Abstract
Inhibitors of the Na+-glucose cotransporter SGLT2 enhance urinary glucose and urate excretion and lower plasma urate levels. The mechanisms remain unclear, but a role for enhanced glucose in the tubular fluid, which may interact with tubular urate transporters, such as the glucose transporter GLUT9 or the urate transporter URAT1, has been proposed. Studies were performed in nondiabetic mice treated with the SGLT2 inhibitor canagliflozin and in gene-targeted mice lacking the urate transporter Glut9 in the tubule or in mice with whole body knockout of Sglt2, Sglt1, or Urat1. Renal urate handling was assessed by analysis of urate in spontaneous plasma and urine samples and normalization to creatinine concentrations or by renal clearance studies with assessment of glomerular filtration rate by FITC-sinistrin. The experiments confirmed the contribution of URAT1 and GLUT9 to renal urate reabsorption, showing a greater contribution of the latter and additive effects. Genetic and pharmacological inhibition of SGLT2 enhanced fractional renal urate excretion (FE-urate), indicating that a direct effect of the SGLT2 inhibitor on urate transporters is not absolutely necessary. Consistent with a proposed role of increased luminal glucose delivery, the absence of Sglt1, which by itself had no effect on FE-urate, enhanced the glycosuric and uricosuric effects of the SGLT2 inhibitor. The SGLT2 inhibitor enhanced renal mRNA expression of Glut9 in wild-type mice, but tubular GLUT9 seemed dispensable for the increase in FE-urate in response to canagliflozin. First evidence is presented that URAT1 is required for the acute uricosuric effect of the SGLT2 inhibitor in mice.
Keywords: GLUT9, proximal tubule, sodium-glucose cotransport, URAT1, urate transport
INTRODUCTION
The Na+-glucose cotransporter SGLT2 is localized in the brush border of the early proximal tubule, where it mediates most of the renal glucose reabsorption (29, 36, 39). In mice, SGLT2 accounts for ~97% of all renal glucose reabsorption in euglycemic conditions; the remaining 3% is reabsorbed by SGLT1 expressed in the later parts of the proximal tubule (28, 36). SGLT2 inhibitors are antidiabetic drugs that are utilized in type 2 diabetic patients and have beneficial effects on renal and cardiovascular outcome (37). However, many nuances of their effects and their consequences on the physiology and pathophysiology of the kidney and the cardiovascular system remain incompletely understood (37). One unexplained phenomenon relates to the well-documented serum uric acid level-lowering effect of SGLT2 inhibitors (2, 7, 12, 24). The phenomenon is reminiscent of the uricosuric effect that was reported five decades ago for the nonselective SGLT inhibitor phloridzin in healthy subjects, which was associated with an increase in fractional renal urate excretion (FE-urate) (30). Phloridzin had a significantly greater effect than mannitol, suggesting a specific effect on the tubular transport of urate, rather than a pure osmotic effect. Tubular perfusion of d-glucose (50 mg/100 ml) or phloridzin [4.4 mg/100 ml (in the absence of glucose in the perfusate)] reduced the fractional urate absorption in in vivo microperfusion studies of the proximal convoluted tubule (19), demonstrating the luminal effect of both glucose and phloridzin.
SGLT2 does not appear to transport urate (8), thus pointing to a potential effect of glucose or SGLT2 inhibitors on renal urate transporters for the uricosuric effect. Glucose transporter 9 (GLUT9) and urate transporter 1 (URAT1) are the primary candidates for urate reabsorption in the tubular system in humans and mice (31). Human GLUT9 is expressed and operates primarily in the proximal tubule, where GLUT9 isoforms 2 and 1 [solute carrier family 2 member 9 (SLC2A9b and SLC2A9a)] are proposed to be expressed in the apical and basolateral membranes, respectively (31). In RNA from microdissected mouse kidney tubules, RT-PCR detected Slc2a9a at low levels in proximal tubules, while Slc2a9b was detected in distal convoluted and connecting tubules (3), where GLUT9 protein expression was detected in the apical and basolateral membranes (26). Thus, in humans, GLUT9-mediated urate reabsorption appears to be restricted to the proximal tubule; in mice, however, reabsorption also includes the distal tubule. In humans and mice, URAT1 is expressed in the luminal membrane of the proximal tubule (31).
Studies using in vitro cell culture models expressing GLUT9 isoform 1, URAT1, or other potentially involved transporters, including organic anion transporter (OAT) 4 or OAT10, showed that the SGLT2 inhibitor luseogliflozin did not affect the urate transport activities of any of these transporters, thus arguing against a direct effect of the SGLT2 inhibitor on these transporters (8). Moreover, studies in healthy subjects and patients with prediabetes and type 2 diabetes indicated that the uricosuric effect of SGLT2 inhibitors may relate to the increase in tubular glucose delivery (1, 8, 21). Based on in vitro transport studies in Xenopus oocytes, it has been hypothesized that the enhanced delivery of glucose in the lumen of the proximal tubule may facilitate the exchange for intracellular urate through GLUT9 isoform 2 (SLC2A9b) in the apical membrane, thereby enhancing urinary urate excretion (8). In addition, previous studies in Xenopus oocytes indicated that GLUT9 isoform 1 (SLCA9a) can mediate the exchange of extracellular glucose for intracellular urate (6).
To better understand the effect of SGLT2 inhibition on the renal handling of urate, studies were performed in nondiabetic mice. The studies used the SGLT2 inhibitor canagliflozin and involved gene-targeted mice lacking Glut9 in the tubular system or mice with whole body knockout of Sglt2, Sglt1, or Urat1. The approach included assessment of renal urate handling by analysis of urate in spontaneous plasma and urine samples and normalization to creatinine concentrations, as well as renal clearance studies under terminal anesthesia with assessment of glomerular filtration rate (GFR) by FITC-sinistrin. In contrast to humans, most mammals, including rodents, express the enzyme uricase. This enzyme breaks down uric acid to allantoin, which is more water-soluble than urate and excreted with the urine. As a consequence, plasma urate levels are somewhat lower in rodents, and the kidneys filter and reabsorb smaller amounts of urate (31). Therefore, in some studies we aimed to increase plasma urate levels by inhibiting uricase with oxonic acid.
METHODS
Animals.
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and approved by the local Institutional Animal Care and Use Committee. Studies were performed in mice with a whole body knockout of Sglt2 (Sglt2 KO), Sglt1 (Sglt1 KO), or Urat1 (Urat1 KO) and in mice with a knockdown of tubular Glut9 (tGlut9 KO) and their respective littermate wild types (WT). Generation of knockout mice for Sglt2 (36) and Sglt1 (14) is described elsewhere; both mouse lines were on a C57BL/6 background. Heterozygote breeders to generate Urat1 KO mice were purchased from Jackson Laboratory (stock no. 005839; strain of origin: 129P2/OlaHsd). tGlut9 KO mice were generated by cross-breeding mice with a floxed Glut9 gene (stock no. 026945, Jackson Laboratories; strain of origin: C57BL/6) (26) and mice heterozygously expressing Cre recombinase under the control of the Pax8 promoter (strain of origin: C57BL/6), as previously described (5). Adult male mice were used for all studies. They were fed standard rodent diet (diet 7001, Teklad Diets, Envigo, Madison, WI), except the Sglt1 KO mice and their WT controls, which were fed a low-carbohydrate diet (%kcal: 65% protein, 33.2% fat, and 1.9% carbohydrate as maltodextrin; 3.2 kcal/g; diet TD.08212, Harlan Laboratories, Madison, WI) to limit glucose/galactose malabsorption in the Sglt1 KO mice. All mice that entered the study also completed the studies: no mice were lost. Treatments were not blinded. Seven series of studies were performed.
Series 1: Does absence of Sglt2 or Sglt1 affect FE-urate?
