Abstract
Aims
Sodium intake has been linked to left ventricular hypertrophy independently of blood pressure, but the underlying mechanisms remain unclear. Primary hyperaldosteronism (PHA), a condition characterized by tissue sodium overload due to aldosterone excess, causes accelerated left ventricular hypertrophy compared to blood pressure matched patients with essential hypertension. We therefore hypothesized that the myocardium constitutes a novel site capable of sodium storage explaining the missing link between sodium and left ventricular hypertrophy.
Methods and results
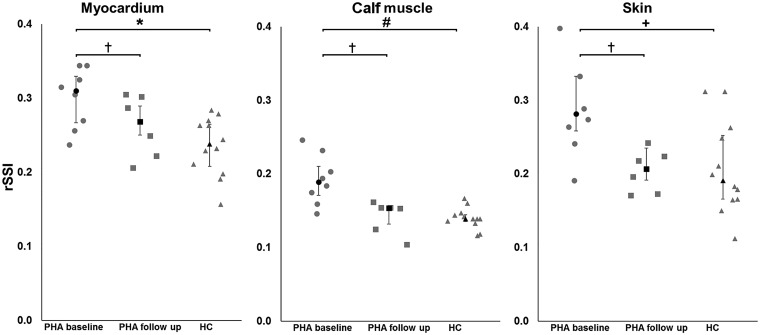
Using 23Na magnetic resonance imaging, we investigated relative sodium signal intensities (rSSI) in the heart, calf muscle, and skin in 8 PHA patients (6 male, median age 55 years) and 12 normotensive healthy controls (HC) (8 male, median age 61 years). PHA patients had a higher mean systolic 24 h ambulatory blood pressure [152 (140; 163) vs. 125 (122; 130) mmHg, P < 0.001] and higher left ventricular mass index [71.0 (63.5; 106.8) vs. 55.0 (50.3; 66.8) g/m2, P = 0.037] than HC. Compared to HC, PHA patients exhibited significantly higher rSSI in the myocardium [0.31 (0.26; 0.34) vs. 0.24 (0.20; 0.27); P = 0.007], calf muscle [0.19 (0.16; 0.22) vs. 0.14 (0.13; 0.15); P = 0.001] and skin [0.28 (0.25; 0.33) vs. 0.19 (0.17; 0.26); P = 0.014], reflecting a difference of +27%, +38%, and +39%, respectively. Treatment of PHA resulted in significant reductions of the rSSI in the myocardium, calf muscle and skin by −13%, −27%, and −29%, respectively.
Conclusion
Myocardial tissue rSSI is increased in PHA patients and treatment of aldosterone excess effectively reduces rSSI, thus establishing the myocardium as a novel site of sodium storage in addition to skeletal muscle and skin.
Keywords: magnetic resonance imaging (MRI), sodium, hypertrophy, primary hyperaldosteronism, myocardial biology
Introduction
In patients with essential hypertension, sodium intake has been studied extensively in the past1,2 and associated with left ventricular hypertrophy (LVH)—a risk factor for adverse cardiovascular events—independent of blood pressure.3–6 In patients with primary hyperaldosteronism (PHA), which is the most common cause of secondary hypertension and characterized by sodium retention by the kidneys due to aldosterone excess,7,8 pronounced LVH was found compared to patients with essential hypertension despite similar blood pressure levels.9 Following treatment of PHA, sodium intake was identified as an independent predictor of reverse remodelling in these patients.10
Tissue sodium accumulation was believed to inevitably be accompanied by retention of free water due to its osmotic activity. However, studies in an animal model of PHA demonstrated that accumulation of sodium in the skin and skeletal muscle occurs in excess of water establishing the concept of water-independent sodium storage.11,12 These experimental findings were confirmed in PHA patients employing sodium magnetic resonance imaging (23Na-MRI), which allows non-invasive quantification of tissue sodium content in humans.13
We hypothesized that sodium accumulates in the myocardium of PHA patients, rendering it an additional site of sodium storage. To test this hypothesis, we established an investigational 23Na-MRI sequence and set up an exploratory proof-of-principle study to compare sodium content in the heart, calf muscle, and skin of PHA patients and healthy controls (HC).
Methods
Subjects and study design
The diagnosis of PHA was based on an aldosterone–renin ratio of ≥25 and a positive saline infusion test after excluding other causes of secondary hypertension. To determine the aldosterone–renin ratio, subjects had to discontinue eplerenone, spironolactone, drospirenone, beta-blockers, aliskiren, angiotensin receptor blockers, and alpha2-antagonists 4 weeks prior testing. Stopping angiotensin-converting enzyme-inhibitors and diuretics was desired but not obligatory. For the saline infusion test, patients received 2000 mL isotonic saline over 4 h. Serum cortisol and plasma aldosterone levels were determined at baseline and after 4 h. Cortisol levels served as an internal validation. Diagnosis of PHA was made if aldosterone levels exceeded a cut-off value of 50 ng/L after 4 h and cortisol levels remained unaltered or declined after 4 h compared to baseline. Cortisol levels were determined by an automated immunoassay (Immulite 2000, Siemens Healthcare Diagnostics GmbH, Germany). Plasma aldosterone and renin levels were determined by automated chemiluminescence immunoassays (ID-iSYS immunodiagnostic systems, Frankfurt am Main, Germany).
