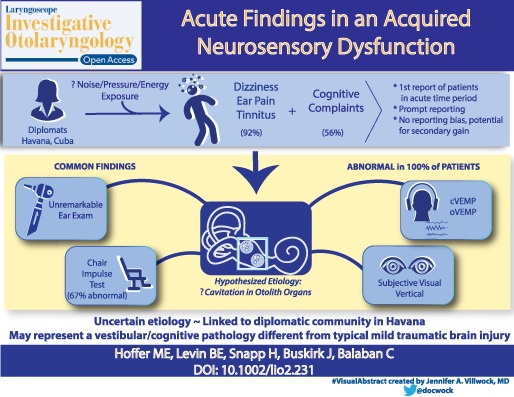
Abstract
Background
In the Autumn of 2016, diplomatic personnel residing in Havana began to present with symptoms of dizziness, ear pain, and tinnitus that emerged after perception of high frequency noise and/or a pressure sensation. Understanding the acute symptoms of this disorder is important for better defining the disorder and developing optimal diagnostic, preventive, and treatment algorithms.
Objectives
To define the presenting symptoms in a cohort of patients in the acute time period after perceiving a noise/pressure exposure in Havana.
Design/Settings/Participants
Review of 25 symptomatic individuals who reported a localized sensation of noise/pressure and 10 asymptomatic individuals (roommates of those affected) who did not experience the sound/pressure.
Results
Immediately after the exposure, the majority of individuals reported intense ear pain in one or both ears and experienced tinnitus. All of the individuals noticed unsteadiness and features of cognitive impairment. On presentation to our center, dizziness (92%) and cognitive complaints (56%) were the most common symptoms. Formal testing revealed that 100% of individuals had an otolithic abnormality and evidence of cognitive dysfunction.
Conclusion and Relevance
This study focuses on the acute presentation of a phenomenon in which symptoms emerge after perception of a localized noise/pressure and in which the acute symptomology includes the universal nature of vestibular injuries and select cognitive deficits. The findings presented in this acute group of patients begin to provide a better picture of the initial injury pattern seen after this exposure and may allow for more accurate diagnosis of this disorder in future cases.
Level of Evidence
Retrospective review
Keywords: Vestibular disorder, Cuba exposure, cognitive disorder, brain injury
A Special Visual Abstract has been developed for this paper. (Visual Abstract 1)
The authors would like to recognize Kurt Yankaskis, PhD (Program Manager at the Office of Naval Research) for his comments helping to clarify this work, Alexander Kiderman, PhD (Chief Technology Officer at NKI) for designing the software and hardware used to analyze these patients, Constanza Pelusso, MD (Research Director in the Department of Otolaryngology at the University of Miami) for her help in filing all necessary IRB forms and reports, and Danierys Font for her assistance in scheduling all of these patients. We would also like to thank Fred Telischi, MD (Chairman), Anthony Etzel (Vice Chairman ), the audiologists, nurses and staff of the Department of Otolaryngology University of Miami, Miller School of Medicine for their help and assistance in caring for these individuals.
BACKGROUND
Beginning in late 2016 and continuing into 2017, a number of diplomats and family members stationed in Havana, Cuba began to report complaints of sudden onset dizziness, ear pain, and tinnitus. Most of the affected individuals reported hearing an unexplained noise before the symptoms began. The affected individuals characterized the sound as being 1) loud, 2) high frequency, 3) very localized, and 4) capable of following them throughout a room. In addition, several individuals reported that if they went outside their front door, the noise immediately stopped. Others reported a sensation of pressure passing through their head and abdomen in certain parts of the room that could be relieved by moving a few feet away.
Swanson et al. reported preliminary findings from 21 exposed individuals who were evaluated an average of 201 days after the perceived exposure; 20 reported persistent symptoms and displayed signs that resembled aspects of mild traumatic brain injury. More precise characterization of their symptom profiles as well as the identification of the sources of these signs and symptoms is limited, though, by the absence of information regarding the acute presentation of the exposed patients and the early course of their treatment response.1 The purpose of this study is to describe the acute presentation of individuals who experienced neurosensory symptoms after exposure to a unique sound/pressure phenomenon.
