Abstract
Introduction:
Electrical impedance myography (EIM) is a noninvasive electrophysiological technique that characterizes muscle properties through bioimpedance. We compared EIM measurements to function, strength, and disease severity in a population with congenital muscular dystrophy (CMD).
Methods:
Forty-one patients with CMD, either collagen 6 related disorders (COL6-RD; n = 21) or laminin α−2-related disorders (LAMA2-RD; n = 20), and 21 healthy pediatric controls underwent 2 yearly EIM exams. In the CMD cohorts, EIM was compared with functional and strength measurements.
Results:
Both CMD cohorts exhibited change over time and had correlation with disease severity The 50-kHZ phase correlated well with function and strength in the COL6-RD cohort but not in the LAMA2-RD cohort.
Discussion:
EIM is a potentially useful measure in clinical studies with CMD because of its sensitivity to change over a 1-year period and correlation with disease severity. For COL6-RD, there were also functional and strength correlations.
Keywords: COL6 related dystrophies, congenital muscular dystrophy, electrical impedance myography, LAMA2 related dystrophies, neuromuscular disorders
Electrical impedance myography (EIM) is a noninvasive, rapid method of measuring changes in muscle composition and integrity through bioimpedance measures.1,2 EIM measurements include resistance, reactance, and phase angle. Resistance and reactance are measured values that are thought to reflect properties of the muscle fiber and surrounding milieu, including the presence of fat and fibrosis. 3 Calculated from the reactance and resistance, the phase angle is a measure of the change in voltage output compared with current input.4 Changes in the phase angle have been used to follow alterations in the disease state of muscle, with lower values suggesting greater pathology. In this capacity, EIM has been proposed as a meaningful biomarker in amyotrophic lateral sclerosis and is currently being investigated in other neuromuscular diseases, including Duchenne muscular dystrophy and spinal muscular atrophy.5–7
To study further the applicability of EIM for following neuromuscular disease progression and its potential use in clinical trials, 2 subtypes of congenital muscular dystrophy (CMD), collagen-6 related disorders (COL6-RDs) and laminin α−2 related disorders (LAMA2-RDs), were evaluated by yearly EIM over 2 years. CMD, a genetically heterogeneous group of degenerative muscle disorders, typically presents at birth or infancy and leads to a variable progression of muscle weakness with possible concomitant joint contractures.8 COL6-RD has mutations encoded by genes COL6A1, COL6A2, and COL6A3 that lead to defects in the collagen VI protein in the interstitial matrix of muscle. LAMA2-RD, also known as merosin-deficient CMD, has mutations in the LAMA2 gene that encodes for laminin α−2, a protein that is present in the basement membrane of muscle and nerves.9,10
In this 2-year cross-sectional study of 2 CMD subpopulations, COL6-RD and LAMA2-RD, and a healthy pediatric population, we present data on (1) changes in EIM over a 1-year period in these 3 populations and (2) correlations of EIM with disease severity, function, and strength measures in the 2 CMD subpopulations.
MATERIALS AND METHODS
Overall Study Design.
The study was performed under clinical protocols NCT01568658 as part of a natural history study in CMD11 and NCT01900132 for EIM. Both protocols were approved by an institutional review board at the National Institutes of Health, and written informed consent for each protocol was obtained from all participants; parents provided written informed consent, and children provided written assent.
Participants.
Individuals with COL6-RD and LAMA2-RD were recruited for the study. Prior to the study, genetic testing or muscle biopsy were required to confirm diagnosis. Participants underwent a medical and neurological exam upon entry into the study and annually. At the present time, all patients have been genetically confirmed except for 2 patients. Of the 2, 1 patient had only a single pathogenic allele identified, and the other patient did not have genetic testing but had a positive family history.
Data from the 2 CMD cohorts, for the purposes of this study, were collected during a 2-year period. Healthy volunteers (HVs), all pediatric, were recruited under clinical protocol for NCT01900132 for EIM and were also followed annually for 2 years. All participants were evaluated at the Clinical Center, National Institutes of Health, Bethesda, MD.
EIM Measurements.
EIM measurements were performed unilaterally in 8 muscle groups, deltoids, biceps, triceps, wrist flexors, quadriceps, hamstrings, medial gastrocnemius, and tibialis anterior, with a handheld electrode array (HEA; HEA-EIM system 1103; Myolex, Inc., San Francisco, CA).
