Abstract
Objectives
Benign paroxysmal positional vertigo (BPPV) is the most common peripheral vestibular end‐organ disease. This article aims to summarize research findings and key discoveries of BPPV. The pathophysiology, diagnosis, nonsurgical, and surgical management are discussed.
Methods
A comprehensive review of the literature regarding BPPV up through June 2018 was performed.
Results
BPPV is typified by sudden, brief episodes of vertigo precipitated by specific head movements. While often self‐limited, BPPV can have a considerable impact on quality of life. The diagnosis can be established with a Dix‐Hallpike maneuver for the posterior and anterior canals, or supine roll test for the horizontal canal, and typically does not require additional ancillary testing. Understanding the pathophysiology of both canalithiasis and cupulolithiasis has allowed for the development of various repositioning techniques. Of these, the particle repositioning maneuver is an effective way to treat posterior canal BPPV, the most common variant. Options for operative intervention are available for intractable cases or patients with severe and frequent recurrences.
Conclusions
A diagnosis of BPPV can be made through clinical history along with diagnostic maneuvers. BPPV is generally amenable to in‐office repositioning techniques. For a small subset of patients with intractable BPPV, canal occlusion can be considered.
Level of Evidence
N/A
Keywords: Benign paroxysmal positional vertigo, particle repositioning maneuver, Dix‐Hallpike, canalith, semicircular canal occlusion
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is the most common peripheral vestibular end‐organ disease and is typified by a sudden, transient gyratory sensation which is accompanied by characteristic nystagmus. Symptoms are provoked by positional changes of the head with respect to gravity and can range in severity from mild dizziness to debilitating episodes that may induce nausea or vomiting, and significantly hinder daily functioning.
Vertigo is defined as the subjective perception of rotational or translational movement in the absence of an external stimulus. In the case of BPPV, aberrant signals from semicircular canals create an illusion of motion which results in vertigo. Patients with vertiginous symptoms will ultimately be diagnosed with BPPV in 17% to 42% of cases, making BPPV the most frequent cause of vertigo.1, 2 One European cross‐sectional study estimated the lifetime prevalence of BPPV to be 2.4%, and data from subsequent research reported the incidence to be 10.7 to 64 cases per 100,000 per year.3, 4, 5 While BPPV can present at any age, its peak onset occurs in the fifth and sixth decades of life.4 Furthermore, the disorders disproportionately affect women at a ratio of 2 or 3 to 1, although the disparity between the sexes is not observed in younger patients or those with a traumatic etiology.2, 4
The high prevalence of BPPV yields a proportionately substantial healthcare burden of approximately $2 billion per year in the United States.6 Moreover, delays in the diagnosis and treatment of BPPV on the order of months are not uncommon and have profound financial and quality of life implications for patients and caregivers.7 A cross‐sectional study of 100 elderly patients found that in those with symptoms not related to balance disorders, 9% had previously unrecognized BPPV.8 Furthermore, studies indicate that as few as 10% to 20% of patients with the condition receive appropriate treatment.3 These data highlight the importance of educating clinicians regarding the disease. Fortunately, in the vast majority of cases, diagnosis is straightforward, and the condition is generally amenable to in‐office treatments. This review aims to lay out salient points and recent advances related to pathophysiology and the treatment of BPPV.
PATHOPHYSIOLOGY
The vestibular system evolved to perceive head motion and position. Specifically, the otolith organs, which are comprised of the utricle and saccule, detect linear acceleration and gravitational forces, whereas the semicircular canals detect rotational acceleration. Endolymph hydrodynamics within the semicircular canals, as well as its influence on the ampullary cupula, allows for the sensation of angular movement in each of the three planes in which the canals are oriented. The symptomatology of BPPV is a direct result of aberrant semicircular canal signalling which creates an illusory sense of motion.
The two prevailing theories regarding BPPV's pathophysiology are the canalithiasis and cupulolithiasis models, and they differ with respect to how endolymphatic debris influences cupular dynamics (Fig 1).
Figure 1.
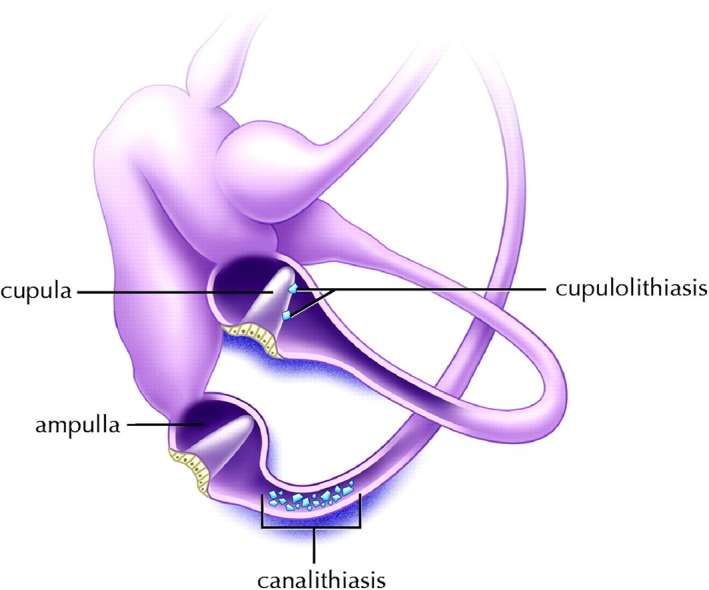
Left inner ear. Demonstration of canalithiasis of the posterior canal and cupulolithiasis of the horizontal canal. Reprinted from Parnes, Agrawal and Atlas. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) Canadian Medical Association Journal September 30, 2003 169 (7) 681–693. © Canadian Medical Association 2003. This work is protected by copyright and the making of this copy was with the permission of the Canadian Medical Association Journal (www.cmaj.ca) and Access Copyright. Any alteration of its content or further copying in any form whatsoever is strictly prohibited unless otherwise permitted by law.
