Abstract
Background
The prevalence of balance and gait deficits increases with age and is associated with the increased incidence of falls seen in the elderly population; these falls are associated with significant morbidity and mortality.
Objectives
To review changes in gait and balance associated with aging and the effect of visual perturbations on gait and balance in the elderly to provide a basis for future research.
Methods
PubMed and Cochrane Library were searched for articles from 1980 to present pertaining to gait and balance in older adults (>60) and younger adults (<60). Search terms included balance, posture, gait, locomotion, gait variability, gait disorders, gait disturbance, elderly, aging, falls, vision, visual, vestibular, and virtual reality. The references section of queried articles was also used to find relevant studies. Studies were excluded if subjects had a diagnosed gait or balance disorder.
Results
Elderly adults show age‐related decline in sensory systems and reduced ability to adapt to changes in their environment to maintain balance. Elderly adults are particularly dependent on vision to maintain postural stability. Distinct changes in spatiotemporal gait parameters are associated with aging, such as slower gait and increased gait variability, which are amplified with exposure to visual perturbations. Increased gait variability, specifically with mediolateral perturbations, poses a particular challenge for elderly adults and is linked to increased falls risk. Virtual reality training has shown promising effects on balance and gait.
Conclusion
Elderly adults show age‐related decline in balance and gait with increased gait variability and an associated increased risk of falls.
Level of Evidence
5
Keywords: Gait, elderly, balance, visual perturbations, virtual reality, spatiotemporal
INTRODUCTION
Balance and gait are important considerations in the health of elderly subjects. It is estimated that 13% of adults self‐report imbalance from ages 65 to 69 and this proportion increases to 46% in those aged 85 and older.1 Similarly, it is estimated that the prevalence of gait disorders in community‐residing elderly adults aged 70 and older is 35%.2 Abnormal gait has been associated with a risk of institutionalization and death that is 2.2 times that seen in elderly adults without gait disorders.2 Additionally, impairments of balance and gait have been implicated in increased risks of falls.3 In adults aged 65 and older, the estimated annual prevalence of falls is 28%.4 Falls are associated with significant morbidity and mortality in the elderly: they are the most common cause of accidental death and nonfatal accidental injury in those 65 and older, accounting for 55.8% of accidental deaths.5
In this review, we aim to discuss current understanding of balance and gait changes in the elderly, defined here to mean those 60 years and older, characterize abnormal gait patterns seen in this population, assess how visual challenges affect gait in the elderly, and discuss interventions that may improve balance and gait in the elderly. To identify the relevant literature on gait and balance in adults, we searched the PubMed and Cochrane Library from 1980 to present using the following search terms: balance, posture, gait, locomotion, gait variability, gait disorders, gait disturbance, elderly, aging, falls, vision, visual, vestibular, and virtual reality. The references section of queried articles was surveyed to identify additional relevant studies. Published studies of subjects with diagnosed gait and/or balance disorders were excluded to focus on the normal consequences of aging. This review provides a basis for future research on gait and balance in the elderly and for the development of novel interventions that can improve gait and balance in the elderly.
BALANCE IN THE ELDERLY
How Is Balance Regulated?
Balance is necessary for an individual to maintain posture, respond to voluntary movements, and react to external perturbations. To maintain balance, an individual's center of mass must stay within the changing base of support. This “limit of stability” depends on an individual's biomechanics, the requirements of the task, and the type of surface the individual is standing on. Animal studies suggest that posture is controlled via supraspinal mechanisms. Specifically, the vestibular system and cerebellum are thought to play primary roles in postural control with the cerebellum important for modifying limb and trunk movements and balancing opposing muscle forces for a required task. Postural control depends on sensory inputs: somatosensory information from muscle and joint proprioceptors, cutaneous sensory information which identifies surface characteristics, vestibular information for head and trunk orientation in space, gravity information from graviceptors in the trunk, and visual input. Situational cues and prior experiences modify these inputs and contribute to balance control.6
Changes in Balance in the Aging Population
Aging is associated with declining balance. Specifically, as task complexity increases through attenuation of sensory feedback, balance impairments can be detected at younger ages. In comparison to young women, elderly women showed a significant increase in sway velocity by the sixth decade when subjects stood on firm ground in vertical configuration with eyes closed in bilateral stance. Overall, decline in postural stability was evident at earlier ages with increased challenges to subjects’ balance, with continued decline in balance with each decade of life. Thus, decreased postural stability was seen with standing on one limb with the eyes open by the sixth decade of life, with standing on a foam surface by the fifth decade of life, and with standing on one limb with the eyes closed by the fourth decade of life.7 Additionally, older subjects were found to have a sway velocity approximately 2.5 times that of younger subjects when standing on a firm surface with their eyes open and four times that of younger subjects with eyes closed; thus, this approximate doubling of sway velocity when switching from eyes open to eyes closed on a firm surface was interpreted as a reflection of the degenerating vestibulocochlear system in elderly adults.8
Older adults may inappropriately activate antagonist muscles more frequently than younger adults while trying to maintain balance. Furthermore, in response to low amplitude perturbations, older adults tended to activate muscles in a proximal to distal sequence and may use a postural stability strategy that involves flexing or extending at the hip. However, younger adults tended not to evoke this strategy and responded to perturbations with a normal distal to proximal muscle activation sequence.9 To maintain postural stability, older adults may also increase postural sway but not necessarily postural instability, representing a change from the small continuous alterations in movement seen in younger adults to larger adjustments.10
Weighting of Sensory Inputs for Balance
Many sensory systems contribute to balance, such as foot/ankle sensory input, visual input, and vestibular input.11, 12 However, many of these systems decline with age. For example, older adults have significantly decreased foot position awareness in comparison with young adults, likely due to decrease plantar mechanoreceptive sensation. This decline is exacerbated by footwear, which impairs awareness of foot position through decreased tactile feedback.13 Similarly, the elderly have decreased mechanoreceptive sensation in the toes and heels.14 Vestibular function also declines with age, with significant decline seen after age 65.15
Several studies have examined the role of different systems in maintaining balance in the elderly. Judge et al.16 found that balance was impaired when visual or foot/ankle proprioceptive input was decreased in elderly adults. Balance was almost four times more impaired by reduced proprioception (swaying platform) than by reduced visual input. However, the relative risk of losing balance was increased 5.7 to 7.4 times when visual input was diminished in addition to proprioception. In addition to sensory input, decreased muscle strength also impairs balance in the elderly: for each Nm/kg increase in lower extremity muscle strength, the adjusted odds of losing balance decreased by 20%.16 In contrast, Pyykko et al.8 suggests a larger importance of visual than proprioceptive input for balance in the elderly, especially in the oldest of adults. They found a 52% decrease in postural stability for those aged 85 and older compared to a 22% decrease in stability in subjects younger than 60 when visual input was removed. In contrast, they found only a 16% decrease in postural stability for those 85 and older compared to a 49% decrease in stability for subjects younger than 60 when misleading proprioceptive input was received.8 The differing importance of visual input found in these two studies may be associated with Pyykko et al.’s8 use of sway velocity to assess sensory inputs to balance while Judge et al.16 measured center of force displacements and loss of balance events to assess sensory inputs to balance. Similarly, the two studies altered proprioceptive input differently: Pyykko et al.8 used calf muscle stimulation to alter proprioceptive input while Judge et al.16 used platform movement to alter proprioceptive input. Additionally, postural sway, which was measured in Pyykko et al.’s study, may not always correlate with postural instability.10 However, Hay17 reiterates the importance of vision in maintaining balance in older adults, concluding that in comparison to young adults, postural stability in older adults is more affected by absence of vision while alteration of proprioceptive information affects older and younger adults similarly. Likewise, in a study of young, healthy older, and fall‐prone older adults attempting to stand still in a virtual environment with visual oscillations in the anteroposterior (AP) direction, Jeka et al.18 found that older adults tended to weight the visual stimulus higher than younger adults, and fall prone older adults tended to weight the visual stimulus higher than healthy older adults; these effects were more apparent at higher visual amplitude perturbations. Thus, it appears that age is the primary factor in differences of visual stimulus weighting during balance maintenance with visual amplitude as a modifying factor.18 Together, these studies suggest that the sensory decline seen in many systems in older adults contributes to instability and older adults rely heavily on vision to maintain balance.