Prior to applying the HEA, the skin overlying the muscle was moistened with isotonic saline to ensure good electrical contact. Three different array sizes, P/N EIM-014–009 adult sensor, P/N EIM 007–002 pediatric sensor, and P/N 2000036 infant sensor, all part of a commercially available product line, were employed (Fig. 1). The adult and pediatric sensors are the same configuration and size, but one is curved and the other is flat surfaced. According to the company, the pediatric and adult sensors are interchangeable for data analysis, and, in this study, the 2 sensors were evaluated as equivalent. Each participant was evaluated with 1 sensor array for all 8 muscles, and the same type of sensor array was used for both year 1 and year 2 studies on each individual. The type of sensor used was determined by the size of each patient’s limbs. Each measurement was repeated 3 times and then averaged. Resistance (R) and reactance (X) were collected, and the phase θ (θ = arctan [X/R]) was then calculated. The 8-muscle average was calculated by taking the mean EIM measurement of all 8 muscle groups. The upper extremity (UE) average was calculated by taking the mean of EIM measurements for the deltoids, biceps, triceps, and wrist flexors. The lower extremity (LE) average was calculated by taking the mean of EIM measurements for the quadriceps, hamstrings, medial gastrocnemius, and tibialis anterior. The 50-kHZ data were preferentially analyzed because this frequency is thought to be most physiologically relevant for muscle. The 8-muscle, UE, and LE averages were used for comparison with functional and strength measures, similarly to other EIM- based studies. Exploratory analyses of the 50:200-kHZ phase ratio and phase slope from 100–500 kHZ were also performed.12,13 The association analysis for the phase slope 100–500 kHZ was limited to COL6-RD and LAMA2-RD participants that were evaluated with the infant sensors. The 50:200-kHZ ratio was calculated by dividing the 50-kHZ data by the 200-kHZ data. Calculation of the phase slope angle for the 100–500-kHZ multifrequency data was limited to participants using the infant sensor because of consistency of frequencies sampled. The phase slope for each muscle was calculated in MATLAB version 2014a (MathLab, Natick, MA).
FIGURE 1.
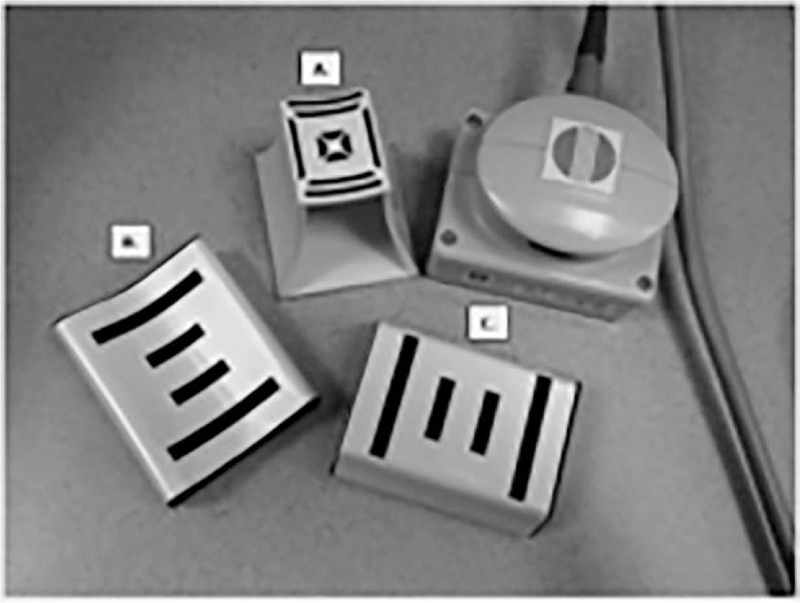
EIM electrode arrays. P/N 20–00036 infant sensor (A), P/N EIM 007–002 pediatric sensor (B), and P/N EIM-014–009 adult sensor (C). The electrode arrays are adjacent to the sensor holder. EIM, electrical impedance myography.
Physician Global Clinical Impression of Severity.
Disease severity was determined by 2 neurologists (C.G.B. and J.C.) with expertise in COL6-RD and LAMA2-RD and was based on the overall clinical presentation of the participant. The Physician Global Clinical Impression of Severity (PGCIS) was rated in severity from 1 to 5: 1, mild; 2, mild-moderate; 3, moderate; 4, moderate-severe; and 5, severe.
Functional and Strength Measures.
Functional and strength measures were performed by a pediatric physical therapist. To assess motor function, participants were evaluated by the Motor Function Measure (MFM32). The MFM is a 32-item scale that assesses 3 dimensions of motor function: (1) standing and transfers, (2) proximal and axial motor function, and (3) distal motor function. The 10-meter run (MFM item 30) of the MFM32 is scored 0–3: 3, runs 10 meters; 2, runs 10 meters with compensatory movements; 1, increases walking speed but cannot run 10 meters; and 0, cannot increase walking speed. For a more robust assessment, the North Star Ambulatory Assessment (NSAA) was performed in ambulatory individuals. Finally, bilateral strength (myometry) measurements of elbow extensors/flexors and knee extensors/flexors were obtained by using a MicroFet handheld dynamometer (HHD, Hoggan Scientific, LLC, Salt Lake City, Utah, http://www.hogganhealth.net/microfet2.php). Three trials for each muscle group were performed. To reflect best performance, the maximum score of each muscle group was used to calculate the myo- metry total, UE, and LE sums.