Spot urine was collected, and then blood was collected by retroorbital puncture under brief isoflurane anesthesia from Sglt1 KO and Sglt2 KO mice and their littermate WT mice to determine concentrations of urate and glucose and calculate FE-urate and renal glucose excretion (n = 6–10/group). Urate was measured using the Amplex Red uric acid/uricase assay kit (catalog no. A22181, Thermo Fisher Scientific), which is based on the uricase-catalyzed conversion of uric acid to allantoin and hydrogen peroxide. Glucose was measured by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity, Thermo Electron, Louisville, CO) and creatinine by mass spectrometry (O’Brien Center for Acute Kidney Injury Research, University of Alabama at Birmingham).
Series 2: Does absence of Sglt1 enhance the glucosuric and uricosuric effects of canagliflozin?
Urine was collected before and at 1.5 h after application of the SGLT2 inhibitor canagliflozin (2 mg/kg ip) or vehicle (1% ethanol, 2 µl/g body wt) in WT and littermate Sglt1 KO mice (n = 8–13/group). Mice were returned to their home cages between injections and second urine collections. This was done because preliminary studies indicated that return to home cages prevented a strong increase in urinary urate-to-creatinine ratios observed in vehicle-treated mice when urine was collected in metabolic cages, which would have confounded any drug effect. At the time of urine collection, a blood glucose meter (Contour, Bayer, Mishawaka, IN) was used to measure blood glucose by tail snip. Urinary urate concentration (measured by uricase-based Amplex Red uric acid assay) was normalized to creatinine (measured by kinetic modification of Jaffe’s reaction; Thermo Fisher Scientific, Waltham, MA) to estimate urate excretion. Since uricase inhibition was used in some of the following studies in Urat1 KO and tGlut9 KO mice, a uricase-independent method to measure urate was needed; therefore, for series 3–7, urate was measured by mass spectrometry.
Series 3: Basal phenotype of mice lacking Urat1 or tubular Glut9.
Spot urine was collected and then blood was collected (by retroorbital puncture under brief isoflurane anesthesia) from Urat1 KO and tGlut9 KO mice and their littermate WT mice to determine urate concentration (by mass spectrometry at the University of California San Diego Core Bio Services) and creatinine (by mass spectrometry at the O’Brien Center for Acute Kidney Injury Research, University of Alabama at Birmingham) and calculate FE-urate (n = 6–7/group).
Series 4: Does canagliflozin given for 7 days enhance FE-urate or change renal mRNA expression of urate transporters, and are any of its responses affected by the absence of tubular Glut9?
After the basal measurements described for series 3, the tGlut9 KO and littermate WT mice were allowed to recover for ≥4 days, and canagliflozin [100 mg/kg diet (same as base diet: diet 7001, Teklad Diets, Envigo)] or vehicle was administered for 7 days. Subsequently, the measurements to determine FE-urate were repeated, as described for series 3, together with measurement of fractional glucose excretion (FE-glucose), and kidneys were harvested under deep and terminal isoflurane anesthesia. The tissue was subsequently used for analysis of tubular urate transporters [including ATP-binding cassette subfamily G member 2 (Abcg2), Glut9, multidrug resistance-associated protein 4 (Mrp4), nicotinate phosphoribosyltransferase 1 and 3 (Npt1 and Npt3), Oat1, Oat3, Oat10, and Urat1] by quantitative RT-PCR. Briefly, frozen kidneys were homogenized using a mechanical tissue homogenizer in lysis buffer provided with the RNeasy Plus mini kit (catalog no. 74136, Qiagen). Total RNA was purified using the RNeasy Plus mini kit according to the manufacturer’s instructions. cDNA was synthesized with 5 µg of RNA using SuperScript IV first-strand synthesis system (catalog no. 18091050, Thermo Fisher Scientific) according to the manufacturer’s instructions. cDNA reactions were diluted fivefold, and 1 μl was used in quantitative PCR. Real-time PCR was carried out on a real-time PCR system (model ABI-7300, Applied Biosystems, Foster City, CA) using 2× TaqMan universal PCR master mix (catalog no. 4304437, Thermo Fisher Scientific) with primers listed in Table 1. The thermal profile was as follows: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, 40 cycles for 15 s at 95°C and 1 min at 60°C. Expression levels were expressed as relative fold increases normalized to the average of two housekeeping genes, ribosomal protein L 19 (rpl19) and hypoxanthine guanine phosphoribosyltransferase (hprt). Each experiment was performed in duplicate.
Table 1.
Real-time PCR primers
| Target | Primer | Source |
|---|---|---|
| Abcg2 | Mm00496364_m1 | Applied Biosystems |
| Ccl2 | Mm00441242-m1 | Applied Biosystems |
| Kim-1 | Mm00506686_m1 | Applied Biosystems |
| Glut9 | Mm00554217_m1 | Applied Biosystems |
| Hprt | Mm00446968_m1 | Applied Biosystems |
| Mrp4 | Mm01226381_m1 | Applied Biosystems |
| Npt1 | Mm01136000_m1 | Applied Biosystems |
| Npt3 | Mm01133465_m1 | Applied Biosystems |
| Oat1 | Mm00456258_m1 | Applied Biosystems |
| Oat3 | Mm00459534_m1 | Applied Biosystems |
| Oat10 | AP7DRZ2 | Thermo Fisher |
| Rpl19 | Mm02601633_g1 | Applied Biosystems |
| Urat1 | Mm01236822_m1 | Applied Biosystems |
Abcg2, ATP-binding cassette subfamily G member 2; Ccl2, C-C motif chemokine ligand 2; Kim-1, kidney injury molecule-1; Glut9, glucose transporter 9; Hprt, hypoxanthine guanine phosphoribosyltransferase; Mrp4, multidrug resistance-associated protein 4; Npt1/3, nicotinate phosphoribosyltransferase 1/3; Oat1/3/10, organic anion transporter 1/3/10; Rpl19, ribosomal protein L 19; Urat1, urate transporter 1.
Series 5: Does absence of tubular Glut9 enhance FE-urate and blunt the acute effect of canagliflozin on FE-urate in renal clearance studies?
Mice were pretreated with oxonic acid in the diet [2% oxonic acid potassium salt (Sigma-Aldrich) in base diet (diet 7001, Teklad Diets, Envigo)] for 7–10 days. Then two-period renal clearance studies were performed in tGlut9 KO and WT littermate mice to determine GFR, FE-glucose, filtered urate, absolute renal excretion of urate (AE-urate), and FE-urate in response to canagliflozin or vehicle under terminal anesthesia. Briefly, mice were anesthetized with thiobutabarbital (100 mg/kg ip, 2 μl/g body wt; Sigma-Aldrich, St. Louis, MO) and ketamine (100 mg/kg im, 2 μl/g body wt; Butler, Dublin, OH). Body temperature was maintained at 37°C with a servo-controlled heating plate. The trachea was cannulated using polyethylene tubing, and 100% oxygen was blown toward the tracheal tube throughout the experiment to stabilize blood pressure in anesthetized mice. The jugular vein was cannulated for continuous infusion of 2.25% bovine serum albumin in 0.85% NaCl at a rate of 0.5 ml·h−1·30 g body wt−1; 5% glucose was added to the infusion to induce moderate hyperglycemic conditions and to attenuate the expected and potentially confounding blood glucose-lowering effect of SGLT2 inhibition. For assessment of GFR, 0.2% FITC-sinistrin (Fresenius-Kabi, Linz, Austria) was added to the infusion. GFR and urinary excretion of glucose and urate were assessed by quantitative urine collection via a bladder catheter in two 30-min periods: after completion of a basal clearance period, canagliflozin (2 mg/kg) or vehicle (20% hydroxypropyl-β-cyclodextrin in saline containing 2% ethanol; 2 µl/g body wt) was applied by intraperitoneal bolus, and 20 min later the second clearance period was started. Blood samples (50 µl) were drawn midway in each period from an arterial catheter, which was also used to monitor blood pressure and heart rate.