METHODS
Participants
This retrospective study has been approved by the IRB at the University of Miami as well as the University's HIPPA compliance office. It has also been approved by the IRB at the University of Pittsburgh. The University of Miami conducted evaluations of all individuals who suspected they were affected by an exposure, as well as a sample of individuals who worked and lived in the same geographic area and denied any exposure. Our group was referred 35 individuals from the same diplomatic mission as the index case. These 35 individuals were selected because they reported that they had either experienced the noise and or a pressure wave and had symptoms similar to the index case or because they were in the same house at the same time as someone experiencing these phenomena. All of these individuals were evaluated at an academic medical center in the United States between 4 and 60 days after exposure. There is some, but not total overlap with the patients described by Swanson et al.1 In addition, this group saw a larger group of 105 embassy workers who denied any “exposure” to noise or a pressure sensation, neither personally nor in anyone who shared their domicile. These individuals were largely referred to us by self‐ request or request of the embassy although the US Marines stationed at the embassy who were not on the initial list, but were seen at the request of the investigators. These individuals were all evaluated in Cuba and underwent the same structured history and physical as those seen in Miami and none of them displayed the symptoms seen in the symptomatic cases, with the exception of some preexisting headache (see Fig. 1). This paper is a review of the presenting symptoms in indiviudals who experienced symptoms after an exposure. The study uses descriptive methods to characterize common symptom patterns that help to better characterize the injury pattern seen after this exposure.
Figure 1.
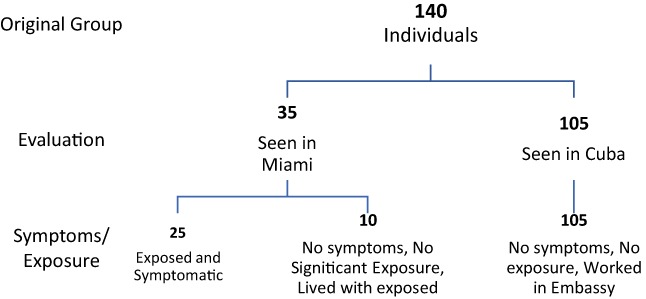
Flow diagram
Intervention
All individuals seen at this academic center underwent a comprehensive history and physical examination that included a standard set of history questions, a physical exam targeted to the head and neck, and a neurologic examination. Standard eye movement testing was performed as part of the neurologic exam and this testing was filmed for more precise computer analysis. These tests included examining the eyes for nystagmus in all fields of gaze, smooth pursuit tests, horizontal saccades, predictive saccades, anti‐saccades, optokinetic response, and vergence measurements. In addition, they underwent tests of visual and auditory reaction time as well as a computerized test of subjective visual vertical (aligning a line straight up and down as a test of the function of the utricle and saccule). A subset of individuals was referred for more formal vestibular and auditory testing and formal neuropsychological testing as allowed by their clinical picture and their health plan.
Statistical Measures
The prevalence of symptoms in the unaffected and affected groups were analyzed with two tailed Fisher Exact tests. Binomial confidence intervals for the prevalence of individual and multiple symptoms were calculated by a the modified Wald method described by Agresti and Coulli.2 Because the Subjective Visual Vertical performance metric (absolute deviation of the subjective setting from earth‐vertical) has a non‐Gaussian distribution, the criterion for abnormal performance were set at 3.45 degrees (deg) from true vertical, the lower fifth percentile in performance for a normal group of 300 subjects tested with the same protocol (subjects described but SVV data were not presented in previous publications3, 4; the 1% criterion score is 4.3 deg from vertical. The fifth percentile criterion was also applied for the anti‐saccade task error rate (at least 43%); the control cumulative distribution has been published.4 The standard clinical criteria for abnormal findings for rotational testing (gain less than 0.8 for a 100 deg/sec impulse), cervical VEMPs (peak amplitude <100 microvolts and/or 35% amplitude asymmetry between sides) and ocular VEMPs (peak amplitude <3 microvolts and/or 35% amplitude asymmetry between sides) were used. The prevalence of abnormal findings was calculated and 99% binomial confidence intervals (modified Wald method,2 calculated directly in MATLAB R2115a, MathWorks, Natick, MA) give expected ranges for prevalence in exposed individuals.
RESULTS
These 35 individuals were examined at the University of Miami, Miller School of Medicine approximately 4 to 60 days after the most recent exposure. There were 21 males and 14 females under the age of 64 years of age (mean: 42.3 ± 11.3 years). The initial history and exam identified 10 individuals (six male and four female) who had no symptoms and simply lived in the same house as a symptomatic individual and were present in the house when the symptomatic induvial was exposed. None of these individuals complained of any new complaints or symptoms and their targeted Otolaryngology and neurologic exams were entirely normal. Only two of these asymptomatic individuals reported extremely brief direct exposure; one reported an extremely brief sensation of exposure to a force wave and a second heard a very brief, high‐pitched noise for a few seconds on a single occasion. The remaining eight unaffected patients reported only indirect exposure, defined as being present in the same house at the time another individual experienced a direct exposure. This group of 10 is designated as the “unaffected group.” The lack of symptoms in the non‐affected “housemates” of affected individuals points to the fact that the exposure showed was both fairly precise and delimited in space and time.
