Abstract
Objective
To use a unique, 41‐question survey to identify patient features distinguishing cervical vertigo from vestibular causes of vertigo and vestibular migraine.
Methods
In this study, a unique, 41‐question survey was administered to 48 patients diagnosed with cervical vertigo (n = 16), migraine (n = 16), and vestibular vertigo (eg, unilateral vestibular paresis, Meniere's disease) (n = 16) to test the hypothesis that a set of distinct symptoms can characterize cervical vertigo. Responses between the three diagnostic groups were compared to identify questions which differentiated patients based on their symptoms.
Results
Eight questions were successful in differentiating vestibular vertigo from migraine and cervical vertigo. Symptoms endorsed by subjects with cervical vertigo overlapped substantially with subjects with well‐established vestibular disturbances as well as symptoms of subjects with migraine. Twenty‐seven percent of cervical vertigo subjects reported having true vertigo, 50% having headache, and 94% having neck pain.
Conclusion
Lacking knowledge of neck disturbance, the symptoms we elicited in our questionnaire suggest that cervical vertigo subjects may resemble migraine subjects who also have evidence of neck injury. Whether or not subjects with “cervical vertigo” also overlap with other diagnoses defined by a combination of symptoms and exclusion of objective findings such as chronic subjective dizziness and other variants of psychogenic dizziness remain to be established.
Level of Evidence
IV
Keywords: Cervical Vertigo, cervicogenic dizziness, vertigo, neck injury, symptoms, migraine
INTRODUCTION
Ryan and Cope defined cervical vertigo as vertigo caused by “disease of the neck.”1 The definition of cervical vertigo that we will use in this paper is “dizziness combined with a neck disorder, where reasonable alternatives have been ruled out.” This definition resembles that of Heikkila2 as well as Wrisley.3 We furthermore use the term “dizziness” to indicate a sensation of spatial disorientation, possibly but not necessarily including an illusion of motion or unsteadiness.
Cervical vertigo by this definition is common in certain contexts. Table 1 summarizes data from seven large studies of whiplash injuries. Out of a total of 1097 subjects, 52% of them reported dizziness. Cervical vertigo is a matter of considerable concern due to the high litigation‐related costs of whiplash injuries.4, 5 Dizziness is also reported by subjects with neck injuries due to other processes than whiplash (Table 1), although fewer studies are available. Animal studies have established that experimental lesions confined to the cervical area can induce nystagmus and ataxia.6, 7, 8, 9 In humans, recent theories suggest that cervical vertigo may be due to discrepancies between vestibular and proprioceptive information, precipitated by inflammation within the cervical intervertebral discs and increased muscle tension.10, 11 However, as of yet, no consensus of expert opinion defines the entity of cervical vertigo, and review articles concerning diagnostic efforts in humans often are mainly concerned with the question as to whether or not cervical vertigo even exists.12, 13, 14, 15
Table 1.
Symptoms Reported in Previous Studies of Whiplash and Non‐Whiplash Subjects.
| % subjective complaints | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | N | Dizziness | Neck Pain | Imbalance/Unsteadiness | Headache | Cognitive | Nausea/GI | Vision | Hearing | Psychological |
| Studies focused on whiplash injury | ||||||||||
| Norris and Watt 1983 | 61 | 3 | 100 | ‐ | 66 | ‐ | ‐ | 8 | 8 | ‐ |
| Hildingsson and Toolanen 1990 | 93 | 23 | 88 | ‐ | 54 | ‐ | ‐ | 9 | 4 | ‐ |
| Oosterveld et al. 1991 | 262 | 85 | 92 | ‐ | 87 | 34 | ‐ | 24 | 15 | 31 |
| Barrett et al. 1995 | 29 | 10–21 | 100 | ‐ | 52–72 | 24–28 | ‐ | ‐ | ‐ | 10–59 |
| Radanov et al. 1992 | 51 | 67 | 31 | 22 | 80 | 33–63 | ‐ | 27–41 | 39 | 20–49 |
| Treleaven et al. 2003 | 105 | 15–60 | 60 | 25–52 | 56 | 21–35 | 40 | 21–38 | 25 | 21 |
| Cobo et al. 2010 | 557 | 45 | 100 | ‐ | 52 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Weighted average | 52 | 96 | 33 | 66 | 34 | 40 | 24 | 18 | 29 | |
| Studies focused on dizziness and neck‐disturbance (non‐whiplash) | ||||||||||
| Karlberg et al. 1996 | 17 | 100 | 100 | 59 | 76 | ‐ | 71 | ‐ | 24 | ‐ |
| Bracher et al. 2000 | 15 | 100 | 87 | ‐ | 47 | ‐ | ‐ | ‐ | ‐ | 67 |
| Malmstrom et al. 2007 | 22 | 100 | 100 | 63 | 77 | ‐ | 23 | ‐ | ‐ | 5 |
| Weighted average | 100 | 96 | 61 | 68 | ‐ | 44 | ‐ | 24 | 30 | |
The main clinical problem in diagnosing cervical vertigo is that symptoms of subjects who have both neck disorders and dizziness may overlap with other entities. In other words, it is difficult to differentiate between the chance coincidence of arthritis of the neck and dizziness, from the situation where arthritis of the neck causes dizziness.16, 17 Additionally, clinicians find similar symptomatology is associated with psychogenic dizziness as well as migraine headache.18, 19, 20
To test the hypothesis that a set of distinct symptoms can characterize cervical vertigo, we administered a questionnaire to subjects with cervical vertigo, vestibular migraine and those with vestibular vertigo. Herein we report the differences in responses from subjects in each group.
