Abstract
Metastasis to bones is determined by both intrinsic traits of metastatic tumor cells as well as properties appertaining to the bone microenvironment. Bone marrow niches are critical for all major steps of metastasis, including the seeding of disseminated tumor cells (DTCs) to bone, the survival of microscopic metastases under dormancy and the eventual outgrowth of overt metastases. In this review, we discuss the role of bone marrow niches in bone colonization. The emphasis is on complicated and dynamic nature of cancer cells-niche interaction, which may underpin the long-standing mystery of metastasis dormancy, and represent a therapeutic target for elimination of minimal residue diseases and prevention of life-taking, overt metastases.
Keywords: Bone Metastasis, Disseminated Tumor Cells, Bone Marrow Niches, Hematopoietic Stem Cells
Common Metastatic Traits vs. Bone Tropism
The survival of cancer patients has been significantly improved with advances in early detection and treatments. However, the spreading of cancers to distant organs, known as cancer metastasis, is often incurable and is the major cause of death in cancer patients. The skeleton is one of the most common metastatic sites, particularly in breast and prostate cancers [1]. Bone metastasis is most likely associated with a unique set of skeletal complications, including bone pain, pathologic fractures, hypercalcemia, and spinal cord compression, which lead to a reduced quality of life or even death in these patients [2]. Similar to metastasis to other organs, bone metastasis is a process of multiple steps, including local migration and invasion, intravasation into the circulation, systemic dissemination, arrest and extravasation into bone marrow, survival under dormancy, reactivation and ultimate outgrowth (Figure 1) [3]. Thus, bone metastasis is partly regulated by processes and pathways commonly related to metastasis in general, such as epithelial-mesenchymal transition (EMT, see Glossary), cancer stem cell (CSC)-like properties and escape of immunosurveillance in metastatic tumor cells [4]. On the other hand, organ tropism of metastasis has also been noticed since over a century ago. About 90% and 70% of patients dying of prostate and breast cancer, respectively, show evidence of skeletal involvement at autopsy [5, 6]. Tumor cells with certain molecular characteristics also appear to more commonly metastasize to the bone. Such metastatic predilection to bones can be intuitively explained by the ‘seed and soil’ hypothesis, that the metastatic tumor cells must interact with the unique bone environments to establish successful metastasis [7].
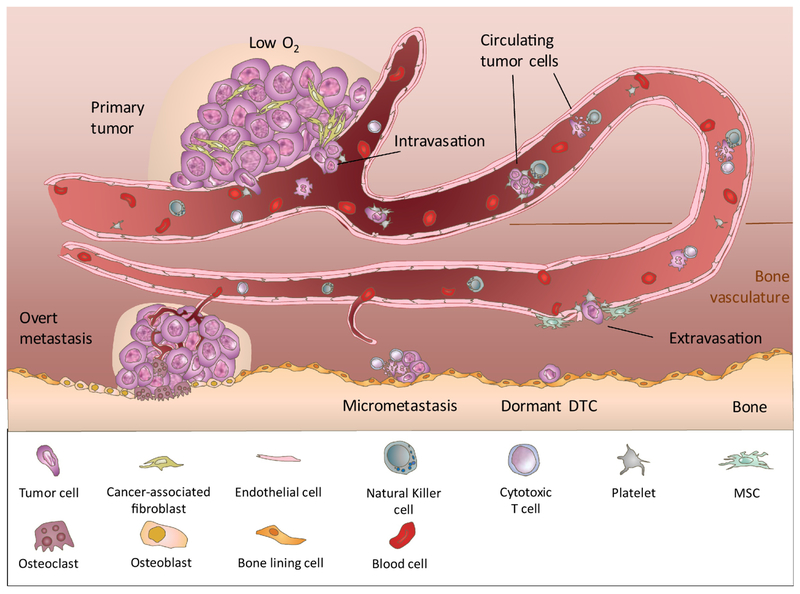
Figure 1. Steps of Bone Metastasis.
Bone metastasis is a multiple-step and lengthy process. Facilitated by EMT and the primary tumor microenvironment (eg. hypoxia and cancer associated fibroblasts), invasive tumor cells may intravasate into the blood vessel as single circulating tumor cells (CTCs) or CTC clusters. While in circulation, CTCs may aggregate with platelets to survive against physiochemical pressure and immunosurveillance. After arrival at the bone marrow vasculature, CTCs attach and adhere to the bone marrow endothelium via intercellular adhesion, and extravasate into bone marrow parenchyma. T umor cells may subsequently enter a dormant state for a prolonged period of time, until they are re-activated under favorable conditions to form micrometastasis. The progression of micrometastases into overt metastases is limited by neo-angiogenesis and immunosurveillance mechanisms. Overt metastases in bone commonly leads to abnormal bone growth or resorption, both of which reduce bone strength and increase the risk of bone fracture.
Origins of Bone Metastatic Traits
Bone is unique for its mineral content, matrix composition, extreme rigidity, high concentration of extracellular calcium, hypoxia and acidic pH [8]. Such unique environment imposes specific requirements of metastatic seeds (Box 1). In addition to these physiochemical properties, bone matrix is also enriched for matrix proteins, cytokines and growth factors, including ligands for integrins, insulin-like growth factors (IGFs), transforming growth factor beta proteins (TGFβs), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs), and bone morphogenetic proteins (BMPs), all of which have been proposed to regulate bone metastasis either directly or indirectly [9–12]. For example, TGFβ is deposited into the mineral bone by osteoblasts and could be released and activated by osteoclasts during bone remodeling [1, 8, 10]. Multiple important functions of TGFβ in bone metastasis have been implicated including induction of local invasion and angiogenesis, suppression of the anti-tumor immune system, regulation of tumor dormancy and initiation of osteolytic vicious cycle [11, 13].
Box 1. Adaptation to the Physiochemical Properties of Bone Environment.
Bone is unique in many aspects, and tumor cells must adapt to the unique environment to successfully colonize in bone.
Firstly, the inorganic phase of bone is mainly composed of the mineral hydroxyapatitea nanocrystals (HA). HA crystals have been shown to promote the mitogenesis and secretion of matrix remodeling enzymes of breast cancer cells [146]. Furthermore, the bone tropic subline of MDA-MB-231 cells showed increased secretion of pro-osteoclastic interleukin-8 in an HA-rich environment, which may therefore contribute to the vicious cycle [147]. In a recent study, the skeletal sites with less mature HA were found to be more likely to develop bone metastasis in a mouse model of breast cancer [148].
Secondly, the extracellular bone matrix is enriched with type-I collagen, osteopontin (OPN), and bone sialoprotein (BSP). Elevated expression of OPN and BSP have been observed in cancers with bone tropism [31]. Overexpression of integrin αvβ3 in breast cancer cells facilitates tumor cell adhesion to collagen and BSP, and is associated with an increased metastatic propensity to bone [149]. Moreover, inhibition of a collagen receptor, discoidin domain receptor-1(DDR1), has been shown to reduce the homing and colonization of lung cancer cells to bone [150].
Thirdly, the high mineralization content of bone gives rise to its rigidity. Bone tropic cancer cells can benefit from the high stiffness by enhancing the production of PTHrP and the response of Fyn kinase to a rigid matrix [151–153].
Fourthly, the bone environment is imbued with calcium ions. A high calcium level has long been proposed to activate pathways that promote the survival and metastatic capacity of tumor cells [66, 154–156]. Additionally, calcium plays a key role in osteolytic vicious cycle by stimulating the production of PTHrP in tumor cells [157].
Fifthly, the bone marrow is known to be a highly hypoxic environment. As a result, the overexpression of hypoxia induced factor 1α(HIF1α) was observed in two thirds of bone metastases [158]. Hypoxia is known to modulate multiple steps of bone metastasis, including the premetastatic niches, dormancy and osteolytic vicious cycles [37, 50, 159].
Lastly, the acidic environment of bone may be further exemplified by abnormal metabolic activities of metastatic cancer cells [160, 161]. An acidic milieu increases bone resorptive activity, which may contribute to the osteolytic vicious cycle [162].