Slower Sensory Integration in the Elderly
Elderly adults also display reduced ability to adapt to new sensory information while maintaining balance. They have more difficulty than younger adults rapidly reintegrating new sensory information for balance control and show increased postural instability with receipt of new sensory information, unlike young adults. However, they do show the ability to partially adapt to reinsertion of visual or proprioceptive information while young adults show the ability to completely adapt to new sensory information.17 Similarly, as subjects get older, they have reduced ability to adapt to diminished or inaccurate visual/proprioceptive input to maintain balance, suggesting an inability to increase the weighting of vestibular input or to minimize the weighting of inaccurate visual input.16 In contrast, rather than differences in the acute response to changes in visual information, Jeka et al.18 found that older adults displayed a trend towards slower reweighting of visual information over longer time periods during exposure to a high amplitude visual stimulus, suggesting a role for vision in ongoing postural stability rather than acute perturbations.18 However, the lack of difference found in short time periods could also be due to limitations in the temporal resolution of their data.
GAIT DISTURBANCE
How Is Gait Controlled?
In bipedal primates, gait is controlled by the spinal cord, brainstem, cerebellum, and forebrain.6 The gait pathway involves projections from the pre‐motor and motor areas of the frontal cortex to the basal ganglia, from there to locomotor control centers in the brainstem and cerebellum, and finally to the spinal pattern generators.19 Neural control of gait is typically divided into separate processes: activation and guidance, regulation and execution. Cortical circuits are involved in the activation and visual guidance involved in navigation. Reciprocal circuits between the cortex‐basal ganglia and cortex‐cerebellum are involved in regulation of gait, which includes postural tone, balance and coordination of limb movement.20 Many disturbances of gait arise from the forebrain, which is important for providing the goal and purpose for walking. This includes the basal ganglia, frontal subcortex, and motor cortex.6
The rhythmic pattern of human gait is controlled by “central pattern generators” which are coordinated groups of interneurons in the spinal cord that allow alternate activation of agonist and antagonist muscles for proper gait. However, afferent somatosensory pathways and locomotor regions in the brain are also involved with the gait cycle, with locomotor regions important for initiating and modulating gait.19 Drew, Prentice, and Schepens21 suggest that the spinal cord integrates inputs from peripheral afferents and supraspinal structures, which is divided between fine control and postural support mechanisms. They conclude that signals descending from supraspinal structures are integrated with signals that generate basic locomotor rhythm as well as peripheral inputs within the spinal cord.21 Posture and movement may be integrated through the presence of descending commands for movement being relayed to brainstem areas involved in postural control, providing an avenue for adjustments of the magnitude and timing of postural changes during movement.21 Table 1 summarizes terminology use to describe different gait parameters and Figure 1 illustrates a few key gait parameters.
Table 1.
Glossary of Gait Terminology.
| Term | Definition |
|---|---|
| Gait speed | Distance walked per unit time |
| Step width | Lateral distance between the heels for consecutive heel strikes of the two feet |
| Stride width | Perpendicular distance from the heel of one foot to the line connecting two consecutive heel strikes of the contralateral foot |
| Step length | Distance between the heels in the anteroposterior direction for consecutive heel strikes of opposite feet |
| Stride length | Distance between the heels in the anteroposterior direction for consecutive heel strikes of the same foot |
| Stride time | Time between consecutive heel strikes of the same foot |
| Cadence | Number of steps taking per unit time |
| Gait variability | Step‐to‐step deviations/variations in gait parameters |
| Gait cycle | One complete cycle of one limb starting when the foot first contacts the ground to when the same foot next contacts the ground |
| Stance phase | Phase of gait cycle from touchdown to liftoff of the same foot |
| Swing phase | Phase of gait cycle during which the foot of interest is not on the ground |
| Double support | Both feet are simultaneously contacting the ground |
| Single support | Only one foot is in contact with the ground |
Figure 1.
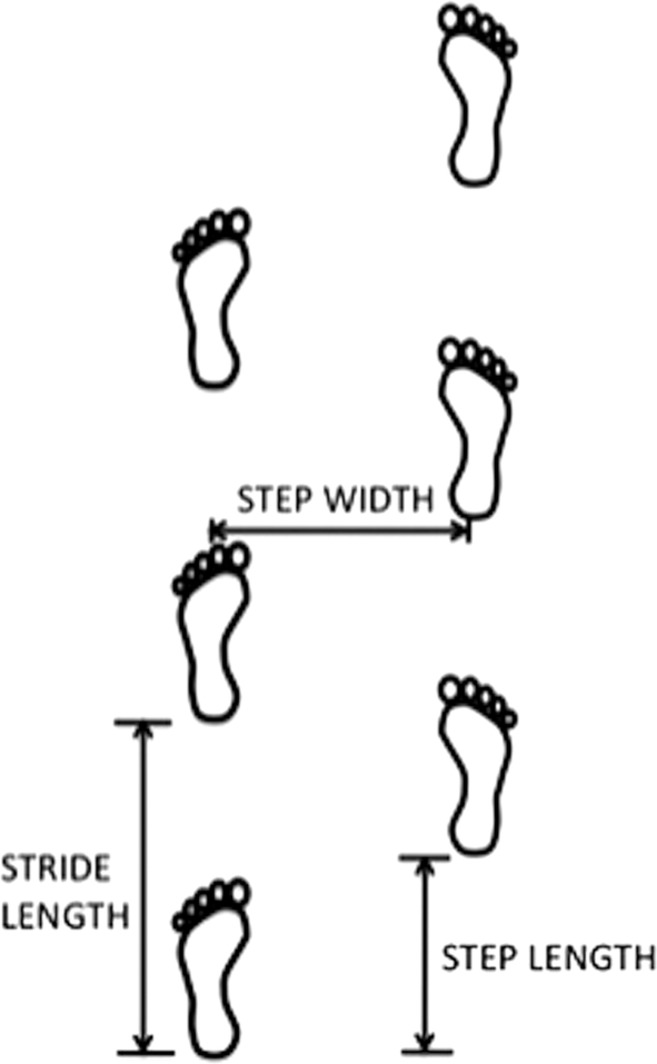
Gait parameters. Illustration of Gait Parameters including step width, step length, and stride length.
