Abstract
Cocaine use disorder is a chronically relapsing disease without FDA-approved treatments. Using a rodent model of cocaine relapse, we and others have previously demonstrated that the beta-lactam antibiotic ceftriaxone attenuates cue- and cocaine-primed reinstatement of cocaine-seeking. Ceftriaxone restores cocaine-induced deficits in both system xc- and GLT-1 expression and function in the nucleus accumbens core (NAc). We recently demonstrated that restoration of GLT-1 expression in the NAc is necessary for ceftriaxone to attenuate reinstatement of cocaine-seeking. Here we used an adeno-associated virus (AAV) to overexpress GLT-1a in the NAc to investigate whether such restoration is sufficient to attenuate cue- and cocaine-primed reinstatement. Rats self-administered cocaine for two weeks and received injections of either AAV-GFAP-GLT-1a or AAV-GFAP-eGFP in the NAc following the last day of self-administration. Rats then underwent three weeks of extinction training (during which time transduction and expression occurred) before undergoing a cue- or cocaine-primed reinstatement test. Microdialysis for the quantification of glutamate efflux in the NAc was conducted during the cocaine-primed test. Rats that received AAV-GFAP-GLT-1a reinstated cue-primed cocaine-seeking in a similar manner as rats that received the control AAV-GFAP-eGFP. Upregulation of GLT-1a attenuated glutamate efflux during a cocaine-primed reinstatement test, but was not sufficient to attenuate reinstatement. We confirmed that GLT-1a upregulation resulted in functional upregulation of glutamate transport and expression, without affecting sodium-independent glutamate uptake, indicating system xc- was not altered. These results indicate that upregulation of NAc GLT-1 transporters alone is not sufficient to prevent the reinstatement of cocaine-seeking and implicate additional mechanisms in regulating glutamate efflux.
Keywords: relapse, microdialysis, xCT, glutamate transport, addiction
1. Introduction
Cocaine addiction is a chronic disorder that affects approximately 1.5 million Americans each year (National Institute on Drug Abuse, 2015). Even after long periods of abstinence from cocaine, the risk of relapse remains high (O’Brien, 2003). Relapse to drug-seeking is modeled in animals with the operant self-administration extinction-reinstatement model (Epstein et al., 2006). Use of this model has consistently demonstrated that the reinstatement of cocaine-seeking is accompanied by synaptic glutamate release in the nucleus accumbens core (NAc) when primed by cocaine (Lutgen et al., 2014; McFarland et al., 2003; Trantham-Davidson et al., 2012) and drug-associated cues (Smith et al., 2017).
Several adaptations in the NAc are proposed to be responsible for the ability of cocaine itself and cocaine-associated cues to induce synaptic glutamate release. Basal, non-synaptic NAc glutamate levels are decreased 2–3 weeks after cessation of cocaine self-administration due to downregulation of system xc- function (Baker et al., 2003; Trantham-Davidson et al., 2012). System xc- is the source of the majority of non-synaptic glutamate levels in the NAc, exchanging extracellular cystine for intracellular glutamate in a sodium-independent manner (Baker et al., 2002; Flynn & McBean, 2000). Its catalytic subunit is xCT, and protein expression of xCT is also decreased 2–3 weeks after the cessation of cocaine self-administration (Knackstedt et al., 2010). Non-synaptic glutamate is important for providing tone on receptors such as group II metabotropic glutamate receptors (mGlu2/3). This tone is vital as both non-synaptic and vesicular glutamate release are regulated by mGlu2/3 in the NAc (Baker et al., 2002; Conn & Pin, 1997; Moran et al., 2005).
Also influencing glutamate homeostasis in the NAc are glutamate transporters which remove glutamate from the synaptic and extrasynaptic compartments. Of the glutamate transporters, GLT-1 (human EAAT2) is responsible for 90% of CNS glutamate uptake (Haugeto et al., 1996). GLT-1 is predominantly located in astrocytes with increased amounts of expression adjacent to synapses, however, GLT-1 expression has been observed in hippocampal and cortical neuronal membranes as well (Chaudhry et al., 1995; Chen et al., 2004; Danbolt, 2001; Murphy-Royal et al., 2017). Glutamate transporters manage the activation of nearby metabotropic receptors, the kinetics of excitatory postsynaptic currents, and control cross-talk between synapses (Arnth-Jensen et al., 2002; Rimmele & Rosenberg, 2016; Tzingounis & Wadiche, 2007). There are two splice variants of GLT-1 in the rat brain, GLT-1a and GLT-1b. GLT-1a is the more prominent isoform expressed in the brain, and is localized close to synapses (Berger et al., 2005; Sullivan et al., 2004). GLT-1b is a C-terminus splice variant of GLT-1a. GLT-1b is predominantly located proximal to the soma, and accounts for only 6–10% of total brain GLT-1 expression (Furness et al., 2016; Holmseth et al., 2009; Sullivan et al., 2004). GLT-1a and GLT-1b protein expression is also decreased following 3 weeks of extinction from cocaine self-administration, contributing to decreased sodium-dependent glutamate uptake at this time (Knackstedt et al., 2010; Lacrosse et al., 2017).
The β-lactam antibiotic ceftriaxone was first identified as having the ability to increase the expression of GLT-1a and GLT-1b in a mouse model of ALS (Rothstein et al., 2005). Ceftriaxone was also demonstrated to increase xCT expression in vitro (Lewerenz et al., 2009). Following cocaine self-administration, chronic daily ceftriaxone administration (5–7 days; 100 and 200 mg/kg) increases expression of GLT-1 and xCT in the NAc and attenuates cue, cocaine- and context-primed reinstatement of cocaine-seeking (Knackstedt et al., 2010; LaCrosse et al., 2016; Sari et al., 2009). Ceftriaxone prevents the increase in synaptically-released glutamate during cocaine-primed reinstatement and restores basal glutamate levels prior to a reinstatement test (Trantham-Davidson et al., 2012).
Using an antisense strategy, we recently showed that GLT-1 upregulation in the NAc is necessary for ceftriaxone to attenuate cue-primed reinstatement of cocaine-seeking (LaCrosse et al., 2017). Here, we upregulate GLT-1a expression using an adeno-associated virus (AAV), utilizing a GFAP promoter to preferentially target overexpression to astrocytes. We hypothesized that upregulation of GLT-1a would increase sodium-dependent glutamate transport, thus diminishing the glutamate efflux that occurs during the reinstatement of cocaine-seeking, and therefore attenuate reinstatement to cocaine seeking.
2. Materials and Methods
2. 1. Subjects
Adult male Sprague Dawley rats (n=107; Charles River, 300–350 g; Raleigh, NC, USA) were housed in a temperature and humidity controlled vivarium. Rats were maintained on a 12 hr reverse-light cycle and all procedures were carried out during the dark phase of the cycle. Animals were provided 20 grams of standard lab chow daily and water ad libitum. Initial experiments were conducted at the Medical University of South Carolina, (Charleston, SC; Fig. 1I, F) using 13 rats. The remainder of the data presented were collected at the University of Florida (Gainesville, FL). All work was approved by the Institutional Animal Care and Use Committees of each university.
Figure 1.
