Abstract
Monoclonal antibodies (mAbs) that target immune co‐signaling pathways have the potential to enable immune mediated tumor eradication. While early adoption of these agents for the treatment of advanced squamous cell carcinoma of the head and neck (SCCHN) has produced some astounding clinical successes, the majority of patients fail to respond to therapy. The purpose of this review is to first provide a broad overview of the immuno‐oncology (I‐O) landscape and to then focus on the current status of mAb‐based I‐O (mAb:I‐O) for the treatment of SCCHN, with particular attention to the development of strategies for improving treatment responses.
Keywords: Immuno‐oncology, head and neck cancer
INTRODUCTION
Harnessing the power of the immune system for the treatment of cancer has tremendous promise—promise based on not only the capacity of immune system to recognize and kill malignant cells, but also, and perhaps more importantly, built on its ability to “remember” prior tumor cell exposure through recall responses. Early attempts to manipulate the immune response through techniques such as intratumoral administration of immune adjuvants, generation and passive administration of tumor infiltrating lymphocytes,1 active vaccination against tumor‐associated/tumor‐specific antigens,2, 3, 4 and cell‐based vaccines,5 showed the promise of cancer immunotherapy. However, in general, these approaches yielded only sporadic success and/or could not be translated into phase III clinical trials to validate their utility.6
Over the past several years, the promise of cancer immunotherapy has started to be realized. For example, there is a burgeoning availability of approved monoclonal antibodies (mAbs) that protect and/or augment the functions of T cells and other cell populations—affording them the opportunity to mediate tumor destruction.7 These advances are occurring in parallel/synergy with cell‐based engineering approaches that allow creation of immune cells that specifically recognize tumor‐associated/specific molecules.8, 9 Finally, the knowledge that tumors have a variety of unique proteins, called neoantigens, that can be recognized and targeted by the immune system, creates new opportunities for both vaccine development and use in combination with passive cell transfer.10, 11, 12 The purpose of this review is to provide a brief overview of the field of I‐O and then to focus on the use of mAbs designed to manipulate co‐stimulatory/co‐inhibitory molecules, with particular attention on immunomodulatory mAbs relevant to the treatment of patients suffering from malignancies of the head and neck.
What Is Immuno‐Oncology? A Brief Overview
In its broadest sense, I‐O can be conceptualized as any treatment that either directly or indirectly modulates the immune system, and whereby the result of such modulation influences the development, maintenance, and/or progression of malignant disease. Many treatments, e.g., radiation/chemotherapy, have broad immunologic effects that likely impact both tumor response rates and potential for disease recurrence. As such, for the purposes of this review, we will restrict use of the term, I‐O, to characterize three broad clinical platforms: active approaches using cancer vaccines, passive approaches using either designer immune cells, and mixed approaches, e.g., antibodies designed to target specific proteins on the tumor cell surface and/or manipulate defined co‐stimulatory/co‐inhibitory pathways.
Active approaches are best exemplified by the administration of vaccines, which induce the immune response to immunogens shared between the vaccine and the tumor. For example, our group has pioneered the use of Trojan Peptide Vaccines (TPV) designed to target the immune response to MAGE‐A3 and HPV‐16 proteins expressed in specific tumor types.6, 13, 14 These peptides are undergoing evaluation in a randomized phase IIb clinical trial to determine their efficacy—in combination with low‐dose Cytoxan, GM‐CSF, and Hiltonol—to prevent recurrent disease in patients with high‐risk oral cavity cancer who have completed standard of care therapy. Currently, others and we are also developing vaccine approaches to target neoantigens—defined as unique tumor‐specific proteins resulting from mutations in the tumor genome.15, 16, 17, 18 While these approaches hold tremendous promise for preventing disease recurrence in high‐risk settings and for therapeutic use as part of combinatorial platforms, in our opinion, it is unlikely that vaccine‐based approaches alone will effectively treat large volume disease.
Active approaches require the immune system to generate antigen responses and to harness those responses as a means to mediate tumor regression. In contrast, passive I‐O approaches, such as cell‐based treatments, deliver agents that, in and of themselves, may be completely able to mediate tumor regression. A complete discussion of modern cell‐based therapies is beyond the scope of this review and several excellent references are available in the literature.19, 20, 21 In general, such engineered cells can be categorized into two broad groups: chimeric antigen receptor (CAR) T cells22, 23 that are engineered to target proteins present on the tumor cell surface, e.g., CD19 and chimeric T cell receptor–expressing cells that are engineered to target intracellular proteins, e.g., MAGE‐A3.24 While clinical use of these cells has resulted in spectacular treatment successes, particularly for patients with B cell lymphomas, significantly more work is required to realize similar acmes in solid malignancies, to limit “on target” side effects mediated by these therapies, and to increase the accessibility of such approaches.25, 26, 27
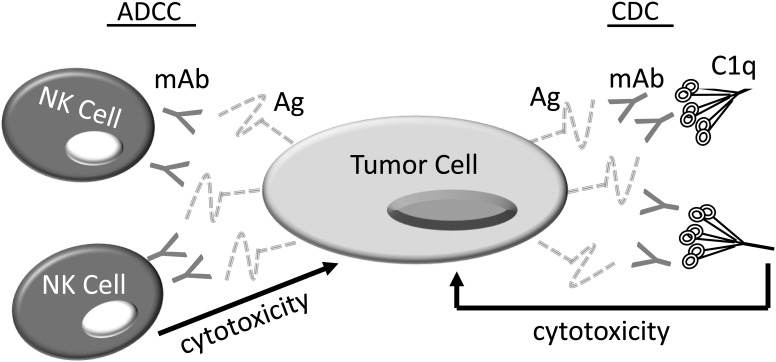
The final type of I‐O approach, mAb therapy, has both active and passive components.28 Historically, the use of Abs for the immunotherapy of squamous cell carcinoma of the head and neck (SCCHN) adhered to Paul Ehrlich's original concept of a “magic bullet”29—where Abs specific for unique protein epitopes could seek out and kill cells bearing these epitopes on their surfaces through processes termed antibody dependent cell mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) (Fig. 1).30, 31 The utility of targeted mAb therapy for SCCHN is best evidenced by the ability of the anti‐EGFR (epidermal growth factor receptor) mAb, cetuximab (Erbitux), in combination with radiation to improve loco regional control and survival in advanced SCCHN when compared to radiation alone.32 Despite the fact that this study proved the therapeutic potential of cetuximab, the impact of cetuximab on clinical outcomes is modest and meta analyses suggest that cetuximab and radiation therapy (XRT) are likely not equivalent to platinum/XRT combinations in the treatment of advanced SCCHN.33
Figure 1.
