Abstract
Background:
Paraquat (PQ) poisoning is a serious public health problem in many countries. In spite of different treatments, the mortality is still high. We performed a meta-analysis to see whether hemoperfusion (HP) in combination with other treatments reduces the mortality more than HP alone in patients with PQ poisoning.
Materials and Methods:
We searched EMBASE, PubMed, Google Scholar, ISI Web of Knowledge, Cochrane Central Register of Controlled Trials, Scopus, Springer, TRIP, ProQuest, and references of the included studies from January 2000 to August 2017. Two reviewers independently searched and extracted data. We measured I2 to determine variance contributed by heterogeneity. To investigate the publication bias, Begg's and Egger's tests were used along with funnel plot analysis.
Results:
Ultimately 12 articles were included in the meta-analysis. Five articles compared HP with conventional therapy with a total of 1311 patients, and seven articles compared mortality of patients received HP versus those received HP in combination with an additional treatment. HP alone reduced the odds of death (odds ratio [OR] = 0.20; 95% confidence interval [CI]: 0.11–0.40, P < 0.0001) compared to conventional therapy. Furthermore, the odds of death was higher in HP group compared to those received HP in combination of additional treatments (OR = 1.24; 95% CI: 1.05–1.46, P = 0.01).
Conclusion:
The mortality was less in HP-treated group compared to those received only conventional therapy. Addition of other treatments with HP reduced the mortality more than HP alone.
Keywords: Hemoperfusion, meta-analysis, mortality, paraquat, poisoning
INTRODUCTION
Paraquat (PQ) poisoning is one of the most serious public health problems.[1] All body systems, depending on the amount of ingested toxin, are involved in PQ poisoning.[2] The kidney has been shown to eliminate PQ very effectively; however, some degree of renal failure is observed with acute PQ poisoning.[3] The toxic compound accumulates in lung tissue where free radicals are formed and produces alveolitis followed by pulmonary fibrosis.[4] In spite of different modalities, the mortality is still high in PQ poisoning.[3,4,5]
There is no gold standard therapy for PQ poisoning. Activated charcoal or fuller earth, radiation therapy, deferoxamine, cyclophosphamide (CP), methylprednisolone (MP), Vitamin E, Vitamin C, hemoperfusion (HP), hemodialysis (HD), continuous venovenous hemofiltration (CVVH), and N-acetyl cysteine (NAC) have been used with varying degrees of success.[6,7,8,9,10,11,12,13,14]
Until now, there are five meta-analyses about treatment of PQ poisoning.[15,16,17,18,19] Agarwal et al., Li et al., and He et al. reported meta-analysis about the effect of pulse immunosuppressive and glucocorticoid therapy, which showed a beneficial effect.[15,16,18] CVVH prolonged the survival time of the patients without significant effect on mortality.[19] Li et al. reported a meta-analysis about the reducing effect of HP on mortality.[17] However, many studies did not show the beneficial effects.[20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] Although the efficacy of pulse immunosuppressive glucocorticoid therapy, CVVH, and HP alone has been shown in above-mentioned meta-analyses, the mortality is still high in single treatment. Since PQ poisoning is a serious public health problem in many countries, we performed a meta-analyses study to see whether HP in combination with other treatments reduces the mortality of PQ poisoning more than HP alone as no reported study yet.
MATERIALS AND METHODS
The project was approved by the Institutional Ethics Committee of our university.
Search strategy
We searched Google Scholar, EMBASE, Pub Med, ISI Web of Knowledge, Cochrane, Scopus, Springer, TRIP, and ProQuest from January 2000 to August 2017. The search strategies included (paraquat) AND (poisoning OR intoxication OR toxicity OR overdose) AND (hemoperfusion OR charcoal hemoperfusion) AND (survival OR death OR mortality OR fatality) in title and abstract based on MESH/subject. The list of references for the studies identified was also examined for more relevant studies.