The complex bone environment is selective for cancer cells with specific molecular traits. It is known that bone tropism is linked to sex steroid hormones and related pathways. Specifically, bone is the predominant metastatic site for estrogen receptor alpha (ERα) positive luminal breast cancer, whereas ERα negative basal-like breast cancer tends to metastasize to lungs and brains but less commonly to bones [14]. How such different organ-tropism develop in different subtypes of breast cancer is largely unknown. We showed in a previous study that Src hyperactivation is tied to latent bone metastasis by supporting cancer cell survival in the bone microenvironment [15]. The mutual activation between Src and ERα may provide one possible mechanism of the bone-tropism of ERα+ breast cancer [16, 17]. However, Src inhibitors, such as dasatinib, exhibited very limited clinical efficacies on heavily pre-treated metastatic breast cancer [18–20], suggesting that additional pathways may drive further metastasis progression and therapeutic resistance. By comparison, the role of androgen-androgen receptor (AR) axis is elusive in bone metastasis of prostate cancer. The metastases of prostate cancer are mainly androgen-independent but remain dependent on the activity of AR [21]. Meanwhile, activation of Src family kinases, which are also directly activated by AR, was found to correlate with the occurrence of metastases in hormone-independent prostate tumors [22], suggesting the potential involvement of AR-Src pathway in prostate cancer bone metastasis.
High-throughput profiling speeds up the discovery of genetic and epigenetic traits associated with bone metastasis [15, 23–28]. Intriguingly, many such traits appear to be also relevant for regulation of bone hemostasis. For instance, the bone metastatic subline of MDA-MB-231 breast cancer cells highly overexpress IL-11, which is produced by bone marrow stromal cells and osteoblastic cells to stimulate osteoclastogenesis [23, 29]. Human prostate cancer bone metastasis specimens and bone-tropic sublines of LNCaP cells show increased expression of bone related matrix proteins, including osteopontin (OPN), bone sialoprotein (BSP) and osteocalcin (OC) [30, 31]. Overexpression of BSP in brain-tropic subline of MDA-MB-231 cells could re-direct them to form metastases in the bone [32]. Thus, bone tropism may be related to the capacity of tumor cells expressing such bone related factors, known as osteomimicry [30, 33]. In addition to osteomimetic properties intrinsic to tumor cells, other components of primary tumors may also display bone stromal cell characteristics and therefore influence the selection of bone metastatic seeds. For instance, mesenchymal stromal/stem cells (MSCs) and cancer associated fibroblasts in breast tumors create a bone marrow-like environment through secreting C-X-C Motif Chemokine Ligand 12 (CXCL-12) and IGF1 [34]. The Src-hyperactive bone metastatic seeds are therefore pre-selected from the heterogenous tumor mass because of their survival advantage conferred by enhanced PI3K-AKT activity in this bone marrow-like environment [34]. This mechanism, termed ‘metastasis seed-preselection’, may help explain why organ-specific metastatic outcome is associated with some gene expression features of primary tumors [34, 35].
It is now clear that the distant organs could be remotely conditioned by the primary tumors ahead of metastatic spread. The tumor-derived factors and extracellular vesicles prepare a ‘fertile soil’ for future metastasis, which is now commonly termed as ‘pre-metastatic niche’ [36]. A special pre-metastatic niche mechanism was recently described in the context of bone colonization of ERα-breast cancer cells. Activation of HIF1α by the hypoxic environment in primary tumors stimulates the secretion of lysyl oxidise (LOX), thereby enhancing the bone resorptive activity of osteoclasts and creating a favorable, osteolytic lesion for arriving tumor cells [37]. In another study, Engblom et al found that lung cancer leads to increased osteoblastic activity and bone mass, even without the presence of bone metastases in mouse model and human patients [38]. This in turn triggers the production and infiltration of a specific population of tumor-promoting neutrophils into the primary tumors [38]. It remains to be determined if such increase of bone mass could be observed in other types of cancer and whether it contributes to bone metastasis. Organ tropism can also arise through extracellular vesicles or exosomes shed from primary tumors. The metastatic distribution of melanoma cells to bones was increased in mice pre-treated with exosomes derived from highly metastatic melanoma cells as compared to mice treated with exosomes from poorly metastatic melanoma cells, suggesting that tumor derived exosomes may educate bone environment to facilitate later metastatic colonization [39]. Furthermore, exosomes with distinct expression of specific integrins may target certain organs, where they mediate organ-specific development of the pre-metastatic niche, yet the exosomes specifically targeting bones have not been characterized so far [40].
The Course of Bone Colonization
Compared to the metastatic diseases in other organs, bone metastases appear to follow a somewhat unique course. Firstly, the dissemination of tumor cells to bone marrow can be an early event, even preceding the formation of invasive primary tumors. In mouse models, cytokeratin-positive epithelial tumor cells were detected in the bone marrow in the pre-malignant phase of breast tumors [41–43]. This was further supported by the presence of disseminated tumor cells (DTCs) in the bone marrow of patients with ductal carcinoma in situ (DCIS) and pathologically localized prostate cancer [41, 44, 45]. Secondly, DTCs are present in the bone marrow of patients with various types of cancers, including ovarian, gastric and colorectal cancers [46–48], yet clinically overt bone metastases were rarely observed in some of these cancer types [2], suggesting that dissemination to bone may not be sufficient for the onset of bone metastases. Lastly, despite their early dissemination, a significant proportion of overt bone metastases are detected very late, even decades after the diagnosis of primary tumors [9, 10, 15]. This is in contrast to the relatively fast progressing visceral metastases, and suggests a long dormancy period in bone metastases.
Clinical evidence and experimental models support that DTCs may remain in a quiescent state for years in the bone marrow [49]. This cellular dormancy is characterized by cell cycle arrest at G0 phase and a lack of proliferating markers [49, 50]. Multiple pathways have been implicated in regulating quiescence of DTCs, such as PI3K-AKT signaling, TGFβ2-p38 axis, hypoxia signaling, BMPs and WNT family [50–54]. Notably, DTCs also express surface markers of stem cell-like population, such as CD44, EpCAM and ALDH1 [55–57]. Moreover, DTCs are resistant to the adjuvant therapies [58–60]. These suggest that DTCs share common features with CSCs.
The dormant DTCs could re-enter cell cycle and form multi-cellular micrometastases when the microenvironment becomes growth permissive. At this stage, although the tumor cells may be dividing, tumor mass could be steady or only slowly growing due to a balance between cell proliferation and apoptosis [49, 50]. Hurdles for tumor expansion may include active immunosurveillance and lack of angiogenic support [49, 50]. This clinically undetectable stage of metastasis was less characterized until recently. Several molecules and pathways have been identified by us and other groups to play important roles at this stage of bone metastasis, including VCAM-1-integrins, adhesion and gap junction proteins, IRF7, MSK1, and nephronectin [61–66]. For instance, we found that bone micrometastases predominantly reside in an osteogenic niche [62]. Tumor cells utilize E-cadherin to interact with N-cadherin on niche cells, and perturbation of this heterotypic junction can block niche-conferred growth advantage [62].
The more advanced stages of bone metastasis have been extensively studied, which involves the accelerating positive feedback between tumor and bone environment. Different molecular mechanisms drive the two different types of bone metastasis, osteoblastic (bone-forming) metastasis and osteolytic (bone-resorbing) metastasis (Figure 2). While prostate cancer predominantly generates osteoblastic lesions, metastases from breast cancer, multiple myeloma and lung cancer mostly result in osteolytic lesions in bone [1, 10, 67]. Despite the distinction, most bone metastases are mixtures of both types and exhibit simultaneously enhanced osteoclast and osteoblast activities [1, 10, 67], probably due to the tight coupling between osteoclast and osteoblast functions.
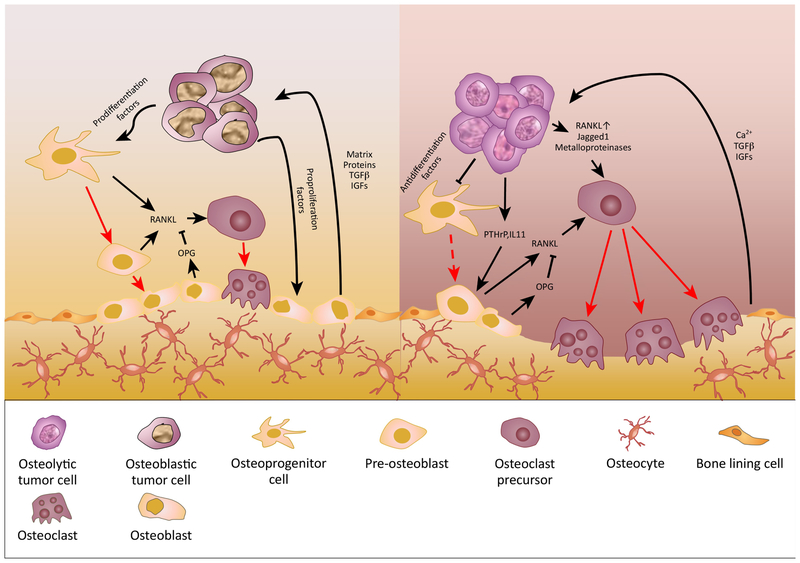
Figure 2. Mechanism of Osteoblastic and Osteolytic Metastasis.