Glomerular filtration of glucose and urate is given by the product of GFR and plasma concentrations of glucose and urate, respectively. FE-glucose and FE-urate were calculated as the absolute urinary excretion rate divided by the glomerular filtration per time of glucose and urate, respectively. For FITC-sinistrin, fluorescence was determined [after plasma dilution 1:10 in 0.5 mol/l HEPES (pH 7.4)] using a fluorospectrometer (model ND-3300, NanoDrop Technologies, Wilmington, DE). Urate was measured by mass spectrometry (University of California San Diego Core Bio Services). Glucose was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity, Thermo Electron).
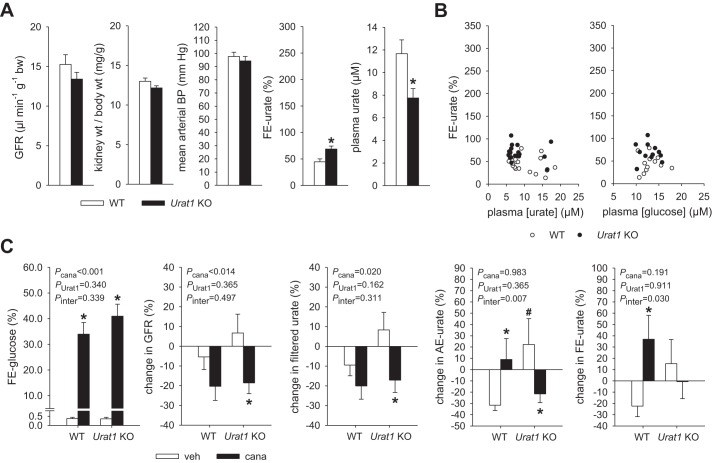
Series 6: Does absence of Urat1 enhance FE-urate and blunt the acute effect of canagliflozin on FE-urate in renal clearance studies?
Mice were fed the oxonic acid-containing diet as in series 5 for 7–10 days. Then two-period renal clearance studies were performed in Urat1 KO and WT littermates to determine GFR, FE-glucose, AE-urate, and FE-urate in response to canagliflozin or vehicle under terminal anesthesia, as described in series 5 for tGlut9 KO mice.
Series 7: Did pretreatment with oxonic acid in series 5 and 6 increase plasma urate, and does absence of Urat1 and tubular Glut9 have additive effects on renal urate handling?
Renal clearance studies were performed as described in series 5 and 6 under terminal anesthesia to determine GFR and the absolute excretion and reabsorption of urate. However, in series 7, the mice were not pretreated with oxonic acid. Studies were performed in tGlut9 KO and littermate WT mice. Moreover, Urat1 KO mice were crossed to tGlut9 KO mice to generate final breeders that were Urat1 KO and homozygote for floxed Glut9, and either heterozygote or wild-type for Pax8. Thus the offspring were either Urat1 KO and tGlut9 WT (i.e., Urat1 KO) or Urat1 KO and tGlut9 KO ( i.e., double knockouts for Urat1 and Glut9 in the kidney), which were tested in the same setting.
Statistical analysis.
Values are means ± SE. Data were log-transformed when so-doing resulted in a distribution that was more normal. Statistical differences were analyzed by two-way ANOVA followed by appropriate post hoc testing (Holm-Sidak) for pair-wise comparisons, by unpaired Student’s t-test when only two groups were compared, or by linear regression analysis to probe for correlations between two parameters. P < 0.05 was considered statistically significant.
RESULTS
Some quantitative differences in values for plasma urate and FE-urate were observed between experimental series. This may be explained by differences in methods, which are briefly summarized again before presentation of actual results. Urate measurements in series 1 and 2 used a uricase-based assay, whereas for series 3–7 in Urat1 KO and tGlut9 KO mice, mass spectrometry was used, because mice in series 5 and 6 were pretreated with a uricase inhibitor to enhance plasma urate levels. Moreover, determination of plasma and urinary urate concentrations and FE-urate in series 1–4 was based on spontaneous urine collections and a blood draw under brief isoflurane anesthesia, as well as normalization of plasma and urinary urate concentrations to creatinine, whereas renal clearance studies under terminal anesthesia with modest volume expansion and measurement of GFR (by FITC-sinistrin) were used in series 5–7 to determine FE-urate.
Series 1: Absence of Sglt2, but not Sglt1, increased FE-urate.
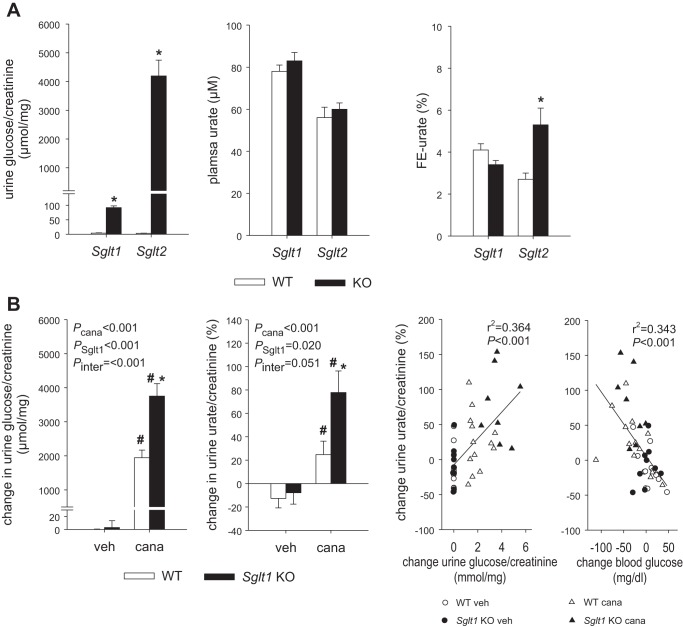
Glycosuria was relatively minor in Sglt1 KO vs. WT mice, and plasma urate and FE-urate were not different between these two groups. In Sglt2 KO mice, urine glucose-to-creatinine ratios were strongly increased compared with WT mice and plasma urate did not change but FE-urate doubled (Fig. 1A).
Fig. 1.
A: absence of the Na+-glucose cotransporter Sglt2, but not Sglt1, increased fractional renal urate excretion (FE-urate). Glycosuria was relatively minor in Sglt1 knockout (KO) compared with wild-type (WT) mice, and FE-urate was not different between these groups. In Sglt2 KO mice, the urine glucose-to-creatinine ratio was strongly increased compared with WT mice and FE-urate was doubled. Values are means ± SE; n = 6–10 mice/group. *P < 0.05 vs. WT, by unpaired Student’s t-test for comparison between 2 groups. B: absence of Sglt1 enhanced glucosuric and uricosuric effects of canagliflozin (cana). In WT mice, canagliflozin increased urinary glucose-to-creatinine and urate-to-creatinine ratios compared with vehicle (veh) application. In Sglt1 KO mice, glucosuric and uricosuric effects of canagliflozin were enhanced. Overall, the change in urinary glucose correlated positively with the increase in urinary urate, whereas the change in blood glucose correlated inversely with urinary urate. Values are means ± SE; n = 8–13 mice/group. *P < 0.05 vs. WT; #P < 0.05 vs. vehicle, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons and linear regression analysis, respectively.
Series 2: Absence of Sglt1 enhanced glucosuric and uricosuric effects of canagliflozin.
In WT mice, application of canagliflozin strongly increased the urinary glucose-to-creatinine and urate-to-creatinine ratios compared with vehicle application (Fig. 1B). In Sglt1 KO mice, the glucosuric and uricosuric effects of canagliflozin were enhanced. Overall, the change in urinary glucose positively correlated with the increase in urinary urate (Fig. 1B). Moreover, the change in blood glucose inversely correlated with urinary urate (Fig. 1B).
Series 3: Basal phenotype of mice lacking Urat1 or tubular Glut9.