Targeted Antibody Therapy. Targeted mAbs, eg, Cetuximab, bind proteins that are uniquely or preferentially expressed on the tumor cell surface. Engagement of the aggregated Fc fragments of these mAbs by Fc receptors (FcR) on NK cells enables NK cell–mediated tumor killing through a process termed antibody dependent cell‐mediated cytotoxicity (ADCC). Alternatively, binding of these Fc aggregates to FcRs on phagocytic cells such as macrophages can induce Ab dependent cell–mediated phagocytosis (ADCP) (not shown). Finally, binding to C1q, which is the first component of the classical complement cascade, can result in complement dependent cytotoxicity (CDC). Importantly, in some cases, these mAbs also block receptor ligand interactions that can potentiate tumor proliferation and survival.
More recently, Abs are being “repurposed” for cancer therapy—not as the targeted agents that Ehrlich originally envisioned, but rather as tools capable of governing the anti‐tumor immune response through inhibition of “immune checkpoints” that mediate immunologic tolerance and by stimulating receptors, which facilitate the proliferation, survival, and trafficking of immune cells into the tumor microenvironment.34 Importantly, the receptors and ligands that compose these co‐inhibitory pathways are, in general, not specifically expressed on tumors and/or in the tumor microenvironment. Rather, in the healthy host, these receptor–ligand interactions tightly control the process known as immune homeostasis—a process that allows for immune mediated surveillance and prevention of malignancy35 while simultaneously regulating aberrant immune responses capable of mediating autoimmunity.36 Malignant cells and cells within the tumor microenvironment “hijack” the same pathways that are integral to our immunologic health and use them as a biologic shield to evade immune destruction.
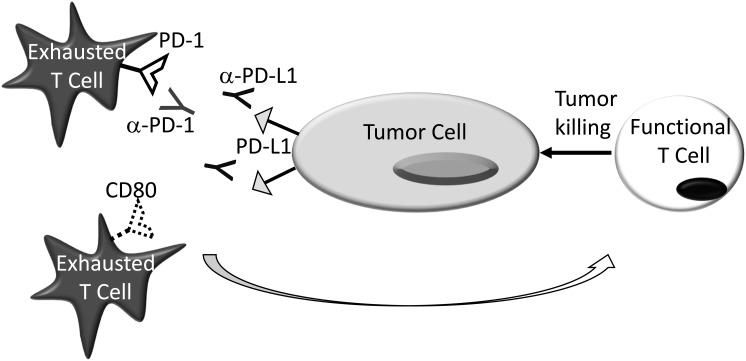
The best studied of the immune checkpoint inhibitors for the treatment of SCCHN are those that block PD‐L1 (B7‐H1, CD274)37 and PD‐1 (CD279)38, 39 interactions.40, 41 In the early 2000s our lab was the first to report that PD‐L1 was expressed on a wide variety of malignancies, including SCCHN, and that blockade of the interactions between PD‐L1 and one of its cognate ligands, PD‐1, could facilitate tumor rejection (Fig. 2).42, 43 These findings were later reproduced and expanded by many other investigative teams, demonstrating both the veracity of these initial data and the potential role of PD‐L1: PD‐1 blockade in the treatment of SCCHN.44, 45, 46, 47
Figure 2.
PD‐1:PD‐L1 Blockade. PD‐L1— expressed either on the tumor cell surface or on cells within the tumor microenvironment—binding to PD‐1 and/or CD80 on the surface of tumor reactive effector T cells, renders them dysfunctional. Blockade of these interactions with α–PD‐1 or α–PD‐L1 mAbs, protects these cells and facilitates their anti‐tumor activity. While α–PD‐1 and α–PD‐L1 mAbs are often considered to be analogous, α–PD‐L1 mAbs block the interactions between PD‐L1 and PD‐1/CD80 while α–PD‐1 mAbs inhibit PD‐1 engagement of both PD‐L1 and PD‐L2 (Not Shown). Both α–PD‐1 and α–PD‐L1 mAbs are further differentiated by the ability of their aggregated Fc fragments to activate complement and mediate ADCC/ADCP.