MATERIALS AND METHODS
Population Under Study
After obtaining approval from the Institutional Review Board (IRB) of Northwestern University, we prospectively surveyed 48 subjects in three diagnostic groups: vestibular disorders (16), migraine‐associated vertigo (16), and cervical vertigo (16). All subjects presented to our tertiary care clinic for care and had diagnoses assigned by an expert in the field of otoneurology (coauthor T.C.H.) using a combination of history, a comprehensive set of vestibular and auditory tests, and imaging. After receiving a final diagnosis, every patient was offered an opportunity to complete our questionnaire until the predetermined cap for subject enrollment was reached. Questionnaires were administered by the author (A.T.H.), who was not involved in the diagnosis of subjects and was not aware of subjects' diagnoses during administration.
In the vestibular disorder group, there were 10 subjects with vestibular neuritis and three subjects with unilateral vestibular loss other than vestibular neuritis (Ramsay‐Hunt syndrome, status‐post posterior fossa surgery for trigeminal nerve decompression, and one idiopathic unilateral vestibular loss). These were all associated with >40% unilateral paresis on electronystagmography (ENG) caloric testing. Three additional subjects had definite Meniere's diagnosed by criteria established by the American Academy of Otolaryngology–Head and Neck Surgery Committee on Hearing and Equilibrium.21
Subjects in the migraine group all met the criteria for vestibular migraine, also termed migraine‐associated vertigo/dizziness, established by the Headache Classification Committee of the International Headache Society (IHS).22 Subjects diagnosed with posttraumatic migraine were not included in this study due to the possibility of an associated neck injury.
For cervical vertigo subjects, we excluded vestibular causes of vertigo by requiring a normal ENG, rotary chair test, and positional testing. History, audiologic testing, and physical examination eliminated conditions such as Meniere's syndrome. Central causes of vertigo were excluded by brain imaging. None of the subjects met criteria for vestibular migraine. Posttraumatic vertigo subjects were included. Although an abnormal cervical magnetic resonance imaging was not an absolute requirement to be diagnosed with cervical vertigo (Table 2), all subjects had an abnormal cervical MRI. Thirteen subjects had a disk or spur touching the anterior spinal cord, and the remaining only showed degenerative disc changes. Eight subjects exhibited severe stiffness of the neck to palpation. Seven subjects related a history of temporal proximity of dizziness to an injury confined to the neck, including five subjects having whiplash and one with a neck injury due to a fall. Four subjects had cervical spondylosis on MRI as their only evidence for a neck injury.
Table 2.
Criteria Used to Diagnose Cervical Vertigo.
|
Development of Questionnaire
We developed a unique, 41‐question survey (Appendix A) using questions from two well‐established questionnaires: the Dizziness Handicap Inventory (DHI)23 and the Neck Disability Index (NDI) questionnaire.24 Our goal in using both questionnaires was to comprehensively identify common factors associated with dizziness and a neck disorder. Questions adapted from the DHI allowed us to capture symptoms found in the dizzy patient reliably. The NDI provided us with a list of functional problems seen in patients with a neck disorder (eg, whiplash injury, cervical radiculopathy). The latter questions were compared to those abstracted from the DHI to ensure all questions assessing pain and activities of daily living in neck disorder patients were also included in those evaluating dizziness.
Out of the original 25 DHI questions, we selected 18 questions. The remaining 21 questions were created by referencing sections of the NDI questionnaire that focused on the patient's response to neck pain concerning their associated symptoms of headache and vertigo. Two questions were duplicates, to determine whether subjects were consistent. Questions #1 and #2 were demographic questions and were not included in further analyses.
