1. Introduction
The shoulder complex is one of the most mobile joints in the human body allowing a great freedom of motion to the upper limb. However, enhanced mobility comes at the cost of making joint stabilisers susceptible to injury in extremes of motion. Unfortunately chronic shoulder pain and weakness is a common cause of a visit to physician's office with its prevalence being as high as 15.4% in men and 24.9% in women with a significant rise in pain and severity in population over 50 years.1 Rotator cuff tendinopathies are believed to be the most common cause of shoulder pain syndromes with one cross-sectional study reporting that 86% of all clinical diagnoses of shoulder pain were that of rotator cuff lesions.2
Sub acromial impingement syndrome (SAIS) is a spectrum of pathologies of the sub acromial space including partial rotator cuff tears (RCT), calcific tendinitis, supraspinatus tendinosis and bursitis. It is estimated from cross sectional studies that it affects over 50% of population over 60 years of age.3 The recurrent and persistent nature of the pathology can result in significant disability and functional loss. The etiopathology of SAIS is complex and multifactorial. The sub acromial space is a tight space between the acromion and the humeral head and contains the rotator cuff tendons, long head of biceps, a bursa and the coracoacromial ligament. A combination of intrinsic factors (degeneration from overuse) and extrinsic factors (compression from anatomic structures) are implicated to cause inflammation and subsequent degeneration of the rotator cuff.4
In his classical study, Neer described the three stages of impingement. Stage I with oedema and haemorrhage of the bursa and cuff which is typical in persons under twenty-five years old. Stage II involves irreversible changes, such as fibrosis and tendinitis of the rotator cuff, and typically occurs in patients who are twenty-five to forty years old. Stage III is marked by partial or complete tears of the rotator cuff and usually is seen in patients over forty years of age.5
Early tendinosis and partial tears are usually treated conservatively with rest, analgesics, physical therapy and steroid injections.6 However, none of the current therapies show uniform improvement in clinical, functional, and radiological outcomes and available evidence suggests that effective intervention for early tendinosis and low-grade tears is still elusive. It has been shown that the major limiting factor is the failed healing response to microtrauma. PRP contains a number of bioactive molecules which theoretically could improve the tissue microenvironment enabling better tendon healing response. The strategy of employing biologic factors like PRP in tendon healing are based on fact that tendinopathy shows reduced stem cell numbers, disorganised matrix and hypoperfusion.7 In a recent current concepts article Singh et al. suggested that orthobiologics have a role to play in the rotator cuff syndrome.8
1.1. Platelet rich plasma (PRP) - contents and formulation
Platelets are small fragments of megakaryocytes and play an important role in coagulation cascade. Apart from a key role in haemostasis, they also play an important function in tissue healing. Platelets contain many dense bodies also known as alpha granules which act as repositories for growth factors. Anabolic growth factors contained in PRP include transforming growth factor(TGF-β1), platelet derived growth factor (PDGF), Insulin-like growth factor(IGF-1), vascular endothelial growth factors(VEGF), human growth hormone (hGH) and fibroblast growth factor (bFGF). These factors are released in presence of activating agents like thrombin or calcium chloride. Most of the factors are released in first ten minutes but leeching of factors continues for as long as several weeks.
Current literature lacks consensus in various terminologies used for platelet preparations. In general PRP is defined as autologous plasma with platelet concentration more than baseline levels of 1,50,000/μl – 3,oo,oo/μl.9 PRP may be leucocyte rich (LR- PRP) or leukocyte poor (LP-PRP). A separation system is required for reduction of leucocytes in preparation. It has been shown by several studies that LR-PRP elicits an inflammatory response. Views of studies regarding value of delivery of concentrated dose of leucocytes remains contradictory. Some studies postulate that heightened inflammatory response might hinder healing by triggering release of catabolic metabolites,10,11 while others highlight the antimicrobial role of leucocytes and a higher level of growth factors.12 At present, literature remains inconclusive in proving a beneficial role of leucocytes in PRP.