HC subjects had to be normotensive (mean 24 h ABPM systolic blood pressure of ≤135 mmHg) and naïve for antihypertensive medication.
All subjects underwent clinical assessment, office blood pressure measurement (Boso medicus uno; Bosch und Sohn, Jungingen, Germany) and 24 h ambulatory blood pressure monitoring (ABPM; Mobil-O-Graph NG ambulatory blood pressure monitor; I.E.M. GmbH, Stolberg, Germany). Patients underwent cardiac 1H-MRI prior to 23Na-MRI of the heart and the calf.
The study was approved by the local Ethics Committee, and written informed consent was obtained from all subjects prior to inclusion. Six PHA patients underwent follow-up examination after at least 4 months of treatment.
Magnetic resonance imaging
MRI studies were performed using a 3 T whole body MRI scanner (Magnetom PRISMA, Siemens, Erlangen, Germany). All subjects underwent cardiac 1H-MR in supine position with an 18-channel coil (Siemens Erlangen, Germany). Left ventricular function was measured with ARGUS volumetry software (Siemens, Erlangen, Germany). To assess extracellular volume (ECV) in the septum, quantitative T1-maps prior to and 10 min after contrast agent injection were acquired using a standard MOLLI technique in 7 HC and 4 PHA patients.14,15
23Na-MRI included a 3D gradient echo sequence [repetition time (TR) 100 ms; echo time (TE) 2.01 ms; flip angle 90°; field of view (FOV) 500 × 500 × 200 mm3; acquisition matrix 128 × 128 × 10 with a resolution of 3.9 × 3.9 × 20 mm3; 8 averages; acquisition time ∼17 min]. A dual tuned 23Na/1H surface coil (Rapid Biomed, Rimpar, Germany) consisting of a loop for 1H (235 × 225 mm), a 23Na transmit coil (single loop 280 × 175 mm) and a combination of a loop (170 × 135 mm) and an 8-shaped coil (180 × 180 mm) for signal reception was used for optimizing signal-to-noise ratio (SNR), as proposed by Haase et al.16 The receive coil sensitivities were determined in a test object and thereafter used to correct for all in vivo measurements. The patients were examined in prone position to minimize breathing motion.17 The acquisition block was positioned double oblique according to the short-axis view. For quantification of septal sodium signal intensities, the region of interest (ROI) was defined to the interventricular septum. The ROI was placed in the centre of the septum to avoid signal contamination from both blood pools.
Sodium signal intensities of skeletal muscle and skin were measured in the calf in supine position with the same sequence and coil as for 23Na imaging of the heart. To assess sodium signal intensity in the calf, two ROIs were placed in the calf muscle and the average of the two ROIs is presented in this study. For sodium signal intensities in the skin, the following parameters had to be adjusted: FOV 500 × 500 × 300 mm3, acquisition matrix 384 × 42 × 10 resulting in a resolution of 1.3 (AP) × 11.8 (RL) × 30 (HF) mm3, and 4 averages. Sodium signal in the skin was also assessed via the average of two different ROIs placed sharply in the skin, to minimize partial volume effects.
Calibration and validation of 23Na-MRI
Vials with predefined sodium concentrations (40, 60, 80, and 100 mmol/L) were placed below the 23Na/1H surface coil and served as external standards for all scans to calibrate for relative tissue sodium. Relative sodium signal intensity (rSSI) as the primary marker of interest was calculated as ratio of tissue sodium intensity to sodium intensity of the 100 mmol/L reference vial. The 100 mmol/L vial was chosen to avoid bias during calibration, as it provided the best SNR. Since 100 mmol/L is a concentration above physiologically expected values, we also investigated the association of measured signal intensity and the sodium concentration gradient throughout the vials. In data from 10 randomly picked examinations, the relationship between the different sodium concentrations in each reference vial and the therefrom obtained signal intensities was investigated by calculating R2.
First results were obtained from animal tissue samples that could be expected to provide different sodium content (bovine kidney, bovine heart, pig kidney, pig neck muscle). These values were compared to results from flame atomic absorption spectrometry (FAAS). Please refer to the Supplementary material online for further details. Since the SNR of 23Na-MRI is rather low, we performed a repeatability analysis of our technique: we examined five healthy volunteers on two different days (time interval between the scans: 2–5 days) and calculated the repeatability coefficient according to Bland and Altman for each investigated compartment.18
Evaluation of imaging data
The cardiac 1H data sets were evaluated by a senior radiologist with 10 years of experience and expert-level certification in cardiac MRI by the national board of radiology (T.K.). Separately, two blinded and independent observers (M.C., A.M.W.) evaluated the sodium data sets. Interobserver variability calculations according to Bland and Altman18 showed close agreement between the two observers with a mean of the differences (95% confidence interval) of −0.01 (−0.05; 0.04) for the septal wall, −0.004 (−0.05; 0.04) for the calf muscle, and 0.02 (−0.05; 0.09) for the skin. Pearson’s correlation coefficients were 0.896, 0.895, and 0.897, respectively.