The remaining 25 individuals reported direct exposure and were symptomatic (Table 1). This “affected group” included 15 males and 10 females with the same age range and with the same mean age as the larger group (mean 43.2 ± 12.6 years of age). The affected individuals all reported direct exposure to either noise or pressure. In many cases, their search for the origin of the noise (with the noise following them) resulted in a more prolonged exposure exceeding a few minutes. A few individuals (four total) had briefer, exposures (approximately 1 to 2 minutes), but these occurred over several nights. Immediately after the exposure the majority of individuals felt intense ear pain in one or both ears and experienced noticeable tinnitus. All of the individuals noticed unsteadiness and cognitive symptoms (feelings of disorientation, lack of mental clarity, slower speed of processing, difficulty sustaining attention) within 18 hours of the exposure that produced ear pain. On presentation at this academic institution, the affected individuals reported a variety of symptoms that could largely be qualified as neurosensory. All of the symptomatic individuals reported some combination of: 1) dizziness/balance difficulty, 2) hearing loss, 3) difficulty concentrating and slowed processing speed, 4) tinnitus, 5) ear pain, and 6) headaches (non‐focal and localized to one side of the head or the entire head ). The symptom distributions are shown in Table 1. Dizziness (23/25, 92%) and cognitive complaints (14/25, 56%) were the most common individual symptoms in the affected group and all of the symptoms except headache were significantly more frequent in the symptomatic patients as compared to the asymptomatic. All of the 25 affected individuals reported either dizziness or cognitive complaints, with 12 of 25 (48%) reporting both symptoms. In addition, the affected group had a very high incidence of two or more symptoms. All but one of the affected individuals (96%) had two or more symptoms (that one individual only had dizziness). Sixteen individuals (64%) in the affected group had three or more symptoms. Even if headache is excluded, 14 patients (56%) in the affected group presented with three or more symptoms.
Table 1.
Numbers of Symptomatic Individuals in the Affected and Unaffected Groups.
| SYMPTOM | Affected Group (N = 25) | Unaffected group (N = 10) | Difference | 99% Confidence Interval | Fisher Exact P (2 tail) |
|---|---|---|---|---|---|
| Dizziness | 23 (92%) | 0 (0%) | 92% | 66–>99% | <.001 |
| Cognitive | 14 (56%) | 0 (0%) | 56% | 32–78% | .002 |
| Hearing loss | 8 (32%) | 0 (0%) | 32% | 14–58% | .073 |
| Tinnitus | 8 (32%) | 0 (0%) | 32% | 14–58% | .073 |
| Ear pain | 7 (28%) | 0 (0%) | 28% | 11–54% | .084 |
| HA | 6 (24%) | 2 (20%) | 4% | 1 | |
| At least 2 symptoms | |||||
| including HA | 24 (96%)* | 0 | 96% | 71–>99% | <.001 |
| Excluding HA | 24 (96%)* | 0 | 96% | 71–>99% | <.001 |
| At least 3 symptoms | |||||
| including HA | 16 (64%)* | 0 | 64% | 39–83% | <.001 |
| Excluding HA | 14 (56%)* | 0 | 56% | 32–78% | .002 |
Data presented with and without headache
HA = headache.
There was substantial covariation between the neuro‐otologic symptoms. Fifteen affected individuals reported either tinnitus or hearing loss (both symptoms reported by only one person), while 14 affected individuals reported either ear pain or tinnitus (one reported both symptoms) and no one displayed all three. Because dizziness was reported by 23 of 25 affected individuals, it is not surprising that it is commonly associated with the other prevalent symptoms. For example, dizziness was also reported by all eight individuals who reported tinnitus, seven of eight individuals who reported hearing loss, and five of seven individuals with ear pain. No patients in the unaffected group had more than one symptom.
All individuals had a normal ear exam with the exception of focal erythema in the symptomatic ears of the seven individuals complaining of ear pain on presentation to the academic medical center. All of the individuals with dizziness/balance disorders had abnormalities on the qualitative vestibular clinical examination either on spontaneous gaze (spontaneous nystagmus) or on rapid head thrust test (Halmagyi head thrust) for more than one passive head motion frequency. Postural instability was not impacted in this group of individuals nor were significant gait abnormalities identified.
Consistent with the standard approach at this facility for symptomatic patients with potential balance disorder or mild concussion, a more specific set of quantifiable tests was administered to the patients with dizziness to clarify the diagnosis. Individual patient results are show in Table 2 and group data is shown in Table 3. There was a high rate of abnormality (22/25, 88%) in the subjective visual vertical test (greater than or equal to 3.2 degrees of deviation from earth‐vertical with 18 of 25 (72%) of these individuals having deviations of greater than 4.3 degrees). This test is used to determine if there is damage to the otolithic organs (utricle and saccule) which are the inner ear organs that sense linear acceleration and orientation of the head relative to gravity. Twelve individuals with abnormal SVV findings and suspected otolith and semicircular canal‐related dysfunction were given rotational vestibulo‐ocular reflex tests (horizontal semicircular canal‐related function) and vestibular‐evoked myogenic potential testing (otolith‐related functional test). The combination of SVV abnormalities and the high prevalence of deficits in both cervical and ocular Vestibular Evoked Myogenic Potential (VEMP) metrics was suggestive of an asymmetric peripheral vestibular pathology affecting the otolithic organs. Central vestibular function testing (including optokinetic testing, saccadic test, and vestibular fixation tests) was only abnormal in nine cases (36%). The rotational chair testing demonstrated aspects of asymmetric peripheral impairment of horizontal semicircular canal pathways.