Recent studies have translated these early findings into the clinic, evaluating the utility of a modified human IgG4 anti‐PD‐1 mAb, nivolumab in the treatment of patients with recurrent, progressive SCCHN, status post‐platinum failure. Patients receiving nivolumab versus standard therapy enjoyed improved response rates, better progression‐free survival, enhanced 1‐year survival rates, and reduced grade 3/4 toxicity. Despite such success, the response rate in the nivolumab group was only 13.3%, and the levels of PD‐L1 expression were not significantly associated with drug‐related benefit.48 Notably, a second PD‐1 inhibitor, pembrolizumab, yielded similar efficacy in a nearly analogous patient cohort.49 Collectively, these studies introduce some of the promise and challenges associated with I‐O based treatments for SCCHN.
How Does mAb:I‐O Differ from Chemotherapy—What Is all the Hype?
Traditional chemotherapy targets tumors by attacking rapidly proliferating cells during distinct phases of the cell cycle. The lack of specificity of such approaches commonly results in “off target” physical morbidity—morbidity which also manifests as psychosocial distress.50 In contrast to traditional chemotherapy, immune modulating mAbs, (Ab:I‐O), harness the patient's own antitumor immune response to mediate disease regression. These Abs block receptor:ligand interactions that would promote anti‐tumor T cell death, and/or stimulate receptors that facilitate effective priming, trafficking, and proliferation of tumor reactive T cells. A complete discussion of these receptor ligand‐co‐signaling pathways can be found elsewhere.51, 52 The fundamental difference between the manipulation of such co‐signaling pathways and traditional chemotherapy is that such I‐O strategies have the potential to “teach” the immune system—allowing the antitumor immune response to evolve and adapt in response to changes in active and passive tumor defense “strategies.” Such I‐O approaches also have the potential to induce memory responses, which enable long‐term tumor stability/remission—potentially without the requirement for continued drug administration.53
Importantly, based on their mechanisms of action, mAb: I‐O–based therapies have both different and overlapping side effects versus traditional chemotherapeutics54—side effects termed immune‐related adverse events (IRAEs).55, 56 For example, patients with SCCHN treated with the anti–PD‐1 mAb, nivolumab, reported fatigue, nausea, and adverse skin effects, while those patients treated with methotrexate, docetaxol, or cetuximab had more profound gastrointestinal complications. Of greater import, patient reported quality of life was improved in nivolumab‐treated individuals versus those receiving standard of care.48
Where Do We Go From Here? Overcoming Biologic Hurdles to Improve Clinical Outcomes
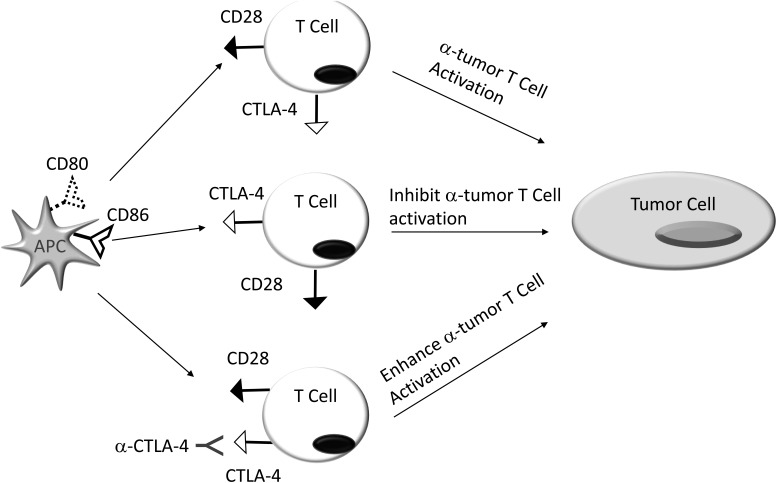
Since the approval of the anti‐CTLA‐4 mAb (Fig. 3), Ipilimumab, by the FDA in 2011, the field of I‐O has witnessed explosive growth.51 However, despite dramatic responses to mAb‐mediated manipulation of co‐signaling pathways in select patients with SCCHN, the overall response rates are disappointingly low. Improving these overall response rates may be facilitated by improved predictive outcome algorithms, rationale therapeutic mAb design, combinatorial treatment approaches, and adoption of alternate clinical trial schemes.
Figure 3.
CTLA‐4 Blockade. Tumor reactive T cells have variable patterns of CD28 and CTLA‐4 expression. Engagement of CD28 on the T cell surface by its cognate ligands CD80 (B7‐1) and/or CD86 (B7‐2), expressed on antigen presenting cells (APC), activates and potentiates these cells. In contrast, binding of these same ligands to the CTLA‐4 counter receptor, inhibits anti‐tumor T cell function. By blocking the binding of CD80/CD86 to CTLA‐4, α‐CTLA‐4 mAbs promote activating CD80/CD86:CD28 interactions that enhance T cell activity.
