Abstract
Cyclocarya paliurus has traditionally been used in medicines and nutraceutical foods. The aims of this study were to determine whether flavonoid accumulation in C. paliurus is dependent on nitrogen (N) availability and to investigate the internal C (carbon)/N balance under controlled conditions. One-year-old seedlings were grown under five increasing available N level treatments (N1–5) and were harvested throughout the 15-d experimental period. The greatest total chlorophyll amount and photosynthetic rate were achieved during the intermediate N treatments (N3 and N4). The greatest starch level was detected in N3. The total C level was relatively stable, but the total N and free amino acid levels increased, which resulted in a decreased C:N ratio. The flavonoid contents in roots and stalks decreased, while leaves showed a different pattern (peaking in N3). The flavonoid level was closely correlated with flavanone-3-hydroxylase activity, which displayed a similar variation pattern, and their levels were significantly positively correlated with those of total C and starch. Thus, the partitioning of C among primary and secondary metabolisms could be responsible for flavonoid biosynthesis and provide the basis for maintaining high yields, which increases the nutritional values of crops and medicinal plants.
Introduction
Secondary metabolites, like flavonoids, have special roles in determining plants quality because they contribute to the colours and flavours of vegetables and fruits, and may have high antioxidant levels that help protect humans from degenerative diseases1–3. Fertilization can increase biomass production in medicinal plant, vegetable and crop cultivation systems; however, it can decrease the biosynthesis and accumulation of physiologically active substances, including flavonoids and terpenes, and further impact the quality of raw materials4,5. As a crucial plant macronutrient that has potential trade-off effects between growth and the secondary metabolism rate, nitrogen (N) has been extensively studied6. Information on the effects of N levels have been acquired using different experimental systems, such as light- and nutrient-controlled tests on two freshwater macrophytes7, feeding different N forms to chamomile plants6, and the gene expression analysis of the flavonoid pathway8.
The biosynthesis of flavonoids, carbon (C)-based secondary substances, is greatly influenced by the N status of the plant, but there is still not a full understanding of the significance of regulating the effects of N. Increasing our knowledge is important not only to clarify the relationship between flavonoid biosynthesis and N metabolism, but also to increase the quality and yield of medicinal plants and crops. Based on the C–nutrient balance hypothesis, fertilization decreases the C:N ratios of plants, reducing surplus C production and decreasing the C-based defences while increasing the utilisation of assimilated N for defence9. The C–nutrient balance hypothesis produces a pattern similar to that predicted by the growth–differentiation balance hypothesis, which predicts that secondary metabolites tend to accumulate under intermediate resource levels owing to the excess pool of assimilates and that the defences can be produced relatively inexpensively9,10. These hypotheses partially form the theoretical basis of quality control in the cultivation of medicinal plants, but they have only partially been supported by studies.
In plants, the main biosynthesis flavonoid pathway is the shikimate pathway, which provides phenylalanine not only for amino acid and protein synthesis but also for the production of secondary metabolites, like flavonoids and terpenes11 (Fig. 1). Therefore, primary and secondary metabolism may compete for the available photosynthetic assimilates, and there is a trade-off in the C allocation10. Generally, plant photosynthesis is less sensitive to nutrient limitations (e.g. N) than growth, which implies that carbohydrate accumulations can exceed growth demands, resulting in their availability for conversion into secondary metabolites. In high available-nutrient environments, large amounts of carbohydrates are allocated to primary metabolism (protein synthesis), while secondary metabolism is limited. Overall, N may regulate the biosynthesis of flavonoids by controlling the C flow allocation between primary and secondary metabolism. This hypothesis has been partially confirmed. For example, high N fertilisation decreases flavonoid accumulation in plants12,13, and N shortage induces carbohydrate (such as starch and fructose) accumulation but decreases amino acid levels within plants14,15.
Figure 1.
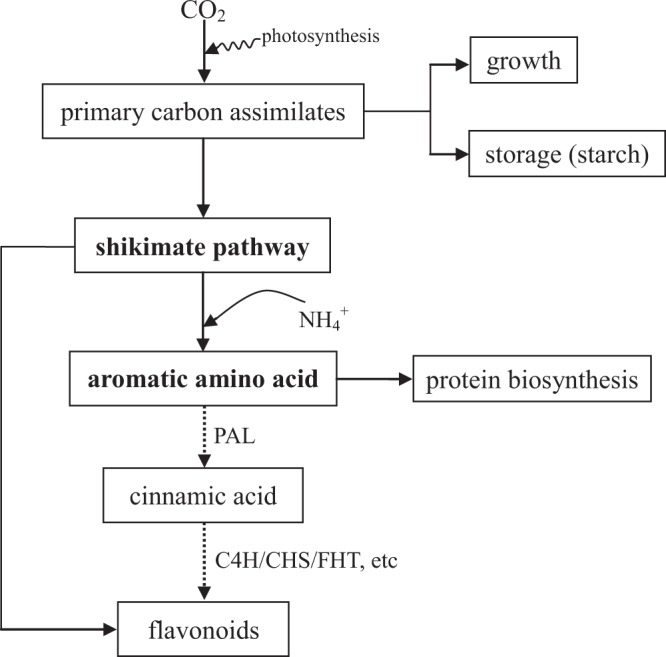
Correlation between flavonoid and amino acid synthesis in Cyclocarya paliurus. The solid and dotted lines indicate primary and secondary metabolism, respectively. PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; CHS, chalcone synthase; FHT, flavanone 3-hydroxylase.
