Graphical abstract
Highlights
-
•
Primary mitral regurgitation (MR) is commonly due to mitral valve (MV) prolapse.
-
•
Off-pump minimally invasive MV repair is an alternative to conventional MV surgery.
-
•
This case describes MV repair using the Harpoon TSD-5 device.
-
•
The technique facilitates image-guided minimally invasive repair.
-
•
Early data suggest the Harpoon procedure is safe and durable and reduces morbidity.
Introduction
Primary mitral regurgitation (MR) is most commonly due to mitral valve (MV) prolapse (Carpentier's classification type II),1 and conventional surgical repair or replacement is the usual surgical treatment.2 Off-pump MV repair with expanded polyfluoroethylene (ePTFE) chordal insertion using the Harpoon TSD-5 device (Harpoon Medical, Baltimore, MD) is an alternative to conventional MV surgery for severe MR in selected cases. The advantages of this procedure include lack of requirement for sternotomy, cardiopulmonary bypass, aortic cross clamp, or cardioplegia.3 However, currently the device is suitable only for highly selected patients, namely, those with MR due to P2 prolapse only who have favorable anterior mitral leaflet anatomy to allow good coaptation following procedure completion. We describe the case of a patient with severe MR due to P2 prolapse who underwent MV repair using the Harpoon TSD-5 device.
Case Report
A 53-year-old university lecturer with breathlessness and severe MR was referred to our center for MV assessment. He underwent quantitative real-time three-dimensional transesophageal echocardiography (RT3DTEE) to assess the etiology of MR, which included potential suitability for mitral repair using the Harpoon device. This revealed a mildly dilated left ventricle (LV) with an estimated ejection fraction of 64%, and severe anterior jet of MR secondary to isolated P2 prolapse. TEE also showed a normal right ventricular size and function but elevated systolic pulmonary arterial pressure of 50 mm Hg. The patient was a candidate for open mitral repair (given his age and lack of significant comorbidities), however, he refused median sternotomy and expressed a preference for a minimally invasive approach as he was keen to return to work soon after his procedure. At the time of assessment, no CE-marked transcatheter MV procedure was available. The patient therefore elected to participate in, and was deemed anatomically suitable for, the mitral Transapical Neochordal Echo-Guided Repair (TRACER) study using the Harpoon system.
The Harpoon Procedure
Following identification of the relevant planes under transthoracic echocardiogram (TTE) guidance (Video 1), transventricular access was gained through a small left anterolateral thoracotomy held open with a tissue retractor. A hemostatic introducer was inserted (Figure 1), through which the delivery system and 21-gauge needle (Figure 2) containing prewound ePTFE sutures could be introduced (Figure 3).
Figure 1.
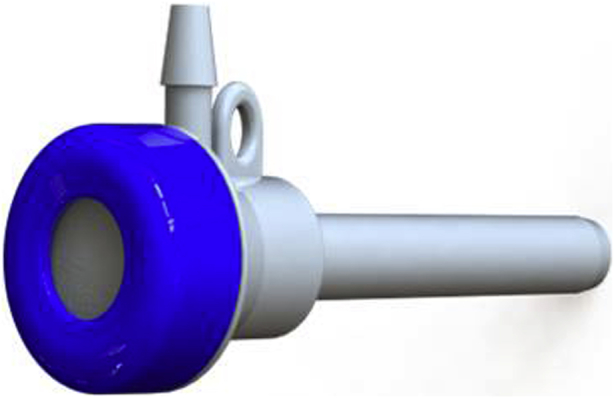
Hemostatic introducer (14 Fr).
Figure 2.

Delivery system (3 mm, 9 Fr).
Figure 3.
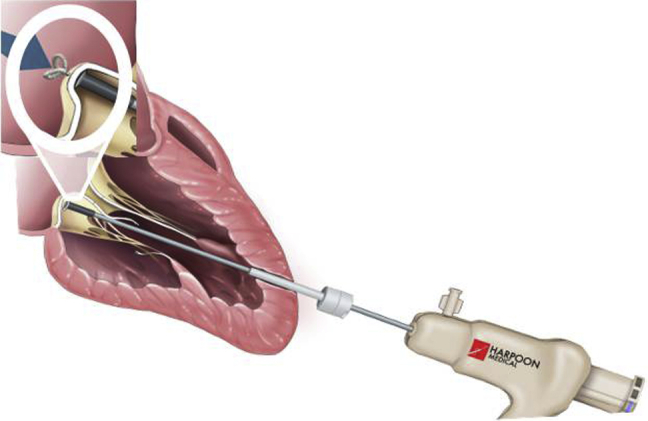
Introduction of delivery system through apex and perforation of posterior leaflet with needle containing preformed ePTFE knot.