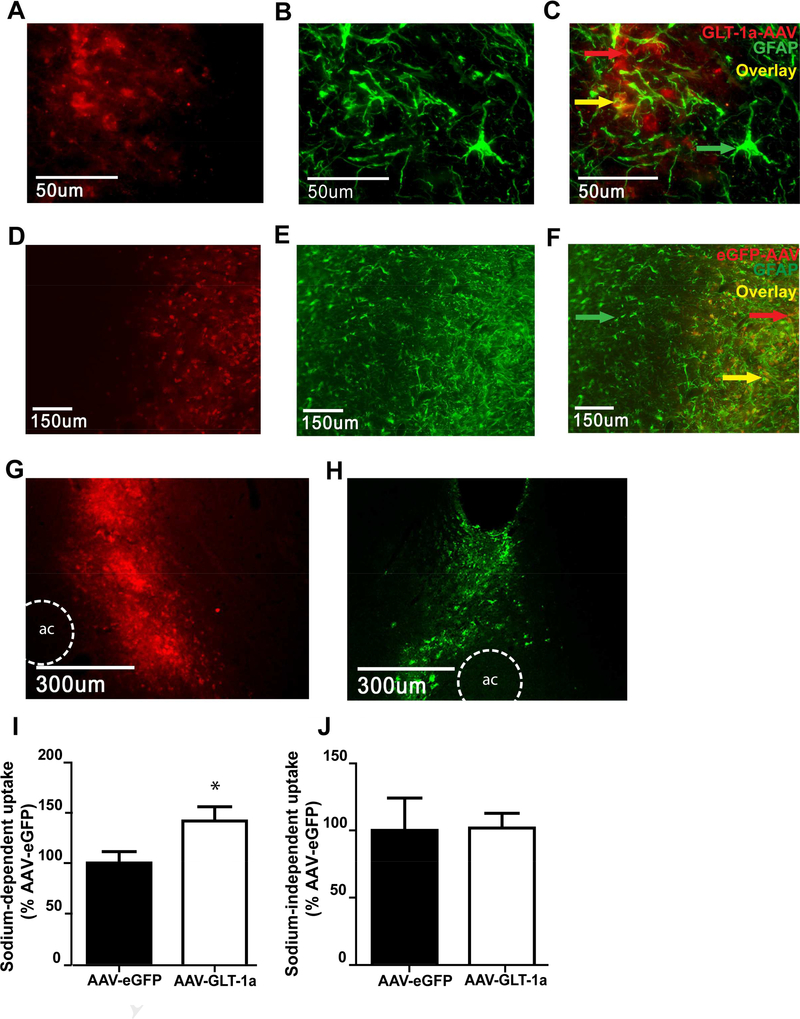
Characterization of GLT-1 and eGFP expression. (A) Immunohistochemistry for the hexa histidine tag on the AAV-GFAP-GLT-1a. (B) Immunohistochemistry for GFAP in the same slice as (A). (C) Overlay of his and GFAP immunoreactivity reveals localization of GLT-1 overexpression to astrocytes in the NAc. (D). Immunohistochemistry for AAV-GFAP-eGFP, (E) and GFAP in the same slice as (D). Overlay of eGFP and GFAP reveals localization of AAV_GFAP-eGFP overexpression in astocytes in the NAc (F). AAV-GFAP-GLT-1a (G) and AAV-GFAP-eGFP (H) overexpression was observed throughout a large portion of the NAc. An ex vivo tritiated glutamate uptake assay revealed that the AAV-GFAP-GLT-1a conferred a functional upregulation of sodium dependent glutamate transport in NAc tissue (I) without affecting sodium independent transport, or system xc- activity (J). * = p<0.05 compared to AAV-GFAP-eGFP.
2.2. Surgical Procedures
Ketamine (87.5 mg/ kg, i.p.) and xylazine (5 mg/kg, i.p.) were administered prior to surgery. Keterolac was administered following surgery to provide analgesia. Catheters (SILASTIC silicon tubing, ID 0.51 mm, OD 0.94 mm, Dow Corning, Midland, MI) were implanted and secured into the jugular vein with suture thread (black braided silk SP117), and passed subcutaneously through the shoulder blades and exited the back. The catheter tubing was connected to a cannula (Plastics One, Roanoke, VA, USA) embedded in a rubber harness (Instech, Plymouth Meeting, PA, USA) worn by the rat for the duration of the self-administration.
Immediately following catheter implantation, all rats received stereotactic surgery for the implantation of cannulae aimed at the NAc. For Experiments 1, 2, and 4, bilateral guide cannulas (Plastics One, Roanoke, VA, USA) were implanted 2 mm above the NAc (AP +1.2 mm, ML +2.5 mm, DV −5.5 mm) and secured using dental cement and stainless-steel skull screws. For Experiment 3, bilateral microdialysis guide cannulae (22 gauge, Synaptech, Marquette, MI, USA) were aimed at the NAc using the same coordinates. Cefazolin (0.1 mL, 100 mg/mL) was administered for three days after surgery. Catheters were flushed with heparinized saline (0.1 mL, 100 Units/mL) prior to and following self-administration sessions.
2. 3. AAV-GFAP-GLT-1a and AAV-GFAP-eGFP Viral Vectors
We employed two adeno-associated viruses (AAVs). The his-tagged AAV-GFAP-GLT1a plasmid (Li et al., 2014) was a kind gift from Dr. David Poulsen (SUNY Buffalo). Initial characterization experiments used vector supplied by Dr. Poulsen. Once we established sufficient overexpression and upregulation of glutamate transport (Fig. 1), we generated larger amounts of the virus by sending the plasmid to be amplified and packaged into an AAV8 by the UNC Viral Vector Core (rAAV8/GFAP-GLT-1a, 2.7 × 10e12 per ml). The control vector, AAVGFAP-eGFP, was also purchased from the UNC Viral Vector Core in the AAV8 serotype (rAAV8/GFAP-eGFP, 1×10e13 per ml). Gfa2 GFAP promoters were utilized to target gene expression exclusively to astrocytes (Lee et al., 2008). Rats received bilateral injections of 1 μL of either AAV-GFAP-GLT-1a or AAV-GFAP-eGFP using a Harvard Apparatus pump connected to Plastics One injectors, at the flow rate of 0.25 μL/min.
2. 4. Cocaine Self-administration and Extinction procedures
Rats were allowed 7 days to recover from surgery before self-administration began. Animals were trained to self-administer cocaine in standard 2-lever operant chambers (Med Associates, St. Albans, VT) using an FR-1 schedule of reinforcement during daily 2 hr sessions. When the active lever was pressed, an intravenous cocaine infusion (0.35 mg/infusion) was delivered. Infusions were paired with a 5 second tone (2900 Hz) and illumination of a stimulus light above the lever. Infusions were followed by a 20 second time-out period, during which time lever presses were recorded, but did not result in delivery of cocaine or cues. Presses on the inactive lever were not reinforced but were recorded. The criteria for self-administration was ten or more infusions for twelve days. All rats in the present experiments met this criterion. Rats were eliminated from data analysis if they exceeded 10 lever presses on the inactive lever after the first day of self-administration. Immediately following the conclusion of the 12-day self-administration period, rats began extinction training, during which time both levers were presented but presses on neither lever resulted in cocaine or cue delivery. Rats received bilateral intra-NAc injections of AAV-GFAP-GLT-1a or the control AAV-GFAP-eGFP immediately following the first session of extinction training. Extinction sessions were 2 hours in length and occurred daily for three weeks.
2. 5. Reinstatement tests
Cue-primed reinstatement tests consisted of a 1 hr test during which the cues that were previously associated with cocaine self-administration were again presented upon presses on the previously active lever. No cocaine was delivered during this test. The amount of lever presses on the previously active lever and inactive lever during the reinstatement test were compared to the respective average number of presses during the final two days of extinction training. A cocaine-primed reinstatement test began with an injection of cocaine (10 mg/kg IP) 5 minutes prior to placement into the operant chamber. Presses on the previously active and inactive levers were recorded but did not result in delivery of drug or cues.