Data Analysis.
Statistical analysis methods were applied to evaluate (1) the reliability and (2) responsiveness (to detect important changes over time and the difference between HV and CMD) of the EIM measurements and (3) the association between the EIM measurements and clinical outcomes (PGCIS, function, and strength scores). The 8-muscle EIM phase average was the primary outcome for the EIM measures. The clinical disease severity (PGCIS) was the primary outcome for the clinical measures. Other analyses were treated as exploratory. The 2 CMD subpopulations, COL6-RD and LAMA2-RD, were analyzed separately because they had slightly different phenotypes and disability severity. The statistical analyses were performed in SAS version 9.4 (Cary, NC).
The interrater reliability study contained 16 HV adult participants and 2 raters. The intrarater reliability study contained 8 HV adult participants and 2 replications. Intraclass correlation coefficients (ICC) and 95% confidence intervals were calculated separately for UE, LE, and 8-muscle averages collected from phase, resistance (R), and reactance (X).
For each EIM measurement outcome, repeated-measures ANOVA analysis was performed to evaluate responsiveness. Year (year 1 vs. year 2) was a within-subject factor, and group (COL6-RD, LAMA2-RD, and HV) was a between-subject factor. The model included group, year, and the interaction between group and year. Multiple comparisons with the Tukey method were performed at the level of the interaction (15 pairwise comparisons for 6 combinations). A Tukey-adjusted P = 0.05 was considered significant for the 8-muscle EIM phase average.
The association between EIM measurements and clinical outcomes was tested for COL6-RD and LAMA2-RD separately. Pearson correlation analysis was applied to functional and strength measurements (continuous); Spearman correlation analysis was applied to PGCIS on a scale of 1–5 and to 10-meter run on a scale of 0, 1, and 2. A significance level of 0.025 was used for testing the association between 8-muscle EIM phase average and PGCIS for COL6-RD and LAMA2-RD cohorts.
To evaluate the sensor effect, the pediatric and adult sensors, which have the same configuration and size, were treated as equivalent. Therefore, the results from the adult and pediatric sensors were combined. For each of the 8-muscle averages, repeated-measures ANOVA was also performed for LAMA2-RD and COL6-RD cohorts separately, in which sensor (adult or pediatric vs. infant) was a between-subject factor and year was a within-subject factor. The model included sensor, year, and the interaction between sensor and year. Multiple comparisons with the Tukey method were performed at the level of the interaction (6 pairwise comparisons for 4 combinations).
RESULTS
Participant Demographics.
In total, 41 CMD patients (21 COL6-RD and 20 LAMA2-RD) and 21 HVs were evaluated (Table 1). The association analysis for the 50-kHZ and the 50:200-kHZ data had the same cohort for COL6-RD, LAMA2-RD, and HV. The COL6-RD and HVs were close in age, but the LAMA2-RD cohort was slightly younger than the COL6-RD cohort. There were more ambulatory patients in the COL6-RD cohort than in the LAMA2-RD cohort, with ambulation defined as the ability to walk 10 meters without the use of assistive devices.
Table 1.
Participant demographics
| COL6-RD |
LAMA2-RD |
HV |
|||
|---|---|---|---|---|---|
| Characteristics | 50-kHZ and 50:200-kHZ analyses |
Phase slope 100–500-kHZ analyses |
50-kHZ and 50:200-kHZ analyses |
Phase slope 100–500-kHZ analyses |
50-kHZ analysis |
| No. of participants | 21 | 13 | 20 | 16 | 21 |
| Mean age (years) | 12.5 63.8 | 12.8 63.9 | 9.4 62.6 | 9.0 62.7 | 11.4 62.5 |
| ±SD (range) | (7–20) | (7–15) | (5–15) | (5–15) | (7–17) |
| Male | 12 | 7 | 9 | 8 | 8 |
| Female | 9 | 6 | 11 | 8 | 13 |
| Ambulatory | 11 | 6 | 4 | 1 | 21 |
COL6-RD, collagen 6-related disorder; HV, healthy volunteer; LAMA2-RD, laminin α-2-related disorder.
Adult sensor interrater and intrarater reliability of 8-muscle, UE, and LE EIM averages had an ICC of ≥0.97 for phase and reactance and an ICC ≥0.94 for resistance (Supporting Information Table 1).
Change in EIM at 50 kHZ Over 1 Year.