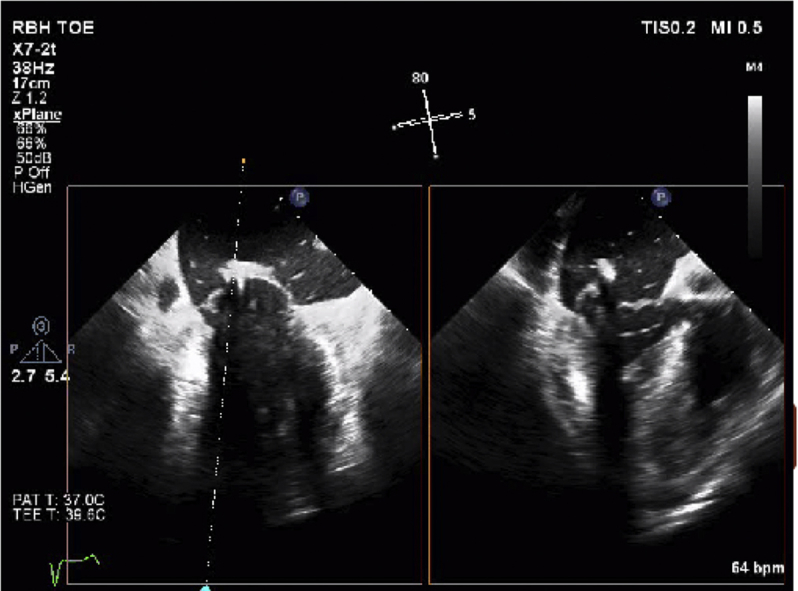
Using RT3DTEE, a series of six ePTFE chords were implanted spanning the whole length of the wide prolapsing P2 segment starting from the lateral aspect of the P2 prolapse (i.e., toward P1; Video 2) and working sequentially toward the medial aspect of the P2 prolapse (i.e., toward P3).
Orthogonal x-plane RT3DTEE views using midcommissural and simultaneous long-axis planes were used to steer the TSD-5 introducer to the ventricular surface of the P2 scallop. Once correct positioning was confirmed in the orthogonal TEE planes (Video 3), the P2 scallop was perforated and a preformed ePTFE knot was deployed on the atrial side of the leaflet under continuous TEE guidance (Video 4).
As each ePTFE knot is approximately 4-5 mm in width, a series of six chords were deployed to cover the length of the prolapsing P2 scallop in this patient (Video 5). The ePTFE knots were placed 4-5 mm from the free edge of the P2 scallop to ensure adequate anchoring and to minimize risk of tear and failure of the ePTFE knots.
En face RT3DTEE imaging using the surgeon's view was used to assess the spacing of the knots on the atrial aspect of the P2 scallop (Videos 6 and 7). Once all the knots were placed, simultaneous chordal tensioning on all knots was then performed under continuous TEE guidance until maximal leaflet coaptation was obtained (Video 8), with no concurrent MR on color Doppler interrogation (Video 9). This resulted in an excellent echocardiographic result with no MR (Videos 10 and 11).
The free ends of the chords were finally fixed epicardially over a stiff Teflon pledget. Routine drains were inserted, and a nerve block was also inserted for pain relief.
The clinical outcome was excellent: the patient was discharged on day 4 postprocedure with no complications (such as bleeding, myocardial infarction, sepsis, or arrhythmia). Follow-up at 30 days with a TTE showed no MR, right ventricular systolic pressure < 20 mm Hg, normal LV cavity size with preserved ejection fraction of 55%-60%, and low LV filling pressures with an E/Lat E' of 10.0. He will continue to be followed up with a repeat TTE at 6 months postprocedure.
