The pulmonary vein (PV) sleeves are extensions of left atrial tissue that cover the proximal surface of the PV adventitia. Despite the importance of the PV sleeves for atrial fibrillation (AF) pathogenesis and as a target of therapy, surprisingly little is known about the variability in their size and electrophysiology. A major factor contributing to this knowledge gap is that noninvasive methods do not currently exist to enable imaging the PV sleeves in a large number of patients; however, studies utilizing electroanatomic voltage mapping during AF ablation have recently demonstrated the feasibility of this approach (1).
Emerging data have found that among all the clinical risk factors, body mass index (BMI) accounts for the greatest contribution to AF risk, and the risk conferred by BMI is greater in men compared with women (2). Given speculation that the effect of BMI on atrial structural remodeling differs by sex, we sought to define the association between BMI, sex, and other clinical characteristics on PV sleeve length using electroanatomic voltage mapping.
The study population consisted of 63 patients referred for de novo AF ablation. Participants were in sinus rhythm, off antiarrhythmic medications, and provided informed consent. A voltage map was created with separate geometries of each PV. Electroanatomic points were taken distally until there was no local PV electrogram. Measurement of the sleeve length was performed offline following a series of quality control steps by staff blinded to the characteristics of the participants. Sleeve length was measured as the surface distance from the PV ostium to the distal margin of the sleeve defined by the color display indicating bipolar voltage <0.1 mV. Four separate measurements were taken for each PV by rotating it 90° along its long axis, and mean sleeve length was calculated. Linear regression models were used to analyze each PV (i.e., left upper PV, right upper PV, left lower PV, and right lower PV). Due to known differences in the sleeve size between upper and lower PVs, inverse variance, fixed-effects meta-analysis techniques were used to combine the data.
Median sleeve length was the following: left upper PV 2.4 (interquartile range [IQR]: 1.9 to 3.0) cm, right upper PV 2.3 (IQR: 1.8 to 3.0) cm, left lower PV 1.9 (IQR: 1.5 to 2.8) cm, and RLPV 1.9 (IQR: 1.3 to 2.6) cm. The upper sleeves were longer than the lower sleeves (p < 0.001). In univariate analysis, age, sex, height, body surface area, BMI, hypertension, and obstructive sleep apnea were significantly associated with sleeve length (all p < 0.05). In multivariable analysis, male sex was associated with a 6.6 mm increase in sleeve length (95% confidence interval: 3.1 to 10.2; p < 0.001), and BMI was associated with a 2.4-mm increase per 5 kg/m2 (95% confidence interval: 1.2 to 3.5; p < 0.001) when adjusted for age (p = 0.10) and height (p = 0.22) (Figure 1).
FIGURE 1. PV Sleeve Length Is Associated With BMI and Male Sex.
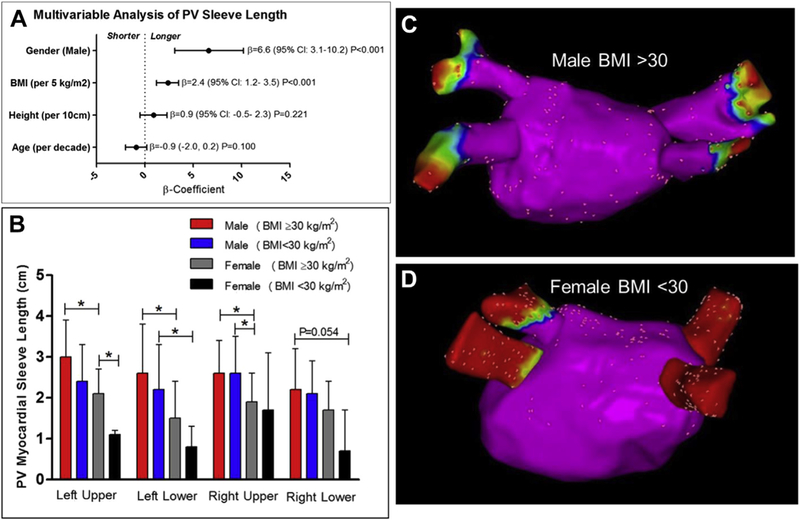
(A) Multivariable results of the overall effect of sex, body mass index (BMI), height, and age on pulmonary vein (PV) sleeve length. (B) A graded decrease in PV sleeve length is displayed according to sex and obese status (BMI ≥30 kg/m2) versus nonobese status (BMI <30 kg/m2). The global test for significance was p < 0.05 (analysis of variance) for the left upper PV, left lower PV, and right upper PV, and p = 0.057 for the right lower PV. An asterisk indicates p < 0.05 for pairwise comparisons (t test). (C, D) Electroanatomic voltage maps of the posterior left atrium and PVs are shown. (C) A man with BMI >30 kg/m2 and a mean PV sleeve length of 3.0 cm. (D) A woman with BMI <30 kg/m2 and a mean PV sleeve length of 0.4 cm (red is bipolar voltage <0.1 mV, indicating absence of myocardium). CI = confidence interval.
The current paradigm is that the PV sleeves primarily contribute to AF pathogenesis as a source of AF triggers. Our study demonstrates that many patients possess large sleeves, up to 3 cm or greater in length, which suggests that in some patients the PV sleeves not only are an important source of PV triggers, but also may significantly contribute to the available AF substrate. Given that men have a higher risk of AF than women do (3), our finding that men with AF have larger PV sleeves than women may partially explain this observation.
The contribution of obesity to the development of AF is thought to be multifactorial, with a complex relationship between obesity, numerous clinical risk factors, and cardiac adipose tissue. Most intriguing is the potential role of cardiac adipose in modulating sleeve size. Cardiac adipose is abundant in the region of the PV antrum (4) and is a rich source of: 1) proinflammatory cytokines; 2) growth factors; and 3) pluripotent mesenchymal stem cells, which have been found to be capable of transforming into atrial cardiomyocytes (5). Further research is needed to elucidate the mechanisms underlying the clinical associations with sleeve size and evaluate whether these differences influence the efficacy of PVI in treating paroxysmal AF.
Acknowledgments
Please note: Dr. Shoemaker was supported in part by the National Institutes of Health grant K23 HL127704. The project was also supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. This study was also the result of work supported with resources and the use of facilities at the Veterans Affairs Medical Center in Nashville, Tennessee. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. Dr. Ellis has received research funding from Medtronic, Atricure, Thoratec, and Boston Scientific; and consulting fees from Medtronic, Sentre Heart, Spectranetics, Biosense Webster, Boston Scientific, and Atricure. Dr. Kanagasundram has served as a speaker for Janssen Pharmaceuticals, Zoll, and Bio-sense Webster. Dr. Crossley has served as a consultant for Medtronic and Boston Scientific and as a speaker for Medtronic.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest that they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administrationguidelines, includingpatientconsentwhereappropriate. Formore information, visit the JACC: Clinical Electrophysiology author instructions page.
REFERENCES
- 1.Teh AW, Kalman JM, Lee G, et al. Electroanatomic remodelling of the pulmonary veins associated with age. Europace 2012;14:46–51. [DOI] [PubMed] [Google Scholar]
- 2.Magnussen C, Nijranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts. Circulation 2017;17:1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 4.Zghaib T, Ipek EG, Zahid S, et al. Association of left atrial epicardial adipose tissue with electrogram bipolar voltage and fractionation: electrophysiologic substrates for atrial fibrillation. Heart Rhythm 2016;13:2333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 2003;75:775–9. [DOI] [PubMed] [Google Scholar]