2. 6. Histology and Immunohistochemistry
To verify viral expression, animals were deeply anesthetized with pentobarbital (100 mg/kg, i.p.), and were transcardially perfused with phosphate-buffered saline (PBS) and then 4% paraformaldehyde (PFA). Brains were extracted and preserved in 4% PFA for 24 hours followed by 20% sucrose in PBS for 48 hours. Brains were frozen and stored at −80°C until they were sliced at 30 μm using a cryostat. Brain slices were washed in PBS-T (PBS +0.2% Triton; 3 × 10 min). The slices were then blocked in 10% Normal Goat Serum in PBS before being probed with primary antibody directed at the his tag (Rabbit anti-hexa histadine, Cell Signaling Technology, Catalog #:2365S, 1:100), GFAP (Mouse anti-GFAP, UC Davis NIH NeuroMab Facility, Catalog #:75–240,1:250) or eGFP (Rabbit anti-GFP, Abcam, Catalog #:Ab290, 1:20,000) overnight at 4° C. Slices were then washed in 3% Normal Goat Serum (4 × 10 min) before they were probed with appropriate Alexa Fluor secondary antibodies (Alexa Fluor 488 goat anti-mouse, 1:200, Alexa Fluor 594 goat anti-rabbit, 1:200, Life Technologies, Catalog #:A11073, A11076). The slices were washed (4 × 10 min) in PBS, then washed (3 × 10 min) in PB (monobasic sodium phosphate and dibasic sodium phosphate in H20) before being placed on slides and coverslipped using antifadent mounting solutions (Citifluor). For Experiment 2 and 3, a separate series of slices from each rat were stained with cresyl violet as a secondary method for confirming probe localization within the NAc.
Images of viral expression were acquired using a Tuscen monochrome CCD camera attached to an Olympus BX51 fluorescent microscope. Rats were eliminated from the behavioral analysis if virus was not localized to the NAc (e.g. only present along the cannula tract). Higher magnification images (Fig. 1) were taken using an Olympus IX81-DSU spinning disc confocal microscope with a monochrome CCD camera using a 40× water immersion objective.
2.7. Methods specific to Experiments 1–4
2.7.1. Experiment 1: Characterization of the viral spread and glutamate transport.
Eighteen rats were implanted with cannulae aimed at the NAc and infused with either AAVGFAP-GLT-1a (n=9) or AAV-GFAP-eGFP (n=9). Three weeks later, two AAV-GFAP-eGFP and three AAV-GFAPGLT-1a rats were overdosed with pentobarbital and perfused as described above for the purposes of characterizing the viral spread. Immunohistochemistry was performed to detect the hexa-histadine tag or GFP as described above. The remaining 13 rats were sacrificed via rapid decapitation to measure sodium dependent and independent glutamate uptake using a tritiated glutamate uptake assay. Glutamate uptake was measured using an invitro slice preparation as described in detail previously (Trantham-Davidson et al., 2012). Rats were decapitated and bilateral punches of the NAc were dissected and sliced into 250 × 250 μm sections using a McIlwain tissue chopper (St. Louis, MO). Slices were incubated at 37°C for 30 min in either oxygenated Krebs-Ringer’s solution phosphate buffer (in mM: 140 NaCl, 1.3 CaCl2, 1.2 KH2PO4, 5 HEPES, 10 glucose and 1 MgCl2, pH 7.4) to quantify sodium-dependent uptake, or an identical buffer in which NaCl was replaced with 140 mM choline chloride to measure sodium-independent uptake. Glutamate uptake was stimulated by the addition of L[3H] glutamate (40 nM, 51 Ci/mM; Perkin-Elmer, Boston MA) to slices in the presence of 3 μM L-glutamate. The reaction was conducted at 37°C for 15 minutes and terminated by ice-cold, sodium-free buffer. Slices were solubilized with 1 % sodium dodecyl sulfate and radioactivity determined using a liquid scintillation counter. Protein content was measured using a bicinchoninic acid assay protein assay (Pierce) and cpm/mg protein calculated.
2.7.2. Experiment 2: Upregulation of GLT-1a and cue-primed reinstatement.
Twenty-eight rats were trained to self-administer cocaine, received bilateral intra-NAc infusions of virus, and underwent three weeks of extinction training as described above (see timeline in Fig. 2A). Following extinction training, rats underwent a 1 hr cue-primed reinstatement test. Rats were sacrificed via transcardial perfusion and brains were taken for immunohistochemistry protocols as described above.
Figure 2.
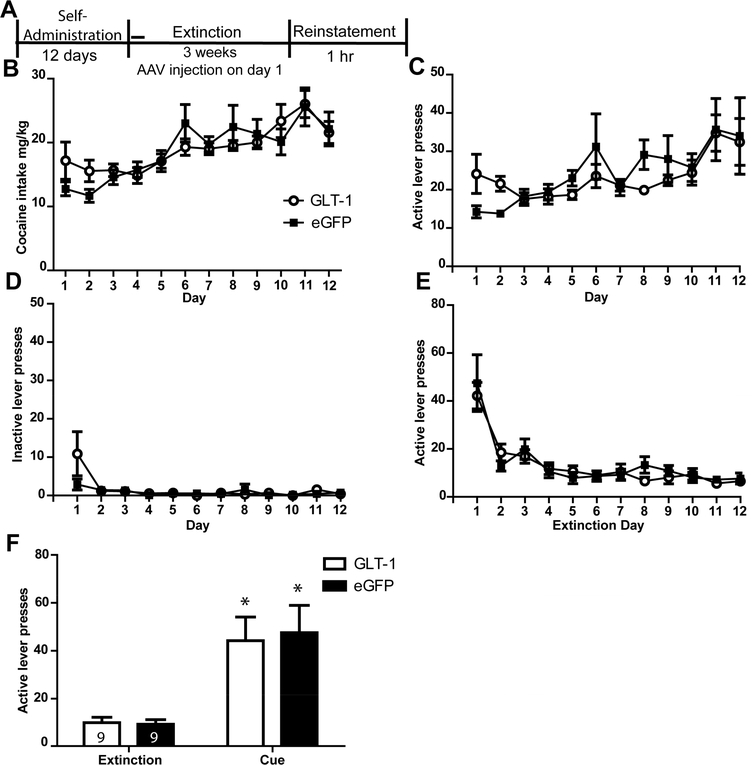
GLT-1a overexpression did not attenuate cue-primed reinstatement of cocaine-seeking. (A) Timeline of experiment. Cocaine intake (mg/kg) (B), active lever presses during self-administration (C), inactive lever presses during self-administration (D) and active lever presses during the first twelve days of extinction (E) did not differ between groups later assigned to receive AAV-GFAP-GLT-1a or AAV-GFAP-eGFP. (F) GLT-1a overexpression did not attenuate cue-primed reinstatement of cocaine-seeking. * = p<0.05 compared to extinction.
2.7.3. Experiment 3: Glutamate efflux during cocaine-primed reinstatement.
Thirty-nine rats underwent cocaine self-administration, bilateral infusion of virus, and extinction training as described above. On the night preceding a cocaine-primed reinstatement test, rats were implanted with microdialysis probes and perfused with artificial cerebral spinal fluid (aCSF) in their home cage overnight, adjacent to the operant chambers. The next morning, rats were placed in the operant chambers and 10-minute baseline samples were collected for 90 minutes prior to and throughout the 90 minute cocaine-primed reinstatement test. Rats were sacrificed via transcardial perfusion and their brains were harvested for immunohistochemistry (as described above).