Cyclocarya paliurus, which is a deciduous tree that is widely distributed in the sub-tropical regions of China, belongs to the Juglandaceae family16. As a valuable medicinal woody tree species, the leaves of C. paliurus have long been used in traditional Chinese medicines and as a food resource17. Extracts from C. paliurus leaves have strong health-promoting effects, such as inhibiting protein tyrosine phosphatase 1B, improving mental efficiency and enhancing antihypertensive actions18,19. Chemical constituent studies have shown the presence of abundant physiologically active compounds, such as flavonoids and triterpenoids, in C. paliurus19,20. N fertilization can decrease the flavonoid accumulations in Cyclocarya paliurus leaves21. However, there is limited knowledge of the regulatory mechanisms involving N.
To obtain the more detailed information about the effects of N on flavonoid biosynthesis in C. paliurus, we investigated flavonoid accumulation and related primary metabolism processes. This study aims at illuminate the metabolic mechanism of N control in photosynthetic C allocation. The information will be of great value for increasing the health-promoting effects and establishing the optimal cropping strategies of C. paliurus plants.
Materials and Methods
Plant material and experimental design
Seeds of C. paliurus were collected from natural forests (a selected single tree) of Anji (Zhejiang, China) in late October, 2014. The collected seeds were firstly subjected to chemical scarification, followed by exogenous gibberellin A3 (GA3) treatments, and then stratification treatments using a method described by Fang et al.22. After stratification treatment for 3 months, the germinated seeds were firstly sown in plastic containers (5 cm in diameter, 15 cm in height) and then transplanted to the field (Baima, Nanjing, China) when the seedlings were about 6 cm in height.
In March 2016, the 1-year old plants were retransplanted to experimental containers (20 cm in diameter and 26 cm in height, 1:1 sand-perlite of planting substrate) in Hefei (Anhui, China) and grown in a controlled environment phytotron with 620 μmol·m−2·s−1 light intensity, a 12 h photoperiod, a 25 °C/15 °C diurnal/night temperature and a constant relative humidity of 65%. All plants were watered 2-days intervals with complete nutrient medium (160 mg/L NH4NO3, 408 mg/L KH2PO4, 136 mg/L CaSO4, 120 mg/L MgSO4, 8.4 mg/L EDTA-Fe (ethylenediaminetetraacetic acid), 3.8 mg/L KCl, 1.6 mg/L H3BO3, 0.3 mg/L MnSO4, 0.3 mg/L ZnSO4, 0.1 mg/L CuSO4, 0.1 mg/L Na2MoO4). After 3-week planting, fertilization treatment were conducted. Five fertilization levels were included in this study: 0 mM NH4NO3 (N1), 0.19 mM NH4NO3 (N2), 0.63 mM NH4NO3 (N3), 1.13 mM NH4NO3 (N4), 2.00 mM NH4NO3 (N5). These plants were irrigated 2-days intervals with different N-concentration solution from then on. Three replications were included in each treatment, and each replication consisted of 5 seedlings. Plants materials were harvested throughout the 15 d experiment.
Measurements of photosynthesis and chlorophyll
The net photosynthesis rate was measured during the 9:00–11:00 under the photoperiod condition over the 15-day experiment. Three seedlings per replication were selected (the fully developed leaflets on the 5-th compound leaf below the apex of the plant) for measurements, using LI-6400XT photosynthetic system (LI-COR, Inc. Lincoln NE, USA). All measurements were conducted on 10 cm2 areas of leaves attached to plants in leaf chambers with forced ventilation. The flow rate of gas was 9 cm3/s.
The leaflets were sampled after the measurements of photosynthetic rate for total chlorophyll assaying. The chlorophyll was extracted using 85% (v/v) acetone solution, and the contents were measured using a colorimetric method at 663 and 645 nm, respectively15.
Measurements of starch and amino acid
Leaf samples for starch and amino acid determinations were taken at the 8 h into a photoperiod. Samples for starch assaying were extracted with 80% (v/v) ethanol, after discarding the extract, the residues were further extracted using 3% HCl solution, and then measured spectrophotometrically at 490 nm using phenol-sulphuric acid method23. Free amino acid was measured after 60% (v/v) methanol extraction using a mixture of ninhydrin in distilled water (2 g in 100 mL) and phosphate buffer solution (pH 6.6). Leaf extracts were added to the mix and heated at 85 °C for 25 min, and the absorbance was measured at 568 nm24. Leucine was used as internal standard.
Measurements of carbon and nitrogen
All plants were divided into three organs types: roots, stalks (including branches) and leaves, and then dried (70 °C) and ground. Afterwards, samples were stored at room temperature until analysis. For measurement, 0.5 mg of samples were warped up with tin can (2 × 5 mm), and the total carbon and nitrogen concentration were determined by combusting in an element analyser (EA3000, Euro Vector, Italy).