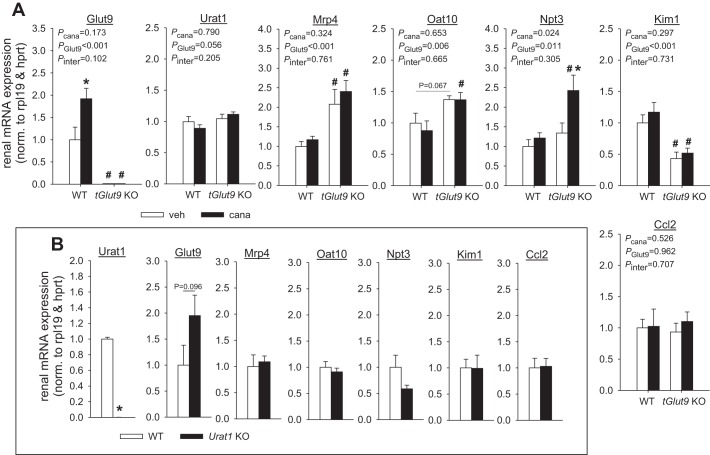
Quantitative RT-PCR confirmed the effective knockdown of renal Glut9 mRNA expression in tGlut9 KO vs. WT mice (Fig. 2A). This was associated with higher renal mRNA expression of the urate transporter Mrp4 and a tendency for higher Oat10 in tGlut9 KO than WT mice, while renal mRNA expression of Urat1 and Npt3 (Fig. 2A), Abcg2, Oat1, Oat3, and Npt1 (data not shown) was not significantly different between tGlut9 KO and WT mice.
Fig. 2.
Canagliflozin increased renal mRNA expression of the urate transporter Glut9. A: quantitative RT-PCR confirmed effective knockdown of renal Glut9 mRNA expression in tubular Glut9 (tGlut9) knockout (KO) vs. wild-type (WT) mice. This was associated with higher expression of the urate transporters multidrug resistance-associated protein 4 (Mrp4) and organic anion transporter 10 (Oat10) and reduced expression of the tubular injury marker kidney injury molecule-1 (Kim-1) in tGlut9 KO mice, while expression of the urate transporter Urat1, nicotinate phosphoribosyltransferase 3 (Npt3), and the proinflammatory marker C-C motif chemokine ligand 2 (Ccl2) was not different from WT mice. Canagliflozin (cana) increased renal Glut9 mRNA expression in WT mice and enhanced Nrp3 expression in tGlut9 KO mice. hprt, Hypoxanthine guanine phosphoribosyltransferase; rpl19, ribosomal protein L 19. Values are means ± SE; n = 4–5 mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. WT, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons. B: quantitative RT-PCR confirmed knockout of renal Urat1 mRNA expression in Urat1 KO mice. This was associated with a tendency for higher renal mRNA Glut9 expression in Urat1 KO mice, while expression of other urate transporters, Kim-1 and Ccl2, was similar between Urat1 KO and WT mice. Values are means ± SE; n = 4–5 mice/group. *P < 0.05 vs. WT, by unpaired Student’s t-test for comparison between 2 groups.
Body weight [24 ± 1 vs. 24 ± 1 g, P = not significant (NS)] and hematocrit (45.9 ± 0.2 vs. 45.4 ± 0.3%, P = NS) were similar in tGlut9 KO and WT mice (n = 10–12/group). In untreated tGlut9 WT mice, plasma urate concentrations and FE-urate varied between 10 and 20 µM and between 10 and 30%, respectively, with mean values of ~14 µM and 18%, respectively (Fig. 3A). In tGlut9 KO mice, mean FE-urate was significantly increased to ~65%, associated with reduced mean plasma urate levels of ~11 µM (Fig. 3A). tGlut9 KO mice presented significantly higher plasma creatinine concentration and a reduced kidney-to-body weight ratio (Fig. 3A). To exclude kidney injury or inflammation as a cause of increased plasma creatinine values in tGlut9 KO mice, potentially due to high tubular urate delivery, markers of kidney injury and inflammation were determined. Renal mRNA expression of the tubular injury marker kidney injury molecule-1 (Kim-1) was actually reduced in tGlut9 KO mice, while the proinflammatory marker C-C motif chemokine ligand 2 (Ccl2) was not different between genotypes (Fig. 2A).
Fig. 3.
Stronger increase in fractional renal urate excretion (FE-urate) in mice lacking tubular glucose transporter 9 (tGlut9) than urate transporter 1 (Urat1). A: in untreated tGlut9 wild-type (WT) mice, plasma urate concentrations and FE-urate (creatinine-based) varied between 10 and 20 µM and between 10 and 30%, respectively, with mean values of ~14 µM and 18%, respectively. FE-urate was strongly increased in tGlut9 knockout (KO) mice, associated with reduced plasma urate levels vs. WT mice. tGlut9 KO mice also showed higher plasma creatinine concentrations and reduced kidney-to-body weight ratios. Values are means ± SE; n = 10–11 mice/group. *P < 0.05 vs. WT, by unpaired Student’s t-test for comparison between 2 groups. B: FE-urate (creatinine-based) tended to be increased in untreated Urat1 KO vs. WT mice, associated with similar plasma urate and creatinine levels and kidney-to-body weight ratios in both groups. Values are means ± SE; n = 5–7 mice/group.
Knockout of renal Urat1 mRNA expression in Urat1 KO mice was confirmed by quantitative RT-PCR (Fig. 2B). This was associated with a tendency for higher (almost double) renal mRNA Glut9 expression in Urat1 KO than WT mice (P = 0.096). Any increase in renal GLUT9 expression would be expected to have attenuated the uricosuric phenotype in Urat1 KO mice. Renal mRNA expression of Mrp4, Oat10, and Npt3 (Fig. 2B) and Abcg2, Oat1, Oat3, and Npt1 (data not shown) was not significantly different between Urat1 KO and WT mice. Body weight (26 ± 1 vs. 28 ± 2 g, P = NS) and hematocrit (47.3 ± 0.7 vs. 48.6 ± 1.0%, P = NS) were similar in Urat1 KO and WT mice (n = 5–7/group). Plasma urate concentration and FE-urate in untreated Urat1 WT mice were similar to values reported above in tGlut9 WT mice (Fig. 3B). FE-urate tended to be slightly increased in Urat1 KO compared with WT mice (P = 0.073), associated with similar plasma urate levels in both groups (Fig. 3B). Plasma creatinine concentration and kidney-to-body weight ratio (Fig. 3B) and renal mRNA expression of Kim-1 and Ccl2 (Fig. 2B) were not different between Urat1 KO and WT mice. Together, the data indicated a robust reduction in renal urate reabsorption in the absence of tubular Glut9, whereas the effect of the Urat1 knockout was very subtle in the tested setting.
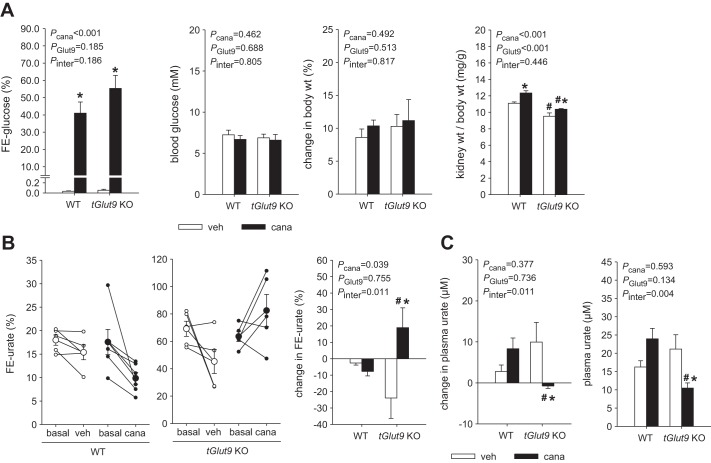
Series 4: Application of canagliflozin for 7 days enhanced renal mRNA expression of Glut9, and absence of tubular Glut9 unmasked a canagliflozin-induced increase in FE-urate and reduction in plasma urate.