Data analysis
Results are expressed as median (quartiles). Non-parametric tests (Fisher’s exact test, Mann–Whitney U test) were used to compare the two groups. P-values of <0.05 were considered statistically significant. To investigate possibly confounding effects on the myocardial rSSI, correlation (Spearman’s rho) and linear regression analyses were performed. To compare the repeat measurements of the PHA patients undergoing follow-up examination, Wilcoxon signed rank test was applied.
The post hoc power analysis based on the difference in rSSI found in this study of 0.300 ± 0.041 (PHA patients) vs. 0.235 ± 0.04 (HC) yielded an effect size of 1.60. The given group sizes of 8 and 12 and an alpha of 0.05 yield a power of 0.95.
All statistical analyses were carried out with SPSS 23 (IBM Corp. Armonk, NY, USA).
Results
Validation of 23Na measurement by MRI
To determine the relationship between sodium concentrations in calibration vials and the respective 23Na-MRI signal, 10 measurements were performed yielding a mean R2 of 0.94 (for details see Methods section and Supplementary material online, Section S1). To evaluate the precision of the measurements in tissue, we plotted 23Na-MRI-derived rSSI from animal tissues against sodium concentrations obtained by FAAS after ashing. Both techniques showed excellent correlation across sodium concentrations ranging from 30 to 90 mmol/L (R2 = 0.986, P = 0.006; Supplementary material online, Section S2 and Figure S1). Obtained repeatability coefficients were 0.043 for septum, 0.046 for calf muscle, and 0.018 for skin (for details see Supplementary material online, Section S3).
Measurement of tissue sodium content in PHA patients and healthy controls
Comparing characteristics of PHA patients and HC, there were no significant differences with regard to age or sex, but PHA subjects had a significantly higher body mass index (BMI) (Table 1). Mean 24 h ABPM systolic and diastolic blood pressures were significantly higher in PHA patients. Further, plasma aldosterone levels were higher in PHA patients, whereas serum potassium was markedly lower, despite potassium supplementation in some patients. In contrast, serum sodium concentrations as well as 24 h urinary sodium excretion did not differ between both groups. Cardiac MRI revealed similar left ventricular ejection fraction (LVEF) in HC and PHA patients. Although left ventricular mass index (LVMi) was significantly increased in PHA, only three PHA patients fulfilled criteria of LVH.
Table 1.
Characteristics of healthy controls (HC) and primary hyperaldosteronism (PHA) patients
| HC (n = 12) | PHA (n = 8) | P-value | |
|---|---|---|---|
| Age (years) | 60.5 (46.3, 63.8) | 54.5 (50.8, 58.5) | 0.263 |
| Male, n (%) | 8 (67%) | 6 (75%) | 1.0 |
| Body mass index (kg/m2) | 26.2 (24.4, 28.4) | 32.0 (26.3, 35.7) | 0.037 |
| Mean systolic 24 h ABPM (mmHg) | 125 (122, 131) | 152 (140, 163) | <0.001 |
| Mean diastolic 24 h ABPM (mmHg) | 80 (76, 88) | 95 (90, 96) | 0.004 |
| Heart rate (b.p.m.) | 67 (61, 74) | 72 (69, 74) | 0.202 |
| Aldosterone (ng/L) | 44.5 (36.3, 70.9) | 185 (157, 358) | <0.001 |
| Renin (ng/L) | 7.8 (5.2, 10.6) | 3.2 (2.5, 6.0) | 0.021 |
| ARR | 8.5 (5.5, 12.0) | 69.7 (56.4, 82.4) | <0.001 |
| Serum sodium (mmol/L) | 141 (139, 142) | 142 (141, 144) | 0.080 |
| Serum potassium (mmol/L) | 4.35 (4.2, 4.5) | 3.9 (3.3, 4.0) | 0.001 |
| Creatinine (µmol/L) | 63.3 (57.2, 77.1) | 65.6 (52.7, 78.6) | 0.938 |
| 24 h urinary sodium excretion (mmol/24 h) | 159 (111, 222) | 233 (179, 355) | 0.165 |
| 24 h urinary potassium excretion (mmol/24 h) | 80.0 (58.2, 111.9) | 98.1 (66.7, 132.9) | 0.440 |
| LVEF (%) | 59 (56, 62) | 57 (47, 63) | 0.510 |
| LVM (g) | 99 (86, 134) | 163 (128, 222) | 0.015 |
| LVM index (g/m2) | 55 (50, 67) | 71 (64, 107) | 0.037 |
| ECV (%) | 27.8 (26.4, 30.5)a | 29.2 (25.8, 31.4)b | 0.935 |
| Myocardial rSSI | 0.24 (0.20, 0.27) | 0.31 (0.26, 0.34) | 0.007 |
| Calf muscle rSSI | 0.14 (0.13, 0.15) | 0.19 (0.16, 0.22) | 0.001 |
| Skin rSSI | 0.19 (0.17, 0.26) | 0.28 (0.25, 0.33) | 0.014 |
Data are median (quartiles). Fisher’s exact test or Mann–Whitney U test, as appropriate.