Glutamate was quantified in dialysate samples using isocratic high-performance liquid chromatography (HPLC) with electrochemical detection (Thermo Fisher Scientific). Microdialysis samples were derivatized with o-pthalaldehyde (Sigma-Aldrich) by an autosampler (Thermo Fisher Scientific) immediately before injection onto a CAPLCELL PAK C18 column (5 μm, 2.0mm I.D. X 50mm; Shiseido). The mobile phase consisted of 100 mM Na2HPO4, 10% (vol/vol) methanol, and 3.5% (vol/vol) acetonitrile (pH=6.5). Glutamate content was quantified by comparing computer-integrated peak areas of samples with those of l-glutamate standards.
2.7.4. Experiment 4: AAV-GFAP-GLT-1a effects on GLT-1 and xCT protein expression.
Thirteen rats self-administered cocaine as described above and received intra-NAc infusions of AAV-GFAP-eGFP (n=7) or AAV-GFAP-GLT-1a (n=6) on day one of extinction training (as described above). Following three weeks of extinction training, rats were sacrificed. Nine age-matched control rats did not undergo surgery or self-administration, but were injected with ketamine/xylazine 6 weeks prior to euthanasia. Brains were extracted and the NAc was dissected on ice. The tissue was homogenized in sucrose buffer and frozen at −80⁰C. Western blotting was performed for GLT-1a and xCT expression. Proteins were separated using10% glycine criterion gels (Bio-rad Hercules, CA, USA) and then transferred to PVDF membrane (Bio-rad Hercules, CA, USA). The membrane was blocked with 5% milk in tris-buffered saline with 1% Tween-20 (TBS-T; 4 × 10 min) and then incubated in primary antibody against either GLT-1a (guinea pig anti-GLT-1a, Millipore Catalog #:AB1783, 1:20,000) or xCT (rabbit anti-xCT, Novus NB300–318, 1:5000) overnight at 4° C. The membrane was washed in TBST (4 × 10 min) before secondary antibody (Jackson Immuno; 1:10,000–1:20,000) was incubated for two hours at room temperature. After washing in TBST (4 × 10 min), the membrane was exposed to Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare Limited, Little Chalfont, United Kingdom) and developed using High Performance chemiluminescence film (GE Healthcare Limited, Little Chalfont, United Kingdom). On the following day membranes were blotted for calnexin to serve as a loading control (rabbit anti-calnexin, Millipore Catalog #:AB2301, 1:40,000).The density of immunoreactive bands were then determined from digitized images using ImageJ software (NIH).
Statistical Analysis
Behavioral data (self-administration, extinction and reinstatement test) was analyzed using SPSS (IBM, Armonk, NY). An alpha level of p<0.05 was set for all statistical analyses. Greenhouse-Geisser adjustments were used when sphericity assumptions were not met. Repeated measure (RM) analyses of variance (ANOVAs) were used to compare the number of lever presses and infusions during self-administration, lever presses during extinction and reinstatement with Group as the between-subjects factor and Time as the RM. Western blot and HPLC data was analyzed using GraphPad Prism (version 5.00, GraphPad Software, La Jolla, CA). Western blot data was first normalized against the density of calnexin immunoreactivity within the same sample, normalized to cocaine-naive controls, and analyzed using independent samples t-tests. In addition to using RM ANOVA to examine group differences in glutamate concentrations prior to and during the cocaine-primed reinstatement test, we also computed area under the curve (AUC) glutamate during the 90 minute test. The formula used was AUC = [0.5(B+S1)d + 0.5(S1+S2)d +……..0.5(Sn-1+Sn)d] – Bdn, where B is average of baseline samples, Sx are the values of each 10-minute sample during the test, n is the total number of samples collected (6) and d is the duration of sample collection (10 min; Chefer et al., 2011). We then conducted an independent–samples t-test on this data to test for group differences.
3. Results
3.1. Experiment 1: AAV-GFAP-GLT-1a Increases sodium–dependent glutamate uptake
Immunohistochemistry for the hexa-histadine tag revealed that the AAV-GFAP-GLT-1a is expressed in the NAc (Fig. 1A). Staining for GFAP (Fig. 1B) and overlaying the hexa-histadine expression with GFAP expression revealed that the AAV-GFAP-GLT-1a was predominantly expressed in astrocytes (Fig. 1C). Immunohistochemistry for AAV-GFAP-eGFP revealed expression in the NAc (Fig. 1D). Overlay of eGFP and GFAP staining (Fig. 1E) revealed that eGFP was also expressed in astrocytes (Fig. 1F). Both AAV-GFAP-GLT-1a (Fig. 1G) and AAV-GFAP-eGFP (Fig. 1H) vectors filled a good portion of the NAc. The ability of the AAV-GFAPGLT-1a to induce a functional upregulation of glutamate transport was assessed in cocaine-naïve rats. We found upregulation of sodium-dependent transport in the AAV-GFAP-GLT-1a infused group relative to rats receiving eGFP [t(1,10)=2.316, p<0.05; Fig. 1I]. There was no group differences in sodium-independent transport [t(1,10)=0.068, p=0.98; Fig. 1J], indicating no effects on system xc- activity.
3.2. Experiment 2: Upregulation of GLT-1a does not attenuate cue-primed reinstatement to cocaine-seeking
Two rats were excluded from data analysis for improper spread of virus. Four rats lost headcaps during self-administration (n=2) or had incorrect cannula placement (n=2) and were therefore eliminated from the study. Four rats experienced catheter failures during self-administration and were excluded from the study. For the remaining 18 rats later assigned to receive AAV-GFAP-GLT-1a (n=9) or AAV-GFAP-eGFP (n=9), there were no differences in number of infusions attained between these groups [F(4.6,16)=0.935, p=0.458]. A significant effect of Time was detected as there was an increase in the number of infusions received across the 12 days of self-administration [F(4.6,16) =10.4, p<0.000]. There was no Group x Time interaction for infusions attained [F(1,16)=1.08, p=0.312]. In addition to infusion number, we also compared the amount (mg/kg) of cocaine intake between groups; there were no Group differences in mg/kg cocaine intake [F(1,176)=0.044, p=0.84; Fig. 2B]. There was a main effect of Time [F(11,176)=8.85, p<0.001] but no Group x Time interaction [F(11,176)=1.01, p=0.44]. There was no Group x Time interaction [F(3.3,176)=1.452, p=0.145] and no effect of Group [F(1,16)=0.056, p=0.815] on active lever presses during self-administration (Fig. 2C). There was a significant effect of Time on active lever presses during self-administration [F(3.5,62.9)=3.42, p=0.018], as lever presses increased for both groups during self-administration. Comparison of inactive lever presses during self-administration for rats that later received AAV-GFAP-eGFP and AAV-GFAP-GLT-1a revealed no effect of Group [F(1.34,16)=1.75, p=0.202; Fig. 2D] or Time [F(1,16)=0.916, p=0.353]. There was a Group x Time interaction for the inactive lever presses during self-administration [F(1.34,16) =4.116, p=0.045] due to the presence of greater pressing in the AAV-GFAP-GLT-1a group on Day 1 which declined by Day 2 (Day 1 mean+/− SEM: AAV-GFAP-eGFP group: 2.88+/−1.4; AAV-GFAP-GLT-1a group: 10.8+/−5.7).
There was a significant effect of Time on active lever presses during the first twelve days of extinction training, as there was a decrease in lever presses for both groups [F(1,28)=19.72, p=0.000; Fig. 2E]. There was no significant effect of Group [F(1,28)=.537, p=0.572]) nor Group x Time interaction [F(1,28)=.537, p=0.867] for active lever presses during extinction training. There was an effect of Time on inactive lever presses during extinction [F(3.57,25)= 19.72, p<0.001; not shown]. There was no significant effect of Group [F(1,16)=1.083, p=0.323], nor a Group x Time interaction [F(3.57,25)=.489, p=0.723] on inactive lever presses during the first twelve days of extinction training.