HPLC-DAD analysis of flavonoids
The fine ground samples for carbon and nitrogen measurements were used for flavonoids analysis. Samples were extracted using an ultrasonic-assisted method with 75% ethanol after removing the fat soluble impurities with petroleum ether. Total flavonoid content was determined using a colorimetric method with detection at 415 nm25. Flavonoid concentration in the extracts was calculated by referencing to a standard rutin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) curve (linearity range: 1.5–52.0 μg rutin/mL, R2 > 0.99) and expressed as milligram rutin equivalent per gram of dry weight (mg/g).
Individual flavonoids, quercetin, isoquercitrin and kaempferol, were determined using high performance liquid chromatograph (HPLC), and all extractions were filtered through a 0.45 μm polytetrafluoroethylene (PTFE) filter prior to HPLC analysis. Quercetin and kaempferol were quantified as aglycones after acid hydrolysis. An Agilent 1200 series system (Waldbronn, Germany) was used, which consisting of an online degasser, a quaternary pump solvent management system, an autosampler, a column heater, an UV/VIS diode array detector (DAD), and a data processing system. Quercetin and kaempferol were separated on an Eclipse Plus C18 column (250 mm × 4.6 mm, 5 μm) at 30 °C, and detected at 365 nm. The mobile phases were methanol (A) and 0.3% phosphoric acid (B) at 55: 45 (VA: VB). For isoquercitrin determination, the mobile phases were methanol (A) and 0.5% phosphoric acid (B). The gradient elution include 0–25 min, 15% A; 15–26 min, 15–90% A; 26–36 min, 90% A; 36–37 min, 90–15% A; and 37–45 min, 25% A. The detection wavelength was 350 nm. The standards quercetin, kaempferol (Sigma-Aldrich Inc., St. Louis, USA), and isoquercitrin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) were used to obtain an external calibration curve.
Measurements of enzyme activity
To measure the levels of PAL, CHS and FHT in leaves of C. paliurus, Plant L-Phenylalanine ammonla-lyase (PAL) ELISA Kit, Plant chalcone synthase (CHS) ELISA Kit, and Plant Flavanone-3-hydroxylase (F3H) ELISA Kit were used, respectively. The purified plant PAL (or CHS, FHT) antibody was used to coat microtiter plate wells, followed by adding PAL to the wells, forming antibody-antigen-enzyme labelled antibody complex. After washing completely, TMB substrate solution was added for color developing at 37 °C for 15 min, and then the reaction was terminated with sulphuric acid solution and the absorbance was performed at 450 nm.
Statistical analysis
For the analysis of variance (ANOVA), Duncan’s multiple-range test was used to calculate significant differences. All statistical analyses were performed at a 95% confidence level. Calculations were conducted using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Effects of N availability on photosynthetic rate and total chlorophyll content
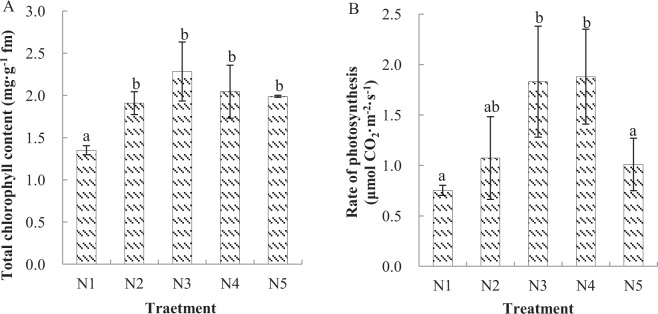
The total chlorophyll contents in leaves of C. paliurus in the phytotron environment were relatively stable when subjected to treatments N2 (0.19 mM NH4NO3) through N5 (2.0 mM NH4NO3), while the contents decreased 41% under N-excluded conditions (Fig. 2A). The greatest net photosynthetic rates, which were significantly greater (p < 0.05) than those during treatments N1 and N5, occurred during treatments N3 and N4 (Fig. 2B).
Figure 2.
Effects of nitrogen treatments on total chlorophyll contents (A) and the photosynthetic rates (B) of Cyclocarya paliurus seedlings. ‘fm’ represents fresh matter. Different letters indicate significant differences among nitrogen treatments in the same category according to Duncan’s test (p < 0.05). N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
Photosynthetic products are the energy bases of primary and secondary metabolisms. Therefore, primary and secondary metabolisms are tightly linked within plants through photosynthesis. Photosynthesis can be influenced by N availability because it affects N partitioning into photosynthetic pigments, enzymes, and the number and composition of chloroplasts26. A positive correlation exists between N availability and photosynthesis27,28, which corroborates our results from treatments N1 to N4. However, the photosynthetic rate during treatment N5 was significantly lower than during treatments N3 and N4. Under natural light conditions, the dry mass accumulation of C. paliurus linearly increases as the fertilization level increases, while the opposite pattern occurs under low light conditions21. Thus, the relative low light intensity conditions in phytotron may partially explain the photosynthetic rate pattern of the present study.