| Classification of PRP | |
|---|---|
| Leucocyte rich - PRP |
|
| Leucocyte poor -PRP |
|
| PRP gel preparations |
|
| PRP by products-Platelet poor plasma Platelet poor gel |
|
1.2. Preparation
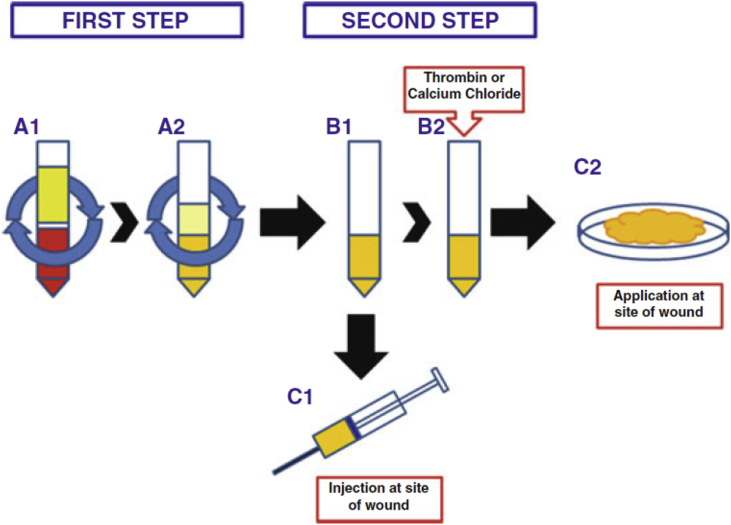
More than 16 systems are available for commercial preparation of PRP.13 All systems have some common preparation steps. First, autologous blood is drawn with anticoagulant and immediately centrifuged to produce a Red Blood Cell (RBC) free and platelet poor plasma (PPP) layer at the top, buffy coat with platelets and leucocytes in the middle and RBC plug at the bottom (see Fig. 1). Through further processing the RBC plug and PPP layer are removed thereby creating a platelet rich concentrate. The platelets are then activated either by calcium chloride, thrombin or by environmental factors and the activated concentrate is applied to the desired site either as a liquid formulation or as a gel which requires additional processing.14
Fig. 1.
Preparation of PRP. Active PRP is prepared immediately before use by a 2-step procedure. Step 1: two centrifugations, the first(A1) designed to eliminate red blood cells, the second (A2) to enrich plasma with platelets (minimum 1 × 10 6/μl to get clinical efficacy). Step 2: Plasma rich in platelets (B1) can be used immediately (C1) or activated with the addition of thrombin or calcium chloride (B2),resulting in fibrin polymerization and production of a gelatinousplatelet gel applied to the surgical site (C2) (Maffulli, 2016).
1.3. Preclinical studies and biological justification
Various basic sciences studies have shown that PRP is rich in growth factors and results of animal experiments look promising. Pre-clinical studies have proved that platelet rich injections help proliferation of different cells including tenocytes and myocytes. Jo et al. observed that PRP increased cell proliferation and expression of gene for collagen synthesis in tenocytes and that addition of thrombin had significant positive effect in accelerating proliferation.15 In-vitro experiments on rat Achilles tendons showed that platelet derived growth factors stimulated healing and improved biomechanical strength by their effects on early stages of healing.
Inherent lack of healing mechanisms and paucity of vasculature are the basic factors which lead to the degenerative cascade in rotator cuff disease. Histopathology shows that tendinosis is characterised by fibrosis, mucoid degeneration, and deposition of disorganised collagen fibrils.16 Pro-angiogenic factors and chemokines contained in PRP supports vascular cell proliferation and migration, endothelial cell rolling and increases vascular permeability.17 In tendon disorders, PRP molecules target tenocytes and fibroblasts promoting migration and collagen deposition. The use of PRP stems from the fact that it might help alter the non-healing mechanisms of a tendon injury by changing the cellular micro-environment.18
In the last decade application of PRP in various musculoskeletal pathologies has fast outpaced the evidence required for its clinical use. Clinicians have exploited the fact that PRP is derived from autologous sources and faces less hurdles from legal and ethical standpoints. Lack of regulatory hurdles, ease of production and administration make it attractive for conservative therapy of chronic shoulder injuries.
2. Discussion
Platelet rich plasma has received much attention in sports medicine and rehabilitation due to reports of its successful use in some celebrity athletes. Application of PRP in clinics has outpaced data from clinical trials. Potential advantages like it being a procedure with low morbidity and relative low cost as compared to surgery make it an attractive option in outpatient setting. Although, several studies in preclinical settings have shown favourable response to PRP therapy. There is however still paucity of data from clinical trials.