Both osteoblastic and osteolytic metastasis involve the interactions between tumor cells, osteoblasts, and osteoclasts. Tumor cells directly and indirectly alter the balance between RANK ligand (RANKL) and its antagonist osteoprotegerin (OPG), which has profound effects on bone homeostasis. In osteoblastic lesions, tumor cells secrete cytokines to promote osteoprogenitor cell recruitment and differentiation, as well as osteoblast proliferation. The activated osteoblasts may create a tumor favorable environment by producing bone matrix proteins and growth factors. Due to the coupling of osteoblast and osteoclast activities, osteoclasts are also stimulated in osteoblastic lesions. However, the overall bone resorption rate is lower than that of bone formation, possibly due to a relatively low ratio of RANKL to OPG in the environment. Thus, the net effect on bone is an abnormal increase in bone mass. On the other hand, osteolytic tumor cells secrete osteolytic factors such as PTHrP and IL-11, which induces osteoblast production of RANKL, therefore promoting osteoclastogenesis. Osteolytic tumor cells can also directly activate osteoclasts through expressing RANKL, Jagged1, and metalloproteinases. Increased osteoclastic activity leads to bone destruction, and releases growth factors and calcium from the bone matrix, which in turn support the expansion of tumor cells. In multiple myeloma, tumor secretions can also inhibit the differentiation of osteoprogenitor cells and contribute to the reduced bone formation.
Overt metastases at distant organs are often incurable because of therapeutic resistance [68]. This resistance might arise in much earlier stages of colonization. Several studies have suggested that DTCs could persist after systemic adjuvant treatments [58–60]. Importantly, the presence of DTCs in bone marrow not only predicts skeletal metastasis but also metastases in other organs [69–72]. As the primary tumors and affected lymph nodes are mostly resected after diagnosis, these persisting minimal residue disease in bone might be the major source of seeds fueling metastases to distant sites. This idea is supported by the following evidence. First, metastatic tumor cells can be recirculated and colonize second distant organs in mouse models [73, 74]. Second, in the clinic, many patients die of metastases at multiple organs rather than single site metastasis [75]. Third, whole genomic sequencing of multiple metastases from the same cancer patient indicate the seeding of bone metastasis to other metastatic sites [76–78]. However, many questions remain unanswered. For instance, what proportion of metastatic lesions are seeded from other metastases? Does such metastasis-to-metastasis spreading utilize distinct mechanisms from primary tumor-to-metastasis seeding? Can we specifically target this process? The answers to these questions will inform the development of new therapeutic strategies against metastatic spreading, especially tertiary metastasis from bone to other visceral organs, as bone-only single site metastasis is usually a confined disease and associated with better survival as compared to metastases in visceral organs [79].
Metastatic Bone Marrow Niches
Bone metastases occur most frequently in the axial skeletons, including the skull, the rib cage and the spine. These sites are also where hematopoiesis mainly occurs [2]. Normal hematopoiesis is sustained by hematopoietic stem cells (HSCs), which reside mainly in specialized bone marrow niches (Box 2) [80, 81]. HSC niches regulate the long-term quiescence, self-renewal and differentiation of residing HSCs. DTCs may compete with HSCs for niche support [82], and therefore, may be regulated by the niche in a similar fashion. Moreover, DTCs utilize similar molecules as HSCs to interact with niche components, such as CXCL12-CXCR4, parathyroid hormone-related protein (PTHrP)-receptor, Jagged1-Notch, and heterotypic adherens junctions [62,67,83–85], suggesting that the knowledge of HSC niches may be applied to metastatic niches for tumor cells. In the following sections we will discuss the candidate niche cells and how they contribute to bone metastasis (Figure 3, Key Figure).
Box 2. Hematopoietic Stem Cell Niches in Bone Marrow.
The mapping of the HSC niche represents an area of ongoing active research. Two anatomically different HSC niches have been proposed based on their location in the bone, namely the endosteal niche and the perivascular niche [80, 81].
The endosteal niche (or osteoblastic niche) is immediately adjacent to the osteoblastic lining cells of the inner bone surface. In an early study, Nilsson et al demonstrated that ex vivo labelled HSCs preferentially seed in the endosteal region after being transplanted into mice [163]. Increase in the number and activity of osteoblasts through genetic modulation of PTH/PTHrP receptors or BMP signaling was reported to correlate with increased number of HSCs [164, 165]. Later, Ang-1, mainly produced by osteoblastic cells, was demonstrated to activate Tie2 on HSCs and enhance the quiescence and survival of HSCs by promoting tight adhesion between HSCs and osteoblasts [166]. However, the direct effects of endosteal niches in regulating HSCs have been challenged later by several studies showing that manipulations of the adhesion molecules either on HSCs or osteoblasts does not affect HSC numbers [167–169].
One the other hand, in a landmark study, Kiel et al proposed that HSCs are localized to the perivascular niche [170]. Subsequent studies suggested that these perivascular HSCs may reside next to sinusoidal blood vessels (the perisinusoidal niche) or arterioles (the periarteriolar niche) [171–176]. In both niches, endothelial cells support HSCs through direct cell-cell contact and paracrine factors, including E-selectin, Notch, CXCL12 and SCF [172–174, 177, 178]. Other perivascular stromal cells broadly defined as MSCs also play a role [171, 172, 179, 180]. Specifically in the perisinusoidal niche, MSCs expressing various markers have been shown in mouse models to support HSC maintenance, including CXCL12-abundant reticular (CAR) cells, Nes-GFP dim, LepR-Cre+ or Prx-1-Cre+ cells [174]. Notably, some of these marker-defined cell populations are overlapping [181]. For example, CXCL12-GFP+ cells are also targeted by Prx-1-Cre and about 80% of LepR-Cre+ cells are also Nes-GFP+ [182]. In the periarteriolar niche, HSCs are regulated by Nes-GFP bright, NG2-Cre+ MSCs, and conditional depletion of NG2-Cre+ cells leads to the exhaustion of HSCs [175].
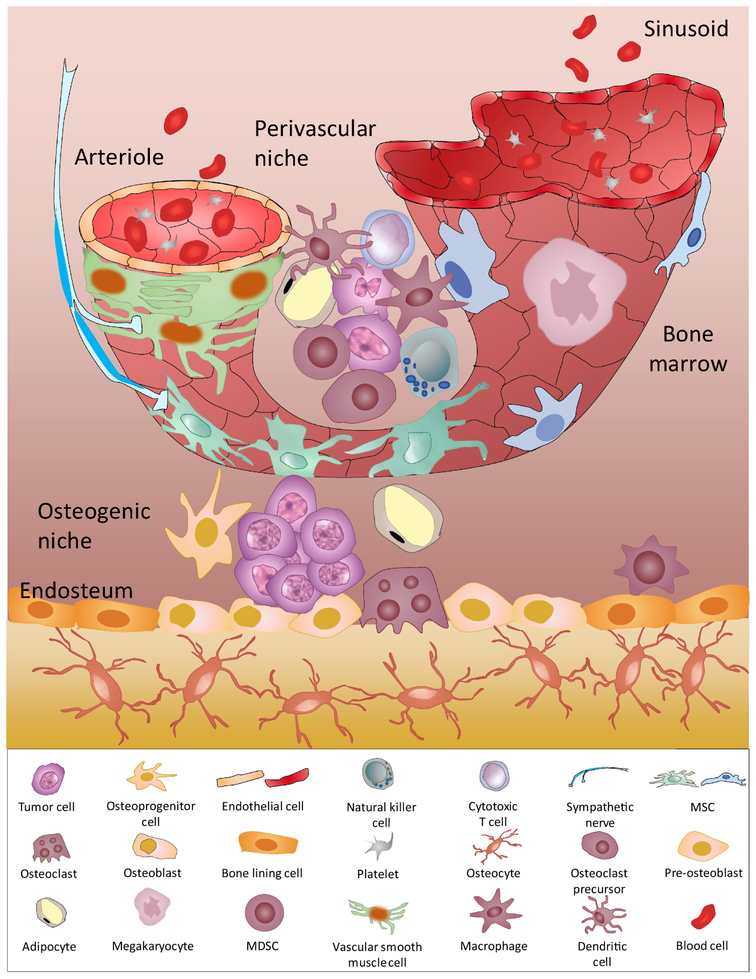
Figure 3, Key Figure Bone Marrow Niches for Metastatic Tumor Cells.