Predicting Treatment Responses
One effective means to improve the clinical response rates for mAb:I‐O is to better understand those individuals most likely to respond to treatment. Using the PD‐L1:PD‐1 pathway as an example, early data from our group—showing that patients with high levels of PD‐L1 either on renal cell carcinomas and/or in their tumor microenvironment was associated with a poor prognosis—led to the hypothesis that levels of PD‐L1 may predict response to anti‐PD‐L1/PD‐1 blockade.57 While it is certainly true that the levels of PD‐L1 expression are associated with treatment responses in patients with certain tumor types, e.g., melanoma, the association between PD‐L1 expression and response to therapy is far from universal.58, 59, 60 Furthermore, even in tumor histologies where high PD‐L1 expression is associated with improved response rates to anti‐PD‐L1/PD‐1, e.g., non–small cell lung cancer (NSCLC), many patients with high PD‐L1 expression do not benefit from therapy.61
Potential causes for the discordance between PD‐L1 expression and treatment outcomes are based on the fact that PD‐L1 is an inducible molecule, so that expression is dynamic.43, 62 Additionally, core/fine needle aspiration biopsies used for analysis may not be representative of PD‐L1 expression levels in the tumor as a whole.63 Furthermore, multiple different assays and different staining protocols may yield different outcomes, particularly with regard to the percentage of tumor cells expressing PD‐L1 and whether PD‐L1 expression in the microenvironment is taken into consideration.64, 65 Moreover, because PD‐L1 can induce early T cell proliferation, it is possible that in certain instances, PD‐L1 expression will actually be beneficial.62 Finally, based on recent data in NSCLC, it is likely that some mAbs targeting the PD‐1 pathway are dependent on PD‐L1 expression while other are not.61, 66
In addition to PD‐L1 expression, intratumoral heterogeneity (ITH) is a valuable tool for predicting response to PD‐L1:PD‐1 blockade.66, 67 Indeed, anti PD‐1 therapy is often effective for patients with mismatch repair deficiency—recognized to have high levels of ITH—independent of tumor type.68, 69, 70 Our understanding of the biologic basis for the improved outcomes to PD‐L1:PD‐1 blockade in patients with high levels of ITH is somewhat limited. However, it is likely that these improved responses are premised on the diversity of potential neoantigens within the tumor microenvironment—diversity that provides more targets for the immune system to attack while also preventing the tumor from evading the immune response by single antigen loss/downregulation.69
Importantly, the role of ITH as a predictor of response may be more nuanced than just the absolute levels of heterogeneity. Specifically, one recent study suggests that response to PD‐L1:PD‐1 blockade is predicated not only on high levels of heterogeneity, but rather on the fact that this heterogeneity is clonal, ie, the mutations are common throughout the tumor. Furthermore, acknowledging the caveats mentioned above, it is interesting to note that select patients with high levels of ITH appear respond to PD‐L1:PD‐1 blockade in the absence of PD‐L1 expression.71 New heterozygosity algorithms such as mutant allele tumor heterogeneity (MATH) developed by Rocco and colleagues, may allow rapid integration of ITH levels into patient selection protocols.72
In addition to PD‐L1 expression and ITH, it is now becoming increasingly apparent that patients whose tumors have inflammatory signature, eg, IFN‐γ, have higher response rates to PD‐L1:PD‐1 blockade versus individuals lacking such phenotypes.73, 74 Indeed, we are now beginning to understand the biologic basis for the lack of inflammation in select tumors—a pathophysiology that appears to rely on the absence of chemokines requisite for the trafficking of BATf3 DCs into the tumor microenvironment and the stimulator of interferon genes (STING) pathway.75 Unfortunately, despite ongoing research efforts to induce immune invasion into the tumor microenvironment through the use of oncolytic viruses,76, 77 STING agonists,78 and modified anti‐PD‐L1 mAbs which trap immunosuppressive cytokines,79 no standardized clinical approaches are currently available.
The most recent efforts to define patients most likely to respond to checkpoint inhibition have focused on the microbiome. Data from three separate studies have now clearly demonstrated that the presence of specific gut microbiota correlate with outcome to PD‐L1:PD‐1 blockade.80, 81, 82 While the biologic basis for the relationship between the gut microbiome and the response to checkpoint inhibition remains uncertain, these data hold promise that the gut microbiome may not only serve as a prognostic tool, but also, that manipulation of the gut microbiota may be an effective strategy to improve treatment outcomes.
Rational Drug Design
In the rush to translate Ab:I‐O into clinical practice for an ever‐increasing number of indications, we as a field have largely lost interest in improving/understanding the tools used to manipulate these co‐signaling pathways. For instance, the majority of mAbs designed to inhibit the PD‐L1:PD‐1 pathway have Fc fragments engineered to eliminate their potential to induce ADCC and CDC.40, 41 While such engineering strategies were likely adopted to mitigate safety concerns, the majority of the original proof of concept studies were performed using rat‐derived Abs that maintain these functional capabilities.43
The importance of these biologic differences is highlighted in a recent murine study showing that engagement of activating FcγRs enhances the activity of mAbs targeting PD‐L1 though modulation of myeloid cell in the tumor microenvironment. In contrast, activating FcγRs are detrimental to the function of anti‐PD‐1 mAbs as they mediate loss of CD8 tumor infiltrating lymphocytes.83 Similarly, using a series of FcγR‐/‐ mice, we showed that the anti‐tumor activity of mAbs targeting the costimulatory molecule, CD137, are dependent on defined Fc:FcR interactions.84 Such observations become even more important when taken in the context of studies from others and us showing that engagement of FcRs through multimeric Fc fusion proteins—somewhat analogous to mAb opsonization of a tumor target—have the potential to induce tolerance.85, 86, 87, 88, 89, 90 Collectively, these initial insights set the stage for future investigations to determine how the interplay between Fc:FcR interactions and ligation of the target molecule influence functions. Furthermore, they serve as a cautionary note for researchers performing rodent studies using immunocompetent mAbs to manipulate co‐signaling pathways, as these studies may have limited relevance to clinically available mAbs.