Patterns of Gait Disturbance
Common patterns of gait disturbance include cautious, stiff‐legged, freezing, frontal, dystonic, cerebellar, sensory ataxic, psychogenic, antalgic, paretic, hypokinetic, and dyskinetic.6, 19 Cautious gait is characterized by shortened stride, widened base, and shortened swing phase but with preserved rhythmic stepping. Spastic/stiff‐legged gait is seen in patients with history of stroke, demyelinating disease, spinal disease, and cerebral palsy and is characterized by leg circumduction, stiffness, and leg scissoring.6, 19 Freezing gait is most associated with Parkinson's disease and is characterized by arrested movement particularly during gait initiation or changes in gait direction. Frontal gait is characterized by impaired stepping and disequilibrium and is most often seen with cerebrovascular disease, particularly of the basal ganglia and periventricular white matter. Dystonic gait is multi‐factorial and has multiple presentations, but the most common focal disorder involves inversion at the ankles sometimes with concurrent extension of the hallux. Cerebellar gait is characterized by a slow, halting gait, widened base of support, and irregular steps of a lurching quality due to truncal instability; it worsens with the eyes closed.6, 19 Sensory ataxic gait is characterized by visual dependence, proprioceptive deficits from peripheral neuropathies, and a high‐stepping, broad‐based gait with diminished swing phase; it worsens with other sensory system deficits.6, 19 Psychogenic gait, such as gait associated with a fear of falling, is associated with extremely slow gait speed, wastage of muscular effort for postural maintenance, and rapid fluctuations in a short time frame.6 Antalgic gait involves a shortened standing phase on the affected leg and is associated with pain and limited passive range of movement. Paretic gait is characterized by asymmetry with a steppage gait and Trendelenburg sign. Hypokinetic gait is characterized by small step length, slow gait speed, shuffling, decreased arm swing, and worsening with cognitive tasks. Finally, dyskinetic gait is characterized by involuntary movements.19 Table 2 summarizes these findings.6, 19
Table 2.
Patterns of Gait Disturbance.
| Type of Gait Disturbance | Characteristics |
|---|---|
| Cautious | Short stride, wide base, short swing phase |
| Stiff‐legged | Leg circumduction, stiffness, scissoring |
| Freezing | Arrested movement, especially at gait initiation or direction change |
| Frontal | Impaired stepping, disequilibrium |
| Dystonic | Often focal with ankle inversion, hallux extension |
| Cerebellar | Slow, halting, wide base, truncal instability |
| Sensory ataxic | High‐steppage, broad‐based, decreased swing phase, associated with sensory deficits |
| Psychogenic | Slow, rapid fluctations, wastage of muscular effort |
| Antalgic | Shortened standing phase on affected leg, associated with pain |
| Paretic | Asymmetric, steppage gait, Trendelenburg sign |
| Hypokinetic | Small step length, slow, shuffling, diminished arm swing |
| Dyskinetic | Involuntary movements |
GAIT DISTURBANCE IN THE ELDERLY
Patterns of Gait Disturbance in the Elderly
Some patterns of gait disturbance arise particularly in old age, including gait related to sensory deficits (polyneuropathy, bilateral vestibulopathy, visual impairment), neurodegenerative disease (Parkinsonism, cerebellar ataxia, dementing syndromes), vascular encephalopathy, normal pressure hydrocephalus (NPH), and anxious gait. Polyneuropathic gait is unsteady, worsened with eye closure, and characterized by loss of ankle‐jerk reflexes. Bilateral vestibulopathy is characterized by unsteady gait in the dark or on uneven surfaces and oscillopsia. Visually impaired gait is unsteady, particularly on uneven ground. Parkinsonism is characterized by hypokinetic gait and decreased capacity for dual‐tasking. Cerebellar gait is ataxic with limb ataxia. Dementing syndromes are characterized by slow gait, increased falls risk, impaired spatial orientation, and decreased dual‐tasking ability. Vascular gait is characterized by a “frontal gait” pattern, which involves small steps, broad‐based gait, and normal arm swing.19 Older individuals with frontal gait disorders walked slower, had shorter stride length, lower cadence, wider support base, and spent longer in double support stance compared to healthy individuals.22 NPH is characterized by apraxic gait. Finally, anxious gait, sometimes classified under psychogenic gait, is characterized by broad‐based gait, improvement with distraction, and normal dual‐tasking ability.19 Additionally, older individuals often adopt a “cautious” gait pattern characterized by slower walking speed, reduced step length and increased variability in step timing. This gait adaptation is more apparent on irregular than level surfaces and may be a mechanism to increase head and pelvis stability in older adults.23 Table 3 summarizes these findings.19, 23 Importantly, although some changes in gait seen in the elderly can be partially attributed to the reduced walking speed seen in older adults, reduced peak hip extension, increased anterior pelvic tilt and both reduced peak ankle plantarflexion and power generation during gait are present in the elderly regardless of walking speed. Thus, hip flexure contractures and ankle plantar flexor concentric weakness may affect gait in elderly individuals.24
Table 3.
Patterns of Gait Disturbance in the Elderly.