Discussion
In patients with degenerative MR, the literature has long shown that MV repair is superior to replacement for both prognostic benefit and minimizing valve-related morbidity.4 MV repair with ePTFE chords has also been proven to have favorable outcomes, introduced clinically in 1986 and most conventionally performed using standard median sternotomy approaches prior to recent minimally invasive methods.5 While initial minimally invasive chordal repairs such as the NeoChord DS1000 have demonstrated successful early results, there have been some shortcomings such as requirements for reoperation and blood transfusions.1 Findings from the Transapical Artificial Chordae Tendinae (TACT) trial demonstrated acute procedural success in 86.7% of the patients undergoing NeoChord implantation (i.e., achieving less than 2+ grade MR from 3+ or 4+), although this number fell to 56.7% at 30 days.6 The TACT trial also recorded 16.7% of patients requiring more than two units of blood transfusions.6
An advantage of the Harpoon device in comparison to NeoChord is the reduced diameter of the size 9-Fr delivery system (a decrease in size by 5 mm) and a hemostatic valve in the introducer, both of which minimize intraoperative bleeding.1 In the initial reports of the mitral TRACER study using the Harpoon system, the authors reported a 100% procedural success rate, and nine out of 11 patients had none/trace or mild MR at 30-day follow-up.3 No blood transfusions were required during the inpatient stay, and only one person required conventional open mitral repair at postoperative day 72. Following on from these results, the authors report prospective data more recently, with 30-day outcomes of mild MR or less in 89% (24 of 27) and moderate in 11% (three of 27) of subjects.7 At 6 months, MR was mild or less in 85% (22 of 26) of patients, with the less than optimal MR reduction in four patients attributed to use of an insufficient imaging platform, improper patient selection (advanced mitral annular calcification), or surgeon error (leaflet perforation). These were deemed to be modifiable risk factors and likely to be improved upon or eliminated with growing clinical experience in the procedure.7 Early LV remodeling was observed at both 30 days and 6 months and was composed of significant reduction in LV end-diastolic volumes and filling pressures, as noted with our patient whose mildly dilated LV cavity size was reported as normal with low LV filling pressures on 30-day follow-up TTE.
Gammie et al. (2018) have also noted that the tensioning of the ePTFE cords allows for significant reduction in mitral annular area at 6 month follow-up, with an average 31% reduction.7 Additionally, systolic anterior motion (SAM), which is often a common and significant complication that can warrant prolonged cardioplegic arrest or MV replacement in conventional open mitral repair, has also been managed with chordal tensioning and shortening of the ePTFE cords, allowing for resolution of systolic anterior motion in two patients intraoperatively with no recurrence.7
Both initial and more recent trial data from the TRACER study have shown low requirement of conversion to open mitral repair following Harpoon repair.3, 7 While the NeoChord system allows for removal of the cords before knotting and securing the chordae, the ePTFE sutures fired using the Harpoon device are not removable and therefore could necessitate resection at future conventional mitral repair if required. Apart from potential risk of resection, if open repair is needed, the fixed length and tension of the chordae that are set based solely on echocardiographic assessment can disrupt the mitral complex if connected inadequately.1 This can affect the leaflet coaptation, the hemodynamics across the MV, or, indeed, with too much tension, dehiscence at the attachment site and rarely subsequent rupture of the knot through the leaflet.8 Yet for both early conversion to open repair and late reoperation for open mitral repair, the three patients in the most recent TRACER trial cohort that required these were noted to have no damage to the mitral leaflets and well-incorporated ePTFE knots at a later stage, allowing for nonresectional repair in all cases and no signs of knot dehiscence.7 Our patient remains free of any evidence necessitating reintervention at present.
RT3DTEE guidance during the Harpoon repair is mandatory and allows for optimal adjustment of the ePTFE cords on a beating heart with physiologic filling pressures to enable accurate leaflet coaptation that is not feasible during open surgical repair on an arrested heart.1, 7 Our case highlights the further importance of minimally invasive off-pump mitral repair as an option for asymptomatic patients with severe MR who can choose an earlier intervention and avoid the comorbid state of the majority of these patients who often present late with atrial fibrillation and impaired left ventricular systolic function.1
Conclusion
Further follow-up is necessary to assess long-term efficacy, as well as direct comparison with conventional open surgical MV repair.7 However, as this case has highlighted, early clinical data suggest the Harpoon procedure is safe and durable and significantly reduces morbidity. The technique facilitates image-guided minimally invasive repair and enables fine-tuning with dynamic ePTFE cord titration/calibration on the beating heart. Our patient has had excellent outcomes and continues to be under follow-up for evaluation of long-term results.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.04.010.
Supplementary Data
Operator balloting LV apex to determine best position for needle insertion, aiming basal to apex and lateral to the left anterior descending artery.