As part of the questions included in the survey, we asked subjects if a head or neck injury caused their dizziness (Question #35) and if acute dizziness medications such as meclizine or diazepam helped (Question #41). Some questions such as “Does chewing affect your symptoms?”(Question #27) were included in an attempt to detect individuals who tended to answer all questions in the affirmative.
Similar to the DHI, answers to questions were scored using “0” for negative responses (No), “1” for partially positive responses (Somewhat), and “2” for exact positive responses (Yes). We decided to use this scoring method to more easily mirror that of the DHI questionnaire, from which many of our questions were adapted. It should be noted that there were no questions taken directly from the NDI questionnaire; thus, scoring was not influenced at all by that of the NDI. Questions developed from the NDI were written so as to be scored like the DHI (ie, 0, 1, 2). No attempt was made to assess the severity of endorsed symptoms.
There were a total of 1842 questions asked throughout all surveys performed in this study. Out of these, 29 (1.6%) were unanswered, either being left blank (n = 14) or deemed N/A (n = 15). Four questions were unanswered in one survey, three questions were unanswered on three surveys, two questions unanswered on two surveys, and one question unanswered on 12 surveys. All 14 questions left blank appeared to be randomly distributed among surveys.
Patients who determined questions about their symptoms did not apply were instructed to write in “N/A” instead of marking an answer. These responses were scored as “0,” as they indicated a response closest to “No.” There were four of these in the cervical vertigo group, five in the vestibular vertigo group, and six in the vestibular migraine group.
There were 14 questions left blank. Out of these, six were in the cervical vertigo group, five in the vestibular vertigo group, and three in the vestibular migraine group. These patients' missing responses were scored as “1″ instead of “0″ or “2″ as an item mean imputation. We used this method of handling the missing data due to the low percentage of missing data and subsequent low likelihood of bias. A multiple imputations model was also used to assess the potential impact of the missing data on results. No differences in the results noted below were seen.
Statistical Analysis
All statistical analysis was done using SPSS v21 (IBM Corporation, Armonk, NY). Standard descriptive statistics were used to study the sample population and the distribution of survey responses. Gender and age differences between groups were studied using a Chi‐square test and Mann–Whitney U nonparametric test, respectively. All analyses were done using results from the three‐response scoring method described previously. The Cronbach alpha coefficient analysis was employed to measure the internal consistency of our questionnaire. Corrected item‐total correlations were performed to measure the strength of the relationship between an individual item and all remaining questions in a subsection. Because the alpha coefficient is a function of the item‐total correlation, questions with high item‐total correlation values contribute more to a scale's reliability and may be considered more representative of the scale's content than the other questions producing low item‐total correlations (r < 0.30). A two‐way contingency table analysis (chi‐square test) was used to identify which questions in our questionnaire produced more symptom endorsements between our diagnostic groups. This analysis was supplemented by Cramér's V test for designating the strength of symptom association.
Additionally, follow‐up pairwise comparisons using the Holm's sequential Bonferroni method were employed to evaluate differences in endorsements across all three diagnostic groups. An alpha level of less than 0.05 was used to establish statistical significance in all analyses. To demonstrate whether a patient endorsed a symptom or not, similar to the NDI, we later combined “Sometimes” and “Yes” as positive responses. These were then calculated solely as item “endorsement” frequencies, reported as a percentage.
RESULTS
Table 3 contains the demographic features of our subjects. Subjects ranged in age from 24 to 85 years, with a mean age of 55 (SD, 13.1 years). There was no overall statistically significant difference in age or gender between the three diagnostic groups.
Table 3.
Demographic Information of Subjects, n = 48.
| Diagnostic group | N | % male | age |
|---|---|---|---|
| Vestibular Vertigo | 16 | 44% | 37–82 |
| Migraine | 16 | 19% | 24–68 |
| Cervical Vertigo | 16 | 31% | 32–85 |
Supplemental Table I (see online supporting information) is a summary of responses to each question, according to diagnostic group. We organized the questions into four content domains: Associated Symptoms, Physical Disability, Functional Disability, and Psychosocial Disability. The overall internal consistency of the questionnaire had a Cronbach alpha value of 0.908. All corrected item‐total correlations were above 0.3 except for six questions (Questions #3, 26, 33–35, and 38). Cronbach alpha values for the four content domains named previously were 0.807, 0.626, 0.680, and 0.727, respectively. No further item reliability analyses were conducted, as the aim of this study was to find differences in responses among diagnostic groups rather than to revise the current questionnaire. Out of 1872 responses, 14 questions were not responded to, and 15 were noted as not applicable, with 77% of the latter arising from asking whether medications for vertigo (Antivert or Valium) had any effect on symptoms (Question #41).