In the cupulolithiasis model, the particulate matter becomes adherent to the cupula itself. This cupular loading renders the system sensitive to gravitational forces, and the resulting alterations in cupular deflection lead to pathological perceptions of motion.9 It has been suggested that this mechanism may represent the more chronic form of BPPV.9
By comparison, the canalithiasis model cites free‐floating particles within the lumen of the canal as the causal factor, a phenomenon that was first demonstrated in vivo by Parnes and McClure.10 A video of the free‐floating particles can be seen in the supplemental material of this article. The aberrant signal results when gravity pulls the particles through the endolymph canal creating a plunger‐like effect which in turn causes ipsidirectional cupular displacement
Clinicopathological research indicates that canalithiasis is the predominant subtype.11 The source of the canaliths responsible for the development of disease has been the subject of much speculation, with many theorizing that displaced otolithic membrane fragments may be the culprit. Recent work by Kao et al. supports this concept.12 Scanning electron micrographs of posterior semicircular canal contents extracted from patients with intractable BPPV show free‐floating otoconia with linking filaments attached to what appears to be a gelatinous matrix (Fig. 2). These images lend credence to the hypothesis that canaliths of utricular origin are the source of the free‐floating particles leading to BPPV. Saccular otoconia are less likely be involved due to their remote position relative to the semicircular canals.
Figure 2.

Scanning electron micrograph image of otoconia within the posterior canal endolymphatic duct removed from a patient with intractable BPPV. Image shown at 20k magnification. Scale bar 20 micrometres. Reproduced with permission from Kao, Parnes, and Chole. Otoconia and otolithic membrane fragments within the posterior semicircular canal in benign paroxysmal positional vertigo. Laryngoscope 90:709–714 (2016)
Most cases of BPPV are idiopathic in origin and probably result from degeneration of the macula. Secondary causes of BPPV refer to identifiable causes of otoconial dislodgement. These include otologic and nonotologic surgery, head trauma, or any means by which a sufficient mechanical force reaches the inner ear.11, 13, 14 Furthermore, the disease can develop via inner ear disorders which ultimately lead to the degradation and disassociation of otoconia from their native gelatinous substrate. These include vestibular neuritis, Meniere's disease and sudden sensorineural hearing loss (SNHL).15, 16, 17
BPPV can develop in any of the three semicircular canals. The posterior canal variant is by far the most common (80–90%)18 because it is the most gravity‐dependent part of the vestibular labyrinth. With regular head movements, free‐floating endolymph debris can migrate into the posterior canal via its nonampullated end (common crus). The cupula creates a partition in the ampulla preventing debris from transgressing the ampulla in any of the canals. The only place for debris to enter or exit the canals is through their nonampullated ends. In contrast, because of the orientation of the superior canal any canaliths particles to the utricle through natural head movements alone, and thus they represent only 10% to 20% and 3% of cases, respectively.9, 18, 19
DIAGNOSIS
History
As with all vestibular patients, detailed clinical history is very important. Clinicians should take care to differentiate the chief complaint from other forms of “dizziness,” such as disequilibrium and pre‐syncope. Other causes of episodic vertigo should also be ruled out, including Meniere's disease, migraine, and semicircular canal dehiscence. Patients with BPPV often perceives the environmental spinning in the pitch plane. These vertigo spells usually last less than 20 seconds (longer for the horizontal canal) but may be accompanied by a lingering, nonspecific imbalance, so patients often overestimate the length of the attacks. Furthermore, the onset of vertiginous symptoms in BPPV is sudden and provoked by specific head movements such as extending the neck, bending forward, or rolling over in bed.9, 20 In posterior canal BPPV, the provoking side to which the head is turned in the supine position usually predicts the affected ear.21 There is a subset of patients who describe atypical histories. This is why we perform Dix Hallpike and horizontal canal testing in all our “dizzy” referrals.
While the majority of BPPV is idiopathic, possible secondary causes include antecedent head trauma, vestibular neuronitis, migraine, Meniere's disease, or prior otologic surgery. Clinicians should also be vigilant for central nervous system etiologies masquerading as BPPV which may represent more pernicious pathology that warrants timely intervention.22 All in all, few diseases mimic the brief, episodic, positional change induced vertigo seen in BPPV, and disparities should prompt consideration of alternative or concurrent peripheral or central vestibular disease.
Diagnostic Maneuvers
The Dix‐Hallpike maneuver is the definitive test for posterior canal BPPV. When performed in the office, the sensitivity and specificity are 79% to 82% and 71% to 75%, respectively.23, 24 The maneuver begins with the patient seated and head turned 45 degrees to the side being tested so as to isolate and vertically orient that side's posterior canal. The patient is then laid back into a supine position with the tested ear down (Fig. 3). Classic teaching suggests hanging the head back over the edge of the bed, but this is not necessary when performing the Dix‐Hallpike test alone. Overextension of the neck may even elicit a false positive response from the contralateral side. Clinicians should also be cautious of patients with neck, back, abdominal, and hip problems as this may require special care during the diagnostic maneuver.25
Figure 3.