Insertion of ePTFE suture with preformed knot through apex.
Positioning of ePTFE chord prior to deployment of knot.
Deployment of first preformed ePTFE knot through P2 scallop.
Simultaneous x-plane RT3DTEE showing deployment of subsequent ePTFE knots to P2 scallop, going from lateral to medial (in this case more medial than Video 4).
Three-dimensional reconstruction of surgeon's view of MV showing first loose ePTFE knot on atrial side of P2 (aorta top, medial on right, lateral on left, posterior on bottom).
Three-dimensional reconstruction of surgeon's view of MV showing all six loose ePTFE knots on atrial side of P2 prior to tensioning.
Chordal tensioning to eliminate P2 prolapse and allow for leaflet coaptation.
Color Doppler flow TEE showing at most trivial MR and excellent coaptation of MV leaflets.
Preoperative three-dimensional reconstruction of surgeon's view of MV showing P2 prolapse and severe regurgitation.
Postoperative three-dimensional reconstruction of surgeon's view of MV showing no prolapse and excellent coaptation of leaflets.
References
- 1.Lancellotti P., Radermecker M., Rodolphe D., Modine T., Oury C., Fattouch K. Transapical beating-heart chordae implantation in mitral regurgitation: a new horizon for repairing mitral valve prolapse. J Thorac Dis. 2016;8:E1665–E1671. doi: 10.21037/jtd.2016.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glower D.D. Surgical approaches to mitral regurgitation. J Am Coll Cardiol. 2012;60:1315–1322. doi: 10.1016/j.jacc.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 3.Gammie J.S., Wilson P., Bartus K., Gackowski A., Hung J., D'Ambra M.N. Transapical beating-heart mitral valve repair with an expanded polytetrafluoroethylene cordal implantation device: initial clinical experience. Circulation. 2016;134:189–197. doi: 10.1161/CIRCULATIONAHA.116.022010. [DOI] [PubMed] [Google Scholar]
- 4.Mohty D., Orszulak T.A., Schaff H.V., Avierinos J.F., Tajik J.A., Enriquez-Sarano M. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation. 2001;104(12 Suppl 1):I1–I7. doi: 10.1161/hc37t1.094903. [DOI] [PubMed] [Google Scholar]
- 5.David T.E., Armstrong S., Ivanov J. Chordal replacement with polytetrafluoroethylene sutures for mitral valve repair: a 25-year experience. J Thorac Cardiovasc Surg. 2013;145:1563–1569. doi: 10.1016/j.jtcvs.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Seeburger J., Rinaldi M., Nielsen S.L., Salizzoni S., Lange R., Schoenburg M. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: The TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol. 2014;63:914–919. doi: 10.1016/j.jacc.2013.07.090. [DOI] [PubMed] [Google Scholar]
- 7.Gammie J.S., Bartus K., Gackowski A., D'Ambra M.N., Szymanski P., Bilewska A. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device: prospective trial. J Am Coll Cardiol. 2018;71:25–36. doi: 10.1016/j.jacc.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 8.Butany J., Collins M.J., David T.E. Ruptured synthetic expanded polytetrafluoroethylene chordae tendinae. Cardiovasc Pathol. 2004;13:182–184. doi: 10.1016/S1054-8807(04)00006-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Operator balloting LV apex to determine best position for needle insertion, aiming basal to apex and lateral to the left anterior descending artery.
Insertion of ePTFE suture with preformed knot through apex.
Positioning of ePTFE chord prior to deployment of knot.
Deployment of first preformed ePTFE knot through P2 scallop.
Simultaneous x-plane RT3DTEE showing deployment of subsequent ePTFE knots to P2 scallop, going from lateral to medial (in this case more medial than Video 4).
Three-dimensional reconstruction of surgeon's view of MV showing first loose ePTFE knot on atrial side of P2 (aorta top, medial on right, lateral on left, posterior on bottom).
Three-dimensional reconstruction of surgeon's view of MV showing all six loose ePTFE knots on atrial side of P2 prior to tensioning.
Chordal tensioning to eliminate P2 prolapse and allow for leaflet coaptation.
Color Doppler flow TEE showing at most trivial MR and excellent coaptation of MV leaflets.
Preoperative three-dimensional reconstruction of surgeon's view of MV showing P2 prolapse and severe regurgitation.
Postoperative three-dimensional reconstruction of surgeon's view of MV showing no prolapse and excellent coaptation of leaflets.