Data extraction and quality assessment
Inclusion criteria were studies: (1) published articles from January 2000 to August 2017, (2) research was performed on patients (both adults and children) with PQ poisoning, (3) all patients or some of them underwent charcoal HP, (4) outcomes of treatment as death or survival had been reported, and (5) the full text of the published papers was available in English.
Exclusion criteria of this study were as follows: (1) case reports and review articles and (2) studies that all patients received the same treatment. With the use of approved keywords in Mesh, the titles of articles were searched. Then, abstracts in English were extracted. Screening process was carried out by two investigators independently who were blinded to the objective of the study. The disagreements between two researchers resolved through the involvement of a third researcher in the discussion meeting by considering the reasons for study inclusion and exclusion criteria. Finally, full texts of articles were selected. In the absence of available full text, the article was requested through an E-mail from the authors, research gate, or by librarians in the Isfahan University of Medical Sciences.
We assessed articles by the Critical Appraisal Skills Program (CASP) score method to guarantee the quality of meta-analysis. In this method, the first two questions are screening questions. If the answers were “yes,” it was proceeding with the remaining questions. Then, it includes 11 critical questions for randomized clinical trials (RCTs) and case–control studies[35,36] and 12 question for cohort studies,[37] evaluating different parts of articles with the answers of “yes” or “no” or “can’t tell.”
Information about the publication such as name of the first author, year of publication, country of study, type of study, age, PQ concentration, sodium dithionite test, time from ingestion to admission, time to performing HP, HP duration and times, mortality, and follow-up were recorded in the tables.
All patients in the evaluated studies had received some of the following conventional therapy including gastric lavage, activated charcoal, fuller earth, diuretic, dexamethasone, Vitamin C, Vitamin E, NAC, other antioxidants, sodium bicarbonate, glutathione, hormones, cathartics, HD for a patient with acute renal failure if necessary, and other supportive care. However, with respect to using HP alone or in combination with other treatments, we categorized and analyzed our studies based on following two sets.
Set 1: Hemoperfusion (conventional therapy + hemoperfusion) versus nonhemoperfusion (conventional therapy)
This set considers those articles compared mortality between two groups of patients; patients received HP and those not received HP. Both groups had also received conventional therapy as mentioned in the method section.
Set 2: Hemoperfuison versus (Hemoperfuison + other treatments)
This set includes those articles compared the mortality between two groups of patients. Patients in control group received HP, and patients in study group received HP in combination with other treatments. Both groups had also received conventional therapy as mentioned in methods section.
Data analysis
The results analyzed using the comprehensive meta-analysis software version 2.0 (Biostat, Englewood, NJ, USA). Cochrane test (Q) of heterogeneity was carried out among the studies. For determining the existence of heterogeneity among studies, the significance level was considered as <0.05. Odds ratios (ORs) or relative risk (RR) with 95% confidence intervals (CIs) using forest plot and with the fixed and random effect approach has been analyzed. Heterogeneity was measured by calculating I2. Substantial heterogeneity was indicated if P < 0.05 and I2 > 50% when a random-effects model should be selected. If homogeneity was suggested (P > 0.05 and I2 < 50%), a fixed-effects model was used. The main outcome was mortality. To investigate the publication bias, Begg's and Egger's tests were used along with funnel plot as a graphical test. 95% CI was estimated and included in forest plot.
RESULTS
An initial search identified 2250 records from databases. Considering exclusion criteria, 154 articles had eligibility for full-text evaluation. One hundred and forty-two full-text articles were further excluded, and ultimately 12 articles were selected for meta-analysis including 8 retrospective, 3 RCT, and 1 case–control studies. A flow diagram that detailed the process is presented in Figure 1. Quality assessment of articles showed that CASP score of all 12 articles was higher than 65 which were acceptable.
Figure 1.