Table 2.
Subject Test Findings.
| Subject | SVV magnitude (degrees) | Antisaccade Task Error Rate (% misdirected) | Chair Impulse (A/N) | cVEMP (A/N) | oVEMP (A/N) |
|---|---|---|---|---|---|
| 1 | 5.8 | 25 | |||
| 2 | 16.7 | 62.5 | |||
| 3 | 5.5 | 6.25 | |||
| 4 | 0.9 | 18.75 | A | N | A |
| 5 | 9.4 | 37.5 | |||
| 6 | 4.7 | 31.25 | |||
| 7 | 4 | 50 | |||
| 8 | 5.6 | 62.5 | |||
| 9 | 14.5 | 37.5 | |||
| 10 | 5.6 | 53 | |||
| 11 | 3.2 | 50 | A | A | N |
| 12 | 5.6 | 80 | |||
| 13 | 6.3 | 31.25 | |||
| 14 | 8.7 | 43 | A | A | N |
| 15 | 4.7 | 43 | N | N | N |
| 16 | 1 | 31.25 | N | A | A |
| 17 | 5.8 | 50 | |||
| 18 | 3.8 | 0 | A | A | A |
| 19 | 8.7 | 28.25 | A | A | A |
| 20 | 4.1 | 40 | A | A | A |
| 21 | 2.8 | 43 | A | A | N |
| 22 | 6 | 88 | A | N | A |
| 23 | 4.5 | 87.5 | A | A | A |
| 24 | 4.7 | 71 | |||
| 25 | 5.8 | 0 | A | N | A |
Subjective Visual Vertical (SVV) magnitude: an abnormal magnitude for Table 2 is greater than or equal to 3.2 degrees, which defines the lower fifth percentile from a group of 300 control subjects. A magnitude less than 3.2 degrees is within normal limits.
Antisaccade Error Rate. An abnormal error rate (incorrect saccade direction) is considered to be a value greater than or equal to 43%, which defines the lower fifth percentile from a group of 300 control subjects. Lower error rates are within normal limits. A zero entry means no errors.
Chair Impulse Test (Horizontal VOR)—HVOR ‐gain less than 0.80 at 100 degrees/sec impulse was termed abnormal (A). Higher values (>0.80) were defined as within normal limits (N).
Cervical Vestibular Evoked Myogenic Potential (cVEMP)—Abnormal (A) if amplitude less than 100 microvolts and/or greater than 35% amplitude asymmetry between sides. Patients not exceeding either threshold are within normal limits (N).
Ocular Vestibular Evoked Myogenic Potential (oVEMP)—Abnormal (A) if amplitude less than 3 microvolts and/or greater than 35% amplitude asymmetry between sides. Patients not exceeding either threshold are within normal limits (N).
Table 3.
Summary of Prevalence of Abnormal Clinical Findings in the Affected Patients
| CLINICAL FINDING (Affected Patients) | Number Tested (N) | Abnormal (Percentage) | Prevalence 99% Confidence Interval |
|---|---|---|---|
| Subjective visual vertical (SVV) | 25 | 22 (88%) | 65–98% |
| Antisaccade test (abnormal error rate) | 25 | 13 (52%) | 31–73% |
| Standard audiometry | 25 | 2 (8%) | 0–31% |
| Central vestibular findings | 25 | 9 (36%) | 18–59% |
| Chair impulse test (HVOR) | 12 | 10 (83%) | 48–98% |
| Cervical vestibular evoked myogenic potential (VEMP) | 12 | 8 (67%) | 34–89% |
| Ocular VEMP | 12 | 8 (67%) | 34–89% |
| At least one VEMP | 12 | 11 (92%) | 56–>99% |
SVV—greater than or equal to 3.2 degrees deviation (lower fifth percentile of normative data from 300 subjects).
Antisaccade—error rate (moving in the wrong direction) greater than or equal to 43% (lower fifth percentile of normative data from 300 subjects).
Standard Audiometry Battery—audiogram, word identification, speech recognition test, tympanometry, reflexes.
Central Vestibular Findings—abnormality on any central vestibular test.
Chair Impulse Test—HVOR gain less than 0.80 at 100 degrees/sec impulse.
Cervical VEMP—abnormal if amplitude less than 100 microvolts and/or greater than 35% amplitude asymmetry between sides.
