Abstract
Purpose:
Breast cancer (BC) in very young women (BCVY) is more aggressive than in older women. The purpose of this study was to evaluate the relevance of a range of clinico-pathological factors in the prognosis of BCVY patients.
Methods:
We retrospectively analyzed 258 patients diagnosed with BCVY at our hospital from 1998 to 2014; the control group comprised 101 older patients with BC. We correlated clinicopathological factors, treatments, relapse and exitus with age and with previously published miRNA expression data.
Results:
We identified some significant differences in risk factors between BCVY and older patients. The age at menarche, number of pregnancies, and age at first pregnancy were lower in the BCVY group and had a greater probability of recurrence and death in all cases. Lymph node-positive patients in the BCVY group are associated with a worse prognosis (P = .02), an immunohistochemical HER2+ subtype, and disease relapse (P = .03). Moreover, there was a shorter time between diagnosis and first relapse in BCVY patients compared with controls, and they were more likely to die from the disease (P = .002). Finally, from our panel of miRNAs deregulated in BC, reduced miR-30c expression was associated with more aggressive BC in very young patients, lower overall survival, and with axillary lymph node metastases.
Conclusions:
Patient age and axillary lymph node status post-surgery are independent and significant predictors of distant disease-free survival, local recurrence-free survival, and overall survival. The HER2+ subtype and lower miR-30c expression are related to poor prognosis in lymph node-positive young BC patients.
Keywords: Breast cancer, young women, risk factors, prognosis
Introduction
Breast cancer (BC) is usually detected in postmenopausal women. Although the average age of BC onset is at 61 years, about 1 in 40 women diagnosed with the disease are very young, and this subset has a higher mortality rate, with 5% to 7% of all deaths from BC belonging to this group.1,2
There are numerous risk factors associated with BC, most of them related to women’s reproductive histories and their lifetime hormonal exposure. These factors include age, age at menarche (the onset of menarche aged less than 11 years),3-5 age at menopause, and age of first pregnancy.6-8 Protective factors include prolonged breastfeeding status7,9-10 and moderated alcohol consumption.11-14 In addition, body mass index (BMI) has been considered as a risk factor in older BC patients but protective in young women.14 Finally, women with a family history of BC and/or BRCA mutations have an increased risk of BC.15-17
The definition of “youth” in BC is controversial, with the age threshold ranging between 30 and 45 years. However, recent studies show that in patients younger than 35 years, the risk of death from BC increased by about 5% per year. Thus, 35 years appears to be the most reasonable cut-off age for clinically defining BC in very young women (BCVY).18,19
The reason for increased mortality in BCVY patients is that their tumors often exhibit more aggressive pathological features. These include larger tumor size, higher histological grades, a higher percentage of lymph node invasion, human epidermal growth factor receptor 2 (HER2) overexpression, and triple negative tumors than their older counterparts.20-29
We performed an exhaustive clinicopathological study on a cohort of 258 Spanish BCVY patients, with the aim of identifying whether BCVY patients exhibit different clinical and/or socio-cultural background characteristics compared with older patients and whether they are likely to have a molecular basis.
Methods
Patient selection
All the subjects were selected from a database of 6072 BC patients diagnosed between 1998 and 2014 at the Hematology and Medical Oncology Unit in the Hospital Clínico Universitario de Valencia, Spain. We identified 258 young (⩽35 years) BCVY women. We used a random selected group of 101 postmenopausal women from the entire group of older patients diagnosed with BC to not bias the comparisons; they were aged between 45 and 95 years as had been used as a control group. The study was approved by the Ethics Board at our hospital and all the subjects gave their written informed consent for their inclusion.
All the patients’ clinical data were collected at their first visit, and information about their pathological characteristics and treatment history was obtained after surgery and following subsequent visits. Tumor size and lymph node involvement were assessed using the seventh edition of the American Joint Committee on Cancer staging manual.30 The patient clinicopathological data used in this study are shown in Table 1. Patients were also classified both according to the type of surgery they underwent and the type of the neoadjuvant/adjuvant treatment they received (Table 2).
Table 1.
The patient clinicopathological data.