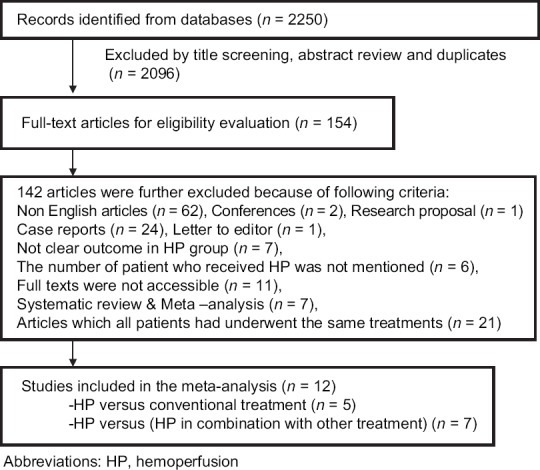
A flowchart of literature searches in this meta-analysis
Hemoperfusion (conventional therapy + hemoperfusion) versus nonhemoperfusion (conventional therapy)
The studies included in this set are summarized in Table 1 and Supplementary Table 1. We found 5 articles with a total of 1311 patients.[38,39,40,41,42] Eight hundred and thirty-seven patients were underwent HP, of which 592 patients died (70.72%). Four hundred and seventy-four patients did not receive HP, of which 428 patients died (90.29%). In a study of Li et al.,[38] because of evaluation of three groups of patients, data of one group were included in this set analysis (HP vs. non-HP) and data of other groups were included in the data set of HP versus (HP + other treatments) which will be explained in the next section.
Table 1.
Information of articles compared patients with paraquat poisoning received hemoperfusion plus conventional therapy (Group 1: Hemoperfusion) versus those received only conventional therapy (Group 2: Nonhemoperfusion)
| First author/year | Study design/area | Sample size (Group 1/Group 2) | Group 1 | Group 2 | Time to HP (h) | Duration/times of HP | Mortality(%) | F |
|---|---|---|---|---|---|---|---|---|
| Rao et al.[39]/2017 | Retrospective/India | 101 (63/38) | HP + Con. (AC, GL) | Con. (AC, NAC, antioxidants) | 33 patients <6 h and 29 patients >6 h |
NM/NM | Group 1=42.9 (HP <6 h=6.1 and in HP >6 h=86.20) | 60 days |
| Group 2=92.10 | ||||||||
| Li et al.[38]/2016 | Retrospective/China | 183 (65/75) | HP + Con. (sodium bicarbonate AC, GL, cathartic, diuresis) | Con. (sodium bicarbonate, AC, GL, cathartic, diuresis) | 1-2 h after admission | 3-4 h/4 times | Group 1=56.90 | 60 days |
| Group 2=78.7 | ||||||||
| Jagadeesan et al.[40]/2017 | Retrospective/India | 10 (5/5) | HP + Con. (AC, GL, HD) | Con. (AC, GL, HD) | NM | NM/NM | Group 1=100 | I h |
| Group 2=100 | ||||||||
| Sun et al.[41]/2016 | Retrospective/Korea | 788 (594/194) | HP + Con. (GL, fuller earth, antioxidant, mannitol) | Con. (GL, fuller’s earth, antioxidant, mannitol) | NM | NM/NM | Group 1=76.26 | 3 months |
| Group 2=95.87 | ||||||||
| Lee et al.[42]/2012 | Retrospective/Korea | 272 (110/162) | HP + Con. (GL, fuller’s earth, mannitol) | Con. (GL, fuller’s earth, mannitol) | NM | NM/NM | Group 1=71.81 | 3 months |
| Group 2=88.27 |
NM=Not mentioned; PQ=Paraquat; HP=Hemoperfusion; AC=Activated charcoal; GL=Gastric lavage; Con=Conventional therapy; NAC=N-acetyl cysteine; HD=Hemodialysis; IH=In hospital; F=Follow-up
Supplementary Table 1.