ARR, aldosterone-renin-ratio; ECV, extracellular volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; rSSI, relative sodium signal intensity.
n = 7.
n = 5.
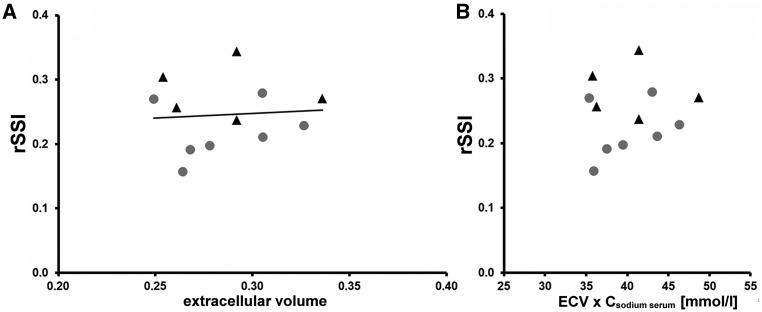
Examples of rSSI of cardiac tissue in a HC subject and PHA patient are shown in Figure 1. PHA patients exhibited significantly higher rSSI in the myocardium compared to HC [0.31 (0.26; 0.34) vs. 0.24 (0.20; 0.27); P = 0.007; Figure 2]. No significant association between the septal rSSI and septal ECV determined from pre- and post-contrast T1-maps was detected (Figure 3A, for pre- and post-contrast T1 data see Supplementary material online, Section S4 and Figure S3). Furthermore, multiplication of serum sodium concentrations with ECV showed no correlation with the rSSI suggesting that the sodium signal was unrelated to individual ECV and serum sodium levels (Figure 3B).
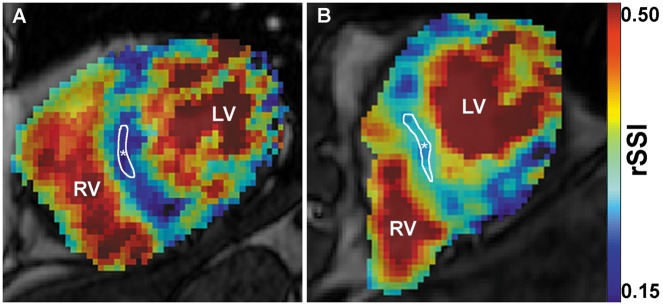
Figure 1.
Short-axis 1H-MRI images of left (LV) and right (RV) ventricle and the interventricular septum merged with corresponding 23Na-MRI images with blue colour indicating low sodium signal and red colour indicating high sodium signal. (A) Representative images of a healthy control subject (60-year-old male; mean 24 h ABPM: 131/90 mmHg), and (B) of a PHA patient (59-year-old male, mean 24 h ABPM: 163/89 mmHg). rSSI of the interventricular septum (*) was 0.21 for the HC and 0.33 for the PHA patient.
Figure 2.
Relative sodium signal intensities (rSSI) in patients with primary hyperaldosteronism (PHA) at baseline (n = 8) and follow-up (n = 6) and in healthy controls (HC; n = 12) in the interventricular septum, calf muscle, and skin. ●, ▪, and ▴ represent medians; error bars represent the 25th/75th percentile (*Pseptum = 0.007; #Pcalf muscle = 0.001; +Pskin = 0.014; †Pbaseline vs. FUP <0.05).
Figure 3.
(A) Relative sodium signal intensities (rSSI) determined by 23Na-MRI as a function of extracellular volume. Data derived from seven healthy controls (●) and five patients with primary hyperaldosteronism (▴). No correlation is apparent (R2 = 0.007; P = 0.802). (B) ECV was multiplied with the corresponding individual serum sodium concentration and plotted against the rSSI to visualize potential outliers or increased sodium signals solely based on higher serum sodium.
Correlation analyses (Spearman’s rho) reveal no association of rSSI with age, sex, or BMI, neither in the whole sample nor within subgroups. In addition, linear regression analyses showed unaffected or only mildly attenuated regression coefficients with univariable or multivariable adjustments for BMI, age, and sex. All P-values indicating a group difference between HC and PHA patients were 0.023 or less.