| Type of Gait Disturbance | Characteristics |
|---|---|
| Polyneuropathic | Unsteady, worse with eye closure |
| Bilateral vestibulopathic | Unsteady in dark and on uneven surfaces |
| Visually impaired | Unsteady, worse on uneven surfaces |
| Parkinsonian | Hypokinetic, decreased dual‐tasking ability while walking |
| Cerebellar | Ataxic |
| Dementing | Slow, increased falls, decreased dual‐tasking ability while walking, impaired spatial orientation |
| Vascular encephalopathic | Small steps, broad‐base, normal arm swing |
| Normal Pressure Hydrocephalus | Apraxic |
| Anxious/psychogenic | Broad‐based, improved with distraction, includes “fear of falling” |
| Cautious | Slow, reduced step length, increased variability in step timing |
Changes in Spatiotemporal Gait Parameters in Elderly Adults
Stability is an important marker of gait in the elderly with local dynamic stability being a valid predictor of falls risk.25 In a study of treadmill walking, Terrier and Reynard26 found that gait instability starts to increase in the fourth to fifth decades of life: mediolateral (ML) dynamic stability is decreased in older individuals while anteroposterior and vertical gait stability appeared to be similar across ages.26 However, treadmill walking imposes a temporal constraint on gait and could mask age‐related differences in gait instability that may be apparent during overground walking. In a study of overground walking in subjects 50 years and older, Verlinden et al.27 found that increasing age is associated with worse gait. Here, worse gait is defined as increased gait variability, shorter stride/step length, slower walking speed, increased percentage of the gait cycle spent in double support and stance, decreased stride width with increased stride width variability, and less accurate tandem walking. The earliest decline and strongest associations with worse gait are seen with gait variability, followed by tandem walking and phases of the gait cycle.27 In assessing falls risk, Figueiro et al.28 found that adults at high risk of falling demonstrate a significantly slower gait speeed than adults at low risk of falling as a result of shorter step length, since step length and cadence are determinants of gait speed. Additionally, Hausdorff et al.29 conducted a prospective cohort study on older subjects whose gait was assessed during overground walking and who were subsequently followed‐up a year later to collect data on the occurrence of any falls. They found that variability of stride time and swing time as well as inconsistency of stride time variance, which measures how variability changes with time, were significantly higher in those who subsequently fell compared to those who did not fall. Thus, a one standard deviation increase in stride time variability or swing time variability increased the likelihood of future falls by 5.3‐ and 2.2‐fold, respectively. They also found that subjects with increased gait variability were more likely to fall sooner than those with less gait variability. In contrast with the findings of Figueiro et al., there was no significant difference in gait speed between those who suffered a fall and those who did not.29 However, this study was a prospective study examining future falls rather than falls risk at the time of the study as in the experiment conducted by Figueiro et al. Regardless, this study provides further evidence for the association between increased gait variability and falls. Together, these studies suggest that older adults who have slower gait speed and increased gait variability may be at increased risk of falls. Fear of falling in elderly adults can also affect gait characteristics. Stride time variability and trunk variability in the AP, ML, and vertical directions were significantly worse in the group with a fear of falling compared to the group without a fear of falling.30
GENERAL EFFECTS OF VISUAL MANIPULATIONS ON GAIT AND BALANCE
Visual Perturbations
Important information about balance can be gathered by analyzing how adults react to visual perturbations. Bugnariu and Fung31 found that tilting of the virtual environment viewed by subjects through a head mounted display did not result in significant postural sway in young or old adults, but older adults did display larger center of mass excursions compared to younger adults. Similarly, Sundermier et al.32 found that when exposed to visual surround moving in the AP direction, young and old subjects displayed increased displacement of their center of pressure (CoP), ranging from minimal to large displacements, but healthy older adults and young adults did not have significantly different CoP displacements; in contrast, older adults with balance problems did have larger CoP displacements in comparison to young adults. However, both young and old adults showed an attenuated response to repeated AP visual movement and oscillation, suggesting adaptation. Interestingly, healthy older adults and younger adults did not differ significantly in terms of shear force created when exposed to AP perturbations but the older adults with balance problems did generate significantly higher shear force than young adults when exposed to AP perturbations, suggesting that older adults with balance problems rely more on hip movements to maintain posture when there is movement of the visual environment.32 The magnitude of perturbations can also have an impact on balance: both young and old adults have decreased sway velocity in lower amplitude than higher amplitude optic flow. Similar to the findings in Sundermier et al.’s study of adaptation to repeated visual perturbations, sway velocity was found to decrease with repeated exposure to anterior‐posterior movement of an image viewed by subjects in the virtual environment while standing on a platform.33 Thus, when exposed to visual perturbations, center of pressure displacements may not differ greatly between old and young adults; however, changes in center of pressure may not fully account for balance differences between older and younger adults.
Insight into the general effects of visual perturbations on gait can be ascertained from studies done in healthy young adults as well. However, it is important to understand how gait can change going from the real to virtual environments. Hollman et al.34 studied changes in spatiotemporal gait parameters in healthy young subjects while they walked at three different speeds on an instrumented treadmill in the virtual environment. Figure 2 illustrates an example of an instrumented treadmill. They found that subjects walked with shorter stride length, increased step width and increased variability of stride velocity and step width in the virtual environment compared to the non‐virtual environment. Thus, they conclude that walking in the virtual environment can, by itself, reduce gait stability in healthy subjects.34 Nonetheless, valuable information about gait can be learned from experiments in the virtual environment. McAndrew et al.35 studied the gait of young adults walking on a treadmill in a Computer Assisted Rehabilitation Environment (CAREN) while exposed to continuous, pseudorandom oscillations of the standing surface or visual field. They found that subjects’ instability worsened more during mediolateral than anteroposterior visual perturbations and that instability was direction specific with subjects experiencing increased instability in the AP direction when AP perturbations but not ML perturbations were applied and increased instability in the ML direction when ML perturbations but not AP perturbations were applied.35 In a similar study using young healthy subjects in the CAREN, McAndrew et al.36 found that subjects had significantly shorter step length and wider step width in both the AP and ML visual perturbation conditions relative to the unperturbed state, but subjects had significantly shorter step length and larger step width during ML than AP perturbations. Interestingly, stride time was significantly shorter during ML perturbations compared to the no perturbation condition, but this pattern was not seen with AP perturbations. Looking at variability data, subjects had significantly greater step length and step width variability for perturbations relative to no perturbations and greater step width variability for ML perturbations than AP perturbations. Stride length variability was significantly greater during ML visual perturbations than AP visual perturbations. Stride time variability was significantly greater during ML visual perturbations than in the no perturbation condition. Thus, the increased variability present in the ML direction suggests that subjects must exert increased control in the ML direction to maintain balance while walking.36 In line with these findings of increased instability with ML perturbations, O'Connor et al.37 found that when healthy young male adults walked on an instrumented treadmill while viewing a virtual hallway with continuous visual perturbations in the ML and AP direction, step variability was most affected by ML rotational disturbances and was associated with increased energy expenditure. They also found that ML translational perturbations were associated with an increased mean step width, net metabolic rate, and step width variability; in contrast to McAndrew et al.’s36 study in the CAREN system, no significant difference in step length or step length variability was found.37 In all, these studies suggest that human gait is most sensitive to perturbations in the mediolateral direction as evidenced through increased gait variability and instability.
Figure 2.

Instrumented Treadmill. Spatiotemporal Gait Parameter Collection for Subject Walking on an Instrumented Treadmill.