| BCVY |
Old BC |
P value | |||
|---|---|---|---|---|---|
| NUM. | % | N° | % | ||
| Age BCVY/old BC | |||||
| <25 years/46-50 years | 12 | 4.7 | 6 | 5.9 | |
| 25-30 years/50-56 years | 60 | 23.2 | 55 | 54.4 | |
| 30-35 years/>65 years | 186 | 72.1 | 40 | 39.6 | |
| Year of diagnosis | |||||
| Before 2000 | 60 | 23 | 50 | 49.5 | .642 |
| After 2000 | 198 | 77 | 51 | 50.5 | |
| Tumor size | |||||
| <2 cm | 94 | 36.4 | 67 | 66.3 | 3.01 × 10−3 |
| 2-5 cm | 112 | 43.4 | 29 | 28.7 | |
| >5 cm | 35 | 13.6 | 5 | 5 | |
| Missing | 17 | 6.6 | 0 | 0 | |
| Lymph node status | |||||
| Node positive | 121 | 46.9 | 38 | 37.6 | .099 |
| Node negative | 120 | 46.5 | 59 | 58.4 | |
| Missing | 17 | 6.5 | 4 | 4 | |
| Grade | |||||
| I | 28 | 11 | 18 | 17.8 | .583 |
| II | 71 | 27.5 | 39 | 38.6 | |
| III | 94 | 36.4 | 39 | 38.6 | |
| Missing | 65 | 25.2 | 5 | 5 | |
| Histologic subtype | |||||
| Ductal | 209 | 81 | 73 | 72.3 | .081 |
| Lobular | 10 | 4 | 7 | 6.9 | |
| Medullary | 8 | 3 | 0 | 0 | |
| Tubular | 6 | 2 | 4 | 4 | |
| Others | 25 | 10 | 17 | 16.8 | |
| Estrogen receptor | |||||
| Positive | 180 | 70 | 72 | 71.3 | .496 |
| Negative | 62 | 24 | 29 | 28.7 | |
| Missing | 16 | 6 | 0 | 0 | |
| Progesterone receptor | |||||
| Positive | 153 | 59.3 | 69 | 68.3 | .319 |
| Negative | 87 | 33.7 | 32 | 31.7 | |
| Missing | 18 | 7 | 0 | 0 | |
| Ki 67 (%) | |||||
| Low <14 | 20 | 7.7 | 18 | 17.8 | .202 |
| 14-30 | 46 | 17.8 | 32 | 31.6 | |
| High >30 | 41 | 15.8 | 16 | 15.8 | |
| Missing | 151a | 58.5 | 35 | 34.6 | |
| Her2 | |||||
| Positive | 73 | 28.3 | 23 | 22.8 | .112 |
| Negative | 138 | 53.5 | 71 | 70.3 | |
| Missing | 47b | 18.2 | 7 | 6.9 | |
| Subtypes | |||||
| Luminal | 131 | 50.7 | 62 | 61.4 | .651 |
| Luminal-Her2 | 46 | 17.8 | 17 | 16.8 | |
| Her2 positive | 26 | 10 | 5 | 5 | |
| Triple negative | 35 | 13.5 | 17 | 16.8 | |
| Missing | 20 | 7.5 | 0 | 0 | |
Abbreviations: BC, breast cancer; BCVY, breast cancer in very young women.
Total number of patients: 258 BCVY and 101 old BC.
The data of Ki 67 were started to be used routinely in the year 2008. Previously lacking these data.
The data of HER2 overexpression are missing because the diagnosis is prior to the knowledge of the role of HER2 in breast cancer.
P values were obtained after a Pearson’s chi-squared test by R/Bioconductor.
Table 2.
Type of surgery, neoadjuvant/adjuvant treatment, and relapse.
| BCVY |
Old BC |
P value | |||
|---|---|---|---|---|---|
| Num. (%) | Num. (%) | ||||
| Surgery | |||||
| Radical mastectomy + lymphadenectomy | 98 | 38 | 30 | 29.7 | 4.7 × 10−03 |
| Radical mastectomy + sentinel node | 87 | 33.7 | 34 | 33.6 | |
| Tumorectomy + sentinel node | 29 | 11.2 | 28 | 27.7 | |
| Missing | 44 | 17 | 9 | 8.9 | |
| Treatment | |||||
| Chemotherapy | 218 | 84.5 | 73 | 72.3 | 3.82 × 10−09 |
| Endocrine therapy only | 10 | 3.9 | 24 | 23.8 | |
| No treatment | 0 | 0 | 4 | 3.9 | |
| Missing | 30 | 11.6 | 0 | 0 | |
| Neoadjuvant treatment | |||||
| Yes | 87 | 33.7 | 17 | 16.8 | 3.4 × 10−03 |
| No | 168 | 65.1 | 84 | 83.1 | |
| Missing | 3 | 1.1 | 0 | 0 | |
| Complete response after neoadjuvant treatment | |||||
| Yes | 9 | 3.5 | 7 | 6.9 | .460 |
| No | 239 | 92.6 | 94 | 93.1 | |
| Missing | 10 | 3.9 | 0 | 0 | |
| Type treatment chemotherapy | |||||
| CMF | 2 | 0.8 | 0 | 0 | 1.08 × 10−05 |
| Schema with antracyclines (AC, FEC, FAC) | 76 | 29.5 | 17 | 16.8 | |
| Taxanes | 28 | 10.8 | 21 | 20.8 | |
| Clinical trial | 74 | 28.7 | 23 | 22.8 | |
| Other | 20 | 7.7 | 12 | 11.9 | |
| No treatment with chemotherapy | 20 | 7.7 | 28 | 27.6 | |
| Missing | 38 | 14.7 | 0 | 0 | |
| Relapse | |||||
| Yes | 91 | 35.3 | 25 | 24.7 | 3.5 × 10−03 |
| No | 105 | 40.7 | 76 | 75.2 | |
| Missing | 62 | 24 | 0 | 0 | |
| Data of relapse | |||||
| <1 year | 24 | 26.4 | 7 | 28 | .296 |
| 1-5 years | 47 | 51.7 | 9 | 36 | |
| >5 years | 20 | 21.9 | 9 | 36 | |
| Missing | 0 | 0 | 0 | 0 | |
| Site of relapse | |||||
| Locoregional | 28 | 30.8 | 9 | 36 | .849 |
| Bone | 19 | 20.9 | 3 | 12 | |
| Brain | 1 | 1.1 | 1 | 4 | |
| Visceral | 37 | 40.7 | 10 | 40 | |
| Other | 6 | 6.6 | 2 | 8 | |
| Missing | 0 | 0 | 0 | 0 | |
| Exitus after relapse | |||||
| Yes | 38 | 41.8 | 8 | 32 | .105 |
| No | 26 | 28.6 | 14 | 56 | |
| Missing | 27 | 29.6 | 3 | 12 | |
Abbreviations: AC: 5-Fluoracil, ciclofosfamide; BC, breast cancer; BCVY, breast cancer in very young women; FAC: 5-Fluoracil, 5-Fluoracil and ciclofosfamide; FEC: 5-Fluoracil, Epirrubicin and Ciclofosfamide.
Total number of patients: 258 BCVY and 101 old BC. The missing patients are those who were sent to their referral centers after treatment and missed follow-up. P values were obtained after a Pearson’s chi-squared test by R/Bioconductor.