In addition to the myocardium, tissue sodium intensities were also determined in the calf muscle and skin. PHA patients compared to HC exhibited a significantly higher rSSI in the calf muscle [0.19 (0.16; 0.22) vs. 0.14 (0.13; 0.15); P = 0.001; Figure 2] as well as the skin [0.28 (0.25; 0.33) vs. 0.19 (0.17; 0.26); P = 0.014; Figure 2].
In conclusion, rSSI of PHA patients at baseline was 127% for cardiac, 138% for calf muscle, and 139% for skin, compared to HC.
Measurement of tissue sodium content in PHA patients after treatment
After employing treatment for PHA (unilateral adrenalectomy: n = 1, medical treatment with mineralocorticoid receptor antagonists: n = 5), patients underwent follow-up examinations after a mean of 6.8 ± 2.6 months. Median 24 h systolic ABPM showed a marked reduction following treatment [158 (138; 164) vs. 132 (127; 163) mmHg; P = 0.027] but LVEF [55 (43; 61) vs. 53 (45; 59) %; P = 0.69] and LVMi [71 (63; 120) vs. 74 (62; 95) g/m2; P = 0.35] remained unchanged (see Table 2 for detailed patient characteristics). All three investigated sodium compartments demonstrated significantly reduced rSSI (Table 2 and Figure 2). Mean rSSI change was highest in the skin with −29% [0.28 (0.23; 0.35) vs. 0.21 (0.17; 0.23), P = 0.028], followed by calf with −27% [0.19 (0.16; 0.24) vs. 0.15 (0.12; 0.17), P = 0.046] and myocardium with −13% [0.31 (0.27; 0.33) vs. 0.27 (0.22; 0.30), P = 0.028]. Compared to HC (n = 12), rSSI of PHA patients at follow-up (n = 6) was 111% for cardiac, 102% for calf muscle, and 98% for skin. A detailed listing of the six PHA patients’ blood pressure measurements, aldosterone levels, and cardiac rSSIs is available in the Supplementary material online (Supplementary material online, Section S5 and Table S1).
Table 2.
Baseline and follow-up characteristics of six primary hyperaldosteronism patients
| Baseline | FUP | P-value | |
|---|---|---|---|
| Age (years) | 55 (48, 61) | 56 (49, 62) | 0.014 |
| Male, n (%) | 5 (83%) | ||
| Body mass index (kg/m2) | 31.6 (24.5, 36.4) | 29.8 (25.8, 34.7) | 0.116 |
| Mean systolic 24 h ABPM (mmHg) | 158 (138, 164) | 132 (127, 163) | 0.027 |
| Mean diastolic 24 h ABPM (mmHg) | 95 (89, 97) | 83 (80, 94) | 0.075 |
| Heart rate (b.p.m.) | 72 (67, 77) | 66 (61, 73) | 0.080 |
| Aldosterone (ng/L) | 265 (156, 445) | 285 (85, 338) | 0.249 |
| Renin (ng/L) | 4.0 (2.3, 7.1) | 4.5 (2.5, 10.6) | 0.753 |
| ARR | 70.4 (52.9, 89.4) | 40.7 (19.3, 76.2) | 0.249 |
| Serum sodium (mmol/L) | 142 (141, 145) | 142 (142, 143) | 0.891 |
| Serum potassium (mmol/L) | 3.9 (3.4, 4.0) | 4.4 (3.5, 4.5) | 0.027 |
| Creatinine (µmol/L) | 76.0 (63.6, 95.5) | 81.4 (69.9, 99.9) | 0.046 |
| LVEF (%) | 55 (43, 61) | 53 (45, 59) | 0.686 |
| LVM (g) | 171 (124, 240) | 184 (121, 188) | 0.500 |
| LVM index (g/m2) | 71 (63, 120) | 74 (62, 95) | 0.345 |
| Myocardial rSSI | 0.31 (0.27, 0.33) | 0.27 (0.22, 0.30) | 0.028 |
| Calf muscle rSSI | 0.19 (0.16, 0.24) | 0.15 (0.12, 0.17) | 0.046 |
| Skin rSSI | 0.28 (0.23, 0.35) | 0.21 (0.17, 0.23) | 0.028 |
Data are median (quartiles). Paired group comparisons were done with Wilcoxon signed rank test.
ARR, aldosterone-renin-ratio; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; rSSI, relative sodium signal intensity.
Discussion
The main finding of this proof-of-principle study is that myocardial sodium signal intensity is increased in PHA patients compared to HC suggesting that the myocardium constitutes a site of sodium storage.