Removal of Visual Input
Inferences about the effects of visual changes on gait can also be assessed from the complete removal of visual input. Wuehr et al.38 studied healthy young subjects walking with both eyes open and eyes closed at five different walking speeds and found that removal of visual input was associated with a significantly slower maximum walking speed than when visual input was present. Additionally, variability of stride time, stride length, and base width recorded on a treadmill were significantly increased without visual input for all walking speeds; base width is defined as the vertical distance from the heel center of one foot to the line of progression formed by two consecutive footprints of the opposite foot. Interestingly, variability in stride time and stride length was significantly higher for slower walking speeds than faster speeds with eyes closed. Thus, it appears that visual information is important for ML gait stabilization (ie, base width) at all speeds, while visual input contributes more to AP gait stabilization (ie, stride length, stride time) at slower speeds than faster speeds.38 Bauby and Kuo39 studied gait in healthy young adults during overground walking with eyes open and closed and found that compared to the eyes open condition, step length decreased by 5.1% while step width increased by 11%. Additionally, the difference between lateral variability and AP variability increased from 79% with visual input to 126% with visual input removed: lateral variability increased by 53% with eyes closed while AP variability increased by only 21% with eyes closed. In all, this suggests that visual‐vestibular feedback is required for lateral stability but is less important for AP stability.39 Similarly, in a study on the effect of removal of visual input on treadmill walking, Saucedo and Yang40 found that for both young and old adults, step length was shorter, stance phase duration was shorter and cadence was increased in the eyes closed compared to eyes open condition. Additionally, they also found that there was a significant increase in step length variability, step width variability and stance phase duration variability for both young and old adults in the eyes closed compared to eyes open condition.40 Partial removal of visual input also provides insight on gait. Specifically, Marigold and Patla41 studied healthy young and old subjects who walked across solid ground and multi‐surface terrain either with normal vision or with their lower visual field blocked. Both young and old adults walked significantly slower when the lower visual field was blocked, but only when walking across a multi‐terrain surface and not normal solid ground. Mean step length was also smaller when the lower visual field was occluded compared to when vision was normal for both young and old adults. Thus they concluded that the lower visual field plays a role in both young and old adults in walking across a multi‐terrain surface.41 Thus in general, like with visual perturbations, removal of visual input is associated with increased gait variability, especially in the ML direction, shortened step/stride length, and wider gait. To summarize, these studies suggest that visual feedback may be used to establish homeostatic mean and tolerance values for spatiotemporal gait parameters. Thus, without visual feedback, mean values are able to vary more from trial to trial and tolerance width may increase, resulting in overall increased gait variability.
EFFECTS OF VISUAL MANIPULATIONS ON GAIT AND BALANCE IN HEALTHY OLDER ADULTS
Visual Perturbations
In addition to the general effects of visual perturbation on gait in the studies discussed above, older adults display some unique characteristics when exposed to visual perturbations. Like in young adults, changes in gait occur in elderly individuals when switching from a real to virtual environment. Such changes are important to understand before evaluating the effects of virtual environment perturbations on spatiotemporal gait parameters. Parijat et al.42 studied healthy older adults walking on a treadmill in the virtual environment (VE). They found a significant decrease in stride length and increase in stride duration in the VE compared to the real environment but noticed normalization after 15 to 20 minutes in the VE. Step width increased in the VE and continued to be significantly different after 15 to 20 minutes of walking. They also found a significant increase of 65% in step length variability after 5 minutes of walking in the VE, which decreased and showed no difference after 25 minutes in the VE. Similar patterns were found in variability of stride velocity (84% increase after 5 minutes in the VE), stride duration (58% increase after 5 minutes in the VE) and step width (21% increase after 5 minutes in the VE); however, step width variability was the only one to show a significant difference after 25 minutes in the VE.42
With regard to virtual reality perturbations, older adults sway more than younger adults in response to AP optic flow and take longer to decrease sway velocity with repeated optic flow exposure than young adults. Unsurprisingly, when optic flow velocity is suddenly increased in amplitude, older adults have a significant increase in sway velocity after the transition but young adults do not.33 In terms of gait, Berard et al.43 studied young and old subjects who were instructed to walk straight in the physical world while watching a VE displayed by a head‐mounted display under three conditions: no visual perturbation, visual perturbation consisting of a 40 degree left or right rotation about a vertical axis, and no visual input (blackness). They found that older adults had a significantly higher mediolateral deviation of their center of mass than younger adults in the visual perturbation conditions but this difference was not seen in the no visual perturbation or blank conditions. Thus, they concluded that elderly individuals have difficultly downweighting erroneous visual information and depend more on visual cues.43 In a similar experiment, Berard et al.44 studied subjects who were instructed to walk straight in the virtual world while watching a virtual environment displayed by a head‐mounted display under three conditions: a left or right focus of expansion (FOE) shift of the virtual scene 40 degrees, 20 degrees, or 0 degrees. For the trials where the FOE was shifted, a mediolateral deviation of the center of mass in the physical environment that is equal in magnitude and opposite in direction to the shift in FOE would result in a straight walking path in the virtual environment. However, although young adults were able to adjust their center of mass, older adults did not alter their center of mass in the physical room regardless of changes in the FOE. Thus, they concluded that older adults may have more difficulty recalibrating their visuomotor systems in a timely fashion to adapt to changing visual cues.44 In an experiment with treadmill walking, Francis et al.45 studied healthy old and young subjects walking on a treadmill while watching a speed‐matched virtual hallway; kinematics data was recorded with a motion capture system and reflective markers. They found that ML visual perturbations significantly increased gait variability (+152% step width variability and + 131% step length variability) in older adults but not younger adults compared to the normal condition, suggesting that older adults depend more on visual feedback for whole‐body positioning. Older adults also walked with 5% shorter step length in the ML visual perturbation condition, a significant change compared to the no visual perturbations condition.45 Figure 3 illustrates an example of a motion capture system using anatomically placed markers. Similarly, Franz et al.46 studied old and young subjects who were instructed to walk on a treadmill at their preferred speed while viewing a speed‐matched virtual reality hallway in the presence and absence of continuous ML visual perturbations. They found that ML visual perturbations were associated with a significantly larger change in ML center of mass in old adults compared to young adults. Similarly, ML visual perturbations affected control of lateral step placement more significantly in old adults than young adults. Thus they concluded that elderly adults rely more on visual feedback to control balance when walking in comparison to young adults due to degradation in somatosensory signaling.46 Lastly, Nyberg47 conducted a study on young and old adults while they walked over normal ground both with and without a virtual environment displayed in a head‐mounted display. They found that although walking in virtual reality decreased walking speed in both young and old subjects, exposure to virtual tilt and virtual snowfall had a larger impact on old adults, decreasing walking speed in all of the old subjects by 114% to 162%, but in only one of the young subjects. However, a second exposure to virtual tilt and snowfall was associated with a faster walking speed than during the first exposure to these events, suggesting adaptation to the virtual environment.47 Thus, it appears that visual perturbations increase gait variability and center of mass movement more in old adults compared with young adults, perhaps due to older adults’ increased reliance on visual input.
Figure 3.

Motion Capture System. Kinematics Data Collection Using Motion Capture System and Anatomic Markers.