A two-way ANOVA conducted on the active lever presses during extinction and test (Fig. 2F) revealed a significant effect of Time [F(1,16)=28.24, p<0.001]. Post-hoc tests using a Bonferroni correction (α= 0.05/2) found that both AAV-GFAP-GLT-1a (p=0.006) and AAV-GFAP-eGFP (p=0.005) rats displayed greater lever presses during the test than during extinction, indicating cue-primed reinstatement of cocaine-seeking. A repeated measure ANOVA revealed no Group x Time interaction [F(1,16)=28.24, p=0.77] indicating reinstatement behavior for AAV-GFAP-GLT-1a and AAV-GFAP-eGFP groups did not differ.
3.3. Experiment 3: Upregulation of GLT-1a does not prevent the increase in glutamate efflux during cocaine-primed reinstatement to cocaine seeking
3.3.1. Self-administration, extinction and reinstatement behavior
Five rats experienced catheter failures during self-administration and were eliminated from the study. Two rats were eliminated because they exhibited more than 10 presses on inactive lever after Day 1 of self-administration and were not included in data analysis. Six animals lost their headcaps before dialysis could be collected and were therefore eliminated from the study. Six rats had improper spread of virus (n=3) or incorrect placement of dialysis cannula (n=3) and were eliminated from all data analysis. Three rats experienced interruptions in collections during microdialysis and were not included in the data analysis. Three rats had multiple microdialysis samples with glutamate concentrations that were more two standard deviations away from the mean (n=2 AAV-GFAP-eGFP; n=1 AAV-GFAP-GLT-1a). There were no significant differences in infusions between groups that were then assigned to receive AAV-GFAP-GLT-1a (n=7) or AAV-GFAP-eGFP (n=7) [F(1,11)=1.659, p=0.273] and no Group x Time interaction [F(1,11)= 6.701, p=0.087)]. A significant effect of Time was detected as there was an increase in infusions received over the course of the 12 days of self-administration [F(1,11)=9.759, p<0.001]. When cocaine intake was calculated by body weight (mg/kg), there was no main effect of Group [F(1,121)=2.13, p=0.17; Fig. 3A], nor was there a Group x Time interaction [F(1,121)=1.38, p=0.19]. There was a main effect of Time when cocaine intake was calculated by body (mg/kg) [F(1,121)=7.7, p<0.001]. There was no main effect of Group [F(1,11)= 3.688, p= 0.074], or Group x Time interaction [F(1,121)= 0.866, p=0.575] on active lever presses during self-administration (Fig. 3B). There was a main effect of Time on active lever presses during self-administration [F(1,11)= 3.79, p= 0.0001; Fig. 3B]. There was no main effect of Group [F(1,11)=3.03 p=0.11], or Group x Time [F(1,121)=0.87 p=0.57] on inactive lever during self-administration (Fig. 3C). There was no effect of Time on inactive lever presses during self-administration [F(11,132)=1.67, p=0.087].
Figure 3.
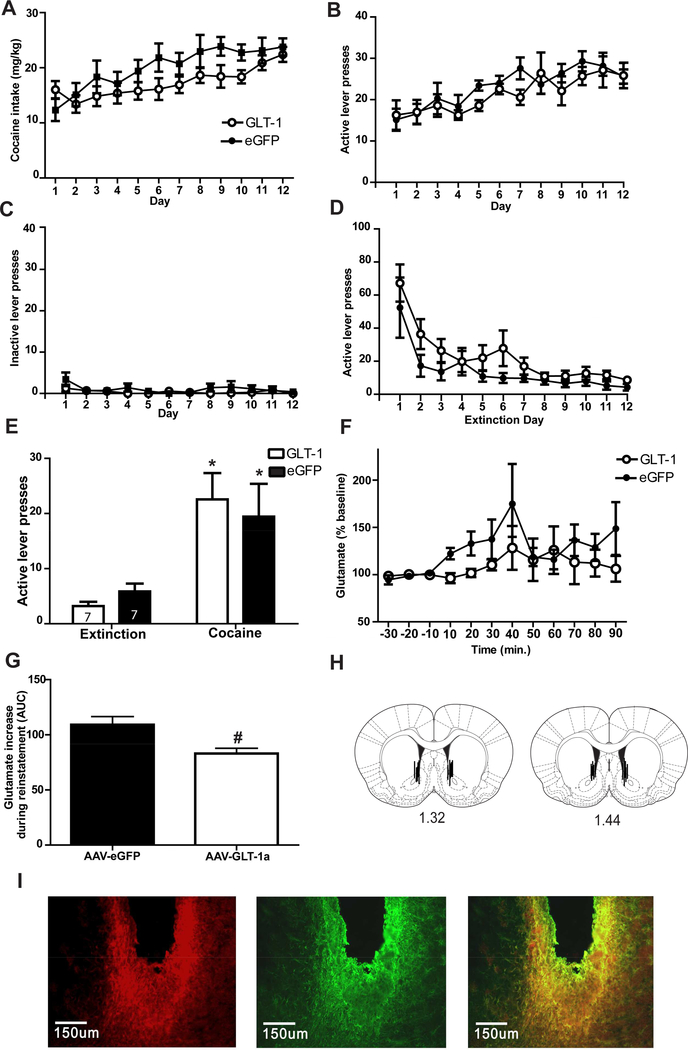
GLT-1a overexpression attenuates glutamate release during cocaine-primed reinstatement but is not sufficient to attenuate reinstatement. Cocaine intake (A), active lever presses during self-administration (B), inactive lever presses during self-administration (C) and lever presses on the previously active lever during the first twelve days of extinction (D) did not differ between groups later assigned to receive AAV-GFAP-GLT-1a or AAV-GFAP-eGFP. GLT-1a overexpression did not attenuate cocaine-primed reinstatement of cocaine-seeking (E). Glutamate efflux in the NAc was observed during the reinstatement of cocaine seeking for both groups (F). Area under the curve analysis of efflux during the full 90 minutes of cocaine-primed reinstatement test was reduced in rats receiving AAV-GFAP-GLT-1a compared to rats receiving AAV_GFAP-eGFP (G). Placement of microdialysis probes (H). (I). Immunohistochemistry for the hexa histidine tag on the AAV-GFAP-GLT-1a and GFAP in the same slice and the overlay of his and GFAP immunoreactivity reveals probe placement within the area of GLT-1a overexpression.* = p<0.05 compared to extinction. # = p<0.05 compared to AAV-GFAP-eGFP.
There was a main effect of Time on active lever presses during the first twelve days of extinction training [F(1,11)=18.66, p<0.001; Fig. 3D] but no effect of Group [F(1,11)=2.54, p= 0.132], indicating an overall decrease in lever pressing. While there was an effect of Time on previously inactive lever presses during extinction [F(1,11)= 6.915, p<0.001; not shown], there was no Group x Time interaction [F(1,11)= 1.09, p=0.372], or Group [F(1,11)=0.009, p=0.29]. Analysis of active lever presses during the 90 minute cocaine-primed reinstatement test (Fig. 3E) revealed an effect of Time, as rats exhibited more presses during the test compared to lever presses on the active lever during extinction [F(1,15)=28.33, p<0.001]. Post-hoc tests using the Bonferroni correction revealed that both AAV-GFAP-GLT-1a (p=0.002) and AAV-GFAP-eGFP (p=0.015) groups reinstated lever pressing (e.g. increase in lever presses from extinction to test). There was no Group x Time interaction on lever pressing [F(1,15)=3.395, p=0.085] indicating that reinstatement did not differ between groups. There was no effect of Group [F(1,15)=.674, p=0.23], Group x Time interaction [F(1,15)=1.43, p=0.89], or effect of Time [F(1,15)=1.39, p=0.11] on inactive lever pressing during the cocaine-primed reinstatement test.