For the 2–year comparisons with repeated-measures ANOVA, 21 COL6-RD patients, 20 LAMA2-RD patients, and 21 HVs were evaluated. The statistical analysis of phase, reactance, and resistance is presented below and in Figure 2. Additionally, the mean and SE of EIM measurements for phase, reactance, and resistance in the 3 populations are presented in Supporting Information Table 2. The EIM sensors used in each group were COL6-RD cohort (8 pediatric sensors and 13 infant sensors), LAMA2-RD cohort (2 adult sensors, 2 pediatric sensors, 16 infant sensors), and HV cohort (10 adult sensors, 8 pediatric sensors, 3 infant sensors).
FIGURE 2.
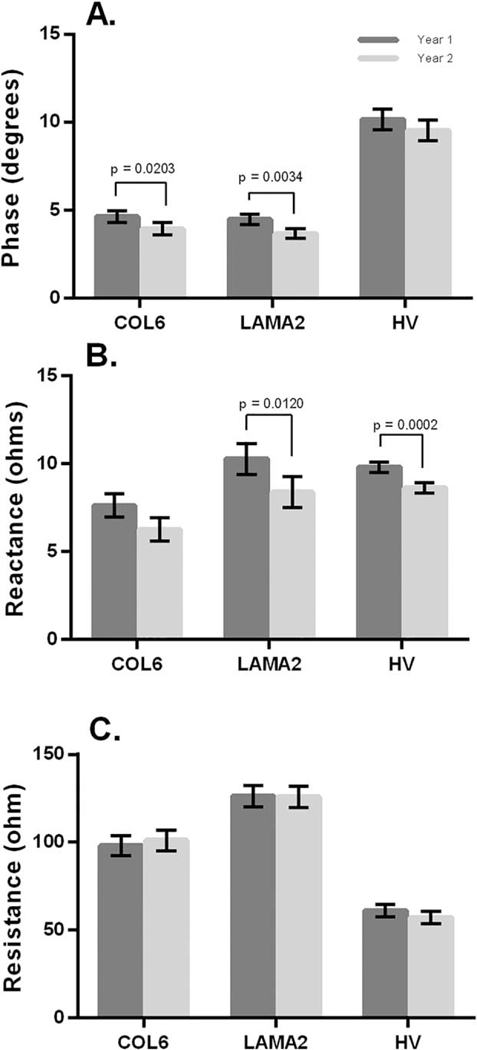
Change in EIM at 50 kHZ in 1-year interval. Comparison of 8-muscle EIM average for COL6-RD, LAMA2 -RD. and HV for phase (A), reactance (B), and resistance (C). Data are mean ± SE. COL6-RD, collagen 6 related disorder; EIM, electrical impedance myography; HV, healthy volunteers; LAMA2-RD, laminin α−2 related disorder.
Phase.
For the 8-muscle EIM phase average, both the COL6-RD and LAMA2-RD groups showed significant decreases in the 1-year interval (Fig. 2A). In the COL6-RD cohort, the UE-EIM phase average also showed a significant decrease in the 1-year interval, but there was not a significant change in the LE-EIM phase average. In contrast, in the LAMA2-RD cohort, the LE-EIM phase average showed a significant decrease in the 1-year interval but not in the UE-EIM phase average. The HV cohort did not have any significant changes over the 1-year interval.
The 8-muscle EIM phase average was significantly different for the HV cohort compared with the COL6-RD and the LAMA2-RD cohorts in both year 1 and year 2 (P< 0.0001 for both cohorts). The 8-muscle EIM average was not significantly different between the 2 CMD cohorts in either year 1 or year 2.
Reactance.
For the LAMA2-RD cohort, there was a significant difference in the 8-muscle EIM average and LE-EIM average but not the UE-EIM reactance average (Fig. 2B). In the COL6-RD cohort, there was a decrease in reactance, but it did not reach significance in the 8-muscle EIM average, LE-EIM reactance average, or UE-EIM reactance. For the HV cohort, the 8-muscle, UE- EIM, and LE-EIM reactance averages all significantly decreased over 1 year, even though a decrease was not reflected in the phase analyses.
The 8-muscle EIM reactance average was significantly different between the HV cohort and the COL6-RD cohort in year 2 only (P = 0.0397) but not the LAMA2-RD cohort in either year. The 8-muscle EIM average was not significantly different between the 2 CMD cohorts.
Resistance.
There were no significant changes in resistance in the COL6-RD, LAMA2-RD, or HV cohorts for the 8-muscle EIM average (Fig. 1C). This was also true for the UE-EIM and LE-EIM averages. The 8-muscle EIM resistance average was significantly different between the HV and the COL6-RD and LAMA2-RD cohorts (P< 0.0001 for both cohorts). The 8-muscle EIM average was significantly different between the 2 CMD cohorts for year 1 (P = 0.0015) only but not for year 2.