Removal of Visual Input
In addition to analyzing the effects of virtual perturbations, insight can be gained from analyzing how removal of visual input affects gait in older adults. Cromwell et al.48 studied community‐dwelling older adults as they walked under conditions with either both eyes open or eyes closed. They found a significant decrease in walking speed in the eyes closed compared to eyes open condition; however, there was not a significant difference in cadence between the eyes closed and eyes open condition. Thus, they concluded that removal of visual input caused older adults to adapt by walking more slowly; walking slowly without a significant change in cadence suggests a shorter step length and increased time spent in the support phase of the walking cycle, which is a more stable state.48 Similarly, Saucedo and Yang40 studied older and younger adults as they walked on a treadmill under both eyes open and eyes closed conditions. Although step width was significantly increased in the eyes closed compared to eyes open condition for older adults, step width was not significantly different in the eyes closed compared to eyes open condition for young adults; however, there was not a significant difference in step width between older and younger adults in either condition. Importantly, there were no significant age by condition interactions for step length, step width, stance phase time, cadence, or variability of step length, step width, or stance phase.40 Thus, they conclude that both older and younger adults undergo similar gait adaptations in the face of visual deprivation. However, this must be interpreted with caution as only 43% of subjects completed the trials so a selection bias towards healthier older subjects is possible. Anderson49 studied young (18–33), young elderly (60–69), and older elderly (70+) adults who walked along a walkway under two conditions: with a full visual field and with the lower visual field blocked from view. They found that the two elderly groups had a significant increase in walking speed in the restricted visual field condition compared to the full visual field condition but the young group's velocity did not change significantly; the two older groups did not differ significantly from each other. Similarly, step length increased in the two older groups in the restricted visual field but was not significantly different in young adults; the two older groups did not differ significantly from each other. Further, step frequency increased significantly in the oldest group in the restricted visual field compared to the full visual field but not in the young elderly or young group. In all groups, the relationship between stride length and frequency (the preferred walking pattern) did not change significantly in the reduced visual field condition. Thus, the increased velocity seen in older subjects suggests that removal of the lower part of the visual field affects subjects’ perceived self‐motion and older adults may be more dependent on vision for regulating walking speed.49 This is somewhat in contrast to the finding in Marigold and Patla's study,41 which found that walking speed on normal ground did not change significantly for older adults when the lower visual field was blocked. In a study assessing multiple manipulations to visual input, Helbostad et al.50 studied older adults as they walked along an electronic gait mat under multiple conditions: full light, dim light, and dim light combined with reduced depth perception, double horizontal vision, blurred vision, or tunnel vision. Figure 4 illustrates an example of an electronic gait mat. They found that dim light alone did not have a significant effect on spatiotemporal gait parameters. However, they found a significant increase in cadence, decrease in step length and increase in step length variability in conditions of reduced depth perception, double horizontal vision, blurred vision, and tunnel vision in dim light compared to full light, with double and tunnel vision having the largest effects. In contrast, there was not a significant effect of altered visual conditions on step width or step width variability.50 Thus, the effect of removal of visual input on spatiotemporal gait parameters in elderly subjects requires further study.
Figure 4.

Electronic Gait Mat. Spatiotemporal Gait Parameter Collection for Subject Walking on Electronic Gait Mat.
Lighting Changes
In addition to direct manipulation of visual input, the light in the environment reaching the eye affects gait. Moe‐Nilssen et al.51 studied community‐dwelling older women who walked along an uneven mat under conditions of normal lighting (>750 lux at floor level) and dimmed lighting (<0.75 lux at floor level). They found that in dim lighting, the walking speed at 3 seconds was 85% of that in normal lighting. They also found that cadence at 3 seconds was 9% higher in the dimmed condition compared to that in the normal lighting condition. However, these differences were not significant after 90 seconds. Thus, they concluded that sudden transition from normal to dim lighting might pose a challenge to balance in elderly women during walking, leading to adoption of a protective gait pattern.51 Similarly, Figueiro et al.28 studied older adults who walked along an instrumented walkway under different lighting conditions. They found that step length was significantly longer under ambient light than with night‐lights alone. When examining standard deviations of step length, they found that subjects had a significantly greater standard deviation under night‐lights alone than under ambient light. Finally, subjects walked significantly faster under ambient light than under night‐lights. Thus, they conclude that lighting has a significant impact on gait parameters in healthy older adults.28 These two studies are in contrast to Helbostad et al.’s50 finding that dim lighting alone does not have a significant effect on gait parameters; however, the dim condition in Helbostad et al.’s study was not as dim as in these two studies. In all, these studies on lighting changes in the elderly suggest that decreased lighting is associated with slower gait and more variable gait, which are risk factors for falls.
THERAPY
Given the importance of gait and balance in normal function, especially in elderly adults, many have studied ways to improve these areas through intervention. Therapies proposed have historically included exercise but new treatment modalities have become available and show promise. Walking as an exercise has been suggested as a way to relieve hip flexure contractures that impair gait in the elderly in order to improve walking.24
Newer training modalities involving virtual reality have also shown promise in improving balance in older adults. Duque et al.52 studied elderly community‐dwelling participants who had suffered from at least one fall in the 6 months prior to assessment and displayed poor balance; they found that subjects who were placed in a virtual‐reality training‐system (Balance Rehabilitation Unit [BRU]) group, consisting of 6 weeks of visual‐vestibular stimulation and postural training, showed significant improvement of balance parameters after 6 weeks and at 9 months maintained significantly better balance across many parameters compared to the control group, who only received standard recommendations for falls prevention. Similarly, BRU‐trained subjects had a significantly lower incidence of falls than the control group.52 Additionally, given the role of sensory decline in balance impairment of elderly subjects, Bugnariu and Fung31 suggested using training involving sensory conflicts to improve balance in the elderly. They found that repeated exposure to sensory conflicts in the elderly was associated with a decrease in the number of steps taken when exposed to perturbations and increased ability to maintain tandem stance.31 Park et al.53 assessed the impact of virtual reality training using a Wii system (Wii Fit soccer, snowboarding, and Table Tilt) and compared this to a ball exercise group over an 8‐week period. They found that average sway speed, sway length and Timed Up and Go (TUG) test time decreased for both interventions, suggesting improved balance. However, the virtual reality exercise resulted in a larger reduction in sway length than the ball exercise.53 Virtual reality can also be used to introduce virtual slips as a means of balance training. Parijat et al.54 studied older adults, half of whom were placed in a control group and half in a virtual reality‐training group. The group in the virtual reality training arm underwent a slip trial on a real slippery walking surface, followed by treadmill training in the virtual environment with inclusion of a virtual slip (induced by tilting of the virtual environment) at random intervals; they then concluded with a second slip trial on the slippery surface. The control group underwent a similar process except instead of treadmill training in the virtual environment they underwent normal walking in the real environment. The virtual reality group had a decrease in falls from the first unexpected slip during the first trial to the unexpected slip post training of 50% to 0% while the control group had a decrease in fall frequency from 50% to 25%.54
Dual‐task training has also been suggested as a way to improve gait in the elderly. Eggenberger et al.55 studied older adults, all with a Mini Mental Status Exam Score of at least 22, who underwent three different 6‐month long training interventions in parallel and were followed up 1‐year post‐intervention. The three programs were a virtual reality dance game training, treadmill memory training using word sequence recall, or basic treadmill walking; strength and balance exercises were given to each group. Gait was analyzed pre‐intervention, at 3‐month and 6‐month during the intervention, and at 1‐year follow up. They found significant improvements in all three interventions from pretest to 6 months in velocity, step length, step length variability, step time, and step time variability. They also found a significant reduction in step time at subjects’ fast walking speed over time in those in the dance group but stable step time in the treadmill memory training group. In contrast, there was a trend towards decreasing step length variability at preferred walking speed while completing a dual‐task (combined cognitive and walking) in those who did treadmill memory training, but step length variability was stable in the dance group. Furthermore, the cost of dual‐task walking on step time variability during fast walking, which is a measure of the decreased walking performance that occurs during completion of a dual‐task compared to a single‐task, was stable in the treadmill memory‐training group and dance group but increased in the normal treadmill walking group. Overall, performance was stable in many of the gait variables at follow up but some gait variables worsened. All interventions were associated with a significant decline in fall frequency at 6 months after training with a trend toward increasing falls at later time periods.55 Thus, combined cognitive and physical training, as seen in the dance game and the treadmill memory training, may have benefits over physical training alone.