An additional consideration when choosing the appropriate Fc fragment to incorporate into the design of mAbs capable of manipulating of specific co‐signaling pathways, is that many co‐signaling molecules function as both receptors and ligands. For example, while PD‐L1 is recognized as a ligand for both CD80 and PD‐1, PD‐1 can also mediate signals through PD‐L1 in a receptor capacity. Indeed, PD‐L1 signaling on tumors protects them from T cell–mediated death.91 Thus while anti‐PD‐L1 mAbs with competent Fcs have the potential to mediate ADCC, ADCP, and CDC of PD‐L1–bearing tumors, if such Fc crosslinking induces protective signals through PD‐L1, the overall anti‐tumor immune response may be attenuated.
In addition to choosing an appropriate Fc fragment—a choice that will likely need to be individualized for each unique mAb—the choice of which receptor:target interactions to block/enhance in each individual pathway requires more study. For example, CTLA‐4—the counter‐receptor for CD28—binds both CD80 and CD86 so that a blocking mAb could potentially be engineered to block either or both interactions.92 Similarly, when considering the PD‐L1:PD‐1 blockade, it is notable that in addition to PD‐1, PD‐L1 also binds CD80, while PD‐1 binds to B7‐DC (PD‐L2).40 While both PD‐L1:PD‐1 and PD‐L1:CD80 interactions are important for PD‐L1 mediated tolerance in mouse models, how this finding will translate into human disease is uncertain, raising the question of whether mAbs which block both interactions are best suited for the treatment of human disease.93 It is also notable that there are molecules, which serve at the interface between multiple co‐signaling pathways, e.g., CD80. As such, targeting specific segments of one pathway will affect receptor:ligand interactions in sister pathways, potentially mediating unanticipated biologic consequences.
In comparison to developing mAbs designed to mediate “checkpoint inhibition,” the development of biologic tools for enhancing co‐stimulating pathways is dramatically more difficult. The genesis of this difficulty is that the ability of a mAb to costimulate T cell proliferation in vitro does not necessarily correlate with its antitumor activity in vivo.94 Furthermore, other factors such as how/whether the ability of such mAbs to block naturally occurring receptor: ligand interactions influences function are difficult to predict using preclinical models. Surgical “window of opportunity trials” may offer a partial solution to these concerns in that it is theoretically possible to test a variety of different mAbs against a particular target and obtain an early readout of their clinical efficacy, while also evaluating tissue‐specific and biomarker data. However, such approaches are limited by factors such as feasibility, expense, and potential treatment delays.95 As such, it is incumbent upon us as a field to better define preclinical parameters predictive of the clinical efficacy of unique mAbs targeting individual co‐signaling pathways.
Combination Therapies
Conceptually, combination approaches, using mAbs targeting diverse co‐signaling pathways, have the potential to support the antitumor immune cell from initial recognition of tumor antigens, to growth/expansion, to mediation of tumor cell death, and, ultimately, generation of a memory pool (not necessarily in that sequence).96 A detailed discussion of these approaches in SCCHN is reviewed elsewhere.40, 41, 97 However, it is critical to understand that trials employing mixed targeting of co‐inhibitory and co‐stimulatory pathways are already demonstrating therapeutic success. For example, combinations of anti‐PD‐1/CTLA‐4 were more effective than either agent alone in the management of untreated PD‐L1 metastatic melanoma.98, 99 Combinations of anti PD‐1 with anti CD137 are currently under study.100, 101 Optimizing such approaches will rely on improved knowledge of the most appropriate combinations for clinical evaluation, a better understanding of how the timing/sequence of drug administration will impact outcomes, and increased availability of clinical grade drugs for evaluation.
In addition to mAb:I‐O combinations, many groups are combining mAb:I‐O with other treatment modalities such as traditional chemotherapeutics and other types of “immunomodulatory” agents such as radiation/cryotherapy/viral therapy. For example, in a phase III trial, coined IMPOWER 150, the combination of the anti‐PD‐L1 inhibitor, Tecentriq, the anti‐VEGF mAb, Avastin, carboplatin, and paclitaxel provided an OS survival benefit in first line therapy of non‐squamous NSCLC versus appropriate control arms. Importantly, this survival benefit was observed in both PD‐L1–positive and negative populations and in patients with a low gene signature for T effector cells—an observation that may provide additional hope for patients lacking an inflammatory infiltrate.102 Alternatively, several groups are using radiation/cryotherapy/viral therapy to induce antigen specific T cell responses within the tumor and then protecting these nascent antitumor T cells with mAb:I‐O.77, 103, 104 We anticipate that these and other combinatorial approaches will be rapidly adopted into the care of patients with SCCHN.
Clinical Trial Design
The majority of new I‐O products were tested and approved in patients with advanced disease.59 However, while data is emerging for patients with SCCHN, findings in other tumor types suggests that mAb:I‐O may have a role in preventing disease recurrence in high‐risk patients. For example, the anti‐CTLA‐4 mAb, Ipilimumab, significantly enhances recurrence free survival and overall survival in patients with surgically treated stage III melanoma.105, 106, 107 Recent studies augment these findings, demonstrating that the anti–PD‐1 mAb, nivolumab, offers a survival advantage over Ipilimumab in patients with stage IIIB, IIIC, or IV melanoma, who have undergone a complete surgical resection.108, 109 Optimistically, ongoing clinical studies will reveal a similarly efficacious role for post‐treatment PD‐L1:PD‐1 blockade in preventing disease recurrence in patients with advanced SCCHN.