3.3.2. HPLC quantification of glutamate levels during cocaine-primed reinstatement to cocaine-seeking
A repeated measures ANOVA detected a significant effect of Time on glutamate levels during baseline collections and a cocaine-primed reinstatement test [F(1,12) =2.107, p=0.018; Fig. 3F]. There was not a main effect of Group, indicating glutamate levels did not differ between groups during cocaine-primed reinstatement [F(1,12)= 0.331, p=0.574]. There was no Group x Time interaction on glutamate levels during cocaine-primed reinstatement [F(1,12)= 0.755, p=0.696]. However, computing AUC for glutamate concentrations during the test revealed a significant reduction in glutamate concentration in the AAV-GFAP-GLT-1a group [t(12) = 2.979, p=0.011; Fig. 3G] compared to AAV-GFAP-eGFP group. Data was only included from rats with microdialysis cannulae within the NAc (see Fig. 3H for placement) and within eGFP or GLT-1a expression as in Fig. 3I.
3.4. Experiment 4: AAV-GFAP-GLT-1a restores protein expression of GLT-1a following cocaine self-administration
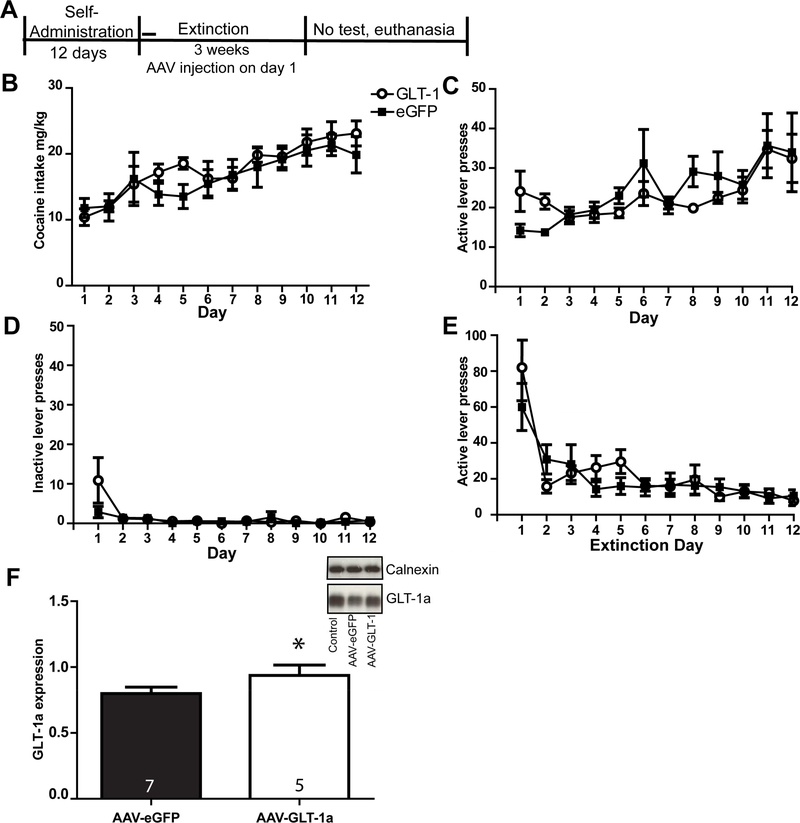
We found that AAV-GFAP-GLT-1a increased sodium-dependent uptake in cocaine-naïve rats (Fig. 1I). In order to understand the inability of GLT-1a overexpression to prevent glutamate efflux during reinstatement, we sought to confirm that AAV-mediated GLT-1a protein upregulation occurred following cocaine self-administration. To that end, GLT-1a and xCT expression were quantified in a separate group of animals which underwent cocaine self-administration, and received either AAV-GFAP-GLT-1a (n=6) or AAV-GFAP-eGFP (n=7). Rats then experienced three weeks of extinction training prior to euthanasia (see timeline in Fig. 4A). Rats were assigned to receive either AAV-GFAP-eGFP or AAV-GFAP-GLT-1a following self-administration such that there was no effect of Group on infusions, active and inactive lever presses during cocaine self-administration (p’s>0.05). There was no Group x Time interaction on infusions received during self-administration [F(11,110)=1.072, p=0.39] and no significant effect of Group [F(1,110)=0.1004, p=0.758]. There was a significant effect of Time on infusions [F(11,110) = 6.25, p=0.000], as both groups increased infusions over the course of self-administration. When cocaine intake was calculated by body weight (mg/kg), there was no main effect of Group [F(1,110)=0.3, p=0.6; Fig. 4B], and no Group x Time interaction [F(11,110)=0.64, p=0.79]. There was an effect of Time when cocaine intake was calculated by body weight [F(11,110)=8.78, p<0.001]. There was no Group x Time interaction on active lever presses during self-administration [F(11,110)=0.549, p=0.865; Fig. 4C] and no main effect of Group [F(1,16)=1.221, p=0.295]. There was no main effect of Group on inactive lever presses during self-administration (F(1,16)=0.0185, p=0.895; Fig. 4D). There was a significant effect of Time on presses on the previously active lever during the first twelve days of extinction training [F(11,110)= 17.13, p<0.0001; Fig. 4E] and no Group x Time interaction [(F(11,110)= 1.547, p=0.125], as both groups decreased lever pressing over the course of extinction. There was no main effect of Group [F(11,110)= 0.1161, p=0.74] on the previously active lever during the first twelve days of extinction training. Total protein expression of GLT-1a was significantly greater in the group that received AAV-GFAP-GLT-1a relative to the AAV-GFAP-eGFP group [t(1,10)=2.44, p=0.3; (Fig.4F)]. No immunoreactivity was detected for the sample from one rat in the AAV-GFAP-GLT-1a group, yielding only 5 data points for this group.
Figure 4.
AAV-GFAP-GLT-1a normalized GLT-1a expression in the NAc following cocaine self-administration and extinction. (A) Timeline of experiment. Mean cocaine intake (mg/kg) (B), active lever presses during self-administration (C), inactive lever presses during self-administration (D) did not differ between groups later assigned to receive AAV-GFAP-GLT-1a or AAV-GFAP-eGFP, nor did active lever presses during extinction (E). Western blotting revealed that GLT-1a was reduced in GFP-infused rats that had self-administered cocaine compared to cocaine rats that had received AAV-GFAP-GLT-1a. * = p<0.05 compared to AAV-GFAP-eGFP.
4. Discussion
AAV mediated GLT-1a expression does not attenuate cue- or cocaine-primed reinstatement to cocaine seeking
Chronic cocaine self-administration alters glutamate homeostasis in the NAc. Pharmacotherapeutic options to reduce relapse, such as ceftriaxone and N-acetylcysteine, attenuate relapse by restoring system xc- and GLT-1a function, increasing basal extrasynaptic glutamate and attenuating glutamate efflux during reinstatement (Baker et al., 2003; Knackstedt et al., 2010; Trantham-Davidson et al., 2012). Utilizing an antisense strategy, we have previously shown GLT-1a to be necessary for both N-acetycysteine and ceftriaxone to attenuate cue-primed reinstatement (Reissner et al., 2014; LaCrosse et al., 2017). Here we found that AAV-mediated upregulation of GLT-1a expression in the NAc was not sufficient to prevent cue- or cocaine-primed reinstatement. Upregulation of GLT-1a is expected to increase removal of synaptically-released extracellular glutamate, thereby reducing the amount of glutamate binding to the post-synaptic receptors during reinstatement. Here we found that in rats with AAV-mediated GLT-1a overexpression in the NAc, glutamate efflux was reduced relative to AAV-GFAP-eGFP controls. However, during a cocaine-primed reinstatement test, glutamate levels still increased from baseline, presumably accounting for reinstatement of cocaine-seeking. This finding is similar to our previous report that when rats undergo abstinence without extinction training after cocaine self-administration, chronic ceftriaxone (200 mg/kg) upregulates NAc GLT1a expression but does not prevent glutamate efflux during a relapse test (LaCrosse et al., 2016).