Risk factors for BC
We studied the age at menarche, age at first pregnancy, lifetime time spent breastfeeding, BMI, family heredity, and the presence of mutations in the BRCA1 and BRCA2 genes in both populations (Table 3). Data began to be collected after finding an increase in the incidence of breast cancer in young women in Spain with the suspicion that the change in reproductive habits was the cause.31,32 The criteria for the genetic study of BRCA1/2 genes are defined in Supplementary Table 1.
Table 3.
The patient reproductive data.
| BCVY |
Old BC |
P value | |||
|---|---|---|---|---|---|
| Num. | % | Num. | % | ||
| Menarche | |||||
| ⩽11 years | 48 | 18.6 | 14 | 13.9 | .531 |
| 12-14 years | 131 | 50.7 | 48 | 47.5 | |
| ⩾15 years | 7 | 2.7 | 7 | 6.9 | |
| Missing | 72 | 27.9 | 32 | 31.7 | |
| Body mass index | |||||
| <25 | 86 | 33.3 | 9 | 8.9 | .078 |
| 25-30 | 27 | 10.4 | 9 | 8.9 | |
| >30 | 3 | 1.2 | 1 | 1 | |
| Missing | 142 | 55 | 82 | 81.2 | |
| Pregnancy | |||||
| Yes | 108 | 41.9 | 63 | 62.4 | 2.74 × 10−07 |
| No | 91 | 35.3 | 6 | 5.9 | |
| Missing | 59 | 22.8 | 32 | 31.7 | |
| Age first pregnancy | |||||
| <20 years | 7 | 2.7 | 6 | 5.9 | 1.88 × 10−05 |
| 20-30 years | 54 | 20.9 | 50 | 49.5 | |
| >30 years | 33 | 12.8 | 7 | 6.9 | |
| Missing | 164 | 63.5 | 38 | 37.6 | |
| Lactation | |||||
| Yes | 59 | 22.9 | 31 | 30.7 | 7.6 × 10−3 |
| No | 23 | 8.9 | 32 | 31.7 | |
| Missing | 176 | 68.2 | 38 | 37.6 | |
| BRCA mutation | |||||
| Yes | 31 | 12.1 | 0 | 0 | 1.76 × 10−06 |
| No | 58 | 22.5 | 8 | 7.92 | |
| Do not meet the study criteria | 169 | 65.5 | 93 | 92.07 | |
| Family heredity | |||||
| First grade | 22 | 8.5 | 4 | 4 | 0.301 |
| Second grade | 33 | 12.8 | 7 | 6.9 | |
| First and second grade | 15 | 5.8 | 3 | 3 | |
| Any heredity | 142 | 55 | 87 | 86.1 | |
| Missing | 46 | 17.8 | 0 | 0 | |
Abbreviations: BC, breast cancer; BCVY, breast cancer in very young women.
Total number of patients: 258 BCVY and 101 old BC. P values were obtained after a Pearson’s chi-squared test by R/Bioconductor.
Data collection
All the tissue samples used were collected after BC surgery or after core needle biopsy (CNB) diagnosis. The formalin-fixed paraffin-embedded tissues were evaluated for their tumor content and sections containing more than 30% tumor cells were defined and cut by a pathologist.
Immunochemistry classification to get the molecular subtypes
Four-micrometer-thick sections were deparaffinized in xylene and rehydrated. Antigen retrieval was done by microwaving in 10 mM citric acid monohydrate for 1 × 5 min at 900 W and for 3 × 5 min at 600 W. Endogenous peroxidase activity was blocked by treatment with 0.5% H2O2. The slides were incubated overnight in a refrigerator at +4°C with appropriate dilutions of the primary antibodies. The reaction was visualized by the Elite ABC Kit (Vectastain, Vector Laboratories, Burlingame, CA, USA) for oestrogen receptor (ER) and by the Envision kit (Dako, Copenhagen, Denmark) for HER2. For progesteron receptor (PR), the sections were subjected to dual colorimetric immunohistochemical (IHC; Envision G/2 Doublestain; Dako).
The result was quantified as the proportion of positively stained tumor cells (range 0%-100%). For the analyses, the tissue samples were classified as positive for ER and PR when ⩾1% of the tumor cells showed positive nuclear staining. In PR dual staining, tumors with ⩾1% Diaminobenzidine (DAB)-stained nuclei or ⩾1% Perm Red-stained cytoplasm were considered positive.
HER2 was scored based on the intensity and percentage of positive cells on a scale of 0 to 3+. Cases were reported 0 (negative) if no staining or membrane staining in less than 10% of invasive tumor cells was seen; 1+ (negative) if faint/barely perceptive membrane staining was detected in more than 10% of invasive tumor cells; 2+ (positive) if weak to moderate complete membrane staining in more than 10% tumor cells or <30% with strong complete membrane staining; or 3+ (positive) if strong complete membrane staining in more than 30% invasive tumor cells was seen. Regarding evaluation of Ki67, the tumor was considered negative when less than 14% of the tumor cells showed positive stained nuclei, othervwise was positive. However, when staining was between 14 to 30% was considered intermediate expression and positive when staining was higher than 30%. We evaluated the entire tumor area from one representative section of the primary tumor.