Rha et al. conducted a prospective double blinded RCT in 39 patients to compare the efficacy of PRP injection with that of dry needling in cases of rotator cuff tendinosis and partial tears. The study concluded that PRP injections led to significant reduction in the Shoulder pain and Disability Index, and improvement in range of motion as compared to dry needling alone. The authors admit that dry needling itself has been shown to have a beneficial response as it elicits an inflammatory response which releases growth factors. Thus, dry needling cannot be considered as a true placebo. Furthermore, quantification of platelet concentration was not done before injection, which, in turn could have affected the quantity of factors release. The study does not state whether a leukocyte rich or poor formulation was used. Despite some shortcomings the study highlights potential of PRP in early rotator cuff disease.19
In their double blinded RCT, Kesikburun et al. compared effect of a single PRP injection with a placebo in 40 patients for patients with diagnosed rotator cuff tendinosis or partial tears. The study concluded that at 1 year, PRP injections had no benefit over placebo in terms of quality of life, disability, pain and range of motion. The study utilised a leukocyte rich formulation of PRP which could have caused a deleterious effect in early stages of healing. The study used a single injection as against serial injections, and it is a matter of debate if successive doses of PRP cause any improvement in healing.20
In a recent single blinded RCT, Nejati et al. studied the effects of PRP injections on patients with a clinical diagnosis of impingement. They concluded that PRP was not superior to exercise therapy in a group of 62 patients randomly allocated in either treatment groups. Patients in PRP group were not advised exercises. At the end of follow-up period, the abduction ROM and the WORC scores were significantly higher in the exercise therapy group. The authors conclude that their findings are in line with several other studies which state that exercise alone is sufficient to improve functional outcome in patients with RCTs. Further studies will be required to assess the possibility of an additive effect of PRP with exercise therapy.21
Ilhanli et al. compared the results of three serial injections of PRP to physical therapy in patients of chronic partial rotator-cuff tears in group of 70 patients. 35 patients received 3 serial doses of PRP along with ROM and stretching exercises while the patients in physical therapy group received 15 sessions of ultrasound therapy, trans-cutaneous electrical stimulation, and exercises. It was observed that ROM was significantly improved in the physical therapy group, however the patients in PRP group showed significantly better DASH scores. The study concludes that PRP therapy shows encouraging clinical results in patients with chronic partial supraspinatus tears.22
In a non-randomised trial, Say and colleagues studied effects of a single dose of activated PRP and methylprednisolone with prilocaine in sixty patients with rotator cuff tendinosis or a partial supraspinatus tear confirmed with imaging. They found that pain and functional scores at six weeks and six months were significantly better in the group with steroid treatment. The lack of randomisation and control group limited the study.23 Contrary to the above study, for patients with a partial supraspinatus tear, Shams et al. reported a statistically significant improvement in both pain and functional scores in PRP group compared to steroid group. The use of leucocyte reduced PRP in this study may have yielded better results.24
Several prospective studies support the use of PRP in chronic rotator cuff tears. Scarpone et al. found improvements in pain, function and radiological scores after single injection of PRP.25 Similarly, Sengodan et al. found significant differences in pre and post injection pain scores after single injection of PRP.26
Apart from application of PRP as a conservative modality, several studies investigated the role of PRP application in arthroscopic rotator cuff repairs. Theoretically biological properties of PRP make it ideal for reducing the re-tear rates and healing of bone tendon interfaces after surgery. A randomised controlled study by Randelli showed improvement in pain and a positive effect on tendon healing.27 Another cohort study failed to show improvements in re tear rates and functional outcomes after use of PRP in surgery.28
| Type of study | No of patients (Control/Int) | Patient diagnosis | Process | Conclusions | |
|---|---|---|---|---|---|
| Kisekburun et al.,2013 (PRP vs saline) | RCT | 40 (20/20) | Rotator cuff tendinosis, partial tear | 60 ml blood, citrate,not activated, 5 ml inj | Not superior to placebo |
| Nejati et al.,2017 (PRP vs exercise) | RCT | 42(20/22) | Shoulder impingement (Clinical diagnosis) | 25 ml blood, citrate, 5 ml inj | Not superior to exercise |
| Rha et al.,2013 (PRP vs needling) | RCT | 39 (20/19) | Supraspinatus tendinosis, partial tear | 25 ml blood, citrate,3 ml | Superior to dry needling |
| Ilhani et al.,2015 (PRP vs physical therapy) | RCT | 62 (32/30) | Partial supraspinatus tear | 15 ml blood, 6 ml PRP, activated by calcium chloride | Improvement of functional score in PRP, Rom better in physical treatment group |
| Shams et al.,2016 (PRP vs steroids) | RCT | 40(20/20) | Partial supraspinatus tear | 10 ml blood, 2.5 ml PRP, Leucocyte reduced |
Not superior at 6 months |
| Say et al.,2016 PRP vs steroids | Nonrandomised trial | 60(30/30) | Rotator cuff tendinosis, partial supraspinatus tear | 30 ml blood, 2.5 ml PRP, Activated by calcium chloride |
Steroids superior |
| Sengodan et al.,2017 | Prospective study | 20 | Partial supraspinatus tear | 20 ml blood, 3 ml PRP | Favourable improvements |
| Scarpone et al.,2013 | Prospective study | 19 | Rotator cuff tendinosis, partial tear | 20 ml blood, 3.5 ml PRP | Favourable improvements |
3. Conclusions
PRP is an excellent autologous source of concentrated bioactive molecules with a potential to accelerate healing. Animal models and its successful application in other chronic musculoskeletal pathologies suggest a possible role in treatment of early rotator cuff tendon tears and shoulder pain syndromes.29 Present literature review concludes that PRP is a safe and effective method in improving pain and function in patients with impingement or partial rotator cuff tears. However, when compared to steroids or physical therapy, evidence is conflicting. Inconsistent results may be explained by lack of standardisation in protocols in preparation and application of PRP. Almost all the studies reviewed had a small sample population. None of the studies examined results in a young adult population and its effect on return to sporting activities. Heterogeneity in literature in respect to different methods to prepare PRP, variability in number and method of injections and individual variation in constituents of PRP make it difficult to interpret and process results conclusively. Current published literature does not address the question whether concentration of leucocytes has any role in outcome. Future studies must clearly state details of processing methods and quantitative data on composition of PRP used.