Astrocytic GLT-1a expression and reinstatement of cocaine-seeking
There are several possibilities that could explain the inability of GLT-1a upregulation to fully attenuate glutamate efflux during reinstatement as is accomplished by ceftriaxone (Trantham-Davidson et al., 2012). Utilizing similar parameters of cocaine self-administration and extinction, Scofield et al. (2016) found that cocaine decreased co-localization of neurons and astrocytes and decreased astrocyte size in the NAc. Ceftriaxone reverts the co-localization of astrocytes and neurons to levels of cocaine-naïve controls, indicating this could be an important factor in its ability to prevent relapse (Scofield et al., 2016). We propose that here, GLT-1a overexpression restored transporter expression and function, however, the transporters may not be positioned sufficiently close to the synapse to perform glutamate uptake efficiently. It is also possible that the AAV-GFAP-GLT-1a employed here did not transduce a sufficient amount of cells within the NAc. However, restoration of GLT-1a expression was observed (Fig. 4F), so inefficient transduction likely not a factor in the inability of this AAV to prevent glutamate efflux during reinstatement.
Possible contributions of neuronal GLT-1a in reinstatement to cocaine seeking prevention
Related to the importance of properly positioned GLT-1 to reduce glutamate efflux and/or attenuate reinstatement, it is possible that our use of the GFAP promoter to target expression to astrocytes contributed to our observations. Although GLT-1 expression is primarily localized to astrocytes (Danbolt, 2001; Murphy-Royal et al., 2017), there is evidence that GLT-1 transporters are also located on axon terminals, at least in the hippocampus (Chaudhry et al., 1995). Ceftriaxone increases the density of GLT-1a in astrocytes, as well as in presynaptic terminals in the hippocampus (Omrani et al., 2009). Increased presynaptic GLT-1 expression, in addition to expression in astrocytes, may be necessary to prevent reinstatement of cocaine-seeking, especially in light of the finding that astrocyte-neuron contacts are reduced are following cocaine (Scofield et al., 2017). Thus, it is possible that GLT-1a was not positioned to perfom sufficient reuptake of synaptically-released glutamate.
While there are numerous reports of the specifity of GFAP-driven transgene expression for astrocytes (e.g. Brenner et al., 1994; Scofield et al., 2015), there are also reports that this promoter can drive expression in neurons (e.g. Fujita et al., 2014). This so called “leaky” expression has been found to be dependent on the transgene expressed (Su et al., 2004). While we show overlap of the his-tagged GLT-1a AAV and GFAP here (Fig. 1), we cannot rule out possible neuronal expression that was not effective at shaping glutamate transmission. Finally, GLT-1 diffuses through the astrocytic membrane to shape glutamatergic transmission (Murphy-Royal et al., 2017), and it is possible that ceftriaxone alters diffusion in a protective manner while GLT-1a overexpression via an AAV does not. Additional work to examine the role of ceftriaxone/GLT-1 upregulation in the formation of contacts between astrocytes and neurons is needed.
Other potential mechanisms of synaptic glutamate regulation by ceftriaxone
Following chronic cocaine use, system xc- is downregulated, leading to decreased basal glutamate and decreased tone on mGlu2/3 (Baker et al., 2003, Moran et al., 2005). Repeated non-contingent cocaine decreases mGlu2/3 function, as does cocaine self-administration (Moussawi et al., 2011; Xi et al., 2002). Following cocaine self-administration and 10 days of extinction, there is a decrease in mGlu2/3 density in the NAc (Pomierny-Chamiolo et al., 2017). Stimulation of mGlu2/3 reduces glutamate efflux during reinstatement (Smith et al., 2017) and is necessary for N-acetylcysteine to attenuate cocaine reinstatement (Moran et al., 2005). Here, AAV-mediated upregulation of GLT-1a did not alter the function of system xc- (Fig. 1G); therefore, it is unlikely it altered basal glutamate levels or mGlu2/3 tone. Future work will determine whether a restoration of mGlu2/3 function is necessary for ceftriaxone to attenuate reinstatement. Ceftriaxone’s therapeutic effects on glutamate and behavior could also be due to its effects in other brain regions. GLT-1 upregulation is observed in the PFC following chronic ceftriaxone administration in rats with a history of alcohol (Dasa et al., 2015) and cocaine (Sari et al., 2009) self-administration. As this region sends a dense glutamatergic projection to the NAc, normalization of glutamate homeostasis in the PFC may contribute to the reduction in glutamate efflux in the NAc.
Absence of co-regulation of xc- and GLT-1
AAV-mediated upregulation of GLT-1 allowed us to investigate the relationship between xCT and GLT-1 expression. Evidence for co-regulation of glutamate transport and system xc-function has been demonstrated in cell culture (Bannai, 1986; Murphy et al., 1989). A history of cocaine self-administration decreases both GLT-1 and xCT expression while N-acetylcysteine and ceftriaxone increase expression of both proteins (Knackstedt et al., 2010). We recently demonstrated that the co-regulation of xCT and GLT-1 expression is only observed following ceftriaxone treatment in rats with a cocaine history, and not in cocaine-naïve rats (LaCrosse et al., 2017). This is consistent with our finding here that AAV-mediated over-expression of GLT-1a did not increase system xc- function. Thus, increasing GLT-1a expression is not sufficient to co-regulate xCT expression or system xc- function. Ceftriaxone may alter a mediator of both xCT and GLT-1a expression, thus increasing both proteins.
A greater understanding of the role of GLT-1 in cocaine relapse
In combination with our previous findings, the present results suggest that while the downregulation of NAc GLT-1 plays a critical role in reinstatement to cocaine-seeking (Lacrosse et al., 2017), the upregulation of GLT-1a in the NAc is not the sole mechanism by which ceftriaxone and N-acetylcysteine attenuate reinstatement to drug seeking. It is likely that other regulatory mechanisms (e.g. transporter localization to membrane or synapse, mGlu2/3 function) or a normalization of activity in regions projecting to the NAc contribute to the ability of ceftriaxone to fully attenuate glutamate efflux and thus the reinstatement of cocaine-seeking. This information will aid in pharmacological treatments for relapse prevention in the future.
Supplementary Material
Highlights.