Studies by Titze’s group established the concept of water-independent tissue sodium storage. Employing 23Na-MRI these studies analysed tissue sodium content in two human organs (skeletal muscle and skin) and showed that (i) both sites act as sodium reservoirs and (ii) their sodium content varies depending on gender, age, and electrolyte dysbalance.13,19,20
We and others used 23Na-MRI in the past to visualize myocardial scars following myocardial infarction.21–23 Rochitte et al.24 used 23Na-MRI to demonstrate that sodium accumulation in reperfusion areas after myocardial infarction is influenced by microvascular obstruction, but myocardial sodium content has not yet been quantified in patients with the focus on disease-related differences. We here demonstrate that the sodium signal in the interventricular septum of PHA patients is increased by almost 30% compared to HC. This result needs to be interpreted with caution. According to Bottomley,25 total cardiac sodium content is a composite of the intracellular (ICV) and extracellular (ECV) compartment:
The 23Na-MRI sequence used in our study does not allow differentiating between the intra- and extracellular compartment and thus, a relative expansion of the sodium-rich ECV would already result in an increased sodium signal reflecting total tissue sodium. A recent study by Kuruvilla et al. suggested that ECV may expand in hypertensive patients with LVH but not in those without.26 Despite the technical challenges inherent to a precise determination of ECV, we believe that a relevant expansion of the ECV is unlikely to explain the observed increase in sodium signal in PHA patients in our study, given that only three of the eight PHA patients had manifest LVH, and that no obvious associations between ECV and the rSSI were observed taking into account individual serum sodium levels.
Sodium is a nucleus with multi-exponential T2 relaxation in biological tissues with a fast (T2f; typical range: 0.5–4 ms) and slow component (T2s; typical range: 12–32 ms) of its spin–spin relaxation time, depending on its binding state.25 With an TE of 2.01 ms, our technique may only capture parts of the T2f signal, thus not accurately measuring total tissue sodium content. For this reason, we deliberately did not present the tissue sodium content as an absolute sodium concentration but rather as rSSI. It is conceivable that merely a change in the binding state of myocardial sodium may cause a change in the sodium signal without a change in total sodium content. However, given the tight association of sodium signal intensities determined by 23Na-MRI with the actual total tissue content in the FAAS experiments, we believe that the rSSI in this study is a good reflection of total sodium content.
Besides the myocardium, we assessed tissue sodium content in calf muscle and skin. In parallel to the myocardium, we detected a 38% higher sodium content in the calf muscle of PHA patients compared to HC which is in good agreement with a previous report by Kopp et al.13 However, in contrast to this report we also detected an almost 39% higher skin sodium content in PHA patients compared to HC. This divergent finding is likely explained by the higher spatial resolution of the sodium MRI sequence used for skin measurements in our study. We used an inplane resolution of 1.3 mm (compared to 3 mm by Kopp et al.), and thereby markedly minimized partial volume effects of the adjacent subcutaneous fat, which is low in sodium.
Clinical implications
Our findings shed new light on tissue sodium storage in the healthy and the diseased. With regard to the skin and skeletal muscle, both organs appear to serve as sodium reservoirs and thereby contribute to sodium homeostasis in the body in addition to the kidney.27 Impaired sodium storage of these sites is considered pathophysiologically relevant mechanisms in the development of salt-sensitive hypertension.20,28
The consequences of myocardial sodium accumulation remain less clear. Observational data in patients with essential hypertension and PHA patients suggested a role for sodium in the pathophysiology of LVH.4,9,10 Findings in patients with chronic kidney disease revealed a strong correlation of skin sodium content and LVM.29 It is likely that skin sodium content in these patients simply reflected tissue sodium content including the myocardium.
Our findings in PHA patients now provide evidence that tissue sodium signal (i) is increased prior to treatment and (ii) decreases close to normal values under treatment in the skin, calf muscle and, importantly, the heart, where sodium deposition relates to LVM.4,9,10
An exchange of intracellular sodium against potassium was identified as the main mechanism of sodium storage in the skeletal muscle,30 which is likely to be paralleled in the myocardium. Evidence from experimental studies support this notion suggesting that intracellular sodium in LVH is increased31,32 and myocardial growth induced via the salt-inducible kinase 1.33 Further evidence for the importance of intracellular myocardial sodium in LVH comes from a recent animal model of hypertrophic cardiomyopathy. Blockade of the late sodium influx by the drug ranolazine was highly efficacious in preventing the development of LVH in these animals by reducing the intracellular sodium content.34
Besides intracellular sodium accumulation, a second mechanism of water-independent sodium storage has been described where sodium accumulates independently of water by binding to negatively charged proteoglycans in the extracellular matrix.35–37 Two members of these proteoglycans, namely syndecan-438,39 and small leucine-rich proteoglycans,40 have been identified to play a critical role in the pathophysiology of myocardial hypertrophy and thus, extracellular sodium accumulation may be another molecular mechanism linking increased myocardial sodium content with cardiac hypertrophy and fibrosis.