Information of articles compared patients with paraquat poisoning received hemodialysis plus conventional therapy (Group 1: Hemodialysis) versus those received only conventional therapy (Group 2: Nonhemodialysis)
| First author/year | Sample size (Group 1/Group 2) | Age (years) | Urine paraquat test | PQ Concentration (μg/ml) | Time from ingestion to arrival (h) |
|---|---|---|---|---|---|
| Rao et al.[39]/2017 | 101/ (63/38) | 26.97±7.679 | Positive | NM | NM |
| Li et al.[38]/2016 | 183/(Group 1.1=65/Group 1.2=43/Group 2=75) | Median age | 5-200 μg/ml | Group 1=22.95±10.41 | Group 1: 7.5 (0.5-20.5)/Group 2:12.2 (0.5-22.0) median ( range) |
| Group 1=37 | |||||
| Group 2=21.56±11.7 | |||||
| Group 2=36 | |||||
| Jagadeesan et al.[40]/2017 | 10/(5/5) | Median age, 28.5 | NM | NM | 2 patients<2 h, 6 patients (2-6 h), 2 patients>6 h |
| Sun et al.[41]/2016 | 788/ (594/194) | S=47±14 | Negative in 26 cases of survival and four cases of nonsurvival group | S=0.4±0.7 | S=8.7±17.2 |
| NS=59±16 | NS=80.3±123.1 μg/ml | NS=6.1±14.4 | |||
| Lee et al.[42]/2012 | 272 (110/162) | Median age | Positive | NM | NM |
| S=49 | |||||
| NS=63 |
NM=Not mentioned in the article; S=Survived, NS=Nonsurvived, PQ=Paraquat
The mortality rate in one study was the same (100%) in both HP and non-HP groups;[40] therefore, it did not presented by the software in the funnel and forest plot. The funnel plot showed no publication bias in the studies [Figure 2]. The meta-analysis of this group showed that I2 was 32.87 (q = 4.46, P = 0.2) indicating no significant heterogeneity. Using the fixed effect, the OR was 0.20 (95% CI = 0.11–0.40) with z = 4.604 (P < 0.0001), which showed that the mortality was less in patients received HP compared to those received only conventional treatment [Figure 3].
Figure 2.
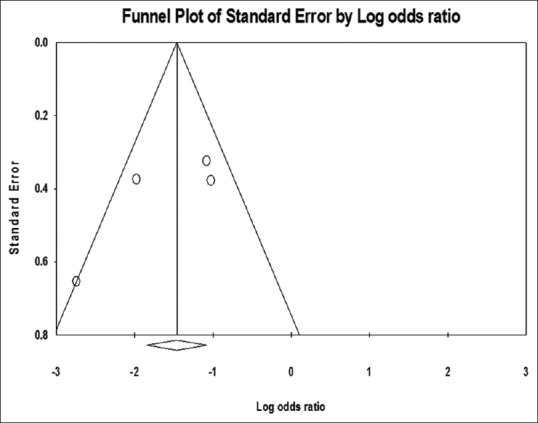
Funnel plot of hemoperfusion and conventional therapy versus conventional therapy in paraquat poisoning mortality
Figure 3.
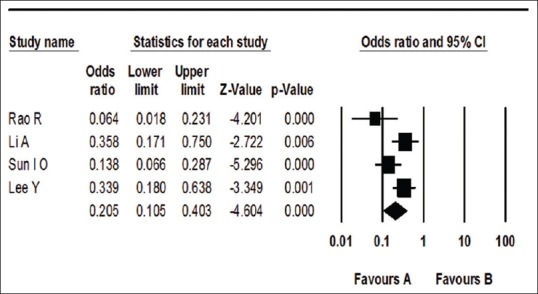
Forest plot for comparison effect of hemoperfusion and conventional therapy (Favor A) versus conventional therapy (nonhemoperfusion) (Favor B) on mortality in paraquat poisoning
Hemoperfuison versus (Hemoperfuison + other treatments)
Seven articles included in this set which their results are summarized in Table 2 and supplementary Table 2.[2,38,43,44,45,46,47] Patients in HP group received conventional therapy and HP. Patients in HP in combination with other treatment group received conventional therapy, HP, and CVVH or continuous renal replacement therapy (CRRT) or CP or/and MP or Chinese medications Xuebijing (XBJ)/Ulinastatin. Analysis of these seven articles evaluated 2881 patients. About 1689 cases were in HP group, and from them, 1174 died (69.50%), and 1192 patients were treated with HP in combination with other treatments, of which 777 died (65.18%).