There were eight questions for which subjects' responses differed significantly across groups on pairwise comparison. Figure 1 shows the performance of these questions.
Figure 1.
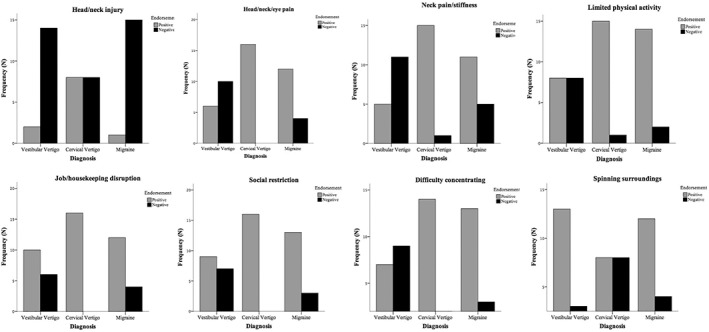
Endorsement frequencies for symptoms that significantly differentiated cervical vertigo (From left to right and top to bottom, Questions #35, 22, 5, 10, 19, 8, 16, and 21, respectively)
Head or Neck Injury
A head or neck injury was reported as a cause of symptoms (Question #3) in 50% of our cervical vertigo subjects (CV), and these subjects were distinguished from migraine (6%) and vestibular vertigo (VV) (13%), Pearson χ2 (2, N = 48) =10.14, P = .006, Cramér's V = 0.46. Compared to CV, responses were significantly less frequent for both VV (P = .022) and migraine (P = .006). Responses between VV and migraine subjects were not statistically significant.
Pain
There were two questions concerning pain. There was a significant difference in how each diagnostic group reported neck pain or stiffness (Question #5), Pearson χ2 (24 N = 48) = 21.79, P = .000, Cramér's V = 0.48, as well as head, neck or eye pain (Question #22), Pearson χ2 (4, N = 48) = 19.64, P = .001, Cramér's V = 0.45. Subjects within each diagnostic group endorsed both pain‐related questions at similar frequencies. Out of the 65% of subjects who reported experiencing only neck pain or stiffness, 94% were CV subjects, 69% were diagnosed with migraine, and 31% had VV. There was no significant difference in responses to more general pain (head, neck or eye) between those diagnosed with CV and migraine (P = .069), but responses were significantly more frequent in migraine (P = .038) and CV (P = .000) when compared to VV. Neck pain/stiffness was significantly more frequent in CV when compared to migraine (P = .014) and VV (P = .000) but showed no difference when migraine patients were compared to VV (P = .093).
Limited Physical Activity
Subjects' responses differed significantly when asked if they were limited by physical activity (Question #10), Pearson χ2 (4, N = 48) = 11.92, P = .018, Cramér's V = 0.352. 94% CV subjects endorsing this item did not differ significantly from migraine subjects but did when compared to VV (P = .010). 88% of migraine subjects reported being significantly limited by physical activity than 50% of VV subjects (P = .027).
Job/Housekeeping Disruption
CV subjects indicated that their problem was interfering with their job or housekeeping activities (Question #19) at a greater frequency (100%) than VV (69%) or migraine (75%), Pearson χ2 (4, N = 48) = 10.63, P = .031, Cramér's V = 0.33. Responses differed significantly between CV and VV (P = .005) but did not significantly differ when comparing VV to migraine (P = .144). The level of this disability did not differ significantly when comparing CV with those diagnosed with migraine (P = .101).
Social Participation
One hundred percent of our CV subjects reported feeling restricted from social participation while 81% of migraine and 56% of VV subjects endorsed the symptom (Question #8), Pearson χ2 (4, N = 48) = 11.16, P = .025, Cramér's V = 0.34. Responses to this question differentiated CV from VV at a statistically significant level (0.007) but not when compared to migraine (0.165). Differences in reported level of social restriction were not seen between VV and migraine (P = .108).
Difficulty Concentrating
CV subjects (88%) endorsed difficulty concentrating (Question #16) as much as migraine subjects (81%). This was less frequent in VV (44%). Differences in difficulty concentrating differed between diagnostic groups at a statistically significant level, Pearson χ2 (4, N = 48) = 12.02, P = .017, Cramér's V = 0.354. We found no significant difference between the mean responses of migraine and CV (P = .865) but showed significantly more frequent concentration difficulty in CV (0.007) and migraine (0.014) when compared to VV.