Limitations
Kopp et al.20 found a sex- and age-related difference in sodium storage of skin and muscle. Our study was not powered to detect respective differences in cardiac sodium content. However, we accounted for these differences by employing a sampling strategy that matched for sex and age. Consistent with previous studies, adjustment for BMI did not materially alter the point estimates, thus indicating a true group difference.20
Regarding medical treatment at baseline, patients were not allowed to take mineralocorticoid receptor antagonists and instructed to avoid RAAS influencing medication including diuretics. The latter was not possible in all patients, due to the need of a sufficient blood pressure control. Although rSSI significantly decreased in all compartments, we could not detect a reduction in LVMi at follow-up. Study duration might have been too short as recent studies revealed effects of PHA on LVM only after 12 months of treatment.41,42
SNR remains a critical methodological issue, but the coefficients found in repeatability experiments were reasonably small allowing to quantify differences observed between patients and controls with confidence. Hence, although statistically significant and internally consistent, our findings await validation in a larger cohort with a longer follow-up period.
Perspectives
This is the first study to show that myocardial sodium signal intensities in PHA patients are elevated compared to HC and sensitive to treatment. 23Na-MRI will have the potential becoming a highly valuable tool to study the role of myocardial sodium in healthy and disease subjects. Technical improvements like ultra-short or zero echo time (UTE/ZTE) sequences will allow quantification of absolute sodium concentration and provide increasing accuracy and insight on the underlying mechanisms.
Supplementary Material
Acknowledgements
We thank the nurses/technicians of the Comprehensive Heart Failure Center (CHFC) and the team of the Clinical Study Unit. Special thanks go to Dr T. Wech for his input on sodium sequence development. We also thank the Institute of Radiology, Dr P. Eder-Negrin and her staff, and Dr T. Reiter, Dr M. Terekhov, and Prof. L. Schreiber for methodological support.
Funding
This study was supported by CHFC-grants to M.C., F.H., and H.K.. The CHFC is supported by the German Ministry of Education and Research (Grant Numbers 01EO1004 and 01EO1504).
Conflict of interest: A.M.W., T.K., and H.K. received a grant from Siemens Healthcare outside the submitted work. All other authors declared no conflict of interest.
References
- 1.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 1988;297:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 3. Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, Porcellati C. et al. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol 2002;39:2005–11. [DOI] [PubMed] [Google Scholar]
- 4. Cailar Du G, Fesler P, Ribstein J, Mimran A.. Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin-angiotensin system. Hypertension 2010;56:865–70. [DOI] [PubMed] [Google Scholar]
- 5. Cailar Du G, Ribstein J, Grolleau R, Mimran A.. Influence of sodium intake on left ventricular structure in untreated essential hypertensives. J Hypertens Suppl 1989;7:S258–9. [DOI] [PubMed] [Google Scholar]
- 6. Ott C, Titze SI, Schwarz TK, Kreutz R, Hilgers KF, Schmidt BMW. et al. High sodium intake modulates left ventricular mass in patients with G expression of +1675 G/A angiotensin II receptor type 2 gene. J Hypertens 2007;25:1627–32. [DOI] [PubMed] [Google Scholar]
- 7. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M. et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266–81. [DOI] [PubMed] [Google Scholar]
- 8. Kaplan NM. Primary aldosteronism. J Hypertens 2012;30:1899–902. [DOI] [PubMed] [Google Scholar]
- 9. Pimenta E, Gordon RD, Ahmed AH, Cowley D, Leano R, Marwick TH. et al. Cardiac dimensions are largely determined by dietary salt in patients with primary aldosteronism: results of a case-control study. J Clin Endocrinol Metab 2011;96:2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catena C, Colussi G, Novello M, Verheyen ND, Bertin N, Pilz S. et al. Dietary salt intake is a determinant of cardiac changes after treatment of primary aldosteronism: a prospective study. Hypertension 2016;68:204–12. [DOI] [PubMed] [Google Scholar]
- 11. Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P. et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 2003;285:F1108–17. [DOI] [PubMed] [Google Scholar]
- 12. Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH. et al. Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol 2005;289:F793–802. [DOI] [PubMed] [Google Scholar]
- 13. Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C. et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension 2012;59:167–72. [DOI] [PubMed] [Google Scholar]
- 14. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP.. Modified Look-Locker inversion recovery (MOLLI) for high-resolutionT1 mapping of the heart. Magn Reson Med 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 15. Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J.. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081–6. [DOI] [PubMed] [Google Scholar]
- 16. Haase A, Odoj F, Kienlin Von M, Warnking J, Fidler F, Weisser A. et al. NMR probeheads for in vivo applications. Concepts Magn Reson 2000;12:361–88. [Google Scholar]