Based on the expression of IHC markers for HER2, ER, PR, and Ki67, tumors were classified as triple negative (TNBC) (ER−/PR−/HER2−), HER2+, luminal A (ER+/PR+/HER2−/Ki-67 < 14%), or luminal B (ER+/PR+/HER2−; ER+/PR+/HER2−/Ki-67 ⩾ 14%), following the St. Gallen International Expert Consensus criteria from 2011.33
BRCA1/2 testing
BC samples from Medical Oncology Unit from “Hospital Universitario Clinico” Valencia that fulfill criteria for BRCA1/2 testing are sent to the Genotyping and Genetic Diagnostic Unit at Central Medical Research Laboratories—University of Valencia to be analyzed. An ethylenediaminetetraacetic acid (EDTA) tube with 8 mL of blood from the patients who complied with the criteria to be screened for BRCA1/2 were sent to the molecular laboratory and after genomic DNA extraction, the samples were sequenced according to manufacturer’s protocol in a specific panel to identify alterations using next generation sequencing technology (Illumina-MiSeq).
microRNA analysis
We utilized microRNA (miR) expression data from a previous study 19 in which the GeneChip miRNA2.0 microarray (Affymetrix (California, USA) was the tool used. raw data are available on GEO database under access number GSE48088.
Statistical analyses
We compared all the different characteristics recorded among BCVY and BC using SPSS, v22.0 (SPSS, Chicago, IL, USA) and Pearson’s chi-squared test using R/Bioconductor. Two-sided P values were calculated using a t test or analysis of variance (ANOVA) depending on the number of groups, and an alpha threshold of 0.05 was set for statistical significance.
Survival analysis was performed using GraphPad Prism v6.0 (GraphPad Software Inc., CA, USA). Progression-free survival (PFS) was defined as the time interval between surgery and the first documented distant relapse. Local recurrence-free survival (LRFS) was defined as the time interval between surgery and the first documented local recurrence. Overall survival (OS) was defined as the time between surgery and death or the last follow-up, whichever occurred first. Kaplan-Meier survival curves were calculated for selected risk factors and the Mantel-Cox test was used to evaluate any differences in OS between groups. We study survival analysis of miRNA expression in patients with axillary lymph node involvement.
Results
Clinicopathological risk factors and patient age
All the clinicopathological data recorded from our sample set are presented in Table 1. From the 11 endpoints investigated, Ki67 staining was not properly recorded in the clinical histories. Ki67 was started to be used routinely in the year 2008; thus, we only had access to Ki67 information in 107 BCVY patients (41.5%) and 66 older patients (65%) and so, results have been calculated with these numbers.
In addition, the results showed that all of the patients included in this study had elevated ER and PR expression levels (70% and 59% in BCVY and 71% and 68% in older, respectively). Even though the data are incomplete for 18% of the BCVY group, HER2 protein overexpression still significantly differed between the groups (28% vs 23% in the BCVY and control groups, respectively). In addition, the lowest proliferation rates (<14 Ki67 expression) were more commonly found in tumors from older patients rather than in BCVY (Table 1).
Our results indicate that BCVY patients exhibit larger tumors in BCVY. If we consider size as a continuous variable, we have a median tumor size of 3.04 cm vs 2.44 cm in BCVY and control group, respectively (P = 7.8 × 10−3). In case of categorizing tumors as less than 2 cm, 2-5 cm, and larger than 5 cm, younger patients have an increase in both categories with large tumors (P = 3.01 × 10−3). Although we observed a trend in presenting an increase of positive nodes in BCVY, this did not reach statistical significance (P = .09). Moreover, these large tumors in BCVY appeared with less cell differentiation and greater axillary lymph node involvement than older BC patients do, although analysis did not reach statistical significance. No other significant differences were detected in the two age subgroups (Table 1).
Treatments and patient age
We could get follow-up data from the entire control sample set (101 patients) and from 192 of the young women with infiltrating BC (74%). Data related to type of surgery, type of treatment relapse, and exitus are presented in Table 2. We observed an increase in radical mastectomy and lymphadenectomy (P = 4.7 × 10−3), chemotherapy (P = 3.8 × 10−9), and neoadjuvant treatment (P = 3.4 × 10−03) in BCVY than old BC patients (data presented in Table 2).
Although we did not detect any statistically significant differences in terms of locoregional breast and armpit surgical treatment between the BCVY and older BC patients, we found statistically significant improvements in terms of the surgery itself, with BCVY patients having received more sentinel node biopsy in recent years, since 2000 (P < .005). When taking the type of chemotherapeutic agent used into account, we detect differences among treatments received between patients younger than 35 years and those older than 45 years, such differences reflect that older patients receive less aggressive treatments with lower comorbidities (greater use of endocrine therapy alone, P = 3.8 × 10−09; no requirement of chemotherapy, P = 1.08 × 10−05) (Table 2).
We observed an increase in neoadjuvant treatment received by the BCVY patients respect to the older group (P = 3.4 × 10−03). We further investigate whether these differences were due to a change in clinical practice; therefore, of the 60 BCVY patients diagnosed prior to the year 2000, only seven received neoadjuvant treatment; therefore, we studied whether there was an increase in the number of patients receiving neoadjuvant treatment in recent years. Of those diagnosed between 2001 and 2007, 24 patients from 96 (25%) were treated with chemotherapy before surgery, while more than half of the patients diagnosed from 2008 to 2014 received neoadjuvant treatment (P < 1 × 10−3). The pattern was similar in older BC patients: 4% of those diagnosed before 2007 received treatment before surgery, but this rose to 29% for those diagnosed between 2008 and 2014 (P = .004). Moreover, there was a higher percentage of complete pathological response (CPR) after neoadjuvant therapy in patients aged more than 45 years than in younger patients, and the highest CPR rate was for pure HER2+ tumors in both groups.
Relapse and exitus and patient age
We studied the association between lymph node status, IHC subtype, axillary lymph node involvement, and relapse in BCVY patients. Our results demonstrate an increase percentage of relapse in BCVY patients compared with the control group (46.4% vs 24.7%, P = 3.5 × 10−03) (Table 2). When we take into account relapse with affected lymph nodes according to immunohistological categories, we see almost half of young patients with luminal tumors (47.3%), 77.3% of luminal/HER2+ tumors, 68% of HER2+, and 40% of TNBC had relapsed (P = .03) (Figure 1). In general, we observed that more than half (56.3%) of BCVY patients relapsed when axillary lymph node involvement was present (P = .02).