Finally, in addition to virtual reality training and exercise, studies have been done on devices that can improve balance in the elderly. Lipsitz et al.56 conducted a randomized single‐blind crossover study on elderly adults who had vibratory insoles place in their shoes set at 0%, 70%, or 85% of their baseline sensory threshold. Compared to no vibratory stimulation, 70% and 85% vibratory stimulation was associated with a significant decrease in elliptical area of postural sway and ML sway when subjects stood on a force plate. However, AP average sway and sway speed were not affected. Additionally, compared to no vibratory stimulation, 70% and 85% vibratory stimulation showed significantly reduced Timed Up and Go (TUG) time, a test of mobility and balance, and decreased variability in stride time and double support time. However, 27 subjects failed vibration screening and were thus excluded from the trial; these subjects tended to be older.56 Thus, although vibratory insoles show promise, their utility may be limited to a subset of the elderly population.
CONCLUSION AND FUTURE DIRECTIONS
In conclusion, balance and gait disturbances are a common occurrence with increasing age and have significant impacts on the well‐being of older adults. Decline of sensory systems in elderly adults has been implicated in the reduced ability of older adults to adapt to changes in their environment and maintain balance; the visual system is particularly important in maintaining postural stability. There are distinct changes in gait associated with aging, such as slower gait and increased gait variability. Exposure to visual perturbations and manipulations emphasizes these changes in gait. Increased gait variability, specifically with mediolateral perturbations, poses a particular challenge for elderly adults, as it has been linked to falls. Thus, treatment to improve balance and gait in the elderly is imperative, with virtual reality training holding particular promise due to the wide range of training available and positive effects seen in early studies.
Future research should focus on better delineating the effects of virtual reality versus virtual reality perturbations on spatiotemporal gait parameters, as there is evidence that walking in virtual reality itself increases gait instability, especially during initial walking. Similarly, given that most studies in the elderly on walking in virtual reality with visual perturbations have been done on a treadmill rather than overground walking, future research should focus on the effect of visual perturbations on spatiotemporal gait parameters during overground walking, as this may better reflect the real‐life walking environment faced by the elderly. Similarly, future studies should assess changes in gait parameters over longer time periods to better characterize gait changes in the elderly and understand gait adaptations to visual perturbations; these may differ from the shorter term changes in gait reported in many of these studies. Future research should also compare how elderly people with and without balance problems are affected by visual perturbations as this review focused on healthy adults without diagnosed balance/gait impairments; such information can provide additional insight into the gait parameters most important for postural stability while walking. Similarly, the effect of visual perturbations on spatiotemporal gait parameters in those with cognitive impairment should be explored, as this review focused on healthy adults without cognitive impairments; this aspect of gait in the elderly is important as a significant proportion of elderly adults suffer from mild to severe cognitive impairment. Lastly, given the association between falls and gait/balance, future research should focus on determining what specific aspects of training improve balance in order to better tailor intervention for older adults; for example, using functional MRI to assess any changes in brain connectivity associated with balance and gait training may give insight into the neural impacts of such training and provide direction for future therapy.
Conflict of Interest: Anil K. Lalwani, MD serves on the Medical Advisory Board of Advanced Bionics Corporation, and the Surgical Advisory Board of Medel Corporation.
Financial Disclosures: None
BIBLIOGRAPHY
- 1. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing 1989;18(1):31–34. [DOI] [PubMed] [Google Scholar]
- 2. Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community‐residing older adults. J Am Geriatr Soc 2006;54(2):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319(26):1701–1707. [DOI] [PubMed] [Google Scholar]
- 4. Prudham D, Evans JG. Factors associated with falls in the elderly: a community study. Age Ageing. 1981;10(3):141–146. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . National Center for Injury Prevention and Control. 2016. Available at: http://www.cdc.gov/injury/wisqars/. Accessed May 20, 2018.