- 17. Geier O, Weng AM, Toepell A, Hahn D, Spindler M, Beer M. et al. Acquisition-weighted chemical shift imaging improves SLOOP quantification of human cardiac phosphorus metabolites. Zeitschrift Für Medizinische Physik 2014;24:49–54. [DOI] [PubMed] [Google Scholar]
- 18. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurment. Lancet 1986;327:307–10. [PubMed] [Google Scholar]
- 19. Kopp C, Linz P, Hammon M, Schöfl C, Grauer M, Eckardt K-U. et al. Seeing the sodium in a patient with hypernatremia. Kidney Int 2012;82:1343. [DOI] [PubMed] [Google Scholar]
- 20. Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN. et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 2013;61:635–40. [DOI] [PubMed] [Google Scholar]
- 21. Sandstede JJW, Pabst T, Beer M, Lipke C, Bäurle K, Butter F. et al. Assessment of myocardial infarction in humans with 23Na MR imaging: comparison with Cine MR imaging and delayed contrast enhancement. Radiology 2001;221:222–8. [DOI] [PubMed] [Google Scholar]
- 22. Sandstede JJW, Hillenbrand H, Beer M, Pabst T, Butter F, Machann W. et al. Time course of 23Na signal intensity after myocardial infarction in humans. Magn Reson Med 2004;52:545–51. [DOI] [PubMed] [Google Scholar]
- 23. Ouwerkerk R, Weiss RG, Bottomley PA.. Measuring human cardiac tissue sodium concentrations using surface coils, adiabatic excitation, and twisted projection imaging with minimal T2 losses. J Magn Reson Imaging 2005;21:546–55. [DOI] [PubMed] [Google Scholar]
- 24. Rochitte CE, Kim RJ, Hillenbrand HB, Chen EL, Lima JAC.. Microvascular integrity and the time course of myocardial sodium accumulation after acute infarction. Circ Res 2000;87:648–55. [DOI] [PubMed] [Google Scholar]
- 25. Bottomley PA. Sodium MRI in human heart: a review NMR Biomed 2015;29:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J. et al. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging 2015;8:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Titze J. A different view on sodium balance. Curr Opin Nephrol Hypertens 2015;24:14–20. [DOI] [PubMed] [Google Scholar]
- 28. Laffer CL, Scott RC, Titze JM, Luft FC, Elijovich F.. Balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 2016;68:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C.. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol 2017;28:1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ziomber A, Machnik A, Dahlmann A, Dietsch P, Beck FX, Wagner H. et al. Sodium-, potassium-, chloride-, and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. AJP: Renal Physiol 2008;295:F1752–63. [DOI] [PubMed] [Google Scholar]
- 31. Chahine M, Bkaily G, Nader M, Al-Khoury J, Jacques D, Beier N. et al. NHE-1-dependent intracellular sodium overload in hypertrophic hereditary cardiomyopathy: prevention by NHE-1 inhibitor. J Mol Cell Cardiol 2005;38:571–82. [DOI] [PubMed] [Google Scholar]
- 32. Boguslavskyi A, Pavlovic D, Aughton K, Clark JE, Howie J, Fuller W. et al. Cardiac hypertrophy in mice expressing unphosphorylatable phospholemman. Cardiovasc Res 2014;104:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Popov S, Venetsanou K, Chedrese PJ, Pinto V, Takemori H, Franco-Cereceda A. et al. Increases in intracellular sodium activate transcription and gene expression via the salt-inducible kinase 1 network in an atrial myocyte cell line. Am J Physiol Heart Circ Physiol 2012;303:H57–65. [DOI] [PubMed] [Google Scholar]
- 34. Coppini R, Mazzoni L, Ferrantini C, Gentile F, Pioner JM, Laurino T. et al. Ranolazine prevents phenotype development in a mouse model of hypertrophic cardiomyopathy. Circ Heart Fail 2017;10:e003565.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15:545–52. [DOI] [PubMed] [Google Scholar]
- 36. Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH. et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol 2004;287:H203–8. [DOI] [PubMed] [Google Scholar]
- 37. Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WHW. et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol 2015;65:378–88. [DOI] [PubMed] [Google Scholar]
- 38. Finsen AV, Lunde IG, Sjaastad I, Østli EK, Lyngra M, Jarstadmarken HO. et al. Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway. PLoS One 2011;6:e28302.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herum KM, Lunde IG, Skrbic B, Louch WE, Hasic A, Boye S. et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc Res 2015;106:217–26. [DOI] [PubMed] [Google Scholar]
- 40. Waehre A, Vistnes M, Sjaastad I, Nygård S, Husberg C, Lunde IG. et al. Chemokines regulate small leucine-rich proteoglycans in the extracellular matrix of the pressure-overloaded right ventricle. J Appl Physiol Am Physiol Soc 2012;112:1372–82. [DOI] [PubMed] [Google Scholar]
- 41. Liao C-W, Chen A, Lin Y-T, Chang Y-Y, Wang S-M, Wu V-C. et al. The relation between the degree of left ventricular mass regression and serum potassium level change in patients with primary aldosteronism after adrenalectomy. J Investig Med 2015;63:816–20. [DOI] [PubMed] [Google Scholar]
- 42. Indra T, Holaj R, Štrauch B, Rosa J, Petrák O, Šomlóová Z. et al. Long-term effects of adrenalectomy or spironolactone on blood pressure control and regression of left ventricle hypertrophy in patients with primary aldosteronism. J Renin Angiotensin Aldosterone Syst 2015;16:1109–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.