Ocular VEMP—abnormal if amplitude less than 3 microvolts and/or greater than 35% amplitude asymmetry between sides abnormal if amplitude typically less than 5 microvolts.
All individuals underwent an audiometric evaluation which included air conducted and bone conductive (sensorineural) testing, word identification scores, a pure tone average, speech reception testing, tympanometry, and acoustic reflex testing. Standard audiometric criteria were utilized to determine normal hearing function including a PTA less than or equal to 20 decibels (dB) and a word identification score of 85% or better. Despite almost one third of individuals reporting hearing loss, only two individuals had abnormal hearing tests. Both cases had at least moderate preexisting hearing loss and while both felt the hearing loss was now worse. However, we did not have comparison audiograms available.
The anti‐saccade task is an eye movement test related to executive function; it requires a subject to suppress and eye movement to a target and, instead, make an eye movement of the same magnitude in the opposite direction. A comparison of the SVV and anti‐saccade abnormalities demonstrated by this group of patients as compared to historical controls collected (but not reported) from an earlier series of patients4 is shown in Figure 2. As can be seen, this group differs significantly from the control population.
Figure 2.
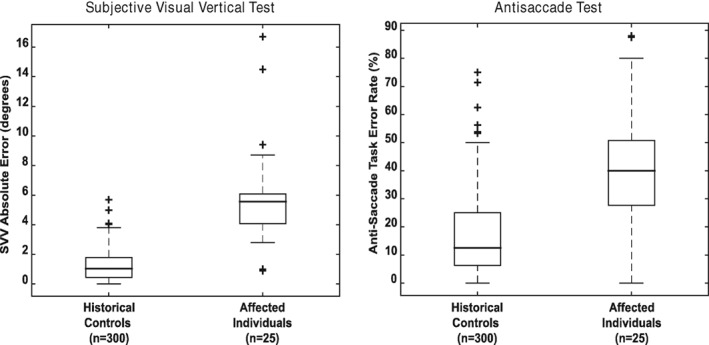
Box plots for distribution of affected individuals as compared to historical controls. Left panel shows subjective visual vertical and right panel shows antisacccade error rate. Historical controls are patients from reference 4.
A subset of nine of the 14 individuals with specific cognitive complaints were administered a battery of neuropsychological measures. These results are shown in Table 4 in order of impairment with the most impaired scores shown first. Most commonly reported neurobehavioral complaints included decreased clarity of thought or “cognitive fog,” inattention, problems retrieving information on demand, especially under distracting conditions, and increased irritability and anxiety as well as overall greater difficulty regulating emotion. Formal neuropsychological testing using a comprehensive battery of tests confirmed these complaints. Decrements indicating performance below expectancy for age and educational level were observed in these individuals on measures of verbal fluency, working memory and sustained attention/vigilance, complex auditory processing requiring the ability to discriminate select stimuli from background noise, grip strength, and organizing sequential material during increasingly high levels of cognitive load. Although all individuals reported emotional distress, half formally endorsed depression and anxiety symptoms on self‐report questionnaires.
Table 4.
Cognitive/Neuropsychological findings.
| Case # | Premorbid estimate of intellect | Subjective complaints | Neuropsychological Findings |
|---|---|---|---|
| A | NART = 114; High Average | • Forgetfulness •Mental fog/Slow performance •Difficulty with complex attention •Reduced motivation |
•Diminished working memory •Slowed processing speed •Inefficient verbal learning •Reduced verbal fluency •Weak grip strength |
| B | NART = 114; High Average | •Forgetfulness •Poor concentration/planning difficulty •Difficulty retrieving words Mood swings •Increased irritability •Lack of motivation |
•Mildly impaired verbal learning and memory •Mildly impaired visual memory •Reduced word finding Mild depression |
| C | NART = 117; High Average | •Slower processing •Difficulty multi‐tasking •Difficulty retrieving words •Greater level of effort required to complete simple tasks |
•Reduced speed of processing Weak grip strength •Diminished sustained attention/ problems sustaining mental set •Difficulty making rapid visual comparisons |
| D | Average | •Slower processing •Attentional problems |
•Slow processing speed |
| E | NART = 117; High Average | •Slower processing •Difficulty concentrating •Difficulty multitasking •Feeling confused •Irritability |
•Reduced ability to focus in the face of competing stimuli •Episodic memory •Working memory difficulties •Weak grip strength. |
| F | NART = 106; Average | •Forgetfulness •Slower processing •Poor concentration •Word finding difficulties •Indecisiveness •Irritability, increased tearfulness decreased interest in activities, anxiety & mood swings |
•Difficulty with verbal memory •Reduced fine motor speed Reduced ability to focus in the face of competing stimuli •Weak Grip Strength •Moderate depression •Mild Anxiety and apathy |
| G | NART = 115; High Average | •Forgetfulness •Slower processing •Difficulty retrieving words •Mood lability & anxiety |
•Decreased visual memory •Reduced verbal fluency •Weak Grip Strength |
| Case # | Premorbid estimate of intellect | Subjective complaints | Neuropsychological Findings |
|---|---|---|---|
| H | NART = 88; Low Average | •Forgetfulness •Slower processing Poor concentration •Difficulties with organization •Difficulty monitoring •Word finding difficulties |