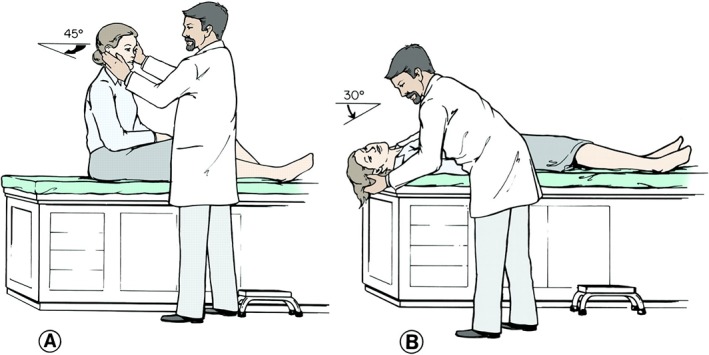
Dix‐Hallpike maneuver (right ear). A, the patient is seated with the head rotated at 45 degrees. B, the patient is quickly lowered into supine position with neck extended below the level of the table. With head extended, examiner observes the patient for nystagmus. Reprinted from Parnes, Agrawal and Atlas. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). Canadian Medical Association Journal September 30, 2003 169 (7) 681‐693. © Canadian Medical Association 2003. This work is protected by copyright and the making of this copy was with the permission of the Canadian Medical Association Journal (www.cmaj.ca) and Access Copyright. Any alteration of its content or further copying in any form whatsoever is strictly prohibited unless otherwise permitted by law.
With a convincing clinical history, a diagnosis of BPPV is made if the Dix‐Hallpike maneuver provokes the appropriate nystagmus.6 Characteristic nystagmus features include latency of onset, limited duration, torsional and upbeat directionality, reversibility, and fatigability. The observed latency between the beginning of the Dix‐Hallpike and the onset of vertigo or nystagmus is due to the inertia of the endolymph particles. This is typically around 2 to 5 seconds. Once the particles migrate down to the most dependent position, the nystagmus and vertigo terminate. Activation of the ipsilateral inferior oblique and contralateral superior rectus during the Dix‐Hallpike leads to a torsional nystagmus (the top pole of the eye beats towards the lowermost ear) along with an up‐beating component. The nystagmus reverses directions when the patient is returned to the upright position. With repeated testing, the nystagmus should fatigue due to the dispersion of the canaliths which reduces their mass effect. The intensity of the nystagmus usually correlates with the severity of a patient's vertigo.
Horizontal canal BPPV should be considered when a patient with a compatible clinical history presents with horizontal nystagmus or no eye movement during the Dix‐Hallpike test.6 Horizontal BPPV is diagnosed with a supine roll test, also referred to as the Pagnini‐McClure maneuver.11 The supine roll test is performed by first positioning the patient supine in a neutral head position with their face directed upwards. The head is then rotated quickly 90 degrees to one side, and the eyes are observed for the presence of nystagmus. Once any elicited nystagmus has abated the head is returned to the neutral position, and the contralateral direction can be tested in similar fashion. Unlike the Dix‐Hallpike maneuver, which isolates one posterior canal during testing, the supine roll test provokes both horizontal canals simultaneously. The affected side is determined by comparing the nystagmus on each side and its direction (Table 1). In general, the provoked vertigo of horizontal canal BPPV is more intense, has a shorter latency, lasts longer and is less prone to fatigue.9, 26
Table 1.
Characteristic Nystagmus Associated With Horizontal Canal BPPV
| Direction of nystagmus | ||
|---|---|---|
| Intensity of nystagmus | Apogeotropic | Geotropic |
| Stronger on the left side | Right cupulolithiasis | Left canalithiasis |
| Stronger on the right side | Left cupulolithiasis | Right canalithiasis |
Superior canal BPPV is comparatively rare.18 Anatomically, the superior semicircular canal lies in the same plane as the contralateral posterior semicircular canal.27 As such, the Dix‐Hallpike maneuver tests for both entities. In a positive Dix‐Hallpike exam of the superior canal BPPV, the torsional component remains the same as for the posterior canal of the undermost ear with the main differentiating element being a down‐beating vertical component.27
Overall, clinical history and characteristic nystagmus findings on provocative maneuvers are sufficient for diagnosing BPPV. Additional investigations such as advanced electrophysiological vestibular and auditory tests are generally not necessary to diagnose isolated BPPV. If concurrent symptoms or atypical findings raise suspicion for other inner ear or posterior fossa abnormalities, clinicians should arrange for further investigations.
NONSURGICAL MANAGEMENT
BPPV is a benign disorder that, in the majority of cases, can be simply and effectively treated using noninvasive means. Hence, patient education and reassurance are essential components of BPPV management. The patients should be informed of BPPV's favourable prognosis, with nearly 25% of patients experiencing spontaneous resolution by 1 month and up to 50% at 3 months.28 While observation is an option, effective treatment can help avoid discomfort and possible injury associated with episodic vertigo.6 Moreover, relapse and remissions can occur unpredictably in both treated and untreated patients.4 As such, education also helps patients anticipate and cope with recurrences.
As our understanding of BPPV improved with time, more antiquated treatments such as rigorous in‐patient physiotherapy have given way to simple, effective in‐office treatments.29 Furthermore, based on current evidence, clinicians should refrain from routinely treating BPPV with vestibular suppressant medications.6, 30 Instead, the preferred treatment now centers around appropriate repositioning maneuvers.