Vertigo (Spinning Surroundings)
Responses of spinning surroundings (Question #21) showed a significantly higher endorsement frequency in VV than CV subjects (P = .008), but no differences were seen when compared to migraine; this did not differentiate CV from migraine. However, this item did not achieve statistical significance when asked in a similar form later in the questionnaire (Question #21).
While one would expect that positional symptoms would be common only in VV subjects, instead, the frequency of endorsement of symptoms elicited by “turning over in bed” (Question #13) was substantial and almost identical across groups (44–56%). However, the frequency of symptoms elicited by “looking up” (Question #3) was the lowest for VV (38%) while symptoms elicited by “getting into/out of bed” (Question #7) was the highest for CV (56%). This suggests that contrary to what one would suppose, positional symptoms do not differentiate between causal categories.
Chewing (Question #27) was indicated as a cause of symptoms by 25% of subjects with CV. This was also reported by 6% of subjects with migraine and 13% of subjects with VV. We included this question in an attempt to detect individuals who responded yes to most questions.
Nausea (Question #28) was more common in CV (50%) and VV (44%) than migraine subjects (25%). This did not differentiate our diagnostic groups, and responses between groups were not significantly different.
DISCUSSION
This study documented that the symptoms endorsed by subjects with cervical vertigo, migraine, and vestibular vertigo overlap. While symptoms endorsed in our questionnaire successfully differentiated vestibular vertigo from migraine and cervical vertigo, lacking knowledge of neck disturbance, they did not differentiate migraine well from cervical vertigo. In other words, in our study, cervical vertigo subjects resembled migraine subjects who also had evidence of neck injury.
There have been no previous attempts to use a questionnaire by combining both the DHI and NDI to differentiate symptoms in cervical vertigo. Reid et al.25 used the DHI to characterize cervical vertigo with a goal of creating a shortened version to assess the diagnostic group. While their study also found CV patients endorsing significantly more job/household disruption, none of the questions they identified for their brief assessment tool (analogous to our Questions #3, 11–12) nor other differentiating questions (analogous to our Questions #7 and 9) reached statistical significance seen in our current study. This may be due to differences in study design, as they compared CV patients with those diagnosed with “general dizziness,” which combined migraine, vestibular neuronitis, among others. In other words, the exact proportions of diagnostic groups within the “general dizziness” group were unclear; wherein there may have been an effect between responses. This study looked to accomplish this while also assessing that attributable to neck disability.
Deng et al.26 attempted to assess symptoms of cervical vertigo by developing a Chinese version of Yardley's Vertigo Symptoms Scale.27 Their questionnaire evaluated both the frequency and duration of symptoms, which ranged from true vertigo to lower back pain. Unfortunately, they did not report the rate of their symptomatic endorsements. Further, their questionnaire focused on patients diagnosed with vertebral artery type of cervical vertigo whereas we did not specify in our study. Their study did show, however, that a questionnaire could be reliable in evaluating changes in the degree of complaints in patients with cervical vertigo.
Although questions regarding neck injury or neck pain were powerful ones to differentiate among subject groups, endorsement of these questions in cervical vertigo patients was required by the structure of this study, as we required evidence of a neck abnormality to be diagnosed as cervical vertigo. Neck injury or neck pain is generally required to diagnose a patient as having cervical vertigo.3, 28 Similarly, the cervical vertigo literature reports that neck pain is widespread in subjects diagnosed with CV (96% for papers summarized in Table 1). Neck pain can also be a feature of migraine.29 As neck disturbance is required to diagnose cervical vertigo subjects, there is no implication of causality or, for that matter, any suggestion that cervical vertigo is different from other entities defined by symptoms alone. In other words, by requiring a neck disturbance, studies of cervical vertigo could be selecting subjects with other causes of vertigo, such as migraine or anxiety that also had a neck disturbance. However, Brandt and Huppert10 recently described patients with vertigo based on head motion–induced misalignment of sensorimotor integration, without any history of head or neck injury.
Recent studies have identified possible objective measures of neck contribution in those diagnosed with cervical vertigo.17, 30, 31 When compared to healthy control subjects, Johnston et al. found that a prolonged reduction in vestibular response to head movement (ie, VOR) when patients attempted to acquire visual targets with cervical soft tissue disorders and normal neuro‐otologic examinations.31 Their results suggest that neck limitations, due to pain or stiffness, manifest an exaggerated proprioceptive response and subsequent disproportionate VOR suppression, which translates into subjective symptoms of dizziness and imbalance.