•Difficulty with simple verbal and visual attention, visual processing •Reduced ability to focus in the face of competing stimuli Reduced vocabulary •Mild depression |
| I | Average | •Poor concentration | •Slow processing speed •Diminished abstract problem solving |
NART = North American Reading Test
DISCUSSION
In this report, the authors describe the acute symptoms and clinical findings in a cohort of individuals who reported neurosensory symptoms after perceiving a loud, high‐pitched sound and/or feeling a pressure sensation in a specific location within a room. The preliminary findings indicate that this group of individuals has specific vestibular and cognitive symptoms. The source of this sound/pressure sensation has not been determined but all of the affected individuals appear to be connected to the diplomatic community in Havana. The disorder appears to be fairly specific for those who actually experienced the sound/pressure sensation because no symptoms were reported by others living in the household or by a group in which no one in the household felt any of these phenomena. Despite the authors’ experience screening over 100 asymptomatic individuals in Havana, it is fair to say that one cannot rule out a similar presentation of symptoms in other individuals who have not reported hearing a sound or perceiving the same pressure sensation. However, the authors have not encountered a comparable clinical presentation in individuals who did report either sensation. Hence, the experience of sound and pressure sensations in these locations appears to be a sufficient condition for the appearance of symptoms and clinically abnormal neurosensory findings. The chronic findings originally reported in JAMA by Swanson et al. are not inconsistent with either a partially compensated vestibulopathy or mild brain trauma.1 The relatively high prevalence of chronic symptoms is not atypical for peripheral vestibular disorders.5 One must exercise considerable caution in the interpretation of a patient's causal attributions for symptoms associated with balance disorders and mild traumatic brain injury (mTBI), including neuropsychological complaints. Attribution is obvious for overt exposure scenarios like a blast wave exposure or blunt impact to the head. However, if dizziness is due to a covert cause, the attribution is not as likely to be accurate. The dizziness, ear pain, and subjective cognitive symptoms are aversive; as in the case of conditioned taste aversion in the presence of nausea and the symptoms may be attributed to irrelevant but novel conditions that merely coincide temporally with the proximate cause. Attribution and misattribution issues for balance disorders and nausea have been reviewed elsewhere.6, 7, 8 More recently, clinical evidence suggests that objective cognitive findings in patients with Otic capsule dehiscence syndrome are resolved by surgical repair.9 As is well‐known for public health concerns, media attention can increase the prevalence of medical consultation by the “worried well.” Recent and continued coverage of this phenomenon therefore makes distinguishing those affected from the worried well even more important.
The exposure responsible for these findings is unknown. It would be imprudent to exclude any potential directed or non‐directed energy sources at this time. For example, perceptions of sound can occur in response to energy exposures that include microwave pulses in the audible ultrasonic range (10–15 kHz peak sensitivity) or as synesthetic effects to light.10, 11 Pulsed microwave stimulation is known to produce ultrasonic cochlear microphonics in guinea pigs, which are suggestive of local propagation of energy in that frequency range.12 The ultrasonic frequency range is represented at the base of the cochlea (“hook portion”) in close proximity to the vestibule. Because sound activation of saccule and utricle produce cervical and ocular VEMPs, respectively, it is not inconceivable that resonant energy in that range could affect vestibular function. In fact, the occupational health literature indicates that intense ultrasonic radiation can produce “a syndrome involving manifestations of nausea, headache, tinnitus, pain, dizziness, and fatigue.13, 14
The potential mechanisms for injury by incident energy include cavitation bubble formation in body fluids. Cavitation bubbles can be produced in aqueous solutions by directed energy sources.15, 16, 17 The energy released by the bubble collapse produces local jet, shock wave, and acoustic emissions.18, 19 Cavitating gas bubble formation also has been associated with local tissue nitrogen accumulation in decompression illness, which may be mimicked by underwater exposure to intense sound sources.20 Hence, internally generated, cavitation‐related effects in blood and intracranial fluids (CSF, perilymph, endolymph, and interstitial fluid) must be considered as possible etiologic factors after unknown energy exposures.