Figure 1.

Percentage of relapses in BCVY according to breast cancer subtype. Light and dark gray bars represent without and without lymph node involvement, respectively. BCVY indicates breast cancer in very young women.
Nearly all imnunochemistry subtypes of BCVY patients (lumnial, Her2 enriched and TNBC) experienced a major relapse compared to control group (old BC) Figure 2A, even though they had smaller tumor sizes which have a priori better prognosis (Figure 2B). Slightly more BCVY patient relapses ended up in death from the disease compared with control patients (42% vs 32%; P < 1 × 10−03). Moreover, we see an increase percentage of death in the more favorable luminal subtypes (Figure 2C). Neither group showed any differences in histological prognostic factors when we took the relapse risk, ie, tumor size, histological grade, and proliferation into account.
Figure 2.
Percentage relapse: according to immunohistochemistry subtypes (A), relapse according to initial tumor size (B), exitus according to immunohistochemistry subtypes (C), and exitus according to period of relapse (D) in BCVY (pale gray) and old BC (dark gray).
Furthermore, our results showed an inverse association between the time from diagnosing the first relapse and the probability of dying from the disease in BCVY patients. The shorter the time, the higher the likelihood that the patient would die from the disease, so that 58% of BCVY patients who relapsed within 1 year died from the disease. This risk reduced to 38% when the relapse was diagnosed at 1-5 years and to 19% when the patient relapsed more than 5 years later (P = 2.00 × 10−3; Figure 2D). Importantly, this trend was not observed in older BC patients. Moreover, we also found an association between risk of death from BC and the location of the first relapse in BCVY patients: patients with a local relapse had a greater chance of survival, but presentation of a visceral or BCVY patients relapsed in major proportion at distant sites, which results in an increased risk and worse prognosis. We see in BCVY patients, 63 with a distant relapse, 13 with local relapse, and 15 with contralateral relapse. In comparison, only 12 old BC had relapse at a distant site (48%), 11 ipsilateral (44%), and finally 2 with contralateral relapse (8%).
Reproductive data and patient age
Finally, the patient’s reproductive data are presented in Table 3. From all the factors described, we encounter high missing data on age at first pregnancy and lactation (63.5% and 68% in BCVY, respectively). Weight is not routinely recorded in the clinical data, therefore, BMI data is composed by only 116 BCVY patients (45%) being highly absent in older patients also. Our results shown that 74% of BCVY patients have normal BMI. Data in older patients are not conclusive.
Although we have information regarding age at menarche only in 86 BCVY patients, we saw that over the last 15 years, the number of BCVY patients with menarche at younger than 11 years has increased (by an average of 8% in the last 7 years with respect to the year 2000). Before the year 2000, at least 68% of BCVY patients carried at least one pregnancy to term; however, in the last 14 years, that number decreased by an average of 16%. In addition, more cases of BCVY were diagnosed during pregnancy, representing a 6% of BCVY cases in the last 6 years compared with 1.7% before the year 2000. The prevalence of BCVY diagnoses in breastfeeding women is also increasing, from 1% before 2000 to a 4% over the last 6 years (Table 3).
Regarding BRCA1/2 mutations, only 31 of the 89 BCVY patients tested (34.8%) were positive. The rest of BCVY patients (169) either did not meet criteria, because they were older than 30 years, or the study was carried out in another center and data are not available. Another eight older patients who met the criteria were also studied but no alterations were found. From the 31 BCVY patients with one BRCA1/2 mutation, 7 (24.4%) presented luminal A/B tumors, 4 (22.2%) were HER2+, 4 (21.4%) were luminal/HER2, and 16 (53.3%) were triple negative (P = .147).
Differential gene expression in tumors from very young or older women based on their miRNA expression profiles
We identified a unique profile of deregulated microRNAs (miR-30c, miR-125a, miR-17, miR-92b, miR-139, and miR-663) associated with BCVY patients.19 Here we investigated any potential links between this differential molecular signature and clinicopathological risk factors in these patients. BCVY patients with axillary lymph node involvement had a significantly higher risk of BC relapse. Thus, we assessed whether any of the aforementioned BCVY- miRs signature was also associated with more aggressive axillary lymph node metastases in BCVY. However, due to the limited patient sample size in this study, we were only able to perform survival analysis in 11 BCVY and 2 control group patients with axillary lymph node involvement.
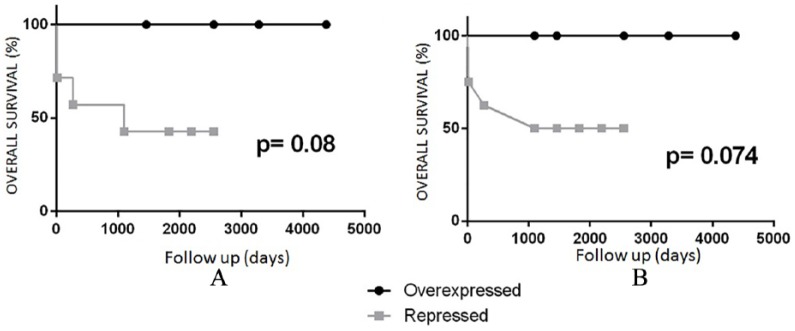
Although we observed no significant differences for any of these miRNAs (probably because of the small sub-sample cohort), we were able to differentiate between the two groups based on hsa-miR-30c expression with a trend to significance (P = .080). Patients with axillary involvement and reduced miR-30c expression levels had a lower OS than those with normal or increased miR-30c expression. The difference was more evident in BCVY patients: seven of them showed a repressed miR-30c status, four of whom died, while control group patients exhibiting miR-30c repression are still alive and have not suffered any relapses (Figure 3).