This study found CV subjects reporting significantly more physical limitation. Although this question (#19) does not help discern an exact reason, CV patients have been reported have an abnormal cervical range of motion32 and some benefit from physical therapy.33 This work further highlights the interplay between functional status and symptomatic involvement, which parallels the emerging neural mismatch concept.10
All three patient groups endorsed work and social disruption with high frequency, but the group who had the least disturbance was the vestibular subjects. Previous literature has also documented disruption of work activities by migraine34 and vestibular disorders.35, 36
All three groups of subjects reported a disturbance of cognition (difficulty concentrating) to various degrees. This is consistent with previous literature that documents disturbance of cognition in all three groups.37, 38, 39, 40 Our vestibular subjects reported a disturbance of concentration much less commonly than did our cervical and migraine subjects. Twenty‐seven percent of our cervical vertigo subjects complained of vertigo—ie, spinning sensations. While less than our migraine or vestibular subjects (both 37%), this suggests that contrary to Brandt,12 true vertigo is endorsed by cervical subjects with a similar prevalence as subjects with well‐documented vestibular disorders endorse it. Additionally, the frequency of symptoms elicited by “turning over in bed” was substantial and almost identical across groups (44–56%). However, the frequency of symptoms elicited by “looking up” was the lowest for vestibular vertigo subjects (44%) while symptoms elicited by “getting into/out of bed” was the highest for cervical vertigo subjects (75%). Thus, a history of positional symptoms does not differentiate between vestibular migraine, vestibular syndromes, and cervical vertigo.
Our study suggested that there is a similar frequency of difficulty reading and blurred vision in migraine (81%) and cervical vertigo subjects (75%). Only 44% of vestibular vertigo subjects had these symptoms. Reports of visual disturbances in these conditions are consistent with previous studies.41, 42, 43, 44
We asked subjects if they had dizziness preceding head pain, expecting that this might differentiate between vestibular subjects whose dizziness is independent of pain and the cervical/migraine group who had both pain and dizziness. This symptom was frequently endorsed in both subjects with migraine and cervical vertigo, less commonly in vestibular vertigo subjects. Previous studies of whiplash or cervical vertigo have not examined the timing of dizziness and head pain. For migraine, it is well known that some variants of vestibular migraine have a vertiginous aura before a headache, and in some, the headache and vertigo occur at the same time.45, 46 Dizziness before head pain, by itself, was not a successful method of separating out subjects.
Other than having a small study population, one of the main limitations of this study is that we did not attempt to differentiate between different categories of cervical vertigo (eg, rotational vertebral artery syndrome (RAS), whiplash‐associated disorder (WAD), degenerative cervical disorders).47 It is possible that responses from CV subjects to questions that overlapped with migraine may be better differentiated when stratified into these categories. As well, as this study affirms a questionnaire may not be the best tool to differentiate cervical vertigo from other entities, more objective measurements remain warranted to characterize cervical vertigo.
CONCLUSION
The symptoms endorsed by subjects with cervical vertigo overlap substantially with subjects with well‐established vestibular disturbances as well as symptoms of subjects with migraine. The history, at least as expressed by this subset of questions from the well‐established dizziness handicap inventory and neck disability index questionnaires, is not sufficient to differentiate cervical vertigo subjects from those with migraine or vestibular disorders.
The responses of subjects with cervical vertigo and migraine overlapped to a much greater extent than that of subjects with well‐established vestibular problems. This supports the conjecture of Yacovino48 that part of the cervical vertigo population, could be viewed as migraine variants. Whether or not subjects with “cervical vertigo” also overlap with other diagnoses defined by a combination of symptoms and exclusion of objective findings such as chronic subjective dizziness and other variants of psychogenic dizziness remain to be established. Particularly helpful to the field at this writing would be a simple objective method of documenting a correlate of “dizziness” after perturbing the neck alone.
Supporting information
Supplementary Table 1: Relative frequencies of reported symptom endorsement, with respect to subjects' presenting problem, n = 48
Appendix A: Dizziness Handicap Inventory‐Cervical
ACKNOWLEDGMENTS
Jessica Buchanan, Sarah Kennedy, Steven Liska, and Alexis Roseman assisted at an early stage of this project.
Conflict of Interest: The authors declare that they have no conflicts of interest.
Funding: There was no funding for this study.