The pattern of findings in the symptomatic group of a vestibulopathy combined with other neurosensory findings could be interpreted as being similar to the presentation of individuals with acute sequelae of mild traumatic brain injury following blast exposure or blunt trauma.21, 22 In addition, it does not seem imprudent to speculate that a highly specific unidentified energy exposure, perceived as a sound or pressure, could be producing an inner ear disturbance or demonstrate findings suggestive of an mTBI. However, this injury pattern does have some differences from the patterns reported in mTBI. The prevalence of individuals presenting with two or more symptoms and the SVV abnormalities seems higher than one would expect after conventional mTBI.23, 24 In addition, the low incidence of headaches (around 25%) is unusual, as many studies of mTBI show that headache is one of the most common and persistent symptoms.25, 26, 27 Perhaps the most important objective clinical feature is the nearly universal evidence of otolithic impairment; such uniformity in symptoms is uncommon in mTBI cases from other sources.28, 29 This frequency of specific vestibular findings is not seen in any control populations. In this work the authors provide the characteristics in a group of patients defined by vestibular pathology in which the clinical presentation seems most consistent with a primary localized neurotologic (largely otolithic) injury with cognitive symptoms.
Because this injury pattern has now been reported elsewhere, it is important for individuals who care for patients to be aware of the presenting symptoms and signs. Objective, tests of otolithic and vestibular function including subjective visual vertical (SVV), vestibular evoked myogenic potentials (VEMPs), and head rotation test (head impulse tests) proved particularly helpful in this population. Given the unknown nature of the type and source of the energy associated with this disorder, careful assessment and documentation of the presenting symptoms as they are seen in future will be critical, since such assessments, at the acute time point after exposure, will the most accurate characterization of the injury. This precise characterization of the acute presentation provides a basis for identifying longer term progression and determining therapeutic efficacy.
LIMITATIONS
This study has several limitations which were imposed by the unusual, novel circumstances surrounding the referral of diplomatic personnel for examination after suspected incidents at an overseas site. First, from a design perspective, we are restricted to retrospective data analysis. Second, the patient examinations were restricted to obtaining clinically necessary diagnostic data. Third, the sample size is limited Nevertheless, this is likely the only opportunity to report the presenting symptoms on even these many patients, seen acutely (without the influence of outside attention or a “pre‐knowledge” of symptomatic complaints). Knowledge of the unbiased presenting symptom patterns reported here is crucial since new cases have been reported all over the globe affecting individuals from many countries.
CONCLUSION
This study focuses on the presenting symptoms of a phenomenon in which symptoms emerge after perception of a localized loud noise or pressure sensation. The unique features of the acute symptomology include the in universal nature of vestibular injuries and the pattern of cognitive findings. The findings presented here are the first report of the acute symptoms in this patient group and begin to provide a better picture of some salient aspects of the initial injury pattern seen after this perceived exposure. This report is intended to facilitate an objective diagnosis of this disorder as new actual or potential cases continue to be reported.
AUTHOR CONTRIBUTIONS
Dr. Hoffer and Dr. Balaban had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Hoffer, Dr. Balaban, and Dr. Levin contributed to the concepts and design of the study. All authors contributed to the acquisition, analysis and interpretation of the data as well as drafting the manuscript. Dr. Hoffer, Dr. Balaban, and Dr. Levin contributed to critical manuscript revisions for important intellectual content.
Conflict of Interest: All authors have completed the ICMJE form for disclosure of potential conflict of interest. No conflicts were reported for this work.
Funding Support: No funding was provided for this work.
Disclaimer: The content and views expressed in this paper are those of the authors and do not represent the official views of the University of Miami, the University of Pittsburgh, the United States Department of State, the United States Department of Defense, or the United States Government.