Posterior Canal BPPV
The French physical therapist Alain Semont and colleagues first described the liberatory maneuver in 1988, now known colloquially as the Semont maneuver.31 Based on the theory of cupulolithiasis, the maneuver aimed to free adherent debris from the cupula with the proposed slingshot effect from the rapid change in head position. Though canalithiasis was unknown at the time, the Semont maneuver also serves to reposition canaliths back into the utricle. Studies have found the Semont maneuver to be efficacious, safe, and advantageous compared to a sham‐control.32 The reported response rate ranges from 70% to 90%, and the recurrence rate is approximately 29%.32, 33 Unfortunately, due to its extreme change in body position at a rapid pace, the Semont maneuver can be challenging and cumbersome in elderly, obese, and infirm patients.
The canalith repositioning procedure (CRP), also known as the Epley maneuver, was devised by Dr. John Epley based on the canalithiasis theory.9 Through a set of controlled head movements, CRP aims to return the canaliths, under the influence of gravity, from the posterior semicircular canal back into the utricle. While the original Epley maneuver described posttreatment activity limitations and mastoid vibration, neither has been shown to enhance the rate of success.34, 35 Over time, a modified form of CRP such as the particle repositioning maneuver (PRM) became commonly used.
First introduced by Parnes and Price‐Jones, PRM offered a more straightforward three‐position maneuver and obviated the need for sedation, mastoid vibration and post‐treatment head position restrictions described by the original CRP9 (FIG. 4). PRM has been shown to have a 70% to 85% success rate after a single treatment.36 More recently, a Cochrane review of 11 relevant studies representing 745 participants, concluded that the Epley maneuver and its modifications are safe and effective.37 Thus, PRM or other variations of CRP have become mainstay treatment in posterior canal BPPV.
Figure 4.
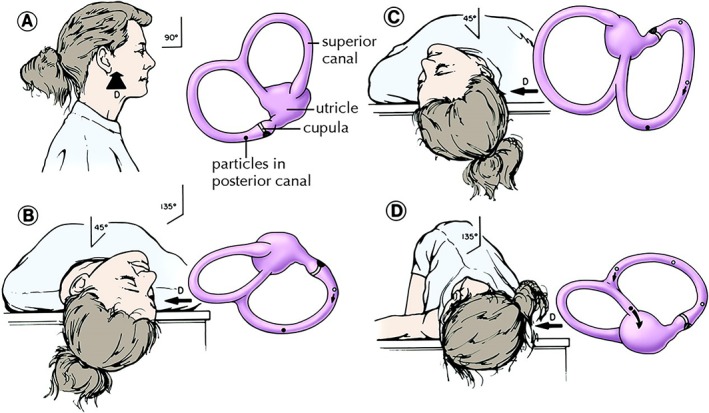
Particle repositioning maneuver (right ear). Sequential movements and the corresponding position of the utricle and semicircular canals. A, the patient is seated as viewed from the right side. B, first step of particle repositioning maneuver and the same position assumed during normal Dix‐Hallpike. This position is maintained for 1‐2 minutes. C, the patient's head is rotated toward the opposite side while neck remains extended. D, in a steady motion, the patient is rolled onto the opposite side. Position D is maintained for another 1‐2 minutes before the patient sits up to position A. D = DIRECTION OF VIEW OF LABYRINTH, DARK CIRCLE = POSITION OF PARTICLE CONGLOMERATE, OPEN CIRCLE = PREVIOUS POSITION. Reprinted from Parnes, Agrawal and Atlas. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). Canadian Medical Association Journal September 30, 2003 169 (7) 681‐693. © Canadian Medical Association 2003. This work is protected by copyright and the making of this copy was with the permission of the Canadian Medical Association Journal (www.cmaj.ca) and Access Copyright. Any alteration of its content or further copying in any form whatsoever is strictly prohibited unless otherwise permitted by law.
The initial steps of the PRM resemble a Dix‐Hallpike maneuver. With the head turned toward the side being treated, the patient is moved into the supine position with the neck in extension, hanging the head back over the edge of the stretcher (Fig. 4B). For those who cannot tolerate neck extension, tilt the stretcher back into Trendelenburg position. This position is maintained for 1‐2 minutes while observing for nystagmus. The patient's head is then turned 90° towards the opposite ear, keeping the neck in full extension. (FIG. 4C) Next, the patient is rolled 90° on to the non‐affected side until the head is diagonally opposite to the first Dix‐Hallpike position. (FIG. 4D) This step should be done fairly swiftly to generate a “secondary stage” nystagmus response. This secondary nystagmus will be ipsidirectional to the Dix‐Hallpilke nystagmus when the canaliths continue in an ampullofugal direction through the common crus and into the utricle. When nystagmus subsides, the patient resumes a seated position. With a successful manoeuvre, there should be no nystagmus or vertigo when the patient returns to the sitting position. Absent secondary stage nystagmus or a reversed nystagmus at the end of the PRM suggests that the canaliths have not returned to the utricle. Controversy exists over whether or not perform a repeat Dix‐Hallpike to test for success. Two main arguments exist for not immediately redoing the Dix‐Hallpike test. First, a negative test may be the result of a fatigued response and not necessarily treatment success. Second, there is a risk of i) undoing the successful PRM by causing the canaliths to reenter the posterior canal or ii) inducing canal conversion when the canaliths enter the horizontal canal.38
Horizontal Canal BPPV
Horizontal canal BPPV leads to horizontal nystagmus beating in either ageotropic or apogeotropic direction during the supine head turns. The geotropic variant (the fast phase of nystagmus towards the ground) is believed to be caused by canalithiasis. It is also more clinically responsive and well‐studied. In comparison, horizontal canal cupulolithiasis manifests as apogeotropic nystagmus where the fast phase of nystagmus is away from the ground.6
When horizontal geotropic nystagmus is encountered, a maneuver known as the barbecue roll or barrel roll can be used to reposition the canalith in the plane of the horizontal canal.39 Starting in a supine position, the head is turned toward the affected ear. The head and body are then turned away from the affected side in 90‐degree increments with each position held for about 1 minute or until nystagmus stops. Once a full 360‐degree rotation is finished, the patient returns to sitting.