Comparison of Adult and Pediatric Versus Infant Sensors.
The sensor effect on EIM measurements was evaluated on both the COL6-RD and LAMA2- RD cohorts (Supporting Information Table 3). For the 8-muscle EIM average in both CMD cohorts, there were no significant differences between the sensors for phase, reactance, or resistance. Only 2 participants in the HV cohort were evaluated with the infant sensor, so sensor effect was not evaluated in the HV cohort.
Association Between 8-Muscle EIM Average (50 kHZ) With PGCIS and Ability to Perform 10-Meter Run (MFM Item 30).
There was a correlation between the 8-muscle EIM phase average with the PGCIS for both the COL6-RD and LAMA2-RD CMD cohorts (Fig. 3). A high severity score in the PGCIS correlated with a low EIM 8-muscle phase average. In addition to the PGCIS, we assessed the association of the EIM 8-muscle average with the 10-meter run (item 30 in the MFM). The COL6-RD cohort showed that the higher 8-muscle EIM phase average was associated with greater ability to perform the 10-meter run (Fig. 4A). There was no association between the 8-muscle EIM phase average and the ability to perform the 10-meter run for the LAMA-RD cohort (Fig. 4B).
FIGURE 3.
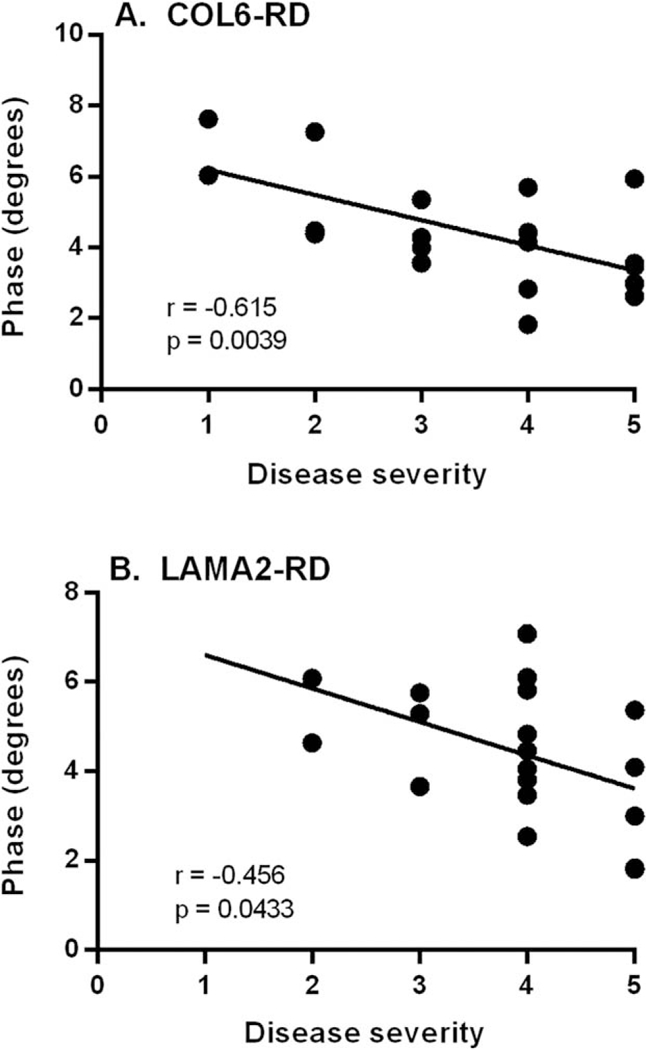
PGCIS vs. 8-muscle EIM phase average. Dotplot representation with best fitted linear line of COL6-RD cohort (n= 19; A) and LAMA2-RD cohort (n= 20; B). The PGCIS is rated on a 5-point scale as 1, mild; 2, mild-moderate; 3, moderate; 4, moderate-severe; and 5, severe. COL6-RD, collagen 6 related disorder; EIM, electrical impedance myography; LAMA2- RD, laminin α−2 related disorder; PGCIS, Physician Global Clinical Impression of Severity.
FIGURE 4.