Figure 3.
Kaplan-Meier curve for overall survival in those BCVY (A) and BC (B) patients who had nodal involvement according to miR-30-c expression deregulation. Overexpression of miRNA is shown in light gray and repression in black. BC indicates breast cancer; BCVY, breast cancer in very young women.
Discussion
BC is the leading cause of cancer death in young women in developed countries; moreover, younger age at diagnosis usually correlates with more aggressive biological tumor characteristics. Based on previously published information,18 we took 35 years as the threshold age for consideration as part of our young patient cohort, which included 258 BCVY patients, representing one of the largest populations of BCVY patients so far studied.
Pathological and clinical differences between BC in younger and older women and survival analysis by subgroup
Over the last 20 years, multiple prospective and retrospective studies have proven that BCVY (⩽35 years) has a worse prognosis than a BC diagnosis in older women,18-20,22-26 Consistent with most previous findings for similar patient cohorts,34-40 our data indicate that BCVY patients tend to present larger, less differentiated BCVY tumors with a higher percentage of axillary lymph node involvement than older BC patients do.
When we look at the molecular prognostic factors, our data show that tumors both in young and older women express a higher percentage of ER than the proportion described in other series such as that by Karihtala and collaborators.1 Moreover, our data demonstrate that a higher percentage of BCVY patient’s present HER2+ tumors compared with their older counterpart controls, whereas published data regarding this latter prognostic factor differ. Some studies found no differences1,34 while others observed increased HER2 amplification.28,41-45
Regarding locoregional breast and armpit treatment, we would like to highlight the fact that the type of breast and sentinel armpit surgery performed on young women over the last two decades tended to be more conservative (P < .005). This practice matches the recent tendency of performing the least aggressive surgery possible to achieve lower patient morbidity.46-49 On the other hand, in agreement with previously published studies,50-52 we also observed the increased implementation of neoadjuvant chemotherapy treatments, which reached significance for BCVY patients.
We found that only 8% of older patients meet criteria for BRCA testing against 35% of young women. Also, in concordance with published literature,31,53 we found that BRCA gene mutations are far more common in young women. Our results also show an even distribution across the molecular cancer subtypes in all the BCVY patients with a BRCA mutation. However, those with triple negative BC subtype showed a higher percentage of BRCA1/2 mutations consistent with data published in 2012 by Criscitiello and colleagues.54
Regarding disease relapse and OS in our studied patients, nearly half of the BCVY women were diagnosed with a BC relapse compared with just a quarter of older women. No IHC subtype seems to be associated with an increased risk of relapse, neither in youngsters nor in older ones, given that the differences are not statistically significant. Nevertheless, it does seem that axillary involvement has a statistically significant relationship with the risk of relapse in young women, and the significance remains if, in addition to axillary involvement, we associate the luminal/HER2 subtype. The axillary involvement thus could be considered as an independent histological prognostic factor.
However, our comparisons of all these clinicopathological factors did not highlight any associations between the tumor subtypes and increased risk of relapse, in either group. Furthermore, there was a statistically significant relationship between axillary lymph node involvement and the risk of relapse in BCVY women. Moreover, our study confirms that axillary lymph node involvement after surgery is the only independent histological prognostic factor in BCVY patients and confirms the results in Asian populations, published by Zhao and colleagues in 2015.55
Regarding death from BC, we concluded that the risk of dying from this disease is significantly associated with the presentation and characteristics of a first relapse, both in our young and older patients. Moreover, if this relapse appears before the year of diagnosis or is in the form of visceral or cerebral relapse, it is associated, in a statistically significant way, with exitus in young women. For this reason, a more thorough follow-up should be considered in young patients, especially in the first year after diagnosis. Furthermore, in agreement with the literature,43 relapse is much more strongly associated with a prognosis of death in BCVY patients when it occurs within the first year after the initial BC diagnosis, or when the relapse presents in visceral or brain tissue.
Reproductive differences between BC in young and older women
We also analyzed whether there were any differences in reproductive factors between our two groups, according to the indications set out in the GRELL study in European women56 and by Pollan and colleagues57 in a Spanish population. Most of our cases were BCVY women diagnosed in recent years (77% of them after the year 2000). Our results reflect the tendency observed in reproductive factors of the Spanish female population over the last 20 years, characterized by a decline in fertility and age at menarche, accompanied by an increase in age at first pregnancy.
Similar to the aforementioned studies, our findings showed an increased BCVY incidence correlated with younger age (<11 years) at menarche. Moreover, 68% of old women (control group) had had at least one pregnancy compared with patients 16% in BCVY. We also detected an increase in the number of young patients diagnosed with BC during pregnancy over the last 10 years (1.7% of diagnoses between 1998 and the year 2000 to vs 6% over the last 6 years). In addition, we observed a similar increase in the diagnosis of BC in breastfeeding women, rising from 1% from 1998 to 2008 to 4% over the last 6 years.