Generally, two different bone marrow niches may host tumor cells. Osteogenic niches (or endosteal niches) are adjacent to the endosteum, which are comprised mainly of osteoblastic cells. Our work suggested that osteogenic cells establish physical connection with residing tumor cells through heterotypic adheren junctions and gap junctions. This interaction activates the mTOR pathway in cancer cells and triggers calcium influx from niche cells, which promotes cancer cell survival and proliferation. By contrast, the perivascular niche is proposed to be a dormancy permissive niche. TSP-1 from endothelial cells maintains tumor cells in a dormant status. Other stromal cells in the perivascular niche are highly heterogenous, including MSCs expressing NG2+, Nes-GFP+, LepR+, or CXCL12 abundant reticular cells (CAR). As such, the perivascular niches may exert complex effects on residing tumor cells. Given that the endosteum is also highly vascularized, it is plausible that osteogenic niches and perivascular niches may spatially overlap in the endosteal region. In addition, MSCs can undergo osteogenic differentiation to generate osteoblastic lineage in vivo. Other cells types found in the bone marrow, including lymphocytes, MDSCs, macrophages, adipocytes, osteoclasts, megakaryocytes, and sympathetic nerves, can directly and indirectly participate in these two niches to orchestrate the progression of bone metastasis.
The endothelium is the first cellular barrier that circulating tumor cells may encounter in the bone marrow. Tumor cells must arrest in blood vessels, adhere to the endothelium, and extravasate into the parenchyma of bone marrow. In vitro cell adhesion experiments showed that bone tropic tumor cells preferentially adhere to bone marrow endothelium as compared to endothelial linings from other organs, suggesting the initial landing of tumor cells in bone is not a completely stochastic event [86]. By intravital imaging, the engraftment of fluorescently tagged tumor cells into bone marrow was found to occur at a specialized perisinusoidal region, which expresses E-Selectin and CXCL12 [87]. Extravasation may not be a rate-limiting step due to the discontinuous and permeable nature of bone marrow endothelium [88]. After extravasation, the tumor cells may remain adjacent to endothelial cells, and be kept dormant by the perisinusoidal environment [89]. Ghajar et al demonstrated that the dormancy of perivascular mammary tumor cells is induced by TSP1 secreted by endothelial cells [90]. A recent study suggests that endothelial cells may transdifferentiate into osteoblasts in prostate cancer [91], adding further complexity to the role of endothelial cells in maintaining dormancy.
While residing in the perivascular niche, tumor cells may also be influenced by other perivascular stromal cells, specifically MSCs. The roles of MSCs in bone metastasis are debatable. MSCs have been demonstrated to facilitate tumor cells extravasation into bone marrow via secreting CXCL12 [92, 93]. However, a subpopulation of MSCs express both endothelial and pericyte markers, and was shown to suppress the homing of cancer cells to bone [94]. While several studies showed that tumor cells may enter a quiescent state when co-coculture with bone marrow derived MSCs [95–99], the proliferation-promoting effects of MSCs were also reported [62, 100–102]. The seemingly contradictory roles of MSCs in bone metastasis may reflect the phenotypic heterogeneity of this cell population, as discussed in the context of perivascular niches for HSCs (Box 2).
The central role of osteoblasts in bone metastasis has been extensively discussed. For instance, prostate cancer cells have been demonstrated to preferentially seed to the osteoblast-rich area in bone [103]. The increased secretion of growth factors by prostate tumor cells may promote recruitment, proliferation, and differentiation of osteoprogenitor cells, leading to osteoblastic metastasis [67]. Tumor-entrained pre-osteoblasts were shown to promote prostate cancer cells growth in a non-contact co-culture system [104]. For breast cancer, our work demonstrated that osteogenic cells form a growth supportive niche for ERα+ breast cancer cells in early stage of bone metastasis [62, 66]. The interaction between osteogenic niches and residing tumor cells is mediated through adherens and gap junctions, which confer survival advantage to tumor cells via activation of mTOR signaling and the calcium influx from niche cells [62, 66]. Osteoblasts also play a role in osteolytic bone lesions. Bone-residing tumor cells secrete factors such as PTHrP and IL-11, which induce RANKL secretion and reduce OPG production in osteoblastic cells [8–10]. These molecular changes subsequently stimulate osteoclast development, drive the osteolytic vicious cycle, and promote bone resorption and tumor growth [10]. Interestingly, despite these above studies, there is also evidence suggesting an opposite role of osteoblasts in promoting dormancy, rather than progression. By intravital imaging, dormant myeloma cells were found to directly contact the endosteal surface, which suggests osteoblastic niches may be dormant niches in the context of myeloma [105]. Similarly, the secretome of osteoblastic cells induced quiescence of DTCs in prostate cancer models [106, 107]. These inconsistent conclusions may reflect the complicated natures of the seed-soil interaction. On one hand, cancer cells that contact osteoblasts using different mechanisms (e.g., adherens junctions vs. focal adhesion) may activate different intrinsic signals. On the other hand, osteogenic cells in variable functional and differentiation statuses may exert different influence on the microenvironment. Thus, the precise effects of microenvironment niche need to be investigated in vivo at a single-cell resolution and in models that recapitulate relevant disease features (e.g., the estrogen receptor status of breast cancer) in the clinic.
Osteoclasts are a main force in remodeling the bone environment. In osteolytic lesions, osteoclasts are highly stimulated by cancer cells and osteoblasts to resorb the bone matrix. This releases deposited growth factors in bone, which in turn support the growth of tumor cells and osteoblasts. Therefore, a positive feedback is established leading to osteolytic vicious cycle [10]. Interestingly, the activity of osteoclasts is also increased in osteoblastic metastasis, suggesting the tight functional connection between osteoblasts and osteoclasts [108]. In multiple myeloma, osteoclasts were reported to remodel the endosteal niches and thereby activate the residing dormant tumor cells [105]. Osteoclast precursors have been shown to share similar markers with myeloid-derived suppressor cells (MDSCs), and exert T-cells suppressive activity in a murine model of rheumatoid arthritis [109, 110]. An et al demonstrated that osteoclasts attenuates T-cell-mediated cytotoxicity via upregulation of a series of immune checkpoint molecules in myeloma [111]. Thus, osteoclasts may play multiple roles other than bone remodeling in the context of bone metastasis.
Immune cells are another major cell population in the bone marrow. Compared to the extensively studied primary tumors, much less is known about the immune landscape in bone metastasis. Since the bone marrow is composed of a wide variety of immune cells [112], both anti-tumor and pro-tumor immune cells may participate in or influence the bone metastatic niche. Active CD4, CD8, Natural Killer T (NKT) cells, and Natural Killer (NK) cells may all contribute to the immune surveillance and eradication of bone metastasis [63, 113–115]. Depletion of T cells and NK cells led to accelerate bone metastasis in a murine breast cancer model [63]. In contrast, active CD4 T cells may also have pro-metastasis roles. They were shown to promote osteoclastogenesis and induce pre-metastatic osteolytic lesions, which may facilitate the colonization of myeloma and breast cancer cells in bone [116, 117]. In addition, regulatory T cells can suppress osteoclast formation as well as cytotoxic T cell function, and therefore, contribute to immune evasion and increased bone deposition in prostate cancer bone metastasis [118, 119].
Myeloid cells mostly assume immunosuppressive roles in the cancer setting. MDSCs are a heterogenous group of immunosuppressive cells. They include immature monocytic cells, neutrophil, and dendritic cells (DCs) [112, 120], and constitute up to 20–30% of all bone marrow stromal cells [112]. The effects of MDSCs on lymphocyte effector were mainly investigated in primary tumors. Their involvement in the bone marrow niche was also observed in multiple myeloma [121]. Besides the immunosuppressive function, MDSCs may be able to differentiate into osteoclasts, which may subsequently promote bone loss in bone metastasis [122, 123]. Plasmacytoid dendritic cells (pDCs) were found to be increased with bone metastasis in a breast cancer model, and depletion of this population could ameliorate bone loss and suppress tumor growth in bone [124]. In addition to MDSCs, macrophages represent another major myeloid cell population. In prostate cancer patients as well as mouse models, CD206+ M2-like macrophages were observed to be enriched in the growing metastatic lesions in bone [125, 126]. Conditional genetic depletion of tissue-resident macrophages reduced tumor burden in bone in mice carrying prostate tumors [126]. In summary, the diversity of immune niches is just beginning to be appreciated, and accordingly, systemic investigation is required to elucidate the link between immune system and bone homeostasis as well as bone metastasis.