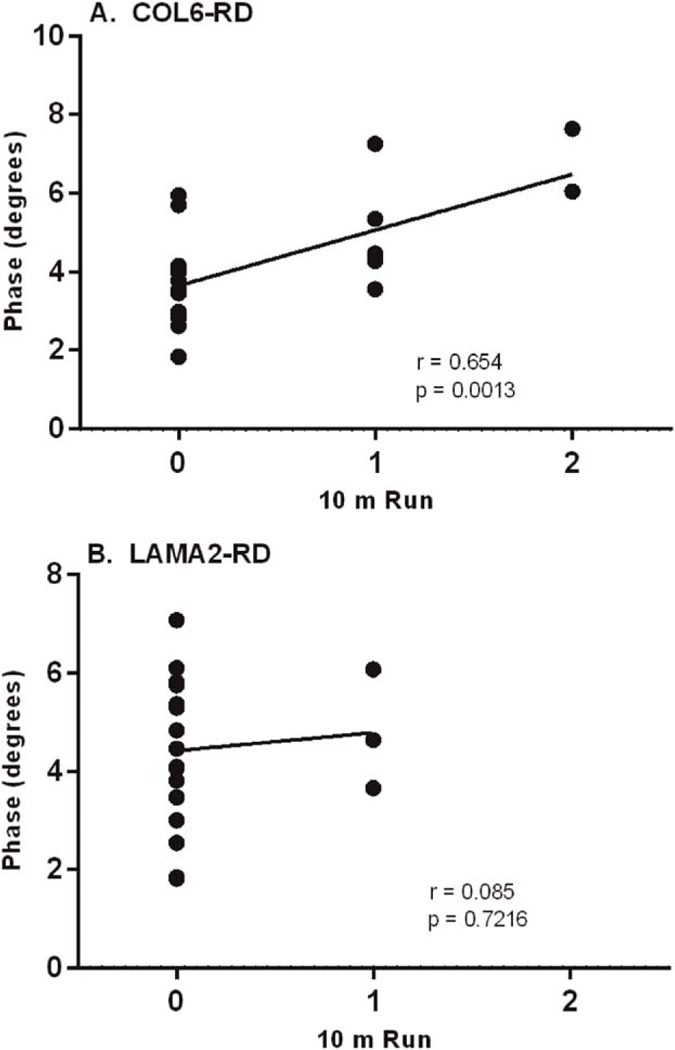
Ability to perform 10-meter run vs. 8-muscle EIM phase average (MFM item 30). Dotplot representation with best fitted linear line of COL6-RD cohort (n = 20; A) and LAMA2-RD cohort (n = 20; B). COL6-RD, collagen 6 related disorder; EIM, electrical impedance myography; LAMA2-RD, laminin α−2 related disorder; MFM, Motor Function Measure.
Association of 50-kHZ Phase EIM With Functional and Strength Measures (Exploratory).
For the COL6-RD cohort, Pearson correlation coefficients indicated that the 8-muscle EIM average at 50 kHZ correlated with the MFM and the NSAA totals (Table 2). The LE-EIM average correlated with both the LE myometry sum and dimension 1 (standing and transfers) of the MFM. The UE-EIM average correlated with dimension 2 (proximal and axial strength) but did not show correlations with dimension 3 (distal strength) of the MFM. For the LAMA2-RD cohort, the 8-muscle EIM, UE-EIM, and LE-EIM averages did not correlate with any of the functional or strength measures (Table 2). No significant correlations for reactance and resistance were observed for either the COL6-RD or LAMA2- RD cohorts (data not shown).
Table 2.
Testing association of 50-kHz EIM with functional and strength scores
| Correlation between |
COL6-RD |
LAMA2-RD |
|||||
|---|---|---|---|---|---|---|---|
| Clinical score | EIM score | r | P | n | r | P | n |
| Myometry total | 8M avg | 0.525 | 0.0174 | 20 | 0.096 | 0.6859 | 20 |
| UE myometry | UE avg | 0.236 | 0.3029 | 21 | 0.011 | 0.9643 | 20 |
| LE myometry | LE avg | 0.616 | 0.0039 | 20 | 0.148 | 0.5327 | 20 |
| NSAA total | 8M avg | 0.792 | 0.0037 | 11 | – | – | 3 |
| MFM total | 8M avg | 0.707 | 0.0003 | 21 | 0.177 | 0.4566 | 20 |
| MFM D1 | LE avg | 0.672 | 0.0009 | 21 | 0.127 | 0.5936 | 20 |
| MFM D2 | UE avg | 0.577 | 0.0061 | 21 | 0.171 | 0.4708 | 20 |
| MFM D3 | UE avg | 0.376 | 0.0933 | 21 | 0.063 | 0.7928 | 20 |
8M avg, 8-muscle average; COL6-RD, collagen 6 related disorder; D1, dimension 1; D2, dimension 2; D3, dimension 3; EIM, electrical impedance myography; LAMA2-RD, laminin2-related disorder; LE avg, 4-muscle lower extremity average; MFM, Motor Function Measure; NSAA, North Star Ambulatory Assessment; P, probability > jrj under H0: p 50; n, No. of participants; r, Pearson correlation coefficient; UE avg, 4-muscle upper extremity average.