An alternative approach is the Gufoni maneuver.40 Here, the patient begins seated upright facing forward and is moved quickly to lay on their side (on unaffected side for geotropic cases, on affected side for apogeotropic cases) while maintaining a neutral head position. After lying in this position for 2 minutes, the patient turns the head 45 degrees towards the ground, maintaining this position for 2 more minutes, and then is returned to a sitting position. A randomized control trial showed comparable success between Gufoni (response rate of 60.9%) and barbecue roll (69.1%).41 In essence, the Gufoni maneuver utilizes linear acceleration and positive inertia to reposition the canaliths. In contrast, Vannucchi and colleagues have proposed a forced prolonged position approach that relies on gravitational sedimentation.42 Here, the patient with geotropic nystagmus assumes a lateral decubitus position on the unaffected side for hours (affected side if nystagmus is apogeotropic). A prospective observational study found the effectiveness of forced prolonged position to be similar to that of Gufoni's maneuver after a single application (with success rates of 76% and 89%, respectively).43 However, the significant time commitment means the forced prolonged positioning is rarely performed.
Superior Canal BPPV
With superior canal BPPV being an uncommon presentation, the data is overall sparse for effective therapeutic maneuvers. A review by Anagnostou and colleagues found mostly case series with the commonly used technique being the reverse Epley maneuver or the Yacovino maneuver.18 The mean success rate for reverse Epley and Yacovino was found to be 75.9% and 78.8%, respectively.
Recurrences, Complications, and Failed Treatment
Spontaneous recurrence following the successful treatment of BPPV is common and should not deter the clinician. Repositioning maneuvers are usually safe and can be repeated at the time of follow up. In the event of a bilateral BPPV, the more symptomatic side should be prioritized with plans to address the other side in follow up. Repositioning one side at a time reduces the theoretical risk of re‐displacing the canaliths of the initially treated side.
During provocative maneuvers, some patients may experience vertigo without nystagmus. This is termed subjective BPPV and may be due to subclinical nystagmus, a fatigued response, and less severe BPPV that activates the sensation of vertigo but not the vestibulo‐ocular reflex.9 Studies have shown repositioning maneuvers in those with subjective BPPV to be nearly as effective as for patients with objective BPPV.44
Overall, repositioning manoeuvres have very few associated side effects. The most frequent sequela is nausea, which is found in 16.7% to 32% of the cases.37 In susceptible patients, prophylactic anti‐emetics and or vestibular suppressants may be necessary. In less than 5% of cases, canal conversion occurs where canaliths may be displaced from the posterior canal into the horizontal canal during repositioning.45 In the event of canal conversion, appropriate treatment of horizontal canal BPPV should ensue. Lastly, clinicians should be mindful of patients with vascular disease or cervical spine disorders as the diagnostic maneuvers and treatments have the potential, albeit very rarely, to cause injury.46
Mastoid oscillation and vestibular rehabilitation therapy may be helpful in cases resistant to conventional repositioning. In instances of multiple failed repositioning maneuvers, a tertiary centre referral should be considered.6 If available, sophisticated patient positioning systems such as the Epley Omniax rotator and the TRV chair may be considered for multi‐canal involvement or patients with mobility issues.47 In the event of refractory disease, surgical intervention may also be appropriate.
SURGERY
It should be stressed that BPPV is a benign disease. Operative intervention should be reserved for intractable cases or patients with severe and frequent recurrences that significantly impact the quality of life. Preoperatively, surgeons should precisely confirm the affected canal and its side. The practitioner should also rule out any secondary causes of BPPV. Preoperative testing should include audiometry given the possible complication of hearing loss from inner ear surgery. Objective vestibular testing is encouraged to establish baseline function in the operative ear and ensure normal vestibular function in the contralateral side. Imaging with CT and MRI should also be arranged for surgical planning and to rule out central lesions that may mimic BPPV.
Overall, less than 1% of BPPV cases require surgical intervention, and the majority involve intractable posterior canal BPPV.48 The primary surgical options are singular neurectomy and posterior semicircular canal occlusion.
Singular Neurectomy
The singular neurectomy for posterior canal BPPV was popularized by Gacek in the 1970s. It involves the transection of the posterior ampullary nerve within the singular canal.49 The surgery removes the resting input from the affected posterior canal ampulla, creating both a dynamic and static vestibular asymmetry. Over time, compensation occurs by central adaptation which can be expedited by vestibular rehab. Large case series of singular neurectomy have shown favorable success with complete resolution of vertigo between 80% to 97%.50, 51 However, with the necessary surgical exposure placing the vestibule at risk, singular neurectomy carries a possible risk of SNHL. Smaller series have reported SNHL complications at 12%, 29%, and 41% among 8, 7, and 12 neurectomies, respectively.52, 53, 54 By comparison, Gacek's updated series of 252 neurectomies reported the rate of SNHL at 3.7%.50 The discrepancy suggests the surgeon's experience is vital to the success of this technically challenging procedure.