In addition to preventing recurrence, mAb–I‐O has a documented role in the neoadjuvant setting. For example, presurgical administration of nivolumab to patients with early NSCLC, mediated major responses in 45% of patients.110 In patients with HPV+ and HPV‐ resectable SCCHN, preoperative administration of nivolumab mediated a reduction in tumor size in approximately 50% of patients.111 Similar findings were also reported using the anti–PD‐1 mAb pembrolizumab, suggesting that these results are not secondary to a use of a particular drug.112 While we as a field continue to await more mature results, the high response rates associated with PD‐L1:PD‐1 blockade in the neoadjuvant setting, hold tremendous promise for patients presenting with advanced SCCHN—particularly those who are HPV negative.113 Of equal import, as previously mentioned, these window of opportunity trials are also an excellent means to get an early indication of how/whether new I‐O targets are worth resource investment.
CONCLUSIONS
A recent study showing the combination of neoantigen specific adoptive transfer, IL‐2, and checkpoint inhibition was effective in curing a patient with widely metastatic breast cancer suggests that we now have I‐O tools capable of treating solid tumors in some patients.12 In this review, we have outlined several focused areas of research that will optimistically allow similar successes in the treatment of patients with cancers of the head and neck and improve both the quality and quantity of life for patients whose disease, as a whole, has proved largely recalcitrant.
Conflict of Interest: Dr. Scott Strome is a cofounder, consultant, and stockholder in Gliknik Inc., a biotechnology company. He receives royalties for intellectual property, related to B7‐H1 (PD‐L1), licensed by the Mayo Clinic College of Medicine to third parties. He receives research support from Pfizer and Gliknik through sponsored research agreements through the University of Maryland, Baltimore. He also serves as a paid consultant to Astra Zeneca and Roche.
BIBLIOGRAPHY
- 1. Dudley ME, Rosenberg SA. Adoptive‐cell‐transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003;3(9):666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10(9):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pardoll DM. Cancer vaccines. Nat Med 1998;4(5 supp):525–531. [DOI] [PubMed] [Google Scholar]
- 4. Romero P, Banchereau J, Bhardwaj N, et al. The Human Vaccines Project: a roadmap for cancer vaccine development. Sci Transl Med 2016;8(334):334ps9–ps9. [DOI] [PubMed] [Google Scholar]
- 5. Gilboa E, Vieweg J. Cancer immunotherapy with mRNA‐transfected dendritic cells. Immunol Rev 2004;199:251–263. [DOI] [PubMed] [Google Scholar]
- 6. Voskens CJ, Strome SE, Sewell DA. Synthetic peptide‐based cancer vaccines: lessons learned and hurdles to overcome. Curr Mol Med 2009;9(6):683–693. [DOI] [PubMed] [Google Scholar]
- 7. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8(6):467–477. [DOI] [PubMed] [Google Scholar]
- 8. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified t cells in chronic lymphoid leukemia. N Engl J Med 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brudno JN, Kochenderfer JN. Chimeric antigen receptor T‐cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15(1):31–46. [DOI] [PubMed] [Google Scholar]
- 10. Lu Y‐C, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol 2016;28(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 12. Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018;24(6):724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voskens CJ, Sewell D, Hertzano R, et al. Induction of MAGE‐A3 and HPV‐16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck 2012;34(12):1734–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strome SE, Savva A, Brissett AE, et al. Squamous cell carcinoma of the tonsils: a molecular analysis of HPV associations. Clin Cancer Res 2002;8:1093–1100. [PubMed] [Google Scholar]
- 15. Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation‐Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science 2014;344(6184):641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linnemann C, van Buuren MM, Bies L, et al. High‐throughput epitope discovery reveals frequent recognition of neo‐antigens by CD4+ T cells in human melanoma. Nat Med 2015;21(1):81–85. [DOI] [PubMed] [Google Scholar]
- 17. Khodadoust MS, Olsson N, Wagar LE, et al. Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature 2017;543(7647):723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassani‐Sternberg M, Bräunlein E, Klar R, et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun 2016;7:13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell 2017;168(4):724–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359(6382):1361–1365. [DOI] [PubMed] [Google Scholar]
- 21. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin‐T‐cell receptor chimeric molecules as functional receptors with antibody‐type specificity. Proc Nat Acad Sci U S A 1989;86(24):10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4‐1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676–684. [DOI] [PubMed] [Google Scholar]
- 24. Morgan RA, Chinnasamy N, Abate‐Daga DD, et al. Cancer regression and neurologic toxicity following anti‐MAGE‐A3 TCR gene therapy. J Immunother 2013;36(2):133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ertl HCJ, Zaia J, Rosenberg SA, et al. Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA advisory committee symposium held June 15, 2010. Cancer Res 2011;71(9):3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guedan S, Posey AD Jr, Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4‐1BB costimulation. JCI Insight 2018;3(1):96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brentjens R, Davila ML, Riviere I, et al. CD19‐targeted T cells rapidly induce molecular remissions in adults with chemotherapy‐refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5(177):177ra38‐ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target‐related effects. Oncologist 2007;12(9):1084–1095. [DOI] [PubMed] [Google Scholar]