Exploratory Analysis of the 50:200-kHZ Ratio and Phase Slope (100–500 kHZ).
Two exploratory analyses were performed as possible alternatives and more sensitive parameters to follow. In the 2-year interval, the 50:200 phase ratio and phase slope did not show any significant changes for either COL6-RD or LAMA2-RD cohorts. Pearson correlation analysis (Supporting Information Table 4) indicated that there was correlation between myo-metry measures and the 50:200-kHZ phase slope for the LAMA2-RD cohort but not for the COL6- RD cohort.
DISCUSSION
For both the COL6-RD and LAMA2-RD cohorts, this study demonstrates a significant change in the EIM 50-kHZ phase for the 8-muscle EIM average over a 1-year period, making it a potential tool for assessing disease progression or response to therapy in clinical studies. In contrast, the HV cohort exhibited no significant change in 50-kHZ phase over the same period. Furthermore, the 50-kHZ phase showed correlation with disease severity measures, particularly the PGCIS, for both CMD subtypes, supporting the clinical relevance of the EIM results. EIM also showed a correlation with the ability to perform a 10-meter run in the COL6-RD cohort but not in the LAMA2-RD cohort, probably because of the low number of ambulatory LAMA2-RD patients. Reactance at 50 kHZ reached significance only in the LAMA2-RD and HV cohorts. The reactance probably contributed to the phase changes and has the advantage of being less susceptible to changes in subcutaneous fat,14 but it was not as robust in clinical correlations for the CMD cohorts. In the HV cohort, the change in reactance did not produce a sufficiently significant impact on change in phase over 2 years. In retrospect, all the HVs appeared to be heavily involved in athletic activities, which could be related to the observed change in reactance rather than reflecting any disease-related changes. Change in reactance for healthy pediatric controls (age > 7 years) has not been observed previously (Rutkove, personal communication). Resistance did not appear to be a sensitive parameter for this study. Also, the multifrequency exploratory analysis of the 50:200-kHZ ratio and 100–500-kHZ phase slope did not enhance the correlations between EIM measurements and strength or functional outcomes.
Exploratory analyses of EIM with functional and strength measures showed that the COL6-RD cohort had the most correlations. Disease severity and LE function may play a role in the different findings between the 2 cohorts. Only 4 of the individuals with LAMA2-RD were ambulatory, whereas roughly half of the COL6-RD cohort were ambulatory. The COL6-RD cohort had good correlations for the 8-muscle EIM phase average with the NSAA score and MFM total. In the EIM subsets, the COL6-RD cohort also had good correlations for the LE-EIM phase average with LE myometry and dimension 1 of the MFM, whereas only dimension 2 of the MFM correlated with UE-EIM. This suggests that the assessments dependent on LE function were more closely captured with the EIM phase measurements. Thus, the less-ambulant LAMA2-RD cohort had fewer EIM correlations with functional and strength measures. Further more, a floor effect is exhibited in dimension 1 of the MFM, standing and transfers, because nonambulatory participants cannot perform most if not all of the items in dimension 1. This also may have limited the LAMA2-RD analysis. We were surprised that there was not a significant difference in 8-muscle phase average between the 2 CMD cohorts even though LAMA2-RD had greater severity in terms of ability to ambulate. The slight difference in reactance between the 2 CMD cohorts also would not account for the difference in severity. There is not a clear explanation for this observation, although it may relate to the distribution of weakness being more marked in specific muscles and not in the 8-muscle EIM average. Also, we are not certain why there were differences in the 2 cohorts with regard to showing decrease in phase in the UE of the COL6-RD cohort and LE of the LAMA2-RD cohort. It is possible that the COL6-RD cohort ambulatory state allowed them to maintain better LE function and less disuse.
EIM was developed to provide a quantitative assessment of muscle by assessing the muscle fiber membrane capacitance properties through reactance measurements and the extracellular milieu through resistance measurements. By inference, the observed phase angle change should reflect change in the muscle pathophysiology. Of the two factors incorporated into phase angle calculations, reactance showed significant change in the LAMA2-RD cohort and mild but not significant decrease in the COL6-RD cohort, whereas the resistance did not change in either group. This may suggest that changes in fiber size and density occur to a greater extent than changes in the amount of connective or fatty tissue surrounding the muscle fibers. Both LAMA2-RD and the later stages of COL6-RD have a combination of muscle fiber loss and fatty tissue changes on muscle biopsies. In support of this, resistance is higher for the LAMA2-RD cohort, particularly in the 8-muscle and LE averages (P> 0.05), than for the COL-6 RD cohort and may relate to the early fibrosis in the LAMA2-RD. The rate of these changes within the muscles of CMD has not been specifically determined, but it is possible that muscle fiber deterioration is an earlier and more rapid occurrence.