Canagliflozin application for 7 days induced a similar increase in FE-glucose in tGlut9 KO and WT mice without significantly changing blood glucose levels or body weight compared with vehicle application (Fig. 4A). Canagliflozin increased the kidney-to-body weight ratio in both genotypes to a similar extent (Fig. 4A). In tGlut9 WT mice, canagliflozin did not significantly alter FE-urate or plasma urate concentration compared with vehicle application (Fig. 4, B and C). In tGlut9 KO mice, however, canagliflozin significantly enhanced FE-urate and reduced plasma urate concentration relative to vehicle application (Fig. 4, B and C). The studies indicated that the absence of tubular Glut9 unmasked a FE-urate-increasing and plasma urate-lowering effect of chronic canagliflozin application in mice.
Fig. 4.
Absence of tubular glucose transporter 9 (tGlut9) unmasked a canagliflozin-induced increase in fractional renal urate excretion (FE-urate) and reduction in plasma urate. A: canagliflozin (cana) application for 7 days induced a similar increase in fractional glucose excretion (FE-glucose) in tGlut9 knockout (KO) and wild-type (WT) mice without significantly changing blood glucose levels or body weight compared with vehicle (veh) application. Canagliflozin increased kidney-to-body weight ratio in both genotypes to a similar extent. Values are means ± SE; n = 5–6 mice/group. B and C: in tGlut9 WT mice, canagliflozin did not significantly alter FE-urate or plasma urate concentration compared with vehicle application. This response differs from acute responses (see Fig. 1B) and may be due to upregulation of renal Glut9 expression in WT mice given canagliflozin for 7 days (see Fig. 2A), which may have attenuated the uricosuric effect. In comparison, in tGlut9 KO mice, canagliflozin significantly enhanced FE-urate and reduced plasma urate concentration relative to vehicle application. Values are means ± SE; n = 5–6 mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. WT, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons.
Canagliflozin significantly enhanced renal mRNA expression of Glut9 in tGlut9 WT mice (Fig. 2A), which may have compensated for reduced urate reabsorption. In comparison and consistent with an effective knockdown, renal Glut9 mRNA expression was not changed by canagliflozin in tGlut9 KO mice, which may have helped unmask a uricosuric effect of canagliflozin in the latter. Possibly as a partial compensation, canagliflozin significantly enhanced renal mRNA expression of Npt3 in tGlut9 KO mice, an effect not observed in tGlut9 WT mice (Fig. 2A). In tGlut9 KO and WT mice, canagliflozin did not significantly alter renal mRNA expression of Urat1, Mrp4, and Oat10 (Fig. 2A), Abcg2, Oat1, Oat3, and Npt1 (data not shown), and Kim-1 and Ccl2 (Fig. 2A).
The data also indicated that a FE-urate-increasing or plasma urate-lowering effect of chronic canagliflozin application could not be demonstrated relative to vehicle application in tGlut9 WT mice in the tested setting, which included intact uricase, with creatinine used to determine FE-urate and application of canagliflozin for 7 days (which upregulated renal Glut9 mRNA). Therefore, this study type was not repeated in Urat1 KO mice; instead, the study design refocused on acute studies with measurement of GFR and pretreatment with oxonic acid.
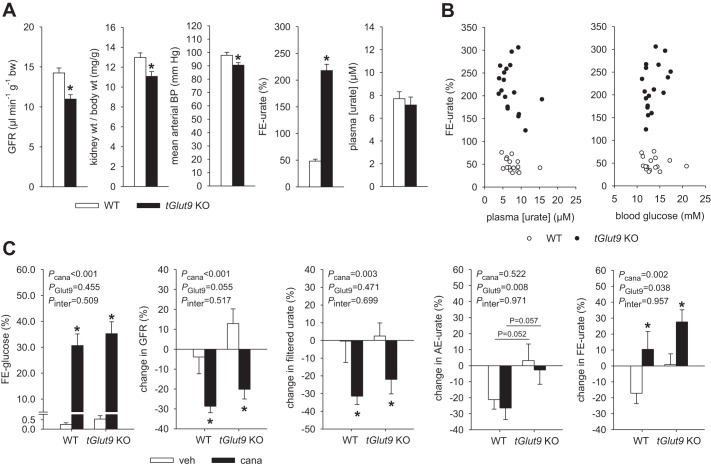
Series 5: Absence of tubular Glut9 enhanced basal FE-urate and preserved the acute increase in FE-urate in response to canagliflozin in renal clearance studies in oxonic acid-pretreated mice.
Mice were pretreated with oxonic acid in the diet for 7–10 days. Body weight (28 ± 1 vs. 29 ± 1 g, P = NS) and hematocrit (41.3 ± 0.7 vs. 41.4 ± 0.5%, P = NS) were similar in tGlut9 KO and WT mice (n = 16–18/group) during the basal period of the renal clearance study. In both groups, hematocrit was ~4% lower than in series 3 studies in awake mice, which may reflect the applied volume infusion protocol to achieve stable experimental conditions for a two-period renal clearance study. The glucose-containing infusion established similar modestly enhanced blood glucose levels in tGlut9 KO and WT mice (13.5 ± 0.5 vs. 13.6 ± 0.6 mM, P = NS), while FE-glucose (0.4 ± 0.1 vs. 0.3 ± 0.1%, P = NS) and fractional excretion of fluid (0.6 ± 0.1 vs. 0.5 ± 0.1%, P = NS) remained low in both genotypes. tGlut9 KO mice showed a modestly lower GFR and kidney-to-body weight ratio (both consistent with data obtained in series 3), associated with a small reduction in mean arterial blood pressure (Fig. 5A), while heart rate was not different from WT mice (data not shown). In tGlut9 WT mice, mean FE-urate was 48% and, thus, somewhat higher than in series 3 in awake, noninfused mice when creatinine-based measurements of FE-urate were used (Fig. 5A). A positive effect of volume expansion on FE-urate has been well documented (19). Furthermore, tubular creatinine secretion accounts for ~45% of urinary creatinine excretion in mice (35). As a consequence, creatinine-based calculation of FE-urate can underestimate the real values due to overestimation of GFR and, thus, filtered urate. The modestly higher FE-urate values in tGlut9 WT mice in this series than in series 3 were associated with modestly lower mean plasma urate concentrations of ~8 µM, despite oxonic acid pretreatment. Mean FE-urate was 218% in tGlut9 KO mice and, thus, also higher than in series 3 and, again, significantly higher than in WT mice (Fig. 5A). Urate is filtered, secreted, and reabsorbed by the kidney (31); values for FE-urate >100% indicated the unmasking of net renal urate secretion by tubular Glut9 knockdown. Plasma urate levels in tGlut9 KO mice were also modestly lower than in series 3 and not different from WT mice (Fig. 5A). The degree of hyperglycemia or plasma urate levels seemed not to determine FE-urate in this series (Fig. 5B; P = NS, by linear regression analysis).
Fig. 5.
Absence of tubular glucose transporter 9 (tGlut9) enhanced basal fractional renal urate excretion (FE-urate) and preserved the acute increase in FE-urate in response to canagliflozin in renal clearance studies in oxonic acid-pretreated mice. A: glomerular filtration rate (GFR) and mean arterial blood pressure (BP) were modestly lower in tubular Glut9 (tGlut9) knockout (KO) than wild-type (WT) mice in the basal clearance period, associated with lower kidney-to-body weight ratios in the former. FE-urate was strongly increased in tGlut9 KO mice, associated with similar plasma urate levels, vs. WT mice. Across all groups, FE-urate was higher than in series 3 studies and associated with lower plasma urate levels. See text for possible explanations. Values are means ± SE; n = 16–18 mice/group. *P < 0.05 vs. WT, by unpaired Student’s t-test for comparison between 2 groups. B: individual data. Degree of hyperglycemia or plasma urate levels seemed not to determine FE-urate. P = not significant, by linear regression analysis within data of either tGlut9 KO or WT. C: canagliflozin (cana) induced a similar increase in fractional glucose excretion (FE-glucose) and reduction in GFR in tGlut9 KO and WT mice. The latter reduced filtered urate, but absolute renal excretion of urate (AE-urate) was maintained, and FE-urate increased in response to canagliflozin relative to vehicle (veh) application in tGlut9 WT and KO mice. Values are means ± SE; n = 8–9 mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. WT, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons.