Other cell types have also been implicated in regulating bone metastasis. For instance, the activation of sympathetic nerve is accompanied by increased infiltration of M2-macrophages in primary tumors and thereby promote metastasis at distant organs including bones [127]. In addition, the sympathetic nervous system has also been suggested to stimulate bone marrow stromal cells and promote breast cancer bone metastasis in mouse. Increasing evidence also shows that bone marrow adipocytes can attract and interact with metastatic tumor cells, and provide an alternative source of growth factors and energy need supporting metastatic growth [128]. When co-cultured with human bone tissues ex vivo, breast cancer cells were observed to preferentially colonize into the adipose tissue compartment [129]. On the other hand, platelets were demonstrated to be critical in the preparation of pre-metastatic niches, however less study has been reported in the context of bone metastasis [36]. Targeting platelet aggregation by inhibiting activated integrin αllBβ3 significantly prevent the onset and later progression of bone metastasis in mouse models [12, 130]. Platelets not only protect circulating tumor cells from immune clearance, but also promote the osteolytic vicious cycle in bone [131]. The lysophosphatidic acid derived from platelets was shown to promote skeletal tumor growth and bone resorption by stimulating pro-osteoclast cytokines [130]. In contrast, their precursor cells, megakaryocytes, have been reported to correlate with increased bone mass and negatively associated with skeletal metastasis in murine prostate metastasis model [132]. In a recent study, increased number of megakaryocytes was found to be associated with the metastatic growth of breast cancer cells in bone in both mouse models and human specimens [133]. However, mice with deficient megakaryocytes developed more aggressive bone metastasis as compared to the wild type hosts, suggesting that the increase of megakaryocytes in bone marrow could be a protective mechanism against bone metastasis in breast cancer [133].
Current Limitations
The interaction between tumor cells and bone cells is crucial for the successful colonization of tumor cells in bone. Bone marrow niches not only provide a ‘sanctuary’ for tumor cells to escape adjuvant therapies but also serve as a ‘cradle’ of evil seeds to fuel local and distant relapse. A lot remains unknown (see outstanding questions) about the bone marrow niches for skeletal metastasis due to several challenges. First, the current preclinical models for bone metastasis are far from ideal. Few models can faithfully recapitulate the natural progress of bone metastasis, particularly the extended latency observed in patients, which poses a major limitation and hinders the translational application of preclinical research. In addition, more clinically relevant models, like syngeneic mouse models with hormone responsive tumors, are required for future study of immune regulation of bone metastasis. Besides better models, new approaches are required to capture the interaction between tumor cells and host cells at the single cell level. Although many cell types may contribute to bone colonization, we need to identify the key ones that directly promote progression and are therapeutically targetable. That requires a much more refined and precise map of where the different niches are located, and their spatial relationship with bone metastases at different stages. The studies on HSCs provide us with a complex map of bone marrow niches (Box 3). Moreover, the map of bone marrow niches is dynamic. The bone constantly undergoes turnover, which can be influenced by many processes including menopause, aging, and drug treatments. It is likely that the metastatic niches evolve during these events, which in turn alter the fate of resident tumor cells. For instance, the decrease in estrogen level either due to menopause or endocrine therapies could significantly enhance osteoclast-mediated bone resorption by increasing the expression of RANKL while reducing the expression of OPG [134]. Src inhibitor dasatinib was shown to accelerate the differentiation of MSCs into osteoblasts but inhibit osteoclast activity [135, 136]. Chemotherapy agents induce Jagged1 expression in osteoblastic lineage through ROS pathway, and subsequently promote the seeding of cancer cells to bone and their resistance to chemotherapy [84]. Finally, a combination of single cell sequencing, lineage tracing and cutting-edge microscopy may help us further refine this map and capture this complex and dynamic interaction between cancer cells and various niches.
Outstanding Questions.
What are the mechanisms underlying the homing of DTCs into a specific niche? Is it a stochastic process or a selective process for tumor cells with specific traits?
Are there different niches for different metastatic seeds? Do different niches carry out different functions during bone metastasis? How do niche components co-evolve with the residing tumor cells?
How will systemic changes, such as the obesity, aging, bone-modifying agents (e.g., bisphosphonates) and cancer treatments (e.g., endocrine therapies and chemotherapies), influence the niches? Can these conditions lead to redistribution of tumor cells among different niches, and consequently alter the kinetics of bone colonization?
Can cancer cells migrate from one niche to another and thereby change their fates? If so, what are the underlying mechanisms?
Do bone marrow niches educate residing tumor cells and promote further dissemination?
What therapeutic opportunities can we get from a better understanding of the interaction between tumor cells and the bone marrow niches in bone metastasis?
Box 3. The Complexity of Hematopoietic Stem Cell Niches.
The complexity of HSC niches is under active investigation.
Firstly, more bone marrow stromal cells are recently implicated to either directly or indirectly regulate HSCs, including osteoclasts, macrophages, adipocytes, megakaryocytes, and sympathetic nerves [183–187].
Secondly, recent studies have revealed the heterogenous nature of bone marrow niches. For instance, HSCS have different reactive oxygen species (ROS) lvels at arteriolar and sinusoidal sites due to the different vascular permeability, which in turn regulates their migration, differentiation and survival [188]. Moreover, endothelial cells at perisinusoidal and periarteriolar niches differ in their expression of surface markers, their secretion of niche factors as well as their functional contribution to HSC niches [189]. Interestingly, the same cytokine factors also have different contributions to HSC maintenances at different perivascular sites [190]. For example, selectively ablation of CXCL12 from arteriolar NG2-Cre+ cells, but not from sinusoidal LepR-Cre+ cells, reduces HSC numbers [190].
Thirdly, the niches and residing HSCs are not static. Endothelial cells are shown to remodel to surround HSCs after their arrival at the perivascular niches [191]. MSCs, which are close to the endothelial pocket, were shown to orientate HSC division [191].
Lastly, the endosteal niches and perivascular niches may overlap to some extent. Perivascular MSCs are known to be precursors of osteoblastic cells, and importantly, the endosteal surface is also highly vascularized, suggesting that the endosteal niche and perivascular niche may be a common niche. This can be supported by a recent study showing that the endosteal but not central bone marrow vasculatures were degraded and correlated with the loss of HSCs in acute myeloid leukemia model [192]. Taken together, all the above suggest the dynamic and complex nature of HSC niches.
Concluding Remarks
Bone metastasis is currently still incurable. Targeting the tumor-niche interaction and the niche components represents a promising direction of future therapeutic strategies to eliminate minimal residue disease and to prevent overt metastasis. For instance, the therapeutic antibody against Jagged1 on tumor cells and osteoblastic niches was shown recently to significantly reduce bone metastasis and overcome niche-induced chemotherapeutic resistance [84]. Our recent work suggests that a short interlude of treatment with everolimus plus arsenic trioxide could diminish the survival advantage of DTCs conferred by osteogenic niches, and significantly prevent the long-latency bone metastasis in mice [66]. Pharmacological modulation of the metastatic ‘soil’ towards a normalized bone environment can also ameliorate bone metastasis. A good example here is the application of bisphosphonates in treating bone metastasis. Although Zoledronic acid plus current standard adjuvant care failed to show additional overall benefit in patients with early breast cancer, it reduced the frequency of bone metastasis and improved disease-free survival in postmenopausal patients or patients with low estrogen level [137–142]. In addition to potential anti-tumor effects, their well-known function is to inhibit the increased bone resorptive activity of osteoclasts [143]. Importantly, bisphosphonates can also repolarize M2-like tumor associated macrophages to M1-like antitumor phenotype and activate T cells [144, 145]. Given the tight link of bone and immune system, it is rational to test the combination of immune checkpoint therapy with current therapies in treating bone metastasis.
Highlights.
Hi Bone metastasis is determined by cancer cell-intrinsic traits as well as their interactions with the unique bone microenvironment.
The bone metastasis niche undergoes dynamic changes during different stages of bone colonization including dormancy, reactivation, and outgrowth.
Various cell types including osteoblasts, osteoclasts and immune cells reside in and regulate the bone metastasis niche by direct cell-cell contact and paracrine signaling.
Parallels with the hematopoietic stem cell (HSC) niche may aid our understanding of the bone metastasis niche.
Bone may harbor a reservoir of tumor cells for further metastatic dissemination.
Therapies targeting cancer-niche interactions may eradicate micrometastases and prevent overt metastases.
Acknowledgement
X.H.-F.Z. is supported by the US Department of Defense DAMD W81XWH-16–1–0073, NCI CA183878, the Breast Cancer Research Foundation, and the McNair Medical Institute. H.W. is supported in part by the US Department of Defense DAMD W81XWH-13-1-0296.