C- and N- metabolisms were changed in response to N availability
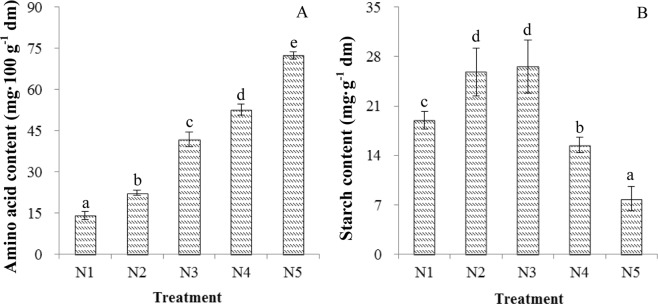
In the present studies, the total C levels in these three organs (leaf, stalk and root) were relatively stable under the five N-level conditions, while the total N levels linearly increased as the N fertilization increased, which resulted in a decreased internal C/N ratio (Table 1). Overall, the internal C:N balance were changed in response to N availability. Furthermore, N availability altered the N and C metabolic levels within plants. The levels of free amino acids linearly increased as the N availability increased (Fig. 3A), while the starch level presented a unimodal curve with the greatest amount occurring during treatment N3 (Fig. 3B). The variation patterns of starch and free amino acid levels indicated that N and C metabolic levels are tightly linked in almost every biochemical pathway within plants. This was confirmed by the total C and total N measurements in roots, stalks and leaves (Table 1).
Table 1.
Variations in the carbon, nitrogen and carbon-to-nitrogen ratio (C/N) in the roots, stalks and leaves of Cyclocarya paliurus seedlings under five different nitrogen fertilization treatments.
| Treatment | Root (%)a | Stalk (%) | Leaf (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| nitrogen | carbon | C/N | nitrogen | carbon | C/N | nitrogen | carbon | C/N | |
| N1 | 1.11a | 42.31a | 38.14c | 0.62a | 41.90a | 68.05d | 2.09a | 42.48b | 20.42c |
| N2 | 1.54ab | 41.63a | 27.28b | 0.87ab | 41.89a | 48.33c | 2.78b | 43.24b | 15.59b |
| N3 | 1.81bc | 41.28a | 23.36ab | 1.07b | 42.06a | 40.58bc | 2.72b | 42.39b | 15.59b |
| N4 | 2.17c | 39.31a | 18.16a | 1.39c | 41.55a | 30.20a | 2.85b | 42.57b | 14.95b |
| N5 | 2.11c | 41.04a | 19.91a | 1.38c | 42.22a | 30.97ab | 3.23c | 39.22a | 12.19a |
aDifferent lowercase letters within a column indicate significant differences among nitrogen treatments in the same category according to Duncan’s test (p < 0.05). N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
Figure 3.
Effects of nitrogen treatments on free amino acid (A) and starch (B) level in leaves measured at 8 h into a photoperiod in Cyclocarya paliurus seedlings. ‘dm’ represents dry matter. Different letters indicate significant differences among nitrogen treatments in the same category according to Duncan’s test (p < 0.05). N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
The C:N balance was influenced by N availability, as observed in other plants, such as grapevine, kale and tobacco2,15,23. The altered internal C:N balance could further affect plants on the following different levels: (1) gene expression involved in N assimilation, photosynthesis and secondary metabolism8,14,15; (2) the concentration and composition of metabolites29,30, which have important roles in determining the qualities of crops and medicinal plants; and (3) the root-to-shoot biomass allocation and morphology of plants23,31. In this study, the total C level was relatively stable, but the photosynthetic C allocation linearly increased with total N as the N availability level increased (Table 1), suggesting that a greater proportion of C was allocated to the N-assimilation pathway. This left less C for C-metabolite biosynthesis under high-N conditions, which was partially demonstrated by the variations in starch and amino acid levels (Fig. 3).
In high-N environments, the induced nitrate reductase activity and nitrate transport typically result in increased N assimilation. Therefore, the levels of N-assimilation (glutamic acid or glutamine) are considered indicators of a high internal N state14. Here, this was mainly evident at the amino acid level, and comparable results have been detected in other experimental systems6,15. Higher amino acid contents under high-N conditions imply that a large amount of C skeletons were allocated to N assimilation and the leaf carbohydrate content declined (Fig. 3B), which re-establishes the equilibrium between photosynthesis and C utilisation15. Starch is the main reserved carbohydrate in plants11, and it is an indicator of the resource pool available for allocation to different metabolic pathways. Primary metabolisms of plants are expected to receive priority for resources over secondary metabolisms. Thus, accumulation of secondary metabolites is usually occurred when there are abundant reserved carbohydrates within plants9, and the allocation trade-offs for C and N may occur among the biochemical pathways within plants.
Even though the C- and N-metabolic levels, which are regulated by the internal C:N balance, have been studied in response to phytochemicals or specific gene expression changes14,15, the mechanistic bases for the regulation remains to be revealed at the global gene express or transcriptional level.