BIBLIOGRAPHY
- 1. Ryan GM, Cope S. Cervical vertigo. Lancet 1955;269(6905):1355–1358. [DOI] [PubMed] [Google Scholar]
- 2. Heikkilä H . The vertebral column In: Boyling J JG, Twomey P, eds. Grieve's Modern Manual Therapy. 3rd ed. Edinburgh: Churchill Livngstone; 2004. [Google Scholar]
- 3. Wrisley DM, Sparto PJ, Whitney SL, Furman JM. Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther 2000;30(12):755–766. [DOI] [PubMed] [Google Scholar]
- 4. Gudmundsson S, Oddsdottir G, Runarsson T, Sigurdsson S, Kristjansson E. Detecting fraudulent whiplash claims by support vector machines. Biomed Signal Proces 2010;5(4):311–317. [Google Scholar]
- 5. Zaloshnja E, Miller TR, Blincoe LJ. Costs of alcohol‐involved crashes, United States, 2010. Ann Adv Automot Med 2013;57:3–12. [PMC free article] [PubMed] [Google Scholar]
- 6. Biemond A, De Jong JM. On cervical nystagmus and related disorders. Brain 1969;92(2):437–458. [DOI] [PubMed] [Google Scholar]
- 7. Igarashi M, Miyata H, Alford BR, Wright WK. Nystagmus after experimental cervical lesions. Laryngoscope 1972;82(9):1609–1621. [DOI] [PubMed] [Google Scholar]
- 8. Manzoni D, Pompeiano O, Stampacchia G. Cervical control of posture and movements. Brain Res 1979;169(3):615–619. [DOI] [PubMed] [Google Scholar]
- 9. de Jong PT, de Jong JM, Cohen B, Jongkees LB. Ataxia and nystagmus induced by injection of local anesthetics in the Neck. Ann Neurol 1977;1(3):240–246. [DOI] [PubMed] [Google Scholar]
- 10. Brandt T, Huppert D. A new type of cervical vertigo: Head motion‐induced spells in acute neck pain. Neurology 2016;86(10):974–975. [DOI] [PubMed] [Google Scholar]
- 11. Peng B. Cervical vertigo: historical reviews and advances. World Neurosurg 2018;109:347–350. [DOI] [PubMed] [Google Scholar]
- 12. Brandt T. Cervical vertigo—reality or fiction? Audiol Neurootol 1996;1(4):187–196. [DOI] [PubMed] [Google Scholar]
- 13. Brandt T, Bronstein AM. Cervical vertigo. J Neurol Neurosurg Psychiatry. 2001;71(1):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yacovino D. Cervical vertigo: myths, facts, and scientific evidence. Neurologia 2012; 10.1016/j.nrl.2012.06.013. [DOI] [Google Scholar]
- 15. Guinand N, Guyot JP. [Cervical vertigo: myth or reality?]. Revue medicale suisse 2009;5(219):1922–1924. [PubMed] [Google Scholar]
- 16. Machaly SA, Senna MK, Sadek AG. Vertigo is associated with advanced degenerative changes in patients with cervical spondylosis. Clin Rheumatol 2011;30(12):1527–1534. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Yang C, Pang X, et al. Mechanoreceptors in diseased cervical intervertebral disc and vertigo. Spine 2017;42(8):540–546. [DOI] [PubMed] [Google Scholar]
- 18. Furman JM, Jacob RG. Psychiatric dizziness. Neurology 1997;48(5):1161–1166. [DOI] [PubMed] [Google Scholar]
- 19. Huppert D, Strupp M, Rettinger N, Hecht J, Brandt T. Phobic postural vertigo—a long‐term follow‐up (5 to 15 years) of 106 patients. J Neurol 2005;252(5):564–569. [DOI] [PubMed] [Google Scholar]
- 20. Staab JP, Eckhardt‐Henn A, Horii A, et al. Diagnostic criteria for persistent postural‐perceptual dizziness (PPPD): Consensus document of the Committee for the Classification of Vestibular Disorders of the Barany Society. J Vestib Res 2017;27(4):191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez‐Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere's disease. J Vestib Res 2015;25(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22(4):167–172. [DOI] [PubMed] [Google Scholar]
- 23. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg 1990;116(4):424–427. [DOI] [PubMed] [Google Scholar]
- 24. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14(7):409–415. [PubMed] [Google Scholar]
- 25. Reid SA, Callister R, Katekar MG, Treleaven JM. Utility of a brief assessment tool developed from the Dizziness Handicap Inventory to screen for cervicogenic dizziness: A case control study. Musculoskelet Sci Pract 2017;30:42–48. [DOI] [PubMed] [Google Scholar]
- 26. Deng Z, Yuan WA, Wang HH, Zhan HS. [Development of Chinese version vertigo symptom scale (VSS): reliability and validity]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015;44(2):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res 1992;36(8):731–741. [DOI] [PubMed] [Google Scholar]