Glossary
- Bone tropism
the propensity of certain tumors, including prostate and breast cancer, to metastasize to the skeleton system.
- Cancer stem cell
a rare subset of cancer cells within tumors with self-renewal, differentiation, and in vivo tumor initiation capacities.
- Ductal carcinoma in situ
the earliest form of breast cancer. The cells have become malignant but have not spread out of the milk duct of the breast. It is considered as non-invasive disease.
- Disseminated tumor cells
tumor cells that have left the primary tumor, survived the circulation and finally landed on the parenchymal of distant organs.
- Epithelial-Mesenchymal transition
a conversion of polarized epithelial cells towards a mesenchymal phenotype, which is considered as the initiating step of metastasis. Tumor cells may lose cell-cell adhesion and gain migrative and invasive capacity during EMT.
- Minimal residue disease
refers to the persistent tumor cells in cancer patients without symptoms or signs of disease after systemic treatments, including circulating tumor cells and disseminated tumor cells.
- Hematopoietic stem cells
a population of progenitor cells that can differentiate into all types of blood and immune cells.
- Immunosurveillance
a process whereby the immune system identifies and clears foreign pathogens and pre-cancerous and cancerous host cells. Tumor cells must escape immunosurveillance to successfully colonize in distant organs.
- Mesenchymal stromal/stem cells
a population of multipotent stromal cells giving rise to the specialized cells in skeletal tissues, including osteoblasts, chondrocytes, fibroblasts, and adipocytes. They are commonly perivascularly located.
- Osteoblasts
bone cells originating from mesenchymal stem cells, which produce bone matrix and form new bones.
- Osteoclasts
a type of large multinucleate cells breaking down bone tissues. They belong to the myeloid lineage, and are derived from HSCs.
- Osteomimicry
a phenomenon of bone tropic tumor cells to express genes and to secret matrix proteins that commonly restrict to bone cells.
- Seed and Soil
a hypothesis raised by Stephen Paget to explain the metastatic preference to specific organs. The metastatic tumor cells (the seed) must interact with the distant organ microenvironment (the soil) to establish the successful colonization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest in this review.
References
- 1.Mundy GR (2002) Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s [DOI] [PubMed] [Google Scholar]
- 3.Obenauf AC and Massagué J (2015) Surviving at a Distance: Organ-Specific Metastasis. Trends in Cancer 1, 76–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massagué J and Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubendorf L et al. (2000) Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol 31, 578–583 [DOI] [PubMed] [Google Scholar]
- 6.Lee YN (Margaret. (1983) Breast carcinoma: Pattern of metastasis at autopsy. J. Surg. Oncol 23, 175–180 [DOI] [PubMed] [Google Scholar]
- 7.Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat. Rev. Cancer 3, 453–458 [DOI] [PubMed] [Google Scholar]
- 8.Kingsley LA et al. (2007) Molecular biology of bone metastasis. Mol. Cancer Ther. 6, 2609–17 [DOI] [PubMed] [Google Scholar]
- 9.Weilbaecher KN et al. (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11, 411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esposito M et al. (2018) The Biology of Bone Metastasis. Cold Spring Harb. Perspect. Med 8, a031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juárez P and Guise TA (2011) TGF-β in cancer and bone: Implications for treatment of bone metastases. Bone 48, 23–29 [DOI] [PubMed] [Google Scholar]
- 12.Bakewell SJ et al. (2003) Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc. Natl. Acad. Sci. U. S. A 100, 14205–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JJ et al. (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest 103, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennecke H et al. (2010) Metastatic behavior of breast cancer subtypes. J. Clin. Oncol 28,3271–3277 [DOI] [PubMed] [Google Scholar]
- 15.Zhang XHF et al. (2009) Latent Bone Metastasis in Breast Cancer Tied to Src-Dependent Survival Signals. Cancer Cell 16, 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W et al. (2001) Potentiation of Estrogen Receptor Activation Function 1 (AF-1) by Src/JNK through a Serine 118-Independent Pathway. Mol. Endocrinol 15, 32–45 [DOI] [PubMed] [Google Scholar]
- 17.Wong C-W et al. (2002) Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc. Natl. Acad. Sci. U. S. A 99, 14783–8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Finn R et al. (2009) Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059. Cancer Res. 69, 3118 [Google Scholar]
- 19.Herold CI et al. (2011) Phase II trial of dasatinib in patients with metastatic breast cancer using real-time pharmacodynamic tissue biomarkers of Src inhibition to escalate dosing. Clin. Cancer Res. 17, 6061–70 [DOI] [PubMed] [Google Scholar]
- 20.Morris PG et al. (2018) Phase II Study of Paclitaxel and Dasatinib in Metastatic Breast Cancer. Clin. Breast Cancer 18, 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman BJ and Feldman D (2001) The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1, 34–45 [DOI] [PubMed] [Google Scholar]
- 22.Tatarov O et al. (2009) Src Family Kinase Activity Is Up-Regulated in Hormone-Refractory Prostate Cancer. DOI: 10.1158/1078-0432.CCR-08-1857 [DOI] [PubMed] [Google Scholar]
- 23.Kang Y et al. (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 [DOI] [PubMed] [Google Scholar]
- 24.Cosphiadi I et al. (2018) Bone Metastasis in Advanced Breast Cancer: Analysis of Gene Expression Microarray. Clin. Breast Cancer 18, e1117–e1122 [DOI] [PubMed] [Google Scholar]
- 25.Savci-Heijink CD et al. (2016) A novel gene expression signature for bone metastasis in breast carcinomas. Breast Cancer Res. Treat 156, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luis-Ravelo D et al. (2013) A gene signature of bone metastatic colonization sensitizes for tumor-induced osteolysis and predicts survival in lung cancer. Oncogene 33, 5090–5099 [DOI] [PubMed] [Google Scholar]
- 27.Casimiro S et al. (2012) Analysis of a bone metastasis gene expression signature in patients with bone metastasis from solid tumors. Clin. Exp. Metastasis 29, 155–164 [DOI] [PubMed] [Google Scholar]
- 28.Xie L et al. (2013) Genome-Wide Identification of Bone Metastasis-Related MicroRNAs in Lung Adenocarcinoma by High-Throughput Sequencing. PLoS One 8, e61212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girasole G et al. (1994) Interleukin-1 1: A New Cytokine Critical for Osteoclast Developmentdihydroxyvita-min D3 * parathyroid hormone * interleukin-l * tumor necrosis factor * interleukin-6, 93 [Google Scholar]
- 30.Koeneman KS et al. (1999) Osteomimetic properties of prostate cancer cells: A hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 39, 246–261 [DOI] [PubMed] [Google Scholar]
- 31.Kruger TE et al. (2014) Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit. Rev. Oncol. Hematol 89, 330–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JH et al. Bone sialoprotein promotes bone metastasis of a non-bone-seeking clone of human breast cancer cells. Anticancer Res. 24, 1361–8 [PubMed] [Google Scholar]
- 33.Awolaran O et al. (2016) Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. The Breast 30, 156–171 [DOI] [PubMed] [Google Scholar]
- 34.Zhang XHF et al. (2013) Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smid M et al. (2006) Genes associated with breast cancer metastatic to bone. J. Clin. Oncol 24, 2261–7 [DOI] [PubMed] [Google Scholar]
- 36.Peinado H et al. (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 [DOI] [PubMed] [Google Scholar]
- 37.Cox TR et al. (2015) The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Engblom C et al. (2017) Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peinado H et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med 18, 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshino A et al. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hüsemann Y et al. (2008) Systemic Spread Is an Early Step in Breast Cancer. Cancer Cell 13, 58–68 [DOI] [PubMed] [Google Scholar]
- 42.Hosseini H et al. (2016) Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper KL et al. (2016) Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Köllermann J et al. (2008) Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J. Clin. Oncol 26, 4928–33 [DOI] [PubMed] [Google Scholar]
- 45.Jung Y-S et al. (2003) Clinical significance of bone marrow micrometastasis detected by nested rt-PCR for keratin-19 in breast cancer patients. Jpn. J. Clin. Oncol 33, 167–72 [DOI] [PubMed] [Google Scholar]
- 46.Juhl H et al. (1994) Immunocytological detection of micrometastatic cells: Comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal and pancreatic cancer patients. Int. J. Cancer 57, 330–335 [DOI] [PubMed] [Google Scholar]
- 47.Banys M et al. (2009) Disseminated Tumor Cells in Bone Marrow May Affect Prognosis of Patients With Gynecologic Malignancies. Int. J. Gynecol. Cancer 19, 948–952 [DOI] [PubMed] [Google Scholar]
- 48.Dardaei L et al. (2011) The detection of disseminated tumor cells in bone marrow and peripheral blood of gastric cancer patients by multimarker (CEA, CK20, TFF1 and MUC2) quantitative real-time PCR. Clin. Biochem 44, 325–330 [DOI] [PubMed] [Google Scholar]
- 49.Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sosa MS et al. (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bragado P et al. (2013) TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/p signalling. Nat. Cell Biol. 15, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carcereri de Prati A et al. (2017) Metastatic Breast Cancer Cells Enter Into Dormant State and Express Cancer Stem Cells Phenotype Under Chronic Hypoxia. J. Cell. Biochem 118, 3237–3248 [DOI] [PubMed] [Google Scholar]