- 29. Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer 2008;8473–480. [DOI] [PubMed] [Google Scholar]
- 30. Taylor R, Chan S‐L, Wood A. FcgRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Can Imm Immunother 2009;58:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López‐Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58(11):1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous‐cell carcinoma of the head and neck. N Engl J Med 2006;354(6):567–578. [DOI] [PubMed] [Google Scholar]
- 33. Petrelli F, Coinu A, Riboldi V, et al. Concomitant platinum‐based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: a systematic review and meta‐analysis of published studies. Oral Oncol 2014;50(11):1041–1048. [DOI] [PubMed] [Google Scholar]
- 34. Kim TK, Herbst RS, Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol 2018;39(8):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Investig 2007;117(5):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuchroo VK, Ohashi PS, Sartor RB, Vinuesa CG. Dysregulation of immune homeostasis in autoimmune diseases. Nat Med 2012;18(1):42–47. [DOI] [PubMed] [Google Scholar]
- 37. Dong H, Zhu G, Tamada K, Chen L. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 38. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11(11):3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pai SI, Zandberg DP, Strome SE. The role of antagonists of the PD‐1:PD‐L1/PD‐L2 axis in head and neck cancer treatment. Oral Oncol 2016;61:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zandberg DP, Strome SE. The role of the PD‐L1:PD‐1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2014;50(7):627–632. [DOI] [PubMed] [Google Scholar]
- 42. Dong H, Strome SE, Salomao DR, et al. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- 43. Strome SE, Dong H, Tamura H, et al. B7‐H1 blockade augments adoptive T‐cell immunotherapy for squamous cell carcinoma. Cancer Res 2003;63(19):6501–6505. [PubMed] [Google Scholar]
- 44. Tsushima F, Tanaka K, Otsuki N, et al. Predominant expression of B7‐H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol 2006;42(3):268–274. [DOI] [PubMed] [Google Scholar]
- 45. Lee Y, Shin JH, Longmire M, et al. CD44+ cells in head and neck squamous cell carcinoma suppress T‐cell–mediated immunity by selective constitutive and inducible expression of PD‐L1. Clin Cancer Res 2016;22(14):3571–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Jie H‐B, Lei Y, et al. PD‐1/SHP‐2 inhibit Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res 2015;75(3):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lyford‐Pike S, Peng S, Young GD, et al. Evidence for a role of the PD‐1:PD‐L1 pathway in immune resistance of HPV‐associated head and neck squamous cell carcinoma. Cancer Res 2013;73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferris RL, Blumenschein GJ, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen E, Harrington KJ, Le Torneau C, et al. LBA45_PR ‐ Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE‐040. Ann Oncol 2017;28(Suppl 5):v605‐v49. [Google Scholar]
- 50. Love RR, Leventhal H, Easterling DV, Nerenz DR. Side effects and emotional distress during cancer chemotherapy. Cancer 1989;63(3):604–612. [DOI] [PubMed] [Google Scholar]
- 51. Hoos A. Development of immuno‐oncology drugs—from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15(4):235–247. [DOI] [PubMed] [Google Scholar]
- 52. Chen L, Flies DB. Molecular mechanisms of T cell co‐stimulation and co‐inhibition. Nat Rev Immunol 2013;13(4):227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366(10):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 55. Michot JM, Bigenwald C, Champiat S, et al. Immune‐related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 56. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 2009;58(5):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7‐H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101(49):17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kluger HM, Zito CR, Turcu G, et al. PD‐L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res 2017;23(15):4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herbst RS, Soria J‐C, Kowanetz M, et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1–positive non–small‐cell lung cancer. N Engl J Med 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 62. Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by By‐H1 autoantibodies in rheumatoid arthritis. J Clin Investig 2003;111(3):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khunger M, Bordeaux J, Dakappagari N, et al. Tumor PD‐L1 heterogeneity in non‐small cell lung cancer: Does biopsy size and volume matter? J Clin Oncol 2018;36(Suppl):12058. [Google Scholar]
- 64. Udall M, Rizzo M, Kenny J, et al. PD‐L1 diagnostic tests: a systematic literature review of scoring algorithms and test‐validation metrics. Diagn Pathol 2018;13(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD‐L1 immunohistochemistry for pulmonary squamous‐cell and adenocarcinomas. Mod Pathol 2016;29(10):1165–1172. [DOI] [PubMed] [Google Scholar]
- 66. Carbone DP, Reck M, Paz‐Ares L, et al. First‐line nivolumab in stage IV or recurrent non–small‐cell lung cancer. N Engl J Med 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD‐1 inhibition. N Engl J Med 2017;377(25):2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Le DT, Uram JN, Wang H, et al. PD‐1 Blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le DT, Durham JN, Smith KN, et al. Mismatch‐repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18(9):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor‐outcome classes of head and neck squamous cell carcinoma. Oral Oncol 2013;49(3):211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non inflamed tumor microenvironment. Semin Oncol 2015;42(4):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ayers M, Lunceford J, Nebozhyn M, et al. IFN‐γ–related mRNA profile predicts clinical response to PD‐1 blockade. J Clin Investig 2017;127(8):2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res 2016;27(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haanen JBAG. Converting cold into hot tumors by combining immunotherapies. Cell 2017;170(6):1055–1056. [DOI] [PubMed] [Google Scholar]