Effects of N availability on flavonoid accumulation and underlying correlation with the C:N balance
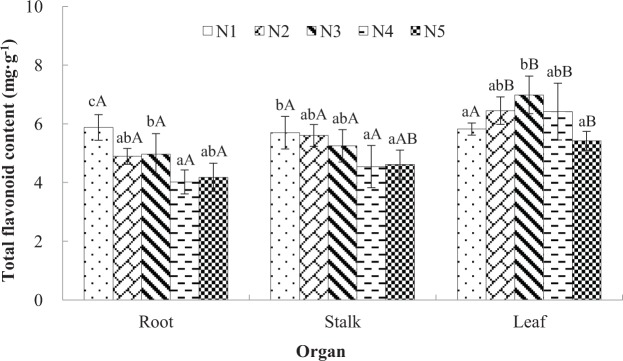
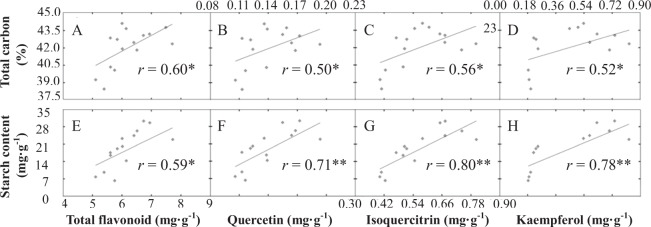
As non-nitrogenous secondary metabolites, flavonoids were significantly influenced by N availability (p < 0.05). The greatest contents of total flavonoid were detected during treatment N1 in roots (5.88 mg/g dry mass (dm) and stalks (5.70 mg/g dm), being 28.9% and 19.1% more than under treatment N5, respectively (Fig. 4). However, flavonoid accumulation in leaves showed a different variation pattern. Intermediate N availability (N3) resulted in the greatest content (6.98 mg/g dm), and the lowest content was detected under treatments N1 (5.82 mg/g dm) and N5 (5.42 mg/g dm). Furthermore, three individual flavonoids were measured in this study. The main individual flavonoid was isoquercitrin, having an average content of 0.22 mg/g dm, followed by kaempferol (0.19 mg/g dm) and quercetin (0.06 mg/g dm) (Table 2). The variation patterns of the three individual flavonoids in different organs under the five N-fertilization conditions were similar to the patterns of total flavonoids. Of the organs evaluated, leaves had the greatest flavonoid content, while roots had the lowest flavonoid content. Along the N gradient, a significant positive correlation existed between flavonoids and the indicators of internal C status (total C and starch), while a stronger correlation existed between flavonoids and starch (Fig. 5A,B).
Figure 4.
Effects of nitrogen treatments on the total flavonoid contents of different organs in Cyclocarya paliurus seedlings. Values within each graph followed by the different letters indicate significant differences among nitrogen treatments (lower case) and organs (upper case) (n = 3) according to Duncan’s test (p < 0.05). N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
Table 2.
Effects of nitrogen availability on the flavonoid contents of different organs in Cyclocarya paliurus seedlings.
| Treatment | Individual flavonoid contents (mg/g)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| quercetin | isoquercitrin | kaempferol | |||||||
| root | stalk | leaf | root | stalk | leaf | root | stalk | leaf | |
| N1 | 0.03dA | 0.06 dB | 0.12aC | 0.06dA | 0.12 dB | 0.50bC | 0.06dA | 0.13 dB | 0.23aC |
| N2 | 0.02cA | 0.04cB | 0.15bC | 0.04cA | 0.09cA | 0.63cB | 0.04cA | 0.11cB | 0.58cC |
| N3 | 0.02bA | 0.03bA | 0.18cB | 0.04cA | 0.07bA | 0.74 dB | 0.04bcA | 0.08bA | 0.76 dB |
| N4 | 0.01 aA | 0.02 aA | 0.15bB | 0.03bA | 0.04 aA | 0.55bB | 0.03abA | 0.06 aA | 0.46bB |
| N5 | 0.01 aA | 0.02 aA | 0.11aB | 0.02 aA | 0.04aB | 0.41aC | 0.03 aA | 0.05aB | 0.19aC |
aDifferent letters indicate significant differences among nitrogen treatments (lower case, within column) and organs (upper case, within row) (n = 3) for the same category according to Duncan’s test (p < 0.05). N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
Figure 5.
Correlations between the total carbon level and the contents of total flavonoid (A), quercetin (B), isoquercitrin (C), and kaempferol (D) in leaves of Cyclocarya paliurus after 15 d of five different N-level fertilization treatments. Correlations between the starch content and the contents of total flavonoid, quercetin, isoquercitrin, and kaempferol were marked (E–H), respectively.
C. paliurus is a relative fast-growing tree species, and it possesses high plasticity levels in anatomical structure, biochemistry and phenotype21,32. The biosynthesis and accumulation of flavonoids in C. paliurus leaves can be significantly induced by environmental factors, such as light intensity, soil nutrients and planting site21,33. Thus, the inducible secondary metabolites in this plant could be significantly influenced by fertilization, which has been partially demonstrated in a previous study21. However, limited information is available regarding the underlying regulatory effects of N fertilization. Understanding the regulatory effects of N on photosynthetic C allocation is important for the development of new agricultural practices that maintain high crop yields as well as the nutritional values of crops and medicinal plants.