- 53.Gao H et al. (2012) The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malladi S et al. (2016) Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 165, 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balic M et al. (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 12, 5615–21 [DOI] [PubMed] [Google Scholar]
- 56.Giordano A et al. (2013) Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann. Oncol 24, 2515–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantel K and Alix-Panabières C (2014) Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 3, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeyama H et al. (2017) Usefulness of CTC and DTC-BM Detection for Adjuvant Therapy Effects and Prognosis Prediction in Early Breast Carcinoma: Results of 8–11 Years of Follow-up Evaluation. Ann. Surg. Oncol 24, 1227–1233 [DOI] [PubMed] [Google Scholar]
- 59.Naume B et al. (2014) Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol 32, 3848–3857 [DOI] [PubMed] [Google Scholar]
- 60.Janni W et al. (2011) Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clin. Cancer Res. 17, 2967–76 [DOI] [PubMed] [Google Scholar]
- 61.Lu X et al. (2011) VCAM-1 Promotes Osteolytic Expansion of Indolent Bone Micrometastasis of Breast Cancer by Engaging α4β1-Positive Osteoclast Progenitors. Cancer Cell 20, 701–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H et al. (2015) The Osteogenic Niche Promotes Early-Stage Bone Colonization of Disseminated Breast Cancer Cells. Cancer Cell 27, 193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bidwell BN et al. (2012) Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med 18, 1224–1231 [DOI] [PubMed] [Google Scholar]
- 64.Gawrzak S et al. (2018) MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+breast cancer. Nat. Cell Biol. 20, 211–221 [DOI] [PubMed] [Google Scholar]
- 65.Wang D et al. (2018) NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche. J. Bone Oncol. 13, 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H et al. (2018) The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell 34, 823–839.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Logothetis CJ and Lin SH (2005) Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 5, 21–28 [DOI] [PubMed] [Google Scholar]
- 68.Greenberg PA et al. (1996) Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol 14, 2197–205 [DOI] [PubMed] [Google Scholar]
- 69.Braun S et al. (2005) A Pooled Analysis of Bone Marrow Micrometastasis in Breast Cancer. N. Engl. J. Med 353, 793–802 [DOI] [PubMed] [Google Scholar]
- 70.Wiedswang G et al. (2003) Detection of Isolated Tumor Cells in Bone Marrow Is an Independent Prognostic Factor in Breast Cancer Pathologist managed FNC out patient clinic View project Cytomegalovirus in cancer View project. Artic. J. Clin. Oncol DOI: 10.1200/JCO.2003.02.009 [DOI] [PubMed] [Google Scholar]
- 71.Ment Bidard F-C et al. (2008) Disseminated Tumor Cells of Breast Cancer Patients: A Strong Prognostic Factor for Distant and Local Relapse. DOI: 10.1158/1078-0432.CCR-07-4749 [DOI] [PubMed] [Google Scholar]
- 72.Domschke C et al. (2013) Prognostic Value of Disseminated Tumor Cells in the Bone Marrow of Patients with Operable Primary Breast Cancer: A Long-term Follow-up Study. Ann. Surg. Oncol 20, 1865–1871 [DOI] [PubMed] [Google Scholar]
- 73.Hoover HC and Ketcham AS (1975) Metastasis of metastases. Am. J. Surg 130, 405–411 [DOI] [PubMed] [Google Scholar]
- 74.Rashidi B et al. (2000) An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin. Cancer Res. 6, 2556–61 [PubMed] [Google Scholar]
- 75.Chen M-T et al. (2017) Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci. Rep 7, 9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gundem G et al. (2015) The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoadley KA et al. (2016) Tumor Evolution in Two Patients with Basal-like Breast Cancer: A Retrospective Genomics Study of Multiple Metastases. PLoS Med. 13, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ullah I et al. (2018) Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Invest 128, 1355–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sherry MM et al. (1986) Metastatic breast cancer confined to the skeletal system: An indolent disease. Am. J. Med 81, 381–386 [DOI] [PubMed] [Google Scholar]
- 80.Morrison SJ and Scadden DT (2014) The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L and Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiozawa Y et al. (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest 121, 1298–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taichman RS et al. (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62, 1832–7 [PubMed] [Google Scholar]
- 84.Zheng H et al. (2017) Therapeutic Antibody Targeting Tumor- and Osteoblastic Niche-Derived Jagged1 Sensitizes Bone Metastasis to Chemotherapy. Cancer Cell 32, 731–747.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirbe AC et al. (2007) Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone, [DOI] [PMC free article] [PubMed]
- 86.Lehr JE and Pienta KJ (1998) Preferential Adhesion of Prostate Cancer Cells to a Human Bone Marrow Endothelial Cell Line. JNCIJ. Natl. Cancer Inst. 90, 118–123 [DOI] [PubMed] [Google Scholar]
- 87.Sipkins DA et al. (2005) In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inoue S and Osmond DG (2001) Basement Membrane of Mouse Bone Marrow Sinusoids Shows Distinctive Structure and Proteoglycan Composition: A High Resolution Ultrastructural Study. DOI: 10.1002/ar.XXXX [DOI] [PubMed] [Google Scholar]
- 89.Price TT et al. (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med 8, 340ra73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghajar CM et al. (2013) The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin S-C et al. (2017) Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Dev. Cell 41, 467–480.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corcoran KE et al. (2008) Mesenchymal Stem Cells in Early Entry of Breast Cancer into Bone Marrow. PLoS One 3, e2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Correa D et al. (2016) Mesenchymal stem cells regulate melanoma cancer cells extravasation to bone and liver at their perivascular niche. Int. J. Cancer 138, 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rossnagl S et al. (2018) A Subpopulation of Stromal Cells Controls Cancer Cell Homing to the Bone Marrow. Cancer Res. 78, 129–142 [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y et al. (2009) Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 23, 925–933 [DOI] [PubMed] [Google Scholar]
- 96.Bliss SA et al. (2016) Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 76, 5832–5844 [DOI] [PubMed] [Google Scholar]
- 97.Lim PK et al. (2011) Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 71, 1550–60 [DOI] [PubMed] [Google Scholar]
- 98.Ono M et al. (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal 7, ra63 [DOI] [PubMed] [Google Scholar]
- 99.Bartosh TJ et al. (2016) Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc. Natl. Acad. Sci. U. S. A 113, E6447–E6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu W et al. (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp. Mol. Pathol 80, 267–274 [DOI] [PubMed] [Google Scholar]
- 101.Yan X et al. (2012) Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res. Treat 132, 153–164 [DOI] [PubMed] [Google Scholar]
- 102.Marlow R et al. (2013) A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 73, 6886–99 [DOI] [PubMed] [Google Scholar]
- 103.Wang N et al. (2014) Prostate Cancer Cells Preferentially Home to Osteoblast-rich Areas in the Early Stages of Bone Metastasis: Evidence From In Vivo Models. J. Bone Miner. Res 29, 2688–2696 [DOI] [PubMed] [Google Scholar]
- 104.Probert C et al. (2018) Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene DOI: 10.1038/s41388-018-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lawson MA et al. (2015) Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun 6, 8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu-Lee L-Y et al. (2018) Osteoblast-Secreted Factors Mediate Dormancy of Metastatic Prostate Cancer in the Bone via Activation of the TGFβRIII-p38MAPK-pS249/T252RB Pathway. Cancer Res. 78, 2911–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yumoto K et al. (2016) Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci. Rep 6, 36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keller ET and Brown J (2004) Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell. Biochem 91, 718–729 [DOI] [PubMed] [Google Scholar]
- 109.Charles JF et al. (2012) Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Invest 122, 4592–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Charles JF and Aliprantis AO (2014) Osteoclasts: more than ‘bone eaters.’ Trends Mol. Med 20, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.An G et al. (2016) Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 128, 1590–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao E et al. (2012) Bone marrow and the control of immunity. Cell. Mol. Immunol 9, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feuerer M et al. (2001) Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer 92, 96–105 [PubMed] [Google Scholar]
- 114.Zhang K et al. (2011) CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer Res. 71, 4799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lode HN et al. (1998) Natural Killer Cell-Mediated Eradication of Neuroblastoma Metastases to Bone Marrow by Targeted Interleukin-2 Therapy, 91 [PubMed] [Google Scholar]
- 116.Colucci S et al. (2004) T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. DOI: 10.1182/blood-2004-02-0474 [DOI] [PubMed] [Google Scholar]
- 117.Monteiro AC et al. (2013) T Cells Induce Pre-Metastatic Osteolytic Disease and Help Bone Metastases Establishment in a Mouse Model of Metastatic Breast Cancer. PLoS One 8, e68171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zaiss MM et al. (2007) Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. 56, 4104–4112 [DOI] [PubMed] [Google Scholar]