Canagliflozin induced a similar increase in FE-glucose and a reduction in GFR in tGlut9 KO and WT mice (Fig. 5C). The lower GFR reduced filtered urate, but AE-urate was maintained in response to canagliflozin, such that canagliflozin increased FE-urate relative to vehicle application in tGlut9 WT and KO mice (Fig. 5C).
Series 6: Absence of Urat1 enhanced basal FE-urate but prevented the acute increase in FE-urate in response to canagliflozin in renal clearance studies in oxonic acid-pretreated mice.
Mice were pretreated with oxonic acid in the diet for 7–10 days. Body weight (29 ± 1 and 28 ± 1 g, P = NS) and hematocrit (42.0 ± 0.6 and 41.4 ± 0.9%, P = NS) were similar in Urat1 KO and WT mice (n = 12/group) during the basal period of the renal clearance study. As in series 5, in both groups, hematocrit was ~5% lower than in series 3. The glucose-containing infusion likewise established similar modestly enhanced blood glucose levels in Urat1 KO and WT mice (12.9 ± 0.6 and 13.1 ± 0.6 mM, P = NS), while FE-glucose (0.3 ± 0.1 and 0.3 ± 0.1%, P = NS) and fluid (0.5 ± 0.1 and 0.5 ± 0.1%, P = NS) remained low. GFR, kidney-to-body weight ratio, and mean arterial blood pressure (Fig. 6A) and heart rate (data not shown) were not different in Urat1 KO vs. WT mice. In Urat1 WT mice, mean FE-urate was 45% (Fig. 6A) and, thus, somewhat higher than in series 3 mice and similar to the values in tGlut9 WT mice in series 5. This was associated with modestly lower mean plasma urate concentrations of ~11 µM, despite oxonic acid pretreatment. Mean FE-urate was 69% in Urat1 KO mice and, thus, higher than in series 3 and also significantly higher than in Urat1 WT mice. Plasma urate levels in Urat1 KO mice were also lower than in series 3 and significantly lower than in Urat1 WT mice (Fig. 6A). As in series 5, the degree of hyperglycemia or plasma urate levels seemed not to determine FE-urate in this series (Fig. 6B; P = NS, by linear regression analysis).
Fig. 6.
Absence of urate transporter 1 (Urat1) enhanced basal fractional renal urate excretion (FE-urate) but prevented the acute increase in FE-urate in response to canagliflozin in renal clearance studies in oxonic acid-pretreated mice. A: glomerular filtration rate (GFR) and mean arterial blood pressure were similar in Urat1 knockout (KO) and wild-type (WT) mice in the basal clearance period, associated with similar kidney-to-body weight ratios. FE-urate was modestly higher in Urat1 KO mice, associated with lower plasma urate levels, than WT mice. Across all groups, FE-urate was higher than in series 3 studies and associated with lower plasma urate levels. See text for explanations. Values are means ± SE; n = 12 mice/group. *P < 0.05 vs. WT, by unpaired Student’s t-test for comparison between 2 groups. B: individual data. Degree of hyperglycemia or plasma urate levels seemed not to determine FE-urate. P = not significant, by linear regression analysis within data of either Urat1 KO or WT. C: canagliflozin (cana) similarly increased fractional glucose excretion (FE-glucose) in Urat1 KO and WT mice. In Urat1 WT mice, canagliflozin numerically lowered GFR and filtered urate, associated with significant increases in absolute renal excretion of urate (AE-urate) and FE-urate relative to vehicle (veh) application. In Urat1 KO mice, canagliflozin also lowered GFR and filtered urate, but this was associated with a reduction in AE-urate and no change in FE-urate relative to vehicle application. Values are means ± SE; n = 5–7 mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. WT, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons.
Canagliflozin induced a similar increase in FE-glucose in Urat1 KO and WT mice (Fig. 6C). In Urat1 WT mice, canagliflozin numerically lowered GFR and filtered urate (although the difference did not reach statistical significance), associated with significant increases in AE-urate and FE-urate relative to vehicle application (Fig. 6C). In Urat1 KO mice, canagliflozin also lowered GFR and filtered urate, but this was associated with a reduction in AE-urate and no change in FE-urate relative to vehicle application (Fig. 6C).
Thus, in series 5 and 6, canagliflozin increased FE-urate in two independent WT groups and in tGlut9 KO mice. However, canagliflozin did not change FE-urate in Urat1 KO mice, despite similar glucosuria and GFR responses.
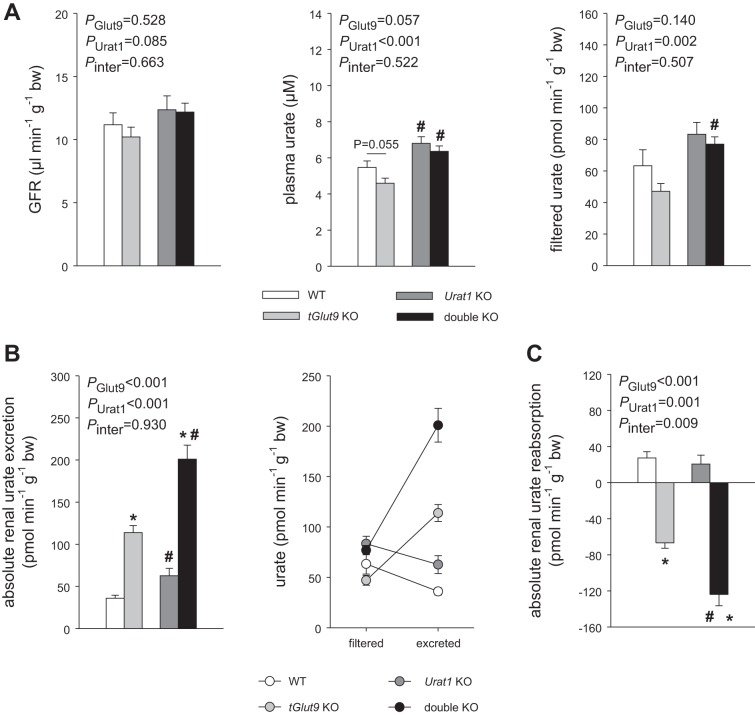
Series 7: Evidence for a modest plasma urate-increasing effect of oxonic acid and additive effects of URAT1 and tubular GLUT9 on tubular urate reabsorption.
To better understand the lower basal plasma urate levels in series 5 and 6 than series 3, clearance studies were performed in the absence of oxonic acid pretreatment. Plasma urate levels were modestly, but significantly, reduced in the absence of oxonic acid in series 3 compared with oxonic acid pretreatment in series 5 and 6 across genotypes: 5.5 ± 0.4 vs. 7.7 ± 0.6 µM in WT mice (P < 0.05), 4.6 ± 0.3 vs. 7.1 ± 0.7 µM in tGlut9 KO mice (P < 0.05), and 6.8 ± 0.4 vs. 11.7 ± 1.2 µM in Urat1 KO mice (P < 0.05). Thus, oxonic acid pretreatment enhanced plasma urate levels by 40–70%. The lower plasma urate levels in clearance studies than in awake mice may have been due to the modest volume expansion in the former, which enhanced FE-urate.
Double-KO mice were included in clearance studies to probe for additive effects of URAT1 and GLUT9. Plasma urate and filtered urate were modestly enhanced in double-KO vs. tGlut9 KO mice (the mice were derived from different breeding colonies; see methods) (Fig. 7A). The increase in filtered urate (~30 pmol·min−1·g body wt−1) may have accounted in part for the increase in urinary urate excretion (~85 pmol·min−1·g body wt−1) in double-KO compared with tGlut9 KO mice (Fig. 7B), but the greatest share was due to a significantly more negative absolute renal urate reabsorption in the double-KO mice (~55 pmol min−1·g body wt−1) (Fig. 7C), indicating that the additional knockout of Urat1 unmasked an even greater net renal urate secretion. In comparison, absolute renal urate reabsorption was not different between WT and Urat1 KO mice, indicating that the tubular knockdown of Glut9 unmasked the contribution of URAT1 to renal urate reabsorption, consistent with the statistically significant interaction (P = 0.009) between the effects of Urat1 knockout and tGlut9 knockout on absolute renal urate reabsorption (Fig. 7C).