Genetic differences between BC in young and older women
Current studies concerning why young women develop more aggressive BC than older patients are insufficient, mildly suggesting that increased tumor aggressiveness is likely due to biological differences. In our previously published work, we identified a signature of differential expression of microRNAs in BCVY patients.19 Here, we checked an association between this signature and axillary lymph node involvement, and the risk of relapse. We decided to study the correlation between miRNAs and axillary lymph node involvement since in the clinical data presented above there was a significant correlation between lymph node involvement and risk of relapse due to the disease. However, our limited sample of BCVY patients with axillary lymph node involvement prevented us from demonstrating any associations with confidence. Nonetheless, it appears that patients in this subgroup with decreased miR-30c expression did have a higher risk of dying from their disease. This is consistent with other descriptions in the scientific literature, where miR-30c is identified as a prognosis biomarker in BC, has been shown to be involved in chemotherapy resistance and cell invasion, and is associated with the epidermal growth factor receptor (EGFR) and erbB4 signaling pathways.58-60
Conclusions
In conclusion, our study shows that the age of BCVY patients with presence of axillary lymph node involvement after surgery is an independent prognostic predictor of PFS and OS. In addition, axillary lymph node involvement and the presentation of a luminal or HER2+ tumor subtype in young women are associated with poor prognosis. Finally, decreased miR-30c expression and axillary lymph node involvement represent a potential prognostic biomarker in BCVY patients. Therefore, more targeted therapies can be specifically invented for and tested in this subgroup.
Supplemental Material
Supplemental material, Supplementarytable_1 for Breast Cancer in Very Young Patients in a Spanish Cohort: Age as an Independent Bad Prognostic Indicator by María Teresa Martínez, Sara S Oltra, María Peña-Chilet, Elisa Alonso, Cristina Hernando, Octavio Burgues, Isabel Chirivella, Begoña Bermejo, Ana Lluch and Gloria Ribas in Breast Cancer: Basic and Clinical Research
Acknowledgments
The Centre of Networked Biomedical Cancer Research (CIBERONC) is a Carlos III Health Institute initiative.[We thank INCLIVA Biobank, which is part of the Spanish Hospital Biobanks Network (ReTBioH)to storage and providing samples for the study. We would like also to thank all the patients and volunteers who gave their consent for inclusion in this study and several patients organizations for their financial support.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This study was partially supported by funds from the Carlos III Health Institute (FIS project PI13/00606), the Fundación LeCadó (Flor de Vida project), European Regional Development (FEDER) and is supported by the Carlos III Health Institute/FEDER (grant number: RD09/0076/00132). SSO is funded by the “Ministry of Education, Culture and Sport” under a FPU fellowship (FPU13/04976). G.R. receives support from a type-II Miquel Servet SNS senior research contract (CP14/00013).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s Note: Elisa Alonso and Octavio Burgues are also affiiliated to Centre of Networked Biomedical Cancer Research (CIBERONC), Valencia, Spain.
Author Contributions: MTM, GR and AL conceived, coordinate and designed all aspects of the study. MTM, SO and MP contribute to the statistical analysis with SPSS and R/Bioconductor Environment. EA and OB contribute in the pathology determinations. MTM, IC, CH and BB selected the patients and collected all the clinical data. MTM, SO and GR wrote the manuscript. All authors revised, read, and approved the final manuscript.
Data Availability Statement: The clinical data used to support the findings of this study are restricted by the ethics board of Hospital Clinico de Valencia to protect patient privacy. Data are available with previous contact with María Teresa Martínez (maitemartinez3@yahoo.es) for researchers who meet the criteria for access to confidential data.
Ethics Approval and Informed Consent: The study was approved by the Ethics Board at Hospital Clínico Universitario de Valencia (Spain), and all the subjects gave their written informed consent for their inclusion.
References
- 1. Karihtala P, Winqvist R, Bloigu R, Jukkola-Vuorinen A. Long-term observational follow-up study of breast cancer diagnosed in women </=40 years old. Breast. 2010;19:456-461. [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48:3355-3377. [DOI] [PubMed] [Google Scholar]
- 3. Hunter DJ, Spiegelman D, Adami HO, et al. Non-dietary factors as risk factors for breast cancer, and as effect modifiers of the association of fat intake and risk of breast cancer. Cancer Causes Control. 1997;8:49-56. [DOI] [PubMed] [Google Scholar]
- 4. Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47-67. [DOI] [PubMed] [Google Scholar]
- 5. Willet WC, Rockhill B, Hankinson SE, Hunter D, Colditz GA. Non genetic factors in the causation of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK. (Eds) Disease of the Breast. 3rd ed. 2004; 223-276. Philadelphia; New York: Lippincott-Raven. [Google Scholar]
- 6. Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379-6391. [DOI] [PubMed] [Google Scholar]
- 7. Collaborative-Group-on-Hormonal-Factors-in-Breast-Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047-1059. [PubMed] [Google Scholar]
- 8. Ewertz M, Duffy SW, Adami HO, et al. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46:597-603. [DOI] [PubMed] [Google Scholar]
- 9. Dumitrescu RG, Cotarla I. Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Med. 2005;9:208-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colditz GA, Baer HJ, Tamini RM. Breast cancer. In: Schoettenfeld D., Fraumeni JF., Jr (Eds) Cancer Epidemiology and Prevention. 3rd ed. 2006; 995-1012. New York: Oxford University Press. [Google Scholar]
- 11. Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2004;111:762-771. [DOI] [PubMed] [Google Scholar]
- 12. Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110-132. [DOI] [PubMed] [Google Scholar]
- 13. Brinton LA, Swanson CA. Height and weight at various ages and risk of breast cancer. Ann Epidemiol. 1992;2:597-609. [DOI] [PubMed] [Google Scholar]
- 14. Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216:28-43. [DOI] [PubMed] [Google Scholar]
- 15. Collaborative-Group-on-Hormonal-Factors-in-Breast-Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389-1399. [DOI] [PubMed] [Google Scholar]