Table 2.
Information of articles compared patients received conventional therapy plus hemoperfusion (Group 1) versus those received conventional therapy + hemoperfusion + other treatments (Group 2)
| First author/year | Study design/area | Sample size (Group 1/Group 2) | Group 1 | Group 2 | Time to HP (h) | HP duration/times | Mortality (%) Group 1/Group 2 | F |
|---|---|---|---|---|---|---|---|---|
| Tsai et al.[43]/2009 | Case-control/Taiwan | 32 (16/16) | HP + Con. (AC) | HP + MP + Con. (AC) | NM | NM | 87.5/87.5 | 7 days |
| Koo et al.[44]/2002 | RCT (prospective)/Korea | 80 (44/36) | HP + Con. (AC or Fuller’s earth, GL, DX, Vit C) | HP + CVVH + Con. (AC or fuller’s earth. HP, DX, Vit C) | Group 1: 6.4±3.4 | 6 h/1 or 2 times (Group 1=7.2±3.5 Group 2=5.5±1.7) | 63.6/66.7 | 3 months |
| Group 2: 5.7±2.9 | ||||||||
| Gao et al.[2]/2015 | Retrospective (RCT)/China | 684 (458/226) | HP + Con. (GL, antioxidant, DX, Vit C) | HP + CVVH + Con. (GL, antioxidants, DX, Vit C) | Group 1: 4.8±2.1 | 6 h/1 or 2 times | 57.4/58.4 | IH |
| Group 1=5.6±2 | ||||||||
| Group 2: 5.2±3.7 | Group 2=5.3±2.3 | |||||||
| Shi et al.[45]/2015 | Retrospective/china | 119 (37/82) | HP + Con. (GL, catharsis, diuresis, hormones, glutathione, and HD for ARF) | HP + XBJ, Ulinastatin + Con. ( GL, catharsis, diuresis, hormones, glutathione, HD for ARF) | NM | 2-3 h/once daily | 70.27/46.34 | 28 days |
| Wu et al.[46]/2014 | Retrospective/Taiwan | 1811 (1046/765) | HP + Con. | HP + MP/CP + Con. (DX) | NM | NM/2.0±1.4 S=2.4±1.6 NS=1.8±1.3 | 75.7/70.7 | NM |
| Ghorbani et al.[47]/2015 | RCT double blind/IRAN | 47 (23/24) | HP + Con. (AC, GL, and HD for renal replacement if necessary | AC, GL, HP, and HD for renal replacement if necessary, CP, MP | NM | 8 h/2 times | 60.9/25 | IH |
| Li et al.[38]/2016 | Retrospective/China | 108 (65/43) | HP + Con. | HP + CVVH + Con. | NM | First day: 3-4 h/2 times next 2 day 6-8 h daily | 56.90/53.50 | 60 day |
NM=Not mentioned; PQ=Paraquat; HP=Hemoperfusion; Con=Conventional; AC=Activated charcoal; GL=Gastric lavage; CVVH=Continues venovenous hemofiltration; HD=Hemodialysis; S=Survived patients; NS=Nonsurvived patients; DX=Dexamethasone; MP=Methyl prednisolone; CP=Cyclophosphamide; CRRT=Continues renal replacement therapy; IH=In hospital; F=Follow-up; Vit=Vitamin
Supplementary Table 2.