Fig. 7.
Evidence for additive effects of urate transporter 1 (URAT1) and tubular glucose transporter 9 (GLUT9) on tubular urate reabsorption. A–C: filtered urate was modestly enhanced in double-knockout (KO) vs. tubular Glut9 (tGlut9) KO mice [mice were derived from different breeding colonies (see methods)], which may have accounted in part for the increase in urinary urate excretion in double-KO vs. tGlut9 KO mice (B), but the greatest share was due to a more negative absolute renal urate reabsorption in the former (C), indicating that additional knockout of Urat1 unmasked a greater net renal urate secretion. In comparison, absolute renal urate reabsorption was not different between WT and Urat1 KO mice. Tubular knockdown of Glut9 unmasked the contribution of URAT1 to renal urate reabsorption, consistent with a significant interaction (P = 0.009) between the effects of Urat1 knockout and tGlut9 knockout (C). Values are means ± SE; n = 6–9 clearance periods in 3–6 mice/group. *P < 0.05 vs. corresponding tGlut9 WT; #P < 0.05 vs. corresponding Urat1 WT, by 2-way ANOVA followed by Holm-Sidak test for multiple comparisons.
DISCUSSION
The current study provides evidence in nondiabetic mice that genetic or pharmacological inhibition of SGLT2 enhances FE-urate. The findings in Sglt2 KO mice argue against the need for a direct effect of the SGLT2 inhibitor on urate transporters to induce a uricosuric effect. This does not exclude any direct interactions but indicates that other mechanisms also contribute. Consistent with a proposed role of increased luminal glucose delivery to induce the uricosuric effect, it was observed that the absence of Sglt1, which by itself had no effect on FE-urate, enhanced the glycosuric and uricosuric effects of the SGLT2 inhibitor canagliflozin. The studies further indicated that tubular GLUT9 seemed dispensable for the uricosuric effect of canagliflozin, at least in mice. Moreover, the renal mRNA expression of Glut9 was upregulated by chronic canagliflozin application in WT mice, which may explain why, in these mice, a FE-urate-increasing and plasma urate-lowering effect of chronic canagliflozin application was not detectable but was unmasked in mice with tubular Glut9 knockdown. Finally, first evidence is presented that URAT1 is required for the uricosuric effect of canagliflozin in mice.
The current studies in mice showed a robust reduction in renal urate reabsorption in the absence of tubular Glut9, whereas the effect of the Urat1 knockout was more subtle, although clearly demonstrated in renal clearance studies with measurement of GFR by FITC-sinistrin and after pretreatment with oxonic acid. This difference in the quantitative contribution of GLUT9 vs. URAT1 for renal urate reabsorption is consistent with previous studies in gene-targeted mice (10, 15, 26), although the current study includes the first characterization of a tubule-specific knockdown of Glut9. The previous study (26) used a whole body knockout of Glut9, which showed increased plasma urate levels due to impaired liver urate uptake via GLUT9 and subsequent degradation via hepatic uricase. The current study allowed a more specific characterization of tubular GLUT9 and showed that the enhanced FE-urate in the absence of tubular Glut9 can be observed with reduced or unchanged plasma urate levels; i.e., the enhanced FE-urate is not simply the consequence of a tubular urate overload. Furthermore, generation of mice with absent Urat1 and knocked down tubular Glut9 demonstrated the additive effect of these two transporters on renal urate reabsorption in the mouse kidney. tGlut9 KO mice also showed a modestly lower blood pressure and GFR, associated with a lower kidney-to-body weight ratio. The involved mechanisms and implications remained unclear, although increased blood urate levels have been linked to increased blood pressure under some conditions (17). A lower GFR may prevent more excessive renal urate loss in tGlut9 KO mice or indicate kidney injury. Renal mRNA expression of the proinflammatory marker Ccl2 (or Mcp1) was not altered in tGlut9 KO vs. WT mice, and the renal mRNA expression of Kim-1, a marker of tubular injury and inflammation (4), was even reduced in the absence of tubular Glut9. In the light of the latter, further studies are needed to determine whether inhibition of GLUT9-mediated tubular urate uptake has nephron-protective potential, possibly beyond lowering hyperuricemia. A functional reduction in GFR can also reduce the general transport burden on the renal tubules.
The current studies argue against a critical role for GLUT9 in the uricosuric effect of canagliflozin in mice. This is based on the findings that tubular knockdown of Glut9 unmasked a FE-urate-increasing and plasma urate-lowering effect following chronic canagliflozin application for 7 days. The latter upregulated renal Glut9 mRNA expression in WT mice, which may have attenuated the uricosuric effect. Such compensation would be lacking in tGlut9 KO mice, thereby unmasking a sustained uricosuric effect of the SGLT2 inhibitor, if the latter was at least in part independent of GLUT9. In this regard, GLUT9 protein expression was also significantly increased in the kidney of streptozotocin-induced diabetic mice (18), which share an increased tubular glucose delivery with SGLT2-inibited mice. Furthermore, the current studies show that the acute FE-urate-increasing effect of canagliflozin in WT mice was likewise preserved in tGlut9 KO mice. The current studies cannot exclude a minor contribution of GLUT9 to the uricosuric effect of canagliflozin in mice. Moreover, the qualitative or quantitative contribution of GLUT9 to the uricosuric effect of SGLT2 inhibition may differ between mice and humans because of the additional expression of GLUT9 in the distal nephron in mice or the higher levels of plasma urate in humans.
The current study provides first evidence that URAT1 is required for the SGLT2 inhibitor canagliflozin to acutely increase FE-urate in mice, inasmuch as the canagliflozin-induced increase in FE-urate was absent in Urat1 KO mice, despite a similar glucosuric response. Any potentially involved mechanism remains unclear. Previous studies using the SGLT2 inhibitor luseogliflozin in stably URAT1-expressing human embryonic kidney (HEK-293) cells argued against a direct inhibition of URAT1 by the drug (8). In studies in Xenopus laevis oocytes, luseogliflozin also had no direct effect on transport mediated by the Na+-coupled monocarboxylate transporter SMCT1 (8). SMCT1 transports monocarboxylates, such as lactate or ketone bodies, which stimulate the exchange transport of urate via URAT1 (20, 33). Thus, inhibition of SMCT1 could lower urate uptake via URAT1. Effects of SGLT2 inhibitors on SMCT2, which also transports lactate and ketone bodies, have not been tested. While SMCT2 is coexpressed with SGLT2 in the early proximal tubule, SMCT1 is coexpressed with SGLT1 in the later proximal tubule (27). Further studies are needed to confirm that SGLT2 inhibitors do not directly interact with URAT1 or SMCT1/2.
URAT1 is expressed in cortical proximal tubules, including the early proximal tubule (15). It is likely also expressed in cortical straight proximal tubules, inasmuch as URAT1 is thought to be responsible for the probenecid-sensitive urate reabsorption (15) and probenecid was shown to inhibit urate reabsorption in the mouse straight proximal tubule (38). The latter is the site of abundant SGLT1 expression in mice (22), thus providing a potential site for functional interactions between SGLT1 inhibition, enhanced luminal glucose, and functional effects on URAT1, which may contribute to the enhanced glucosuric and uricosuric effects of canagliflozin in the absence of SGLT1. While direct inhibitory effects of glucose on proximal tubular urate reabsorption have been established (19) and are consistent with results of the current studies, indirect effects of SGLT2 inhibition on URAT1 may also contribute as a consequence of the blood glucose reduction on endogenous insulin or therapeutic insulin dosage. In this regard, insulin has been shown to decrease urinary urate excretion in humans (11, 23, 32). Moreover, studies in rats indicated that the antiuricosuric effect of insulin is associated with and, potentially, due to upregulation of URAT1 (34). In other words, one may speculate that the degree of glucosuria induced by SGLT2 inhibition determines the suppression of blood glucose and endogenous insulin release, which disengages URAT1-mediated renal urate reabsorption, thereby enhancing urate excretion. In this regard, we observed in series 2 studies that the increase in urinary urate-to-creatinine ratios in response to canagliflozin was not only positively associated with the glucosuric response but was also inversely related to the changes in blood glucose levels measured before and after the experiment (Fig. 1B). Further studies are needed to test the hypothesis that SGLT2 inhibition reduces URAT1-mediated urate reabsorption indirectly by suppressing insulin release. Moreover, it remains to be tested whether an indirect coupling exists between SGLT2 and URAT1, such that primary inhibition of SGLT2 induces secondary inhibition of URAT1, as hypothesized for the interaction between SGLT2 and the Na+/H+ exchanger NHE3 in the early proximal tubule (9, 13, 16, 25).