Despite intensive research on PRP we hardly understand its biology, constitution, and ideal method of delivery. However, in principle PRP has immense potential in field of regenerative medicine and might offer a new possibility for efficient treatment of shoulder tendinopathies. Further large randomised controlled trials are necessary to establish protocols and prove its benefits in terms of clinical outcomes.
Conflicts of interest
Nil.
References
- 1.Hasvold T., Johnsen R. Headache and neck or shoulder pain--frequent and disabling complaints in the general population. Scand J Prim Health Care. 1993;11(3):219–224. doi: 10.3109/02813439308994834. http://www.ncbi.nlm.nih.gov/pubmed/8272656 Available from: [DOI] [PubMed] [Google Scholar]
- 2.Östör A.J.K., Richards C.A., Prevost A.T., Speed C.A., Hazleman B.L. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology. 2005;44(6):800–805. doi: 10.1093/rheumatology/keh598. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.M., Dahiya N., Teefey S.A.S. Location and initiation of degenerative rotator cuff tears: an analysis of three hundred and sixty shoulders. J Bone Joint Surg Am. 2010;92(5):1088–1096. doi: 10.2106/JBJS.I.00686. http://www.ncbi.nlm.nih.gov/pubmed/20439653%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2945926 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LU Bigliani, Levine W.N. Subacromial impingement syndrome. J Bone Joint Surg Am. 1997;79(12):1854–1868. [PubMed] [Google Scholar]
- 5.Neer C.S. Impingement lesions. Clin Orthop Relat Res. 1983 http://www.ncbi.nlm.nih.gov/pubmed/6825348 Mar [cited 2017 Nov 22];(173):70–7. Available from: [PubMed] [Google Scholar]
- 6.Umer M., Qadir I., Azam M. Subacromial impingement syndrome. Orthop Rev. 2012;4(2):18. doi: 10.4081/or.2012.e18. http://www.pagepress.org/journals/index.php/or/article/view/or.2012.e18 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andia I., Maffulli N. Meeting current musculoskeletal health demand through deeper insights into tissue homeostasis and regeneration. Expert Opin Biol Ther. 2015 doi: 10.1517/14712598.2015.1026324. http://www.ncbi.nlm.nih.gov/pubmed/25772645 Jun 3 [cited 2017 Dec 27];15(6):767–71. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Singh B., Bakti N., Gulihar A. Current concepts in the diagnosis and treatment of shoulder impingement. Indian J Orthop. 2017 doi: 10.4103/ortho.IJOrtho_187_17. http://www.ncbi.nlm.nih.gov/pubmed/28966374 [cited 2018 Jan 15];51(5):516–23. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001 doi: 10.1097/00008505-200110000-00002. http://www.ncbi.nlm.nih.gov/pubmed/11813662 [cited 2017 Nov 23];10(4):225–8. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Dragoo J.L., Braun H.J., Durham J.L. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40(6):1274–1281. doi: 10.1177/0363546512442334. http://journals.sagepub.com/doi/10.1177/0363546512442334 Available from: [DOI] [PubMed] [Google Scholar]
- 11.Roman-Blas J.A., Stokes D.G., Jimenez S.A. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthritis Cartilage. 2007;15(12):1367–1377. doi: 10.1016/j.joca.2007.04.011. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2153443&tool=pmcentrez&rendertype=abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann R., Jakubietz R., Jakubietz M. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41(10):1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]
- 13.Chahla J., Cinque M.E., Piuzzi N.S. A call for standardization in platelet-rich plasma preparation protocols and composition reporting. J Bone Jt Surg. 2017;99(20) doi: 10.2106/JBJS.16.01374. http://insights.ovid.com/crossref?an=00004623-201710180-00009 1769–79. Available from: [DOI] [PubMed] [Google Scholar]
- 14.Maffulli N. 2016. Platelet Rich Plasma in Musculoskeletal Practice.http://link.springer.com/10.1007/978-1-4471-7271-0 Available from: [Google Scholar]