Posterior Semicircular Canal Occlusion
Posterior semicircular canal occlusion was first introduced by Parnes and McClure in 1990 to treat intractable BPPV.55 By occluding the canal, the cupula is rendered mostly unresponsive to stimulation from natural head movements or gravitational pull on the canaliths. The resultant dynamic vestibular asymmetry leads to an initial period of postoperative imbalance. The imbalance is corrected through central adaption and postoperative vestibular physiotherapy.
The procedure is performed through a standard postauricular incision and limited mastoidectomy.56 The posterior canal otic capsule is blue‐lined, and a 1 X 3mm fenestration is created. A plug is formed using dry mastoid cortex bone chips mixed with fibrinogen sealant and thereafter inserted through the fenestration thus collapsing the membranous labyrinth and occluding the canal lumen. Afterwards, the fenestra and surrounding bone are covered with temporalis fascia and fibrinogen glue to prevent a postoperative perilymph fistula. With time, the bone chips within the canal ossify resulting in a permanent occlusion of the canal.
Initial experience of Parnes and McClure and subsequent case series found posterior semicircular canal occlusion to be effective in abolishing BPPV while preserving hearing.57 Authors have also used canal occlusion in the treatment of intractable horizontal and superior canal BPPV.58, 59 Other variations include utilizing a laser to blue‐line the canal or produce fibrous occlusions.56 Compared to singular neurectomy, the posterior canal occlusion carries fewer risk and less technical challenges. Of 74 posterior canal occlusions, Parnes and Agrawal showed only one case with a significant postoperative sensorineural hearing loss, which occurred three months postoperatively and was believed to be a result of labyrinthitis. The same patient had also undergone two previous unsuccessful singular neurectomies in the same ear.56
CONCLUSION
Benign paroxysmal positional vertigo is the most common peripheral vestibular disorder and presents as brief, episodic, positionally provoked vertigo. The diagnosis can be made through clinical history along with diagnostic maneuvers, and typically does not require additional ancillary testing. While benign and often self‐limiting, BPPV can have a considerable impact on quality of life. Fortunately, an improved understanding of the underlying pathophysiology has allowed for the creation of various repositioning techniques to help clinicians tackle this common affliction. The particle repositioning maneuver is a simple and effective way to treat posterior canal BPPV, the most common variant. For a small subset of patients with intractable BPPV, posterior canal occlusion is safe, straightforward, and highly efficacious.
Supporting information
Supplemental Video: Intraoperative video of canaliths in the semicircular canal. The video showcased selected cases of fenestrated posterior semicircular canal during posterior semicircular canal occlusion for recalcitrant BPPV. The white conglomerate mass seen is the canaliths within the posterior canal endolymphatic duct. Video also includes an additional cases of canalith seen within the utricle during a translabyrithine approach to vestibular schwannoma surgery.
The authors declare no funding or conflicts of interest.
BIBLIOGRAPHY
- 1. Hanley K, O'Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001;51(469):666–671. [PMC free article] [PubMed] [Google Scholar]
- 2. Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post‐traumatic. Acta Otolaryngol 1999;119(7):745–749. [DOI] [PubMed] [Google Scholar]
- 3. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry 2007;78(7):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim J‐S, Zee DS. Benign Paroxysmal Positional Vertigo. N Engl J Med 2014;370(12):1138–1147. [DOI] [PubMed] [Google Scholar]
- 5. Mizukoshi K, Watanabe Y, Shojaku H, Okubo J, Watanabe I. Epidemiological studies on benign paroxysmal positional vertigo in Japan. Acta Otolaryngol Suppl 1988;447:67–72. [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg 2017;156(Suppl 3):S1–S47. [DOI] [PubMed] [Google Scholar]
- 7. Fife D, FitzGerald JE. Do patients with benign paroxysmal positional vertigo receive prompt treatment? Analysis of waiting times and human and financial costs associated with current practice. Int J Audiol 2005;44(1):50–57. [DOI] [PubMed] [Google Scholar]
- 8. Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg 2000;122(5):630–634. [DOI] [PubMed] [Google Scholar]
- 9. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ 2003;169(7):681–693. [PMC free article] [PubMed] [Google Scholar]
- 10. Parnes LS, McClure JA. Free‐floating endolymph particles: a new operative finding during posterior semicircular canal occlusion. 1992. Laryngoscope 2015;125(5):1033. [DOI] [PubMed] [Google Scholar]