In summary, the studies confirmed the contribution of URAT1 and GLUT9 to renal urate reabsorption, showing a greater contribution of the latter and additive effects. Both genetic and pharmacological inhibition of SGLT2 enhanced FE-urate. The absence of SGLT1 enhanced the glycosuric and uricosuric effects of the SGLT2 inhibitor, which would be expected for a proposed role of increased luminal glucose delivery in the uricosuric effect. The SGLT2 inhibitor enhanced renal mRNA expression of GLUT9 in WT mice, but tubular GLUT9 seemed dispensable for the increase in FE-urate in response to canagliflozin. First evidence is presented that URAT1 is required for the acute uricosuric effect of the SGLT2 inhibitor in mice.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-112042 and R01-DK-106102, University of Alabama at Birmingham/University of California San Diego O’Brien Center of Acute Kidney Injury National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-079337, the Department of Veterans Affairs, and an investigator-initiated grant from Janssen Pharmaceutical.
DISCLOSURES
Over the past 36 mo, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Eli Lilly, Intarcia Therapeutics, Janssen Pharmaceutical, and Merck and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.N., Y.F., H.K., M.B., and V.V. conceived and designed research; A.N., Y.F., W.H., B.F., R.P., C.v.G., A.O., J.N., and V.V. performed experiments; A.N., Y.F., and V.V. analyzed data; A.N., Y.F., and V.V. interpreted results of experiments; V.V. prepared Figs.; V.V. drafted manuscript; A.N., Y.F., W.H., B.F., R.P., C.v.G., H.K., M.B., A.O., J.N., and V.V. edited and revised manuscript; A.N., Y.F., W.H., B.F., R.P., C.v.G., H.K., M.B., A.O., J.N., and V.V. approved final version of manuscript.
REFERENCES
- 1.Andrade JA, Kang HC, Greffin S, Garcia Rosa ML, Lugon JR. Serum uric acid and disorders of glucose metabolism: the role of glycosuria. Braz J Med Biol Res 47: 917–923, 2014. doi: 10.1590/1414-431X20143878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233, 2010. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 3.Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, Bonny O. Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol 297: F612–F619, 2009. doi: 10.1152/ajprenal.00139.2009. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc 125: 293–299, 2014. [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. doi: 10.1002/gene.20008. [DOI] [PubMed] [Google Scholar]
- 6.Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 5: e197, 2008. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382: 941–950, 2013. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 8.Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, Tamai I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 35: 391–404, 2014. doi: 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe JY. MAP17 is a necessary activator of renal Na+/glucose cotransporter SGLT2. J Am Soc Nephrol 28: 85–93, 2017. doi: 10.1681/ASN.2015111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, Nigam SK. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33: 180–192, 2008. doi: 10.1152/physiolgenomics.00207.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266: 3008–3011, 1991. doi: 10.1001/jama.1991.03470210076036. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 15: 721–728, 2013. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Gerasimova M, Mayoux E, Masuda T, Vallon V. SGLT2 inhibitor empagliflozin increases renal NHE3 phosphorylation in diabetic Akita mice: possible implications for the prevention of glomerular hyperfiltration (Abstract) Diabetes 63, Suppl 1: A132, 2014. [Google Scholar]
- 14.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosoyamada M, Ichida K, Enomoto A, Hosoya T, Endou H. Function and localization of urate transporter 1 in mouse kidney. J Am Soc Nephrol 15: 261–268, 2004. doi: 10.1097/01.ASN.0000107560.80107.19. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Patel R, Onishi A, Crespo-Masip M, Soleimani M, Freeman B, Vallon V. Tubular NHE3 is a determinant of the acute natriuretic and chronic blood pressure lowering effect of the SGLT2 inhibitor empagliflozin (Abstract) FASEB J 32, Suppl 1: 620.17, 2018. [Google Scholar]
- 17.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 71: 851–865, 2018. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keembiyehetty C, Augustin R, Carayannopoulos MO, Steer S, Manolescu A, Cheeseman CI, Moley KH. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol 20: 686–697, 2006. doi: 10.1210/me.2005-0010. [DOI] [PubMed] [Google Scholar]
- 19.Knight TF, Senekjian HO, Sansom S, Weinman EJ. Effects of intraluminal d-glucose and probenecid on urate absorption in the rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 236: F526–F529, 1979. doi: 10.1152/ajprenal.1979.236.6.F526. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Nakanishi T, Tamai I. Functional cooperation of SMCTs and URAT1 for renal reabsorption transport of urate. Drug Metab Pharmacokinet 28: 153–158, 2013. doi: 10.2133/dmpk.DMPK-12-RG-070. [DOI] [PubMed] [Google Scholar]
- 21.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol 308: F77–F83, 2015. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 22.Madunić IV, Breljak D, Karaica D, Koepsell H, Sabolić I. Expression profiling and immunolocalization of Na+-d-glucose-cotransporter 1 in mice employing knockout mice as specificity control indicate novel locations and differences between mice and rats. Pflugers Arch 469: 1545–1565, 2017. doi: 10.1007/s00424-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, Frascerra S, Ciociaro D, Ferrannini E. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens 9: 746–752, 1996. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 44: 375–393, 2012. doi: 10.3109/07853890.2011.560181. [DOI] [PubMed] [Google Scholar]
- 25.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014. doi: 10.1681/ASN.2013060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preitner F, Bonny O, Laverrière A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA 106: 15501–15506, 2009. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 33: 5, 2017. doi: 10.1002/dmrr.2886. [DOI] [PubMed] [Google Scholar]
- 28.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skeith MD, Healey LA, Cutler RE. Effect of phloridzin on uric acid excretion in man. Am J Physiol 219: 1080–1082, 1970. doi: 10.1152/ajplegacy.1970.219.4.1080. [DOI] [PubMed] [Google Scholar]
- 31.So A, Thorens B. Uric acid transport and disease. J Clin Invest 120: 1791–1799, 2010. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 92: 51–58, 1997. doi: 10.1042/cs0920051. [DOI] [PubMed] [Google Scholar]
- 33.Thangaraju M, Ananth S, Martin PM, Roon P, Smith SB, Sterneck E, Prasad PD, Ganapathy V. c/ebpdelta Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J Biol Chem 281: 26769–26773, 2006. doi: 10.1074/jbc.C600189200. [DOI] [PubMed] [Google Scholar]
- 34.Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, Uchida S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 313: F826–F834, 2017. doi: 10.1152/ajprenal.00012.2017. [DOI] [PubMed] [Google Scholar]
- 35.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol 302: F1293–F1299, 2012. doi: 10.1152/ajprenal.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Völkl H, Geibel J, Rehwald W, Lang F. A microelectrode for continuous monitoring of redox activity in isolated perfused tubule segments. Pflugers Arch 400: 393–397, 1984. doi: 10.1007/BF00587538. [DOI] [PubMed] [Google Scholar]
- 39.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radović N, Jadrijević S, Aleksic I, Walles T, Sauvant C, Sabolić I, Koepsell H. Localizations of Na+-d-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467: 1881–1898, 2015. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]