- 15.Jo C.H., Kim J.E., Yoon K.S., Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012 doi: 10.1177/0363546512437525. http://www.ncbi.nlm.nih.gov/pubmed/22366517 May 23 [cited 2017 Nov 23];40(5):1035–45. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Lyras D.N., Kazakos K., Agrogiannis G. Experimental study of tendon healing early phase: is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res. 2010;96(4):381–387. doi: 10.1016/j.otsr.2010.03.010. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Brill A., Elinav H., Varon D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc Res. 2004;63(2):226–235. doi: 10.1016/j.cardiores.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Andia I., Rubio-Azpeitia E., Maffulli N. Platelet Rich Plasma in Musculoskeletal Practice. Springer London; London: 2016. Potential links between tendon pathology and platelet rich plasma biology.http://link.springer.com/10.1007/978-1-4471-7271-0_10 [cited 2017 Nov 23]. p. 223–40. Available from: [Google Scholar]
- 19.Rha D., Park G.-Y., Kim Y.-K., Kim M.T., Lee S.C. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(2):113–122. doi: 10.1177/0269215512448388. http://journals.sagepub.com/doi/10.1177/0269215512448388 Available from: [DOI] [PubMed] [Google Scholar]
- 20.Kesikburun S., Tan A.K., Yılmaz B., Yaşar E., Yazıcıoğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy. Am J Sports Med. 2013;41(11):2609–2616. doi: 10.1177/0363546513496542. http://journals.sagepub.com/doi/10.1177/0363546513496542 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Nejati P., Ghahremaninia A., Naderi F., Gharibzadeh S., Mazaherinezhad A. Treatment of subacromial impingement syndrome: platelet-rich plasma or exercise therapy? A randomized controlled trial. Orthop J Sport Med. 2017;5(5) doi: 10.1177/2325967117702366. http://journals.sagepub.com/doi/10.1177/2325967117702366 232596711770236. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilhanli I., Guder N., Gul M. Platelet-rich plasma treatment with physical therapy in chronic partial supraspinatus tears. Iran Red Crescent Med J. 2015;17(9) doi: 10.5812/ircmj.23732. http://www.ircmj.com/?page=article&article_id=23732 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Say F., Gurler D., Bulbul M. Platelet-rich plasma versus steroid injection for subacromial impingement syndrome. J Orthop Surg. 2016;24(1):62–66. doi: 10.1177/230949901602400115. [DOI] [PubMed] [Google Scholar]
- 24.Shams A., El-Sayed M., Gamal O., Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26(8):837–842. doi: 10.1007/s00590-016-1826-3. [DOI] [PubMed] [Google Scholar]
- 25.Scarpone M., Pritchard M., Rabago D. Effectiveness of platelet-rich plasma Injection for rotator cuff tendinopathy: a prospective Open-label study. @BULLET Glob Adv Heal Med. 2013 doi: 10.7453/gahmj.2012.054. www.gahmj.com [cited 2017 Nov 22];262(22):26–31. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengodan V.C., Kurian S., Ramasamy R. Treatment of partial rotator cuff tear with ultrasound-guided platelet-rich plasma. J Clin Imaging Sci. 2017 doi: 10.4103/jcis.JCIS_26_17. http://www.ncbi.nlm.nih.gov/pubmed/28900553 [cited 2017 Nov 26];7:32. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randelli P., Arrigoni P., Ragone V., Aliprandi A., Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective rct study, 2-year follow-up. J Shoulder Elb Surg. 2011;20(4):518–528. doi: 10.1016/j.jse.2011.02.008. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Bergeson A.G., Tashjian R.Z., Greis P.E., Crim J., Stoddard G.J., Burks R.T. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286–293. doi: 10.1177/0363546511424402. http://journals.sagepub.com/doi/10.1177/0363546511424402 Available from: [DOI] [PubMed] [Google Scholar]
- 29.Kaux J.F., Drion P., Croisier J.L., Crielaard J.M. Tendinopathies and platelet-rich plasma (PRP): from pre-clinical experiments to therapeutic use. J Stem Cells Regen Med. 2015;11(1):P7–P17. doi: 10.46582/jsrm.1101003. [DOI] [PMC free article] [PubMed] [Google Scholar]