59.Tanimoto H., Itoh S., Tabata T. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell. 2000;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- 60.Box G.E.P., Cox D.R. An analysis of transformations. J. R. Stat. Soc. B. 1964;26:211–252. [Google Scholar]
- 61.Lecuit T., Brook W.J., Cohen S.M. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- 62.Wieser R., Wrana J.L., Massagué J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goberdhan D.C., Paricio N., Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oldham S., Stocker H., Hafen E. The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development. 2002;129:4103–4109. doi: 10.1242/dev.129.17.4103. [DOI] [PubMed] [Google Scholar]
- 65.Gao X., Neufeld T.P., Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- 66.Hackley C.R., Mazzoni E.O., Blau J. cAMPr: a single-wavelength fluorescent sensor for cyclic AMP. Sci. Signal. 2018;11:eaah3738. doi: 10.1126/scisignal.aah3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Y., Alégot H., Irvine K.D. The dynamics of Hippo signaling during Drosophila wing development. Development. 2018;145:dev165712. doi: 10.1242/dev.165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dye N.A., Popović M., Eaton S. Cell dynamics underlying oriented growth of the Drosophila wing imaginal disc. Development. 2017;144:4406–4421. doi: 10.1242/dev.155069. [DOI] [PubMed] [Google Scholar]
- 69.West G.B., Brown J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- 70.West G.B., Brown J.H., Enquist B.J. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 71.Martín F.A., Morata G. Compartments and the control of growth in the Drosophila wing imaginal disc. Development. 2006;133:4421–4426. doi: 10.1242/dev.02618. [DOI] [PubMed] [Google Scholar]
- 72.Praktiknjo S.D., Saad F., Hipfner D.R. Activation of Smoothened in the Hedgehog pathway unexpectedly increases Gαs-dependent cAMP levels in Drosophila. J. Biol. Chem. 2018;293:13496–13508. doi: 10.1074/jbc.RA118.001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofer A.M. Interactions between calcium and cAMP signaling. Curr. Med. Chem. 2012;19:5768–5773. doi: 10.2174/092986712804143286. [DOI] [PubMed] [Google Scholar]
- 74.Klatt Shaw D., Gunther D., Grunwald D.J. Intracellular calcium mobilization is required for sonic hedgehog signaling. Dev. Cell. 2018;45:512–525.e5. doi: 10.1016/j.devcel.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loew L.M. Where does all the PIP2 come from? J. Physiol. 2007;582:945–951. doi: 10.1113/jphysiol.2007.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu C., Watras J., Loew L.M. Kinetic analysis of receptor-activated phosphoinositide turnover. J. Cell Biol. 2003;161:779–791. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vinayagam A., Kulkarni M.M., Perrimon N. An integrative analysis of the InR/PI3K/Akt network identifies the dynamic response to insulin signaling. Cell Rep. 2016;16:3062–3074. doi: 10.1016/j.celrep.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martín-Castellanos C., Edgar B.A. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development. 2002;129:1003–1013. doi: 10.1242/dev.129.4.1003. [DOI] [PubMed] [Google Scholar]
- 79.Baumgartner R., Poernbacher I., Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Genevet A., Wehr M.C., Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu J., Zheng Y., Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finn R.D., Attwood T.K., Mitchell A.L. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 2017;45:D190–D199. doi: 10.1093/nar/gkw1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weir M.P., Lo C.W. Gap junctional communication compartments in the Drosophila wing disk. Proc. Natl. Acad. Sci. USA. 1982;79:3232–3235. doi: 10.1073/pnas.79.10.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levin M. Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 2007;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richard M., Hoch M. Drosophila eye size is determined by Innexin 2-dependent decapentaplegic signalling. Dev. Biol. 2015;408:26–40. doi: 10.1016/j.ydbio.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Hoptak-Solga A.D., Klein K.A., Iovine M.K. Zebrafish short fin mutations in connexin43 lead to aberrant gap junctional intercellular communication. FEBS Lett. 2007;581:3297–3302. doi: 10.1016/j.febslet.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahu A., Ghosh R., Prasad M. A gap junction protein, Inx2, modulates calcium flux to specify border cell fate during Drosophila oogenesis. PLoS Genet. 2017;13:e1006542. doi: 10.1371/journal.pgen.1006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ralston A., Blair S.S. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 2005;280:187–200. doi: 10.1016/j.ydbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Milán M., Diaz-Benjumea F.J., Cohen S.M. Beadex encodes an LMO protein that regulates Apterous LIM-homeodomain activity in Drosophila wing development: a model for LMO oncogene function. Genes Dev. 1998;12:2912–2920. doi: 10.1101/gad.12.18.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fletcher A.G., Osterfield M., Shvartsman S.Y. Vertex models of epithelial morphogenesis. Biophys. J. 2014;106:2291–2304. doi: 10.1016/j.bpj.2013.11.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aegerter-Wilmsen T., Heimlicher M.B., Basler K. Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development. 2012;139:3221–3231. doi: 10.1242/dev.082800. [DOI] [PubMed] [Google Scholar]
- 92.Kursawe J., Brodskiy P.A., Fletcher A.G. Capabilities and limitations of tissue size control through passive mechanical forces. PLoS Comput. Biol. 2015;11:e1004679. doi: 10.1371/journal.pcbi.1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nematbakhsh A., Sun W., Alber M. Multi-scale computational study of the mechanical regulation of cell mitotic rounding in epithelia. PLoS Comput. Biol. 2017;13:e1005533. doi: 10.1371/journal.pcbi.1005533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barrack D.S., Thul R., Owen M.R. Modelling cell cycle synchronisation in networks of coupled radial glial cells. J. Theor. Biol. 2015;377:85–97. doi: 10.1016/j.jtbi.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 95.Barrack D.S., Thul R., Owen M.R. Modelling the coupling between intracellular calcium release and the cell cycle during cortical brain development. J. Theor. Biol. 2014;347:17–32. doi: 10.1016/j.jtbi.2014.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nub-Gal4>UAS-GCaMP6f, in ZB media + 0% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 2.5% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 5% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 10% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 20% FEX.
nub-Gal4>UAS-GCaMP6f, in ZB media + 40% FEX.
nub-G4>UAS-GCaMP6f, UAS-GαqRNAi, ex vivo, BL# 36775.
nub-G4>UAS-GCaMP6f, UAS-IP3RRNAi, ex vivo, BL# 25937.
nub-G4>UAS-GCaMP6f, UAS-Plc21CRNAi, ex vivo, BL# 31269.
nub-Gal4>UAS-GCaMP6f, in vivo, spike.
nub-Gal4>UAS-GCaMP6f, in vivo, ICT.
nub-Gal4>UAS-GCaMP6f, in vivo, ICW.
nub-Gal4>UAS-GCaMP6f, in vivo, fluttering.
nub-G4>UAS-GCaMP6f, UAS-IP3RRNAi, in vivo, BL# 25937.
nub-G4>UAS-GCaMP6f, UAS-SERCARNAi, in vivo, BL# 44581.
nub-G4>UAS-GCaMP6f, UAS-Inx2RNAi, in vivo, BL# 29306.
nub-G4>UAS-GCaMP6f, UAS-Plc21CRNAi, in vivo, BL# 31269.
nub-G4>UAS-GCaMP6f, UAS-norpARNAi, in vivo, BL# 31113.
nub-G4>UAS-GCaMP6f, UAS-slRNAi, in vivo, BL# 32906.
nub-G4>UAS-GCaMP6f, UAS-SmoRNAi, ex vivo, BL# 43134.
nub-G4>UAS-GCaMP6f, UAS-DppRNAi, ex vivo, BL# 25782.
nub-G4>UAS-GCaMP6f, UAS-Inx2RNAi, ex vivo, BL# 29306.
nub-G4>UAS-GCaMP6f, UAS-PtenRNAi, ex vivo, BL# 25841.
nub-G4>UAS-GCaMP6f, UAS-mCherry, ex vivo, BL# 35787.