- 16. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee EH, Park SK, Park B, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122:11-25. [DOI] [PubMed] [Google Scholar]
- 18. Han W, Kang SY. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119:193-200. [DOI] [PubMed] [Google Scholar]
- 19. Peña-Chilet M, Martínez MT, Pérez-Fidalgo JA, et al. MicroRNA profile in very young women with breast cancer. BMC Cancer. 2014;14:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18-e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Partridge AH, Hughes ME, Ottesen RA, et al. The effect of age on delay in diagnosis and stage of breast cancer. Oncologist. 2012;17:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Q, Ma B, Kang M. A retrospective comparative study of clinicopathological features between young and elderly women with breast cancer. Int J Clin Exp Med. 2015;8:5869-5875. [PMC free article] [PubMed] [Google Scholar]
- 23. Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23-33. [DOI] [PubMed] [Google Scholar]
- 24. Elkum N, Dermime S, Ajarim D, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer—histopathological and prognostic considerations. Br J Cancer. 1997;75:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker RA, Lees E, Webb MB, Dearing SJ. Breast carcinomas occurring in young women (< 35 years) are different. Br J Cancer. 1996;74:1796-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartley MC, McKinley BP, Rogers EA, et al. Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: a case-control study. Am Surg. 2006;72:1189-94; discussion 1194-1195. [PubMed] [Google Scholar]
- 28. Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273-279. [DOI] [PubMed] [Google Scholar]
- 29. Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer,2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014;219:19-28. [DOI] [PubMed] [Google Scholar]
- 30. AJCC. Breast. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:347-376. [Google Scholar]
- 31. Wang F, Fang Q, Ge Z, Yu N, Xu S, Fan X. Common BRCA1 and BRCA2 mutations in breast cancer families: a meta-analysis from systematic review. Mol Biol Rep. 2011;39:2109-2118. [DOI] [PubMed] [Google Scholar]
- 32. Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145:567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng R, Wang S, Shi Y, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011;20:568-573. [DOI] [PubMed] [Google Scholar]
- 35. Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3:65-72. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez-Angulo AM, Broglio K, Kau SW, et al. Women age < or = 35 years with primary breast carcinoma: disease features at presentation. Cancer. 2005;103:2466-2472. [DOI] [PubMed] [Google Scholar]
- 37. Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zabicki K, Colbert JA, Dominguez FJ, et al. Breast cancer diagnosis in women < or = 40 versus 50 to 60 years: increasing size and stage disparity compared with older women over time. Ann Surg Oncol. 2006;13:1072-1077. [DOI] [PubMed] [Google Scholar]
- 39. Fredholm H, Magnusson K, Lindstro LS, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat. 2016;160:131-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Villarreal-Garza C, Mohar A, Bargallo-Rocha JE, et al. Molecular subtypes and prognosis in young Mexican women with breast cancer. Clin Breast Cancer. 2017;17:e95-e102. doi: 10.1016/j.clbc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 41. McAree B, O’Donnell ME, Spence A, Lioe TF, McManus DT, Spence RA. Breast cancer in women under 40 years of age: a series of 57 cases from Northern Ireland. Breast. 2010;19:97-104. [DOI] [PubMed] [Google Scholar]
- 42. Kheirelseid EH, Boggs JM, Curran C, et al. Younger age as a prognostic indicator in breast cancer: a cohort study. BMC Cancer. 2011;11:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol. 2009;100:248-251. [DOI] [PubMed] [Google Scholar]
- 45. Vrieling C, Collette L, Fourquet A, et al. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients? Eur J Cancer. 2003;39:932-944. [DOI] [PubMed] [Google Scholar]
- 46. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-1232. [DOI] [PubMed] [Google Scholar]
- 47. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241. [DOI] [PubMed] [Google Scholar]
- 48. Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13:412-419. [DOI] [PubMed] [Google Scholar]
- 49. Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567-575. [DOI] [PubMed] [Google Scholar]
- 50. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672-2685. [DOI] [PubMed] [Google Scholar]
- 51. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778-785. [DOI] [PubMed] [Google Scholar]
- 52. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96-102. [DOI] [PubMed] [Google Scholar]
- 53. Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2005;23:9-15. [DOI] [PubMed] [Google Scholar]
- 54. Criscitiello C, Azim HA, Jr, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23:vi13-vi18. [DOI] [PubMed] [Google Scholar]
- 55. Zhao Y, Dong X, Li R, Song J, Zhang D. Correlation between clinical-pathologic factors and long-term follow-up in young breast cancer patients. Transl Oncol. 2015;8:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leclere B, Molinie F, Tretarre B, Stracci F, Daubisse-Marliac L, Colonna M. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2012;37:544-549. [DOI] [PubMed] [Google Scholar]
- 57. Pollan M, Michelena MJ, Ardanaz E, Izquierdo A, Sanchez-Perez MJ, Torrella A. Breast cancer incidence in Spain before, during and after the implementation of screening programmes. Ann Oncol. 2010;21:iii97-iii102. [DOI] [PubMed] [Google Scholar]
- 58. Rodriguez-Gonzalez FG, Sieuwerts AM, Smid M, et al. MicroRNA-30c expression level is an independent predictor of clinical benefit of endocrine therapy in advanced estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2011;127:43-51. [DOI] [PubMed] [Google Scholar]
- 59. Bockhorn J, Dalton R, Nwachukwu C, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun. 2013;4:1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bockhorn J, Yee K, Chang YF, et al. MicroRNA-30c targets cytoskeleton genes involved in breast cancer cell invasion. Breast Cancer Res Treat. 2013;137:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementarytable_1 for Breast Cancer in Very Young Patients in a Spanish Cohort: Age as an Independent Bad Prognostic Indicator by María Teresa Martínez, Sara S Oltra, María Peña-Chilet, Elisa Alonso, Cristina Hernando, Octavio Burgues, Isabel Chirivella, Begoña Bermejo, Ana Lluch and Gloria Ribas in Breast Cancer: Basic and Clinical Research