- 28. Heikkilä H . Cervical vertigo In: Boyling J JG, Twomey P, ed. Grieve's Modern Manual Therapy. 3rd ed. Edinburgh: Churchill Livngstone; 2004:233–240. [Google Scholar]
- 29. Calhoun AH, Ford S, Millen C, Finkel AG, Truong Y, Nie Y. The prevalence of neck pain in migraine. Headache 2010;50(8):1273–1277. [DOI] [PubMed] [Google Scholar]
- 30. L'Heureux‐Lebeau B, Godbout A, Berbiche D, Saliba I. Evaluation of paraclinical tests in the diagnosis of cervicogenic dizziness. Otol Neurotol 2014;35(10):1858–1865. [DOI] [PubMed] [Google Scholar]
- 31. Johnston JL, Daye PM, Thomson GT. Inaccurate saccades and enhanced vestibulo‐ocular reflex suppression during combined eye‐head movements in patients with chronic neck pain: possible implications for cervical vertigo. Front Neurol 2017;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reid SA, Callister R, Katekar MG, Rivett DA. Effects of cervical spine manual therapy on range of motion, head repositioning, and balance in participants with cervicogenic dizziness: a randomized controlled trial. Arch Phys Med Rehab 2014;95(9):1603–1612. [DOI] [PubMed] [Google Scholar]
- 33. Lystad RP, Bell G, Bonnevie‐Svendsen M, Carter CV. Manual therapy with and without vestibular rehabilitation for cervicogenic dizziness: a systematic review. Chiropr Man Therap 2011;19(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lerner DJ, Amick BC, 3rd , Malspeis S, et al. The migraine work and productivity loss questionnaire: concepts and design. Qual Life Res 1999;8(8):699–710. [DOI] [PubMed] [Google Scholar]
- 35. Breivik CN, Nilsen RM, Myrseth E, Finnkirk MK, Lund‐Johansen M. Working disability in Norwegian patients with vestibular schwannoma: vertigo predicts future dependence. World Neurosurg 2013;80(6):e301–305. [DOI] [PubMed] [Google Scholar]
- 36. Aratani MC, Perracini MR, Caovilla HH, Gazzola JM, Gananca MM, Gananca FF. Disability rank in vestibular older adults. Geriatr Gerontol Int 2011;11(1):50–54. [DOI] [PubMed] [Google Scholar]
- 37. Nascimbeni A, Gaffuri A, Penno A, Tavoni M. Dual task interference during gait in patients with unilateral vestibular disorders. J Neuroeng Rehabil 2010;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bell BD, Primeau M, Sweet JJ, Lofland KR. Neuropsychological functioning in migraine headache, nonheadache chronic pain, and mild traumatic brain injury patients. Arch Clin Neuropsychol 1999;14(4):389–399. [PubMed] [Google Scholar]
- 39. Barrett K, Buxton N, Redmond AD, Jones JM, Boughey A, Ward AB. A comparison of symptoms experienced following minor head injury and acute neck strain (whiplash injury). J Accid Emerg Med 1995;12(3):173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gimse R, Bjorgen IA, Straume A. Driving skills after whiplash. Scand J Psychol 1997;38(3):165–170. [DOI] [PubMed] [Google Scholar]
- 41. Gimse R, Tjell C, Bjorgen IA, Saunte C. Disturbed eye movements after whiplash due to injuries to the posture control system. J Clin Exp Neuropsychol 1996;18(2):178–186. [DOI] [PubMed] [Google Scholar]
- 42. Foroozan R. Visual dysfunction in migraine. Int Ophthalmol Clin. 2009;49(3):133–146. [DOI] [PubMed] [Google Scholar]
- 43. Parker DM, Besson JA, McFadyen M. Intermittent alexia. Cortex 1990;26(4):657–660. [DOI] [PubMed] [Google Scholar]
- 44. Braswell J, Rine RM. Evidence that vestibular hypofunction affects reading acuity in children. Int J Pediatr Otorhinolaryngol 2006;70(11):1957–1965. [DOI] [PubMed] [Google Scholar]
- 45. Kayan A, Hood JD. Neuro‐otological manifestations of migraine. Brain 1984;107(Pt 4):1123–1142. [DOI] [PubMed] [Google Scholar]
- 46. Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56(4):436–441. [DOI] [PubMed] [Google Scholar]
- 47. Hain TC. Cervicogenic causes of vertigo. Curr Opin Neurol 2015;28(1):69–73. [DOI] [PubMed] [Google Scholar]
- 48. Yacovino DA, Hain TC. Clinical characteristics of cervicogenic‐related dizziness and vertigo. Semin Neurol 2013;33(3):244–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Relative frequencies of reported symptom endorsement, with respect to subjects' presenting problem, n = 48
Appendix A: Dizziness Handicap Inventory‐Cervical