- 77. Samson A, Scott KJ, Taggart D, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med 2018;10(422):7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corrales L, Gajewski TF. Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. Cytokine 2016;77:245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD‐L1 and TGF‐β. Sci Transl Med 2018;10(424):5488. [DOI] [PubMed] [Google Scholar]
- 80. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD‐1 efficacy in metastatic melanoma patients. Science 2018;359(6371):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD‐1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD‐1–based immunotherapy against epithelial tumors. Science 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 83. Dahan R, Sega E, Engelhardt J, Selby M, Korman Alan J, Ravetch Jeffrey V. FcgRs modulate the anti‐tumor activity of antibodies targeting the PD‐1/PD‐L1 axis. Cancer Cell 2015;28(3):285–295. [DOI] [PubMed] [Google Scholar]
- 84. Sallin M, Zhang X, So E, et al. The anti‐lymphoma activities of anti‐CD137 monoclonal antibodies are enhanced in FcγRIII−/− mice. Cancer Immunol Immunother 2014;63(9):947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sun H, Olsen HS, Mérigeon EY, et al. Recombinant human IgG1 based Fc‐multimers, with limited FcR binding capacity, can effectively inhibit complement‐mediated disease. J Autoimmun 2107;84:97–108. [DOI] [PubMed] [Google Scholar]
- 86. Zhou H, Olsen H, So E, et al. A fully recombinant human IgG1 Fc multimer (GL‐2045) inhibits complement‐mediated cytotoxicity and induces iC3b. Blood Adv 2017;1(8):504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jain AJ, Olsen HS, Vyzasatya R, et al. Fully Recombinant Murine Stradomers™ effectively prevent idiopathic thrombocytopenic purpura and treat arthritis in mice. Arthritis Res Ther 2012;14(4):R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Levin D, Golding B, Strome SE, Sauna ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol 2016;33(1):27–34. [DOI] [PubMed] [Google Scholar]
- 89. Tradtrantip L, Felix CM, Spirig R, Morelli AB, Verkman AS. Recombinant IgG1 Fc hexamers block cytotoxicity and pathological changes in experimental in vitro and rat models of neuromyelitis optica. Neuropharmacology 2018;133:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Spirig R, Campbell IK, Koernig S, et al. rIgG1 Fc hexamer inhibits antibody‐mediated autoimmune disease via effects on complement and FcγRs. J Immunol 2018;200(8):2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7‐H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008;111(7):3635–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Buchbinder EI, Desai A. CTLA‐4 and PD‐1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park J‐J, Omiya R, Matsumura Y, et al. B7‐H1/CD80 interaction is required for the induction and maintenance of peripheral T‐cell tolerance. Blood 2010;116(8):1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4‐1BB T‐cell activation molecule eradicate established tumors. Nat Med 1997;3(6):682–685. [DOI] [PubMed] [Google Scholar]
- 95. Schmitz S, Duhoux F, Machiels J‐P. Window of opportunity studies: do they fulfil our expectations? Cancer Treat Rev 2016;43:50–57. [DOI] [PubMed] [Google Scholar]
- 96. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 2014;14(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bauman JE, Cohen E, Ferris RL, et al. Immunotherapy of head and neck cancer: Emerging clinical trials from a National Cancer Institute Head and Neck Cancer Steering Committee Planning Meeting. Cancer 2016;123(7):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med 2013;69(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perez‐Ruiz E, Etxeberria I, Rodriguez‐ruis M, Melero I. Anti‐CD137 and PD‐1/PD‐L1 antibodies in route towards clinical synergy. Clin Cancer Res 2017;23(18):5326–5328. [DOI] [PubMed] [Google Scholar]
- 101. Tolcher AW, Sznol M, Hu‐Lieskovan S, et al. Phase Ib study of utomilumab (PF‐05082566), a 4‐1BB/CD137 agonist, in combination with pembrolizumab (MK‐3475) in patients with advanced solid tumors. Clin Cancer Res 2017;23(18):5349–5357. [DOI] [PubMed] [Google Scholar]
- 102. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 103. Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, et al. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol 2017;90(1076):20170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers. Front Oncol 2018;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eggermont AMM, Chiarion‐Sileni V, Grob J‐J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375(19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Eggermont AMM, Chiarion‐Sileni V, Grob J‐J, et al. Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): a randomised, double‐blind, phase 3 trial. Lancet Oncol 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 107. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double‐blind, multicentre, phase 2, dose‐ranging study. Lancet Oncol 2010;11(2):155–164. [DOI] [PubMed] [Google Scholar]
- 108. Schuchter LM. Adjuvant melanoma therapy—head‐spinning progress. N Engl J Med 2017;377(19):1888–1890. [DOI] [PubMed] [Google Scholar]
- 109. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 110. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med 2018;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ferris RL, Gonçalves A, Baxi SS, et al. LBA46 An open‐label, multicohort, phase 1/2 study in patients with virus‐associated cancers (CheckMate 358): safety and efficacy of neoadjuvant nivolumab in squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol 2017;28(Suppl 5):mdx440.041–mdx440.041. [Google Scholar]
- 112. Uppaluri R, Zolkind P, Lin T, et al. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2017;35(15 Suppl):6012. [Google Scholar]
- 113. Hanna GJ, Adkins DR, Zolkind P, Uppaluri R. Rationale for neoadjuvant immunotherapy in head and neck squamous cell carcinoma. Oral Oncol 2017;73:65–69. [DOI] [PubMed] [Google Scholar]