- 11. Lee S‐H, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol 2010;6(2):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kao WTK, Parnes LS, Chole RA. Otoconia and otolithic membrane fragments within the posterior semicircular canal in benign paroxysmal positional vertigo. Laryngoscope 2016;90:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kansu L, Aydin E, Gulsahi K. Benign paroxysmal positional vertigo after nonotologic surgery: case series. J Maxillofac Oral Surg 2015;14(Suppl 1):113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park S‐K, Kim SY, Han K‐H, Hong SK, Kim JS, Koo J‐W. Benign paroxysmal positional vertigo after surgical drilling of the temporal bone. Otol Neurotol 2013;34(8):1448–1455. [DOI] [PubMed] [Google Scholar]
- 15. Lee N‐H, Ban J‐H, Lee K‐C, Kim SM. Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg 2010;143(3):413–417. [DOI] [PubMed] [Google Scholar]
- 16. Balatsouras DG, Ganelis P, Aspris A, Economou NC, Moukos A, Koukoutsis G. Benign paroxysmal positional vertigo associated with Meniere's disease: epidemiological, pathophysiologic, clinical, and therapeutic aspects. Ann Otol Rhinol Laryngol 2012;121(10):682–688. [DOI] [PubMed] [Google Scholar]
- 17. El‐Saied S, Joshua B‐Z, Segal N, Kraus M, Kaplan DM. Sudden hearing loss with simultaneous posterior semicircular canal BPPV: Possible etiology and clinical implications. Am J Otolaryngol 2014;35(2):180–185. [DOI] [PubMed] [Google Scholar]
- 18. Anagnostou E, Kouzi I, Spengos K. Diagnosis and treatment of anterior‐canal benign paroxysmal positional vertigo: a systematic review. J Clin Neurol 2015;11(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hornibrook J. Benign Paroxysmal Positional Vertigo (BPPV): history, pathophysiology, office treatment and future directions. Int J Otolaryngol 2011;2011:835671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med 1952;45(6):341–354. [PMC free article] [PubMed] [Google Scholar]
- 21. Shim DB, Ko KM, Kim JH, Lee W‐S, Song MH. Can the affected semicircular canal be predicted by the initial provoking position in benign paroxysmal positional vertigo? Laryngoscope 2013;123(9):2259–2263. [DOI] [PubMed] [Google Scholar]
- 22. Shoman N, Longridge N. Cerebellar vermis lesions and tumours of the fourth ventricle in patients with positional and positioning vertigo and nystagmus. J Laryngol Otol 2007;121(2):166–169. [DOI] [PubMed] [Google Scholar]
- 23. Halker RB, Barrs DM, Wellik KE, Wingerchuk DM, Demaerschalk BM. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix‐hallpike and side‐lying maneuvers: a critically appraised topic. Neurologist 2008;14(3):201–204. [DOI] [PubMed] [Google Scholar]
- 24. López‐Escámez JA, López‐Nevot A, Gámiz MJ, et al. [Diagnosis of common causes of vertigo using a structured clinical history]. Acta Otorrinolaringol Esp 2000;51(1):25–30. [PubMed] [Google Scholar]
- 25. Humphriss RL, Baguley DM, Sparkes V, Peerman SE, Moffat DA. Contraindications to the Dix‐Hallpike manoeuvre: a multidisciplinary review. Int J Audiol 2003;42(3):166–173. [DOI] [PubMed] [Google Scholar]
- 26. Hornibrook J. Horizontal canal benign positional vertigo. Ann Otol Rhinol Laryngol 2004;113(9):721–725. [DOI] [PubMed] [Google Scholar]
- 27. Balatsouras DG, Koukoutsis G, Ganelis P, Korres GS, Kaberos A. Diagnosis of single‐ or multiple‐canal benign paroxysmal positional vertigo according to the type of nystagmus. Int J Otolaryngol 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lynn S, Pool A, Rose D, Brey R, Suman V. Randomized trial of the canalith repositioning procedure. Otolaryngol Head Neck Surg 1995;113(6):712–720. [DOI] [PubMed] [Google Scholar]
- 29. Soto Varela A, Bartual Magro J, Santos Pérez S, et al. Benign paroxysmal vertigo: a comparative prospective study of the efficacy of Brandt and Daroff exercises, Semont and Epley maneuver. Rev Laryngol Otol Rhinol (Bord) 2001;122(3):179–183. [PubMed] [Google Scholar]
- 30. Sundararajan I, Rangachari V, Sumathi V, Kumar K. Epley's manoeuvre versus Epley's manoeuvre plus labyrinthine sedative as management of benign paroxysmal positional vertigo: prospective, randomised study. J Laryngol Otol 2011;125:572–575. [DOI] [PubMed] [Google Scholar]
- 31. Semont A, Freyss G, Vitte E. Curing the BPPV with a liberatory maneuver. Adv Otorhinolaryngol 1988;42:290–293. [DOI] [PubMed] [Google Scholar]
- 32. Chen Y, Zhuang J, Zhang L, et al. Short‐term efficacy of Semont maneuver for benign paroxysmal positional vertigo: a double‐blind randomized trial. Otol Neurotol 2012;33(7):1127–1130. [DOI] [PubMed] [Google Scholar]
- 33. Haynes DS, Resser JR, Labadie RF, et al. Treatment of benign positional vertigo using the semont maneuver: efficacy in patients presenting without nystagmus. Laryngoscope 2002;112:796–801. [DOI] [PubMed] [Google Scholar]
- 34. Devaiah AK, Andreoli S. Postmaneuver restrictions in benign paroxysmal positional vertigo: an individual patient data meta‐analysis. Otolaryngol Head Neck Surg 2010;142(2):155–159. [DOI] [PubMed] [Google Scholar]
- 35. Sargent EW, Bankaitis AE, Hollenbeak CS, Currens JW. Mastoid oscillation in canalith repositioning for paroxysmal positional vertigo. Otol Neurotol 2001;22(2):205–209. [DOI] [PubMed] [Google Scholar]