- 119.Zhao E et al. (2012) Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 1, 152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Youn J-I et al. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol 181, 5791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramachandran IR et al. (2013) Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J. Immunol 190, 3815–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhuang J et al. (2012) Osteoclasts in Multiple Myeloma Are Derived from Gr-1+CD11b+Myeloid-Derived Suppressor Cells. PLoS One 7, e48871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sawant A et al. (2013) Myeloid-Derived Suppressor Cells Function as Novel Osteoclast Progenitors Enhancing Bone Loss in Breast Cancer. DOI: 10.1158/0008-5472.CAN-12-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sawant A et al. (2012) Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol 189, 4258–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roca H et al. (2009) CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem 284, 34342–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soki FN et al. (2015) Bone marrow macrophages support prostate cancer growth in bone, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sloan EK et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morris EV and Edwards CM (2016) The role of bone marrow adipocytes in bone metastasis. DOI: 10.1016/j.jbo.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Templeton ZS et al. (2015) Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia 17, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boucharaba A et al. (2004) Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Invest 114, 1714–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leblanc R and Peyruchaud O (2016) Metastasis: new functional implications of platelets and megakaryocytes. Blood 128, 24–31 [DOI] [PubMed] [Google Scholar]
- 132.Li X et al. Inhibitory Effects of Megakaryocytic Cells in Prostate Cancer Skeletal Metastasis. DOI: 10.1002/jbmr.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jackson W et al. (2017) Role of Megakaryocytes in Breast Cancer Metastasis to Bone. Cancer Res. 77, 1942–1954 [DOI] [PubMed] [Google Scholar]
- 134.Hadji P et al. (2012) The impact of menopause on bone, zoledronic acid, and implications for breast cancer growth and metastasis. Ann. Oncol 23, 2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Id Boufker H et al. (2010) The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer 10, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vandyke K et al. (2010) The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J. Bone Miner. Res 25, 1759–1770 [DOI] [PubMed] [Google Scholar]
- 137.Coleman R et al. (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet. Oncol 15, 997–1006 [DOI] [PubMed] [Google Scholar]
- 138.Coleman RE et al. (2011) Breast-Cancer Adjuvant Therapy with Zoledronic Acid. N. Engl. J. Med 365, 1396–1405 [DOI] [PubMed] [Google Scholar]
- 139.Gnant M et al. (2015) Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol 26, 313–320 [DOI] [PubMed] [Google Scholar]
- 140.Gnant M et al. (2011) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. www.thelancet.com/oncology 12, 631–672 [DOI] [PubMed] [Google Scholar]
- 141.Coleman R et al. (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann. Oncol 24, 398–405 [DOI] [PubMed] [Google Scholar]
- 142.Eidtmann H et al. (2010) Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann. Oncol 21, 2188–2194 [DOI] [PubMed] [Google Scholar]
- 143.Roelofs AJ et al. (2006) Molecular Mechanisms of Action of Bisphosphonates: Current Status. DOI: 10.1158/1078-0432.CCR-06-0843 [DOI] [PubMed] [Google Scholar]
- 144.Coscia M et al. (2010) Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J. Cell. Mol. Med 14,2803–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santini D et al. (2009) In vivo effects of zoledronic acid on peripheral yS T lymphocytes in early breast cancer patients. Cancer Immunol. Immunother 58, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morgan MP et al. (2001) Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol. Carcinog 32, 111–117 [DOI] [PubMed] [Google Scholar]
- 147.Pathi SP et al. (2010) A Novel 3-D Mineralized Tumor Model to Study Breast Cancer Bone Metastasis. PLoS One 5, e8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He F et al. (2017) Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis. Proc. Natl. Acad. Sci. U. S. A 114, 10542–10547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.PÉCHEUR I et al. (2002) Integrin αvβ3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 16, 1266–1268 [DOI] [PubMed] [Google Scholar]
- 150.Valencia K et al. (2012) Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin. Cancer Res. 18, 969–80 [DOI] [PubMed] [Google Scholar]
- 151.Kostic A et al. (2009) Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruppender NS et al. (2010) Matrix rigidity induces osteolytic gene expression of metastatic breast cancer cells. PLoS One 5, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Page JM et al. (2015) Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin p3 and TGF-β receptor type II. Biomaterials 64, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao J et al. (2006) Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 66, 9065–9073 [DOI] [PubMed] [Google Scholar]
- 155.Saidak Z et al. (2009) Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 315, 2072–2080 [DOI] [PubMed] [Google Scholar]
- 156.Joeckel E et al. (2014) High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol. Cancer 13, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanders JL et al. (2000) Extracellular Calcium-Sensing Receptor Expression and Its Potential Role in Regulating Parathyroid Hormone-Related Peptide Secretion in Human Breast Cancer Cell Lines1. Endocrinology 141, 4357–4364 [DOI] [PubMed] [Google Scholar]
- 158.Zhong H et al. (1999) Overexpression of hypoxia-inducible factor 1a in common human cancers and their metastases. Cancer Res. 59, 5830–5835 [PubMed] [Google Scholar]
- 159.Hiraga T et al. (2007) Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 67, 4157–4163 [DOI] [PubMed] [Google Scholar]
- 160.Colegio OR et al. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoneda TN et al. (2014) Acidic Extracellular Microenvironment in Myeloma-Colonized Bone Contributes to Bone Pain. Blood 124, [Google Scholar]
- 162.Krieger NS et al. (1992) Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am. J. Physiol. Physiol 262, F442–F448 [DOI] [PubMed] [Google Scholar]
- 163.Nilsson SK et al. (1997) Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood 89, 4013–20 [PubMed] [Google Scholar]
- 164.Calvi LM et al. (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 [DOI] [PubMed] [Google Scholar]
- 165.Zhang J et al. (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 [DOI] [PubMed] [Google Scholar]
- 166.Arai F et al. (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–61 [DOI] [PubMed] [Google Scholar]
- 167.Kiel MJ et al. (2007) Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1, 204–17 [DOI] [PubMed] [Google Scholar]
- 168.Kiel MJ et al. (2009) Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4, 170–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Greenbaum AM et al. (2012) N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood 120, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kiel MJ et al. (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–21 [DOI] [PubMed] [Google Scholar]
- 171.Méndez-Ferrer S et al. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ding L et al. (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ding L and Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greenbaum A et al. (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kunisaki Y et al. (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Acar M et al. (2015) Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Winkler IG et al. (2012) Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med 18, 1651–1657 [DOI] [PubMed] [Google Scholar]
- 178.Butler JM et al. (2010) Endothelial Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell Stem Cell 6, 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Crisan M et al. (2008) A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 3, 301–313 [DOI] [PubMed] [Google Scholar]
- 180.Zhou BO et al. (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hanoun M and Frenette PS (2013) This niche is a maze; An amazing niche. Cell Stem Cell 12, 391–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pinho S et al. (2013) PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med 210, 1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ludin A et al. (2012) Monocytes-macrophages that express a-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat. Immunol DOI: 10.1038/ni.2408 [DOI] [PubMed] [Google Scholar]
- 184.Hur J et al. (2016) CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell Stem Cell DOI: 10.1016/j.stem.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 185.Mohamad SF et al. (2017) Osteomacs interact with megakaryocytes and osteoblasts to regulate murine hematopoietic stem cell function. DOI: 10.1182/bloodadvances.2017011304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou BO et al. (2017) Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mansour A et al. (2012) Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med 209, 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Itkin T et al. (2016) Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu C et al. (2018) Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun 9, 2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Asada N et al. (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tamplin OJ et al. (2015) Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell DOI: 10.1016/j.cell.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duarte D et al. (2018) Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell 22, 64–77.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]