This study supports the future use of EIM in clinical studies in the COL6-RD and LAMA2-RD CMD subpopulations. The 50-kHZ phase angle proved to be the most sensitive parameter for following the 2 CMD cohorts serially. It also had the better correlation with strength and functional outcome measures, although this is more evident for the COL6-RD cohort. The exploratory analyses with the 50:200-kHZ phase ratio and the 100–500- kHZ phase slope did not provide additional information or sensitivity in this study. The findings are limited by the small population size, and the short follow-up period did not allow for fuller understanding of the effect of disease natural history on EIM changes. Additional studies are required to understand further the pathophysiological basis for the interplay of reactance and resistance changes on phase angle. Part of the evolution of this technique has been the changes in the types of sensors used. A new adult sensor configuration that resembles the infant sensor has replaced the prior adult and pediatric sensors and will require evaluation to determine whether it provides similar results in other populations. Additional validation of the EIM data with clinical outcome measures is important as the technique receives more consideration as a possible biomarker for clinical trials in neuromuscular disorders. Because it is noninvasive, does not require any volitional effort, and is not painful, it is particularly well suited for pediatric cohorts.
Supplementary Material
Acknowledgments
Funding: This work was funded by the Intramural Research Programs of the National Institutes of Neurological Disorders and Stroke, National Institute of Nursing Research, and Mark O. Hatfield Clinical Research Center. The Congenital Muscular Dystrophy-Comparative Outcome Study, a five-year natural history study, was also supported by Cure CMD.
Abbreviations:
- CMD
congenital muscular dystrophy
- COL6-RD
collagen related disorder
- EIM
electrical impedance myography
- HEA
handheld electrode array
- HHD
handheld dynamometer
- HV
healthy volunteers (pediatric)
- ICC
intraclass correlation coefficients
- LAMA2-RD
laminin α−2 related disorder
- LE
lower extremity
- MFM
motor function measure
- NSAA
North Star Ambulatory Assessment
- PGCIS
physician global clinical impression of severity
- UE
upper extremity
Footnotes
Conflicts of Interest: None of the authors have any financial relationship with the company that manufactures the product used in this study or any other conflict of interest.
Additional supporting information may be found in the online version of this article.
The authors thank the patients and families for their involvement in this study and Dr Seward Rutkove for his thoughtful comments related to EIM in this study.
Ethical Publication Statement:We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Nandedkar SD. Emerging techniques in the electrodiagnostic laboratory. PM R 2013;5:S115–S122. [DOI] [PubMed] [Google Scholar]
- 2.Rutkove SB. Electrical impedance myography: background, current state, and future directions. Muscle Nerve 2009;40:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Geisbush TR, Rosen GD, Lachey J, Mulivor A, Rutkove SB. Electrical impedance myography for the in vivo and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle Nerve 2014;49: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 2004;23:1226–1243. [DOI] [PubMed] [Google Scholar]
- 5.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler 2012;13:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in duchenne muscular dystrophy and healthy controls: a multicenter study of reliability and validity. Muscle Nerve 2015;52:592–597. [DOI] [PubMed] [Google Scholar]
- 7.Rutkove SB, Shefner JM, Gregas M, Butler H, Caracciolo J, Lin C, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve 2010;42:915–921. [DOI] [PubMed] [Google Scholar]
- 8.Bonnemann CG, Wang CH, Quijano-Roy S, Deconinck N, Bertini E, Ferreiro A. et al. Diagnostic approach to the congenital muscular dystrophies. Neuromuscul Disord 2014;24:289–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol 2011;7:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schessl J, Zou Y, Bonnemann CG. Congenital muscular dystrophies and the extracellular matrix. Semin Pediatr Neurol 2006;13:80–89. [DOI] [PubMed] [Google Scholar]
- 11.Meilleur KG, Jain MS, Hynan LS, Shieh CY, Kim E, Waite M, et al. Results of a two-year pilot study of clinical outcome measures in collagen VI-and laminin alpha2-related congenital muscular dystrophies. Neuromuscul Disord 2015;25:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: a longitudinal study. Muscle Nerve 2012; 45:642–647. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz S, Geisbush TR, Mijailovic A, Pasternak A, Darras BT, Rutkove SB. Optimizing electrical impedance myography measurements by using a multifrequency ratio: a study in Duchenne muscular dystrophy. Clin Neurophysiol 2015;126:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz DP, Dastgir J, Salman A, Lear B, Bonnemann CG, Lehky TJ. Electrical impedance myography discriminates congenital muscular dystrophy from controls. Muscle Nerve 2016;53(3):402–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