The shikimate pathway, which links flavonoid biosynthesis and N metabolism within plants, catalyses the carbohydrates that come from the glycolysis and pentose phosphate pathways to synthesise aromatic amino acids (phenylalanine, tyrosine and tryptophan)11. The biosynthesis of flavonoids is catalysed by a series of enzymes, such as phenylalanine ammonia lyase (PAL), cinnamate 4-hydroxylase, 4-coumaroyl:CoA-ligase, chalcone synthase (CHS) and flavanone 3-hydroxylase (FHT). Lillo et al.8 revealed that the supply from the shikimate pathway is important for C flux into the flavonoid pathway, in which flavonoids are synthesised from phenylalanine. Many biotic and abiotic factors can trigger the activities of the above enzymes, such as nutrient limitation, light intensity and fungal infection34–36. Consequently, it is not surprising that flavonoid biosynthesis is regulated by N availability through photosynthetic C allocation among different biochemical pathways.
PAL, which functions at the intersection of primary and secondary metabolism and catalyses phenylalanine to form cinnamic acid, is frequently studied. Here, the PAL activities at low N levels (N1 and N2) were significant greater than at relatively high N levels (N3, N4 and N5) (Table 3). Kováčik and Klejdus6 also found that Matricaria chamomilla plants cultured under high N conditions had increased PAL activity levels and flavonoid contents in shoots. However, the greater PAL activity during low N availability did not result in a high flavonoid accumulation in C. paliurus leaves. This phenomenon was also observed in Pyrus communis37 and Vitis vinifera38. Furthermore, the CHS activity was significant greater at high N levels (N4 and N5), and its lowest activity level occurred in N3 (Table 3). Thus, the upstream genes in the flavonoid biosynthetic pathway (PAL and CHS) may not be key enzyme-encoding genes. Interestingly, the variation pattern of FHT activity was completely consistent with the flavonoid accumulation pattern in leaves of C. paliurus, which suggested that FHT is the key enzyme in the flavonoid biosynthetic pathway. FHT expression can be activated by carbohydrates and promote flavonoid accumulation39. Flavonoid and carbohydrate accumulations occurred under intermediate N levels, which indicated that N availability can impact the C:N balance and influence flavonoid accumulation within plants. Thus, the internal C:N balance can control not only the partitioning of C within the amino acid and carbohydrate biosynthesis pathways but also in the flavonoid biosynthesis pathway. However, more convincing work is needed to integrate biochemical and global gene expression changes related to C allocation between primary and secondary metabolisms in the future. In the present study, a strong positive correlation between flavonoids and reserved starch was evidenced by the Pearson’s correlation coefficients (p < 0.01, Fig. 5), which indicated that the C surplus within plants was a good indicator of the flavonoid accumulation. Indeed, plants experiencing intermediate to high N availability should have relative stable photosynthetic C, as shown by the variations in photosynthetic rate and total C (Fig. 2B and Table 1). Under the relative high-N conditions, a greater proportion of C was allocated to the N-assimilation, and the left less C was allocated to secondary metabolisms or reserve (Figs 3 and 4).
Table 3.
Enzyme activities of PAL, CHS and FHT in leaves of Cyclocarya paliurus.
| Treatment | Enzyme activity (OD450/g/min)a | ||
|---|---|---|---|
| PAL | CHS | FHT | |
| N1 | 40.5c | 40.3ab | 179.2b |
| N2 | 45.2d | 39.0ab | 219.5c |
| N3 | 25.4ab | 33.2a | 372.3d |
| N4 | 25.6ab | 42.8c | 206.1b |
| N5 | 22.7a | 51.6c | 108.8a |
aDifferent lowercase letters within a column indicate significant differences among nitrogen treatments in the same category according to Duncan’s test (p < 0.05). PAL, phenylalanine ammonia-lyase; CHS, chalcone synthase; FHT, flavonoid 3′-hydoxylase. N1, N2, N3, N4 and N5 represent the concentrations of NH4NO3 were 0, 0.19, 0.63, 1.13 and 2.00 mM, respectively.
The growth–differentiation balance hypothesis cannot be discounted based on the different variation patterns in response to N availability between leaf and stalk (or root) (Table 2). Saito40 showed that chloroplasts can participate in the primary synthesis of flavonoids. Additionally, Arabidopsis roots grown in complete darkness do not accumulate flavonoids because the expression levels of genes encoding flavonoid biosynthesis-related enzymes are light dependent41. Thus, leaves could be the sole tissue in which flavonoid biosynthesis occurs in higher plants. Buer et al.42 demonstrated that flavonoids can be transported long distances from their synthetic sites to distant tissues (root tips) through the vascular system. Therefore, the effects of N on flavonoid biosynthesis may be reflected only by the flavonoid accumulation in leaves (or in whole plants) rather than non-photosynthetic tissues. In addition, the long-distance movement of flavonoids has profound developmental effects, such as regulating auxin transport, controlling root branching and gravitropism42. The root-to-shoot biomass allocation induced by the C:N balance21,22 may partially explain the different patterns of flavonoid accumulations between leaf and stalk (or root) under the five different N treatments.