- 36. White J, Savvides P, Cherian N, Oas J. Canalith repositioning for benign paroxysmal positional vertigo. Otol Neurotol 2005;26(7):704–710. [DOI] [PubMed] [Google Scholar]
- 37. Hilton M, Pinder D. The Epley manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev 2014;(12):1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parnes LS, Robichaud J. Further observations during the particle repositioning maneuver for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 1997;116(2):238–243. [DOI] [PubMed] [Google Scholar]
- 39. Lempert T, Tiel‐Wilck K. A positional maneuver for treatment of horizontal‐canal benign positional vertigo. Laryngoscope 1996;106(4):476–478. [DOI] [PubMed] [Google Scholar]
- 40. Ciniglio Appiani G, Catania G, Gagliardi M. A liberatory maneuver for the treatment of horizontal canal paroxysmal positional vertigo. Otol Neurotol 2001;22(1):66–69. [DOI] [PubMed] [Google Scholar]
- 41. Kim JS, Oh S‐Y, Lee S‐H, et al. Randomized clinical trial for geotropic horizontal canal benign paroxysmal positional vertigo. Neurology 2012;79(7):700–707. [DOI] [PubMed] [Google Scholar]
- 42. Vannucchi P, Giannoni B, Pagnini P. Treatment of horizontal semicircular canal benign paroxysmal positional vertigo. J Vestib Res 1997;7(1):1–6. [PubMed] [Google Scholar]
- 43. Korres S, Riga MG, Xenellis J, Korres GS, Danielides V. Treatment of the horizontal semicircular canal canalithiasis: pros and cons of the repositioning maneuvers in a clinical study and critical review of the literature. Otol Neurotol 2011;32(8):1302–1308. [DOI] [PubMed] [Google Scholar]
- 44. Tirelli G, D'Orlando E, Giacomarra V, Russolo M. Benign positional vertigo without detectable nystagmus. Laryngoscope 2001;111(6):1053–1056. [DOI] [PubMed] [Google Scholar]
- 45. Herdman SJ, Tusa RJ. Complications of the canalith repositioning procedure. Arch Otolaryngol Head Neck Surg 1996;122(3):281–286. [DOI] [PubMed] [Google Scholar]
- 46. Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol 2010;124(5):575–576. [DOI] [PubMed] [Google Scholar]
- 47. West N, Hansen S, Møller MN, Bloch SL, Klokker M. Repositioning chairs in benign paroxysmal positional vertigo: implications and clinical outcome. Eur Arch Otorhinolaryngol 2016;273(3):573–580. [DOI] [PubMed] [Google Scholar]
- 48. Shaia WT, Zappia JJ, Bojrag DI, LaRouere ML, Sargent EW, Diaz RC. Success of posterior semicircular canal occlusion and application of the dizziness handicap inventory. Otolaryngol Head Neck Surg 2006;134(3):424–430. [DOI] [PubMed] [Google Scholar]
- 49. Gacek RR. Transection of the posterior ampullary nerve for the relief of benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol 1974;83(5):596–605. [DOI] [PubMed] [Google Scholar]
- 50. Gacek RR, Gacek MR. Results of singular neurectomy in the posterior ampullary recess. ORL J Otorhinolaryngol Relat Spec 2002;64(6):397–402. [DOI] [PubMed] [Google Scholar]
- 51. Silverstein H, White DW. Wide surgical exposure for singular neurectomy in the treatment of benign positional vertigo. Laryngoscope 1990;100(7):701–706. [DOI] [PubMed] [Google Scholar]
- 52. Pournaras I, Kos I, Guyot J‐P. Benign paroxysmal positional vertigo: a series of eight singular neurectomies. Acta Otolaryngol 2008;128(1):5–8. [DOI] [PubMed] [Google Scholar]
- 53. Fernandes CM. Singular neurectomy in South African practice. S Afr J Surg 1993;31(2):79–80. [PubMed] [Google Scholar]
- 54. Epley JM. Singular neurectomy: hypotympanotomy approach. Otolaryngol Head Neck Surg (1979). 1980;88(3):304–309. [DOI] [PubMed] [Google Scholar]
- 55. Parnes LS, McClure JA. Posterior semicircular canal occlusion for intractable benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol 1990;99(5 Pt 1):330–334. [DOI] [PubMed] [Google Scholar]
- 56. Parnes LS, Agrawal SK. Transmastoid semicircular canal occlusion for benign paroxysmal positional vertigo and other disorders In: Brackmann D, Clough S, Arriag M, eds. Otologic Surgery 4th Ed. 4th ed. Elsevier Health Sciences; 2015:408–416. [Google Scholar]
- 57. Agrawal SK, Parnes LS. Human experience with canal plugging. Ann N Y Acad Sci 2001;942(519):300–305. [DOI] [PubMed] [Google Scholar]
- 58. Zhu Q, Liu C, Lin C, et al. Efficacy and safety of semicircular canal occlusion for intractable horizontal semicircular benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol 2015;124(4):257–260. [DOI] [PubMed] [Google Scholar]
- 59. Brantberg K, Bergenius J. Treatment of anterior benign paroxysmal positional vertigo by canal plugging: a case report. Acta Otolaryngol 2002;122(1):28–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video: Intraoperative video of canaliths in the semicircular canal. The video showcased selected cases of fenestrated posterior semicircular canal during posterior semicircular canal occlusion for recalcitrant BPPV. The white conglomerate mass seen is the canaliths within the posterior canal endolymphatic duct. Video also includes an additional cases of canalith seen within the utricle during a translabyrithine approach to vestibular schwannoma surgery.