- 6. Viswanathan A, Sudarsky L. Balance and gait problems in the elderly. Handb Clin Neurol 2012;103:623–634. [DOI] [PubMed] [Google Scholar]
- 7. Choy NL, Brauer S, Nitz J. Changes in postural stability in women aged 20 to 80 years. J Gerontol Series A Biol Sci Med Sci 2003;58(6):525–530. [DOI] [PubMed] [Google Scholar]
- 8. Pyykko I, Jantti P, Aalto H. Postural control in elderly subjects. Age Ageing 1990;19(3):215–221. [DOI] [PubMed] [Google Scholar]
- 9. Manchester D, Woollacott M, Zederbauer‐Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 1989;44(4):M118–127. [DOI] [PubMed] [Google Scholar]
- 10. Panzer VP, Bandinelli S, Hallett M. Biomechanical assessment of quiet standing and changes associated with aging. Arch Phys Med Rehabil 1995;76(2):151–157. [DOI] [PubMed] [Google Scholar]
- 11. Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc 1991;39(12):1194–1200. [DOI] [PubMed] [Google Scholar]
- 12. Zalewski CK. Aging of the human vestibular system. Semin Hear 2015;36(3):175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robbins S, Waked E, McClaran J. Proprioception and stability: foot position awareness as a function of age and footwear. Age Ageing 1995;24(1):67–72. [DOI] [PubMed] [Google Scholar]
- 14. Patel M, Magnusson M, Kristinsdottir E, Fransson PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol 2009;105(2):167–173. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh LC, Lin HC, Lee GS. Aging of vestibular function evaluated using correlational vestibular autorotation test. Clin Interv Aging 2014;9:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Judge JO, King MB, Whipple R, Clive J, Wolfson LI. Dynamic balance in older persons: effects of reduced visual and proprioceptive input. J Gerontol A Biol Sci Med Sci 1995;50(5):M263–270. [DOI] [PubMed] [Google Scholar]
- 17. Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res 1996;108(1):129–139. [DOI] [PubMed] [Google Scholar]
- 18. Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall‐prone older adults. J Mot Behav 2010;42(4):197–208. [DOI] [PubMed] [Google Scholar]
- 19. Jahn K, Zwergal A, Schniepp R. Gait disturbances in old age: classification, diagnosis, and treatment from a neurological perspective. Deutsch Arztebl Int 2010;107(17):306–315; quiz 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao A. Gait disorders In: Louis E, Mayer S, Rowland L, eds. Merritt's Neurology. 13th ed Philadelphia, PA: Volters‐Kluwer; 2016:107–117. [Google Scholar]
- 21. Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 2004;143:251–261. [DOI] [PubMed] [Google Scholar]
- 22. Danoudis M, Ganesvaran G, Iansek R. Disturbances of automatic gait control mechanisms in higher level gait disorder. Gait Posture 2016;48:47–51. [DOI] [PubMed] [Google Scholar]
- 23. Menz HB, Lord SR, Fitzpatrick RC. Age‐related differences in walking stability. Age Ageing 2003;32(2):137–142. [DOI] [PubMed] [Google Scholar]
- 24. Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehab 1998;79(3):317–322. [DOI] [PubMed] [Google Scholar]
- 25. Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 2013;10(83):20120999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross‐sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait Posture 2015;41(1):170–174. [DOI] [PubMed] [Google Scholar]
- 27. Verlinden VJA, van der Geest JN, Hoogendam YY, Hofman A, Breteler MMB, Ikram MA. Gait patterns in a community‐dwelling population aged 50 years and older. Gait Posture 2013;37(4):500–505. [DOI] [PubMed] [Google Scholar]
- 28. Figueiro MG, Plitnick B, Rea MS, Gras LZ, Rea MS. Lighting and perceptual cues: effects on gait measures of older adults at high and low risk for falls. BMC Geriatr 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community‐living older adults: a 1‐year prospective study. Arch Phys Med Rehab 2001;82(8):1050–1056. [DOI] [PubMed] [Google Scholar]
- 30. Sawa R, Doi T, Misu S, et al. The association between fear of falling and gait variability in both leg and trunk movements. Gait Posture 2014;40(1):123–127. [DOI] [PubMed] [Google Scholar]
- 31. Bugnariu N, Fung J. Aging and selective sensorimotor strategies in the regulation of upright balance. J Neuroengineering Rehabil 2007;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sundermier L, Woollacott MH, Jensen JL, Moore S. Postural sensitivity to visual flow in aging adults with and without balance problems. J Gerontol A Biol Sci Med Sci 1996;51(2):M45–52. [DOI] [PubMed] [Google Scholar]
- 33. O'Connor KW, Loughlin PJ, Redfern MS, Sparto PJ. Postural adaptations to repeated optic flow stimulation in older adults. Gait Posture 2008;28(3):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hollman JH, Brey RH, Robb RA, Bang TJ, Kaufman KR. Spatiotemporal gait deviations in a virtual reality environment. Gait Posture 2006;23(4):441–444. [DOI] [PubMed] [Google Scholar]
- 35. McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech 2011;44(4):644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo‐random oscillations of the support surface and visual field. J Biomech 2010;43(8):1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait Posture 2012;36(1):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wuehr M, Schniepp R, Pradhan C, et al. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res 2013;224(2):287–294. [DOI] [PubMed] [Google Scholar]
- 39. Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 2000;33(11):1433–1440. [DOI] [PubMed] [Google Scholar]
- 40. Saucedo F, Yang F. Effects of visual deprivation on stability among young and older adults during treadmill walking. Gait Posture 2017;54:106–111. [DOI] [PubMed] [Google Scholar]
- 41. Marigold DS, Patla AE. Visual information from the lower visual field is important for walking across multi‐surface terrain. Exp Brain Res 2008;188(1):23–31. [DOI] [PubMed] [Google Scholar]
- 42. Parijat P, Lockhart TE, Liu J. Effects of perturbation‐based slip training using a virtual reality environment on slip‐induced falls. Ann Biom Eng 2015;43(4):958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berard J, Fung J, Lamontagne A. Impact of aging on visual reweighting during locomotion. Clin Neurophysiol 2012;123(7):1422–1428. [DOI] [PubMed] [Google Scholar]
- 44. Berard JR, Fung J, McFadyen BJ, Lamontagne A. Aging affects the ability to use optic flow in the control of heading during locomotion. Exp Brain Res 2009;194(2):183–190. [DOI] [PubMed] [Google Scholar]
- 45. Francis CA, Franz JR, O'Connor SM, Thelen DG. Gait variability in healthy old adults is more affected by a visual perturbation than by a cognitive or narrow step placement demand. Gait Posture 2015;42(3):380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franz JR, Francis CA, Allen MS, O'Connor SM, Thelen DG. Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Hum Mov Sci 2015;40:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nyberg L, Lundin‐Olsson L, Sondell B, et al. Using a virtual reality system to study balance and walking in a virtual outdoor environment: a pilot study. Cyberpsychol Behav 2006;9(4):388–395. [DOI] [PubMed] [Google Scholar]
- 48. Cromwell RL, Newton RA, Forrest G. Head stability in older adults during walking with and without visual input. J Vestib Res 2001;11(2):105–114. [PubMed] [Google Scholar]
- 49. Anderson PG, Nienhuis B, Mulder T, Hulstijn W. Are older adults more dependent on visual information in regulating self‐motion than younger adults? J Mot Behav 1998;30(2):104–113. [DOI] [PubMed] [Google Scholar]
- 50. Helbostad JL, Vereijken B, Hesseberg K, Sletvold O. Altered vision destabilizes gait in older persons. Gait Posture 2009;30(2):233–238. [DOI] [PubMed] [Google Scholar]
- 51. Moe‐Nilssen R, Helbostad JL, Akra T, Birdedal L, Nygaard HA. Modulation of gait during visual adaptation to dark. J Mot Behav 2006;38(2):118–125. [DOI] [PubMed] [Google Scholar]
- 52. Duque G, Boersma D, Loza‐Diaz G, et al. Effects of balance training using a virtual‐reality system in older fallers. Clin Interv Aging 2013;8:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park EC, Kim SG, Lee CW. The effects of virtual reality game exercise on balance and gait of the elderly. J Phys Ther Sci 2015;27(4):1157–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parijat P, Lockhart TE, Liu J. EMG and kinematic responses to unexpected slips after slip training in virtual reality. IEEE Trans Biomed Eng 2015;62(2):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eggenberger P, Theill N, Holenstein S, Schumacher V, Bruin E. Multicomponent physical exercise with simultaneous cognitive training to enhance dual‐task walking of older adults: a secondary analysis of a 6‐month randomized controlled trial with I‐year follow‐up. Clin Interv Aging. 2015;10:1711–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehab 2015;96(3):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]