Acknowledgements
We sincerely thank Xiaoniu Xu and Xiao Tao for contributing to the laboratory work and technical assistance. This study was funded by the National Natural Science Foundation of China (31800528), and the project of Introduce and Stability of Talents (yj2016–03).
Author Contributions
D.B. and G.L. conceived and designed the experiments, and are the lead investigators. D.B., Y.L., D.X. and Q.Y. performed the experiments and analysed the data. D.B. and G.L. contributed with data interpretation. D.B. and G.L. drafted the paper. All authors have read, edited and approved the final version of the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.André CM, et al. Influence of environment and genotype on polyphenol compounds and vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.) J Food Compos Anal. 2009;22:517–524. doi: 10.1016/j.jfca.2008.11.010. [DOI] [Google Scholar]
- 2.Taleon V, Dykes L, Rooney WL, Rooney LW. Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J Cereal Sci. 2012;2:470–475. doi: 10.1016/j.jcs.2012.05.001. [DOI] [Google Scholar]
- 3.Reyes TH, et al. Physiological effects of short acute UVB treatments in Chenopodium quinoa willd. Sci Rep. 2018;8:371–382. doi: 10.1038/s41598-017-18710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenbaek M, et al. Nitrogen split dose fertilization, plant age and frost effects on phytochemical content and sensory properties of curly kale (Brassica oleracea L. var. sabellica) Food Chem. 2016;197:530–538. doi: 10.1016/j.foodchem.2015.10.108. [DOI] [PubMed] [Google Scholar]
- 5.Tshivhandekano I, Mudau FN, Soundy P, Ngezimana W. Effect of cultural practices and environmental conditions on yield and quality of herbal plants: prospects leading to research on influence of nitrogen fertilization, planting density and eco-physiological parameters on yield and quality of field-grown bush tea (Athrixia phylicoides DC.) J Med Plants Res. 2013;7:2489–2493. [Google Scholar]
- 6.Kováčik J, Klejdus B. Induction of phenolic metabolites and physiological changes in chamomile plants in relation to nitrogen nutrition. Food Chem. 2014;142:334–341. doi: 10.1016/j.foodchem.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 7.Cronin G, Lodge DM. Effects of light and nutrient availability on the growth, allocation, carbon/nitrogen balance, phenolic chemistry, and resistance to herbivory of two freshwater macrophytes. Oecologia. 2003;137:32–41. doi: 10.1007/s00442-003-1315-3. [DOI] [PubMed] [Google Scholar]
- 8.Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plnat Cell Environ. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 9.Stamp N. Out of the quagmire of plant defense hypotheses. Q Rev Biol. 2003;78:23–55. doi: 10.1086/367580. [DOI] [PubMed] [Google Scholar]
- 10.Stamp N. Can the growth-differentiation balance hypothesis be tested rigorously? Oikos. 2004;107:439–448. doi: 10.1111/j.0030-1299.2004.12039.x. [DOI] [Google Scholar]
- 11.Taiz, L. & Zeiger, E. Plant physiology (Fourth edition). Sinauer Associates, Inc, Sunderlan, MA, USA (2002).
- 12.Shiwakoti S, et al. Nitrogen, phosphorus, and potassium effects on biomass yield and flavonoid content of American skullcap (Scutellaria lateriflora) J Plant Nutr. 2016;39:1240–1249. doi: 10.1080/01904167.2015.1050509. [DOI] [Google Scholar]
- 13.Ballizany WL, Hofmann RW, Jahufer MZZ, Barrett BA. Genotype × environment analysis of flavonoid accumulation and morphology in white clover under contrasting field conditions. Field Crop Res. 2012;128:156–166. doi: 10.1016/j.fcr.2011.12.006. [DOI] [Google Scholar]
- 14.Coruzzi G, Bush DR. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001;125:61–64. doi: 10.1104/pp.125.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul MJ, Driscoll SP. Sugar repression of photosynthesis: the role of carbohydrates in signaling nitrogen deficiency through source: sink imbalance. Plant Cell Environ. 1997;20:110–116. doi: 10.1046/j.1365-3040.1997.d01-17.x. [DOI] [Google Scholar]
- 16.Fang SZ, et al. Provenance and temporal variations in selected flavonoids in leaves of Cyclocarya paliurus. Food Chem. 2011;124:1382–1386. doi: 10.1016/j.foodchem.2010.07.095. [DOI] [Google Scholar]
- 17.Xie JH, et al. Isolation, chemical composition and antioxidant activities of a watersoluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010;119:1626–1632. doi: 10.1016/j.foodchem.2009.09.055. [DOI] [Google Scholar]
- 18.Wu ZF, et al. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apoliprotein B48 in Caco-2 cells. Phytochemistry. 2017;142:76–84. doi: 10.1016/j.phytochem.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 2010;119:1491–1496. doi: 10.1016/j.foodchem.2009.09.031. [DOI] [Google Scholar]