BIBLIOGRAPHY
- 1. Swanson RL, Hampton S, Green‐McKenzie J, et al. Neurological manifestations among US government personnel reporting directional audible and sensory phenomena in Havana, Cuba. JAMA 2018;319(11):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agresti A, Coull BA. Approximate is better than “exact’ for interval estimation of binomial proportions. Am Stat 1998;52:119–126. [Google Scholar]
- 3. Balaban CD, Hoffer ME, Szczupak M, et al. Oculomotor, vestibular, and reaction time tests in mild traumatic brain injury. PLoS ONE 2016;11:e0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffer ME, Balaban CD, Szczupak M, et al. The use of oculomotor, vestibular, and reaction time tests to assess mild traumatic brain injury (mTBI) over time. Laryngoscope Investig Otolaryngol 2017;2:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel M, Arshad Q, Roberts RE, Ahmad H, Bronstein AM. Chronic symptoms after vestibular neuritis and the high velocity vestibulo‐ocular reflex. Otol Neurotol 2016;37(2):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balaban CD, Thayer JF. Neurological bases for balance‐anxiety links. J Anxiety Disord 2001;15:53–79. [DOI] [PubMed] [Google Scholar]
- 7. Balaban CD, Yates BJ. What is nausea? A historical analysis of changing views. Auton Neurosci 2017;202:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coelho CM, Balaban CD. Visuo‐vestibular contributions to anxiety and fear. Neurosci Biobehav Rev 2015;48:148–159. [DOI] [PubMed] [Google Scholar]
- 9. Wackym PA, Balaban CD, Mackay H, et al. Longitudinal cognitive and neurobehavioral functional outcomes before and after repairing otic capsule dehiscence. Otol Neurotol 2016;37 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tyazhelov VV, Tigranian RE, Khizhniak EO, Akoev IG. Some peculiarities of audiotory sensations evoked by pulsed microwave fields. Radio Sci 1979;14:259–263. [Google Scholar]
- 11. Fassnidge C, Marcotti CC, Freeman E. A deafening flash! Visual interference of auditory signal detection. Conscious Cogn 2017;49:15–24. [DOI] [PubMed] [Google Scholar]
- 12. Chou C‐K, Galambos R, Guy AW, Lovely RH. Cochlear microphonics generated by microwave pulses. J Microw Power 1975;10:361–367. [DOI] [PubMed] [Google Scholar]
- 13. Curthoys IS, MacDougall HG, Vidal J‐P, de Waele C. Sustained and transient vestibular systems: a physiological basis for interpreting vestibular function. Front Neurol 2017;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Health Canada , 1991. Guidelines for the Safe Use of Ultrasound: Part II – Industrial and Commercial Applications, in: Environmental Health Directorate, H.P.B., Ministry of Natinal Health and Welfare (Ed.). Canadian Communication Group – Publishing, Ottawa, Canada: Available at: http://www.hc‐sc.gc.ca/ewh‐semt/pubs/radiation/safety‐code_24‐securite/index‐eng.php#a2.2.2. Accessed May 22, 2018. [Google Scholar]
- 15. Apfel RF Acoustic Cavitation, in PJ Edmonds (ed). Ultrasonics, Methods of Experimental Physics, Vol. 19, Academic Press, San Diego, CA 1981: 355–411. [Google Scholar]
- 16. Lauterborn I, Bolle H. Experimental investigations of cavitation‐bubble collapse in the neighborhood of a solid boundary. J Fluid Mech 1975;72(2):391–399. [Google Scholar]
- 17. Akhatov I, Lindau O, Topolnikov A, Mettin R, Vakhitova N, Lauterborn W. Collapse and rebound of a laser‐induced cavitation bubble. Phys Fluids 2001;13:2805. [Google Scholar]
- 18. Brujan E‐A, Vogel A. Stress wave emission and cavitation bubble dynamics by nanosecond optical breakdown in a tissue phantom. J Fluid Mech 2006;558:281–308. [Google Scholar]
- 19. Brujan EA, Ikeda T, Matsumoto Y. Jet formation and shock wave emission during collapse of ultrasound‐induced cavitation bubbles and their role in the therapeutic applications of high‐intensity focused ultrasound. Phys Med Biol 2005;50:4797–4809. [DOI] [PubMed] [Google Scholar]
- 20. Tal D, Sachar‐Bener H, Hershkovitz D., Arieli Y, Shupak A. Evidence for the initiation of decompression sickness by exposure to intense underwater sound. J Neurophysiol 2015;114:1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffer ME, Balaban C, Gottshall K, Balough BJ, Maddox MR, Penta JR. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol 2010;31:232–236. [DOI] [PubMed] [Google Scholar]
- 22. McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One 2017;12:e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowley DA, Rogish M, Alexander T, Riggs KJ. Cognitive correlates of pragmatic language comprehension in adult traumatic brain injury: a systematic review and meta‐analyses. Brain Inj 2017;31:1564–1574. [DOI] [PubMed] [Google Scholar]
- 24. Losoi H, Silverberg ND, Wäljas M, et al. Recovery from mild traumatic brain injury in previously healthy adults. J Neurotrauma 2016;33:766–776. [DOI] [PubMed] [Google Scholar]
- 25. Lucas S, Blume HK. Sport‐related headache. Neurol Clin 2017;35:501–521. [DOI] [PubMed] [Google Scholar]
- 26. Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014;34:93–102. [DOI] [PubMed] [Google Scholar]
- 27. Silverberg ND, Iverson GL, Panenka W. Cogniphobia in mild traumatic brain injury. J Neurotrauma 2017;34:2141–2146. [DOI] [PubMed] [Google Scholar]
- 28. Julien J, Tinawi S, Anderson K, et al. Highlighting the differences in post‐traumatic symptoms between patients with complicated and uncomplicated mild traumatic brain injury and injured controls. Brain Inj 2017;17:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Mercier E, Tardif PA, Emond M, et al. Characteristics of patients included and enrolled in studies on the prognostic value of serum biomarkers for prediction of postconcussion symptoms following a mild traumatic brain injury: a systematic review. BMJ Open 2017;7:e017848. [DOI] [PMC free article] [PubMed] [Google Scholar]