NAc GLT-1a upregulation does not attenuate cue- or cocaine-primed reinstatement
GLT-1a overexpression reduces glutamate efflux during cocaine-primed reinstatement
AAV-mediated GLT-1a overexpression increases sodium-dependent glutamate uptake
Acknowledgements:
This work was funded by NIH grant DA033436 and DA037270 awarded to L.A.K.. The authors would like to thank Lizhen Wu for her excellent technical assistance on this project. We would also like to thank John Shallcross for assistance with imaging.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnth-Jensen N, Jabaudon D, Scanziani M, 2002. Cooperation between inde- pendent hippocampal synapses is controlled by glutamate uptake. Nat. Neu- rosci. 5, 325e331. [DOI] [PubMed] [Google Scholar]
- Baker, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, & Kalivas PW (2003). Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature Neuroscience, 6(7), 743–9. 10.1038/nn1069 [DOI] [PubMed] [Google Scholar]
- Baker, Xi ZX, Shen H, Swanson CJ, & Kalivas PW (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 22(20), 9134–9141. http://doi.org/22/20/9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S (1986). Exchange of cystine and glutamate across plasma mem- brane of human fibroblasts. J Biol Chem 261:2256–2263. [PubMed] [Google Scholar]
- Berger UV, DeSilva TM, Chen W, Rosenberg PA, (2005). Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J. Comp. Neurol 492, 78e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, & Messing A (1994) GFAP promoter directs astrocytespecific expression in transgenic mice. J Neurosci. 1994 March;14(3 Pt 1):1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD, & Lilly E (2000). Regulation of Neurotransmitter Release by Metabotropic Glutamate Receptors. [DOI] [PubMed]
- Chaudhry FA, Lehre KP, Lookeren Campagne M van, Ottersen OP, Danbolt NC, & Storm-Mathisen J (1995). Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron, 15(3), 711–720. 10.1016/0896-6273(95)90158-2 [DOI] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippen- berg T, 2011. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict. Biol. 16, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, Desilva T, Tanaka K, … Rosenberg PA (2004). The Glutamate Transporter GLT1a Is Expressed in Excitatory Axon Terminals of Mature Hippocampal Neurons, 24(5), 1136–1148. 10.1523/JNEUROSCI.1586-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ and Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. [DOI] [PubMed] [Google Scholar]
- Danbolt NC (2001). Glutamate uptake. Progress in Neurobiology, 65(1), 1–105. 10.1016/S0301-0082(00)00067-8 [DOI] [PubMed] [Google Scholar]
- Dasa S, Yamamotob B, Hristovc A,& Saria Y (2015) Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 97:67–74 10.1016/j.neuropharm.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y (2006): Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006, 189:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ, & Flynn J (2001) Molecular mechanisms of cystine transport, Biochemical Society Neurotransmitter Transporters: Molecular Mechanism and Regulation, Volume 29, part 6 [DOI] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M (2014) Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci. 2014 December 10;34(50):16594–604. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness, Danbolt, & Zhou, (2016). Neuronal vs glial glutamate uptake: Resolving the conundrum. Neurochemistry International 98:29–45 [DOI] [PubMed] [Google Scholar]
- Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, … Danbolt NC (1996). Brain Glutamate Transporter Proteins Form Homomultimers *, 271(44), 27715–27722. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC, 2009. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 162, 1055e1071. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, & Kalivas PW (2010). Ceftriaxone Restores Glutamate Homeostasis and Prevents Relapse to Cocaine Seeking. Biological Psychiatry, 67(1), 81–84. 10.1016/j.biopsych.2009.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, & Kalivas PW (2010). Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci, 30(23), 7984–7992. 10.1523/JNEUROSCI.1244-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrosse AL, Hill K, & Knackstedt LA (2016). Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. European Neuropsychopharmacology, 26(2), 186–194. 10.1016/j.euroneuro.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrosse AL, O’Donovan SM, Sepulveda-Orengo M, McCullumsmith R, Reissner K, Schwendt M, & Knackstedt LA (2017). Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. The Journal of Neuroscience,June 14,2017 • 37(24):5809–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. (2008). GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008 April;56(5):481–93. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML., Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, & Methner A, (2009) Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro, J Neurochemistry. 2009 October;111(2):332–43. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Li K, Charles Nicaise C, Sannie D, Hala TJ, Javed E, Parker JL, Putatunda R, Regan KA, Suain V, Brion J, Rhoderick F, Wright MC, Poulsen DJ, & Lepore A, (2014). Overexpression of the Astrocyte Glutamate Transporter GLT1 Exacerbates Phrenic Motor Neuron Degeneration, Diaphragm Compromise, and Forelimb Motor Dysfunction following Cervical Contusion Spinal Cord Injury. The Journal of Neuroscience, May 28, 2014, 34(22):7622–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgen V, Kong L, Kau KS, Madayag A, Mantsch JR, & Baker DA (2014) Time course of cocaine-induced behavioral and neurochemical plasticity. Addict Biol. 2014 July;19(4):529–38. doi: 10.1111/j.1369-1600.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ, & Flynn J (2001). Molecular mechanisms of cystine transport Inhibition of sodium-dependent L-cystine transport [DOI] [PubMed]
- McFarland K, Lapish CC, & Kalivas PW (2003). Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(8), 3531–3537. http://doi.org/23/8/3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, Mcfarland K, Melendez RI, Kalivas PW, & Seamans JK (2005). Cystine / Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking, 25(27), 6389–6393. 10.1523/JNEUROSCI.1007-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass J, Lavin A, & Kalivas PW, (2009) NAcetylcysteine reverses cocaine-induced metaplasticity, Nature Neuroscience volume 12, pages 182–189 (2009) doi: 10.1038/nn.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM., See RE, Carr DB, Kalivas PW (2011) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011 January 4;108(1):385–90. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis J, Groc L, & Oliet SHR (2017). Astroglial glutamate transporters in the brain: Regulating neurotransmitter homeostasis and synaptic transmission. Journal of Neuroscience Research, 0(January). 10.1002/jnr.2402 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2015). Nationwide Trends, (June), 5–8.
- O’Brien CP (2003) Research advances in the understanding and treatment of addiction. Am J Addict 12 [Suppl 2]:S36–S47. [PubMed] [Google Scholar]
- Omran A, Melone M, Bellesi M, Safiulina V, Aida T, Tanaka K, Cherubini E, & Conti F (2009) Up-regulation of GLT-1 severely impairs LTD at mossy fibre–CA3 synapses. J. of Physiology. 587, 4575–4588 10.1113/jphysiol.2009.177881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C, Bell K, Duffy P, and Kalivas P, (1996) Repeated Cocaine Augments in the Nucleus Accumbens Excitatory Amino Acid Transmission Only in Rats Having Developed Behavioral Sensitization, The Journal of Neuroscience, February 15, 1996, 16(4):1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Mizera J, & Filip M (2017). Neuroadaptive changes in metabotropic glutamate mGlu2/3R expression during different phases of cocaine addiction in rats. Pharmacological Reports, 10.1016/j.pharep.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, & Kalivas PW (2014). Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement, 316–323. 10.1111/adb.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele TS, & Rosenberg PA (2016). GLT-1: The elusive presynaptic glutamate transporter. Neurochemistry International, 98, 19–28. 10.1016/j.neuint.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. (2005): Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, & Rebec GV (2009). Upregulation of Glt1 Attenuates Cue-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Journal of Neuroscience, 29(29), 9239–9243. 10.1523/JNEUROSCI.1746-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW (2015) Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking, Biological Psychiatry October 1, 2015 Volume 78, Issue 7, Pages 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, … Reissner KJ (2016). Archival Report Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core, 1–9. 10.1016/j.biopsych.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Scofield M,Heinsbroek J, Gipson C,Neuhofer D,Roberts-Wolfe D,Spencer S,GarciaKeller C, Stankeviciute N,Smith J,Allen N,Lorang M,Griffin W III, Boger B,& Kalivas P.(2017) Accumbens nNOS Interneurons Regulate Cocaine Relapse The Journal of Neuroscience, 37(4):742–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M (2004) Expression specificity of GFAP transgenes. Neurochem Res. 2004 November;29(11):2075–93. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Rauen F, Fischer F, Weibner M, Grewer C, Bicho A, & Pow D(2004) Cloning, Transport Properties, and Differential Localization of Two Splice Variants of GLT-1 in the Rat CNS: Implications for CNS Glutamate Homeostasis, Glia 45:155–169. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, & Knackstedt LA (2012). Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci, 32(36), 12406–12410. 10.1523/JNEUROSCI.1976-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, & Wadiche JI (2007). Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci, 8(12), 935–947. 10.1038/nrn2274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.