- 20.Lin Z, et al. The chloroform extract of Cyclocarya paliurus attenuates high-fat diet induced non-alcoholic hepatic steatosis in Sprague Dawley rats. Phytomedicine. 2016;23:1475–1483. doi: 10.1016/j.phymed.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Deng B, et al. Integrated effects of light intensity and fertilization on growth and flavonoid accumulation in Cyclocarya paliurus. Journal of Agricultural and Food Chem. 2012;60:6286–6292. doi: 10.1021/jf301525s. [DOI] [PubMed] [Google Scholar]
- 22.Fang SZ, Wang JY, Wei ZY, Zhu ZX. Methods to break seed dormancy in Cyclocarya paliurus (Batal.) Iljinskaja. Sci Hortic. 2006;110:305–309. doi: 10.1016/j.scienta.2006.06.031. [DOI] [Google Scholar]
- 23.Grechi I, et al. Effect of light and nitrogen supply on internal C: N balance and control of root-to-shoot biomass allocation in grapevine. Environ Exp Bot. 2007;59:139–149. doi: 10.1016/j.envexpbot.2005.11.002. [DOI] [Google Scholar]
- 24.Buysse J, Broeck HVD, Merckx R. The effect of different levels of N limitation on sugars, amino acids, growth and biomass partitioning in broadbean (Vicia faba L.) Ann Bot. 1996;78:39–44. doi: 10.1006/anbo.1996.0092. [DOI] [Google Scholar]
- 25.Bao JS, et al. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J Agric Food Chem. 2005;52:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- 26.Bassi D, Menossi M, Mattiello L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci Rep. 2018;8:2327. doi: 10.1038/s41598-018-20653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchiori PER, Machado EC, Ribeiro RV. Photosynthetic limitations imposed by self-shading in field-grown sugarcane varieties. Field Crop Res. 2014;155:30–37. doi: 10.1016/j.fcr.2013.09.025. [DOI] [Google Scholar]
- 28.Theobald JC, Mitchell RAC, Parry MAJ, Lawlor DW. Estimating the excess investment in ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of spring wheat grown under elevated CO2. Plant Physiol. 1998;118:945–955. doi: 10.1104/pp.118.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariz I, et al. Changes in the C/N balance caused by increasing external ammonium concentrations are driven by carbon and energy availabilities during ammonium nutrition in pea plants: the key roles of asparagine synthetase and anaplerotic enzymes. Physiol Plantarum. 2013;148:522–537. doi: 10.1111/j.1399-3054.2012.01712.x. [DOI] [PubMed] [Google Scholar]
- 30.Kruse J, et al. Elevated pCO2 affects C and N metabolism in wild type and transgenic tobacco exhibiting altered C/N balance in metabolite analysis. Plant Biol. 2003;5:540–549. doi: 10.1055/s-2003-44792. [DOI] [Google Scholar]
- 31.Wang LH, et al. Biomass allocation, compensatory growth and internal C/N balance of Lolium perenne in response to defoliation and light treatments. Pol J Ecol. 2016;64:485–499. doi: 10.3161/15052249PJE2016.64.4.004. [DOI] [Google Scholar]
- 32.Liu Y, et al. Effect of light regime and provenance on leaf characteristics, growth and flavonoid accumulation in Cyclocarya paliurus (Batal) Iljinskaja coppices. Bot Stud. 2016;57:1–13. doi: 10.1186/s40529-016-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng B, et al. Variation and stability of growth and leaf flavonoid content in Cyclocarya paliurus across environments. Ind Crop Prod. 2015;76:386–393. doi: 10.1016/j.indcrop.2015.07.011. [DOI] [Google Scholar]
- 34.Zhang LL, et al. Seasonal variation and gender pattern of phenolic and flavonoid contents in Pistacia chinensis Bunge inflorescences and leaves. J Plant Physiol. 2016;191:36–44. doi: 10.1016/j.jplph.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Logemann E, Parniske M, Hahlbrock K. Modes of expression and common structural features of the complete phenylalanine ammonia-lyase gene family in parsley. P Nati Acad Sci USA. 1995;92:5905–5909. doi: 10.1073/pnas.92.13.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheible WR, et al. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopis in response to nitrogen. Plant Physiol. 2004;136:2483–2499. doi: 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyn WJ, et al. Regulation of pear color development in relation to activity of flavonoid enzymes. J. Am. Soc. Hortic. Sci. 2004;129:6–12. doi: 10.21273/JASHS.129.1.0006. [DOI] [Google Scholar]
- 38.Hiratsuka S, et al. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Sci. Hortic. 2001;90:255–264. doi: 10.1016/S0304-4238(00)00266-1. [DOI] [Google Scholar]
- 39.Zheng YJ, et al. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009;58:251–260. doi: 10.1007/s10725-009-9373-0. [DOI] [Google Scholar]
- 40.Saito K. Possible site of flavonoid synthesis in the photosynthetic apparatus. Biochem J. 1974;144:431–432. doi: 10.1042/bj1440431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins GI, et al. UV and blue light signalling: pathways regulating chalcone synthase gene expression in. Arabidopsis. New Phytol. 2001;151:121–131. doi: 10.1046/j.1469-8137.2001.00151.x. [DOI] [PubMed] [Google Scholar]
- 42.Buer CS, Muday GK, Djordjevic MA. Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol. 2007;145:478–490. doi: 10.1104/pp.107.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.