Information of articles compared patients received conventional therapy plus hemoperfusion (Group 1) versus those received conventional therapy + hemoperfusion + other treatments (Group 2)
| First author/year | Study design/area | Sample size (Group 1/Group 2) | Age (years) | Urine paraquat test | PQ concentration μg/ml) | Time from ingestion to arrival (hours) | CASPscore |
|---|---|---|---|---|---|---|---|
| Tsai et al.[43]/2009 | Case-control/Taiwan | 32 (16/16) | Group 1=44.2±15.6 | Positive | Group 2: 47.8±97.3 | Group 2: 6.4±4.4 | 82 |
| Group 2=43.5±4.2 | Group 1: 17.5±20.5 | Group 1: 6.7±4.8 | |||||
| S=30.25±6.24 | S=0.25±0.19 | S=3±1.41 | |||||
| NS=45.79±14.56 | NS=37.29±74.77 | NS=7.04±4.57 | |||||
| Koo et al.[44]/2002 | RCT (prospective)/Korea | 80 (44/36) | Group 1=47±16 | 2.3% in Group 1 and 2.8% in Group 2 was negative | NM | NM | 83 |
| Group 2=43±17 | |||||||
| Gao et al.[2]/2015 | Retrospective/ (RCT) China | 684 (458/226) | Group 1=39±15 Group 2=37±19 | Group 1=0.1% | NM | NM | 87 |
| Group 2=0.2% was Negative | |||||||
| Shi et al.[45]/2015 | Retrospective/china | 119 (Group 1=37/Group 2.1=43 Group 2.2=39) | Group 1=35.25±8.75 Group 2=33.29±7.66 | NM | Group 1=12.26±4.59 | Group 1: 12.31±8.21 | 87 |
| Group 2=11.87±6.63 | Group 2: 10.32±6.47 | ||||||
| Wu et al.[46]/2014 | Retrospective/Taiwan | 1811 (1046/765) | S=38.6±14.9 | NM | NM | NM | |
| NS=50.4±17.5 | |||||||
| Ghorbani et al.[47]/2015 | RCT double-blind, IRAN | 47 (23/24) | Group 1=23.7±5 | Navy or dark | NM | Group 1=11 | 81 |
| Group 2=22.5±5 | Group 2=15 | ||||||
| Li et al.[38]/2016 | Retrospective/China | 183 (65/43) | NM | Urine paraquat concentration 5-200 μg/ml | Group 1: 22.95±10.41 | Group 1: 7.5 (0.5-20.5) | 88 |
| Group 2: 7.8 (0.5-19.0) (median and range) | |||||||
| Group 2: 20.82±9.26 |
S=Survivals; NS=Nonsurvivals; NM=Not mentioned in the article
Begg's tests showed no asymmetry among studies (Kendall's S statistic: 13, P = 0.07) indicating no publication bias. The meta-analysis showed that I2 was 45 (Q = 11, P = 0.1) showing no heterogeneity, so using fixed effect model, OR was 1.24 (95% CI = 1.05–1.46 and z = 2.58; P = 0.01) which showed that mortality was higher in HP group compared to HP + other treatments [Figure 4].
Figure 4.
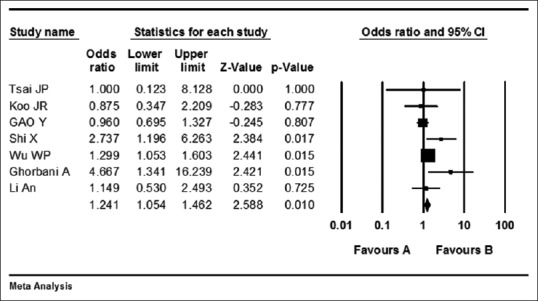
Forest plot of comparison effect of hemoperfusion (Favor A) versus hemoperfusion with other treatments (Favor B) on mortality in paraquat poisoning
DISCUSSION
Hemoperfusion versus nonhemoperfusion
Our meta-analysis results showed that mortality was less in patients underwent HP besides conventional therapy compared with those only received conventional treatment (OR: 0.20; 95% CI: 0.10–0.40). Our result is similar to previous meta-analysis.[17] In Li et al. study, HP also reduced mortality significantly (RR: 0.60; 95% CI: 0.54–0.66) and the mortality rate was 38.4% in HP-treated patients which is lower than our analysis. Limited resources of their study which included PubMed, Web of Science and one Chinese database (China National Knowledge Infrastructure), or publication bias may be the reason.[17] Although HP reduced mortality, the mortality is still high which seems that other treatments for PQ poisoning should be considered.
Hemoperfusion versus (hemoperfusion + other treatments)
Our results showed patients received HP in combination with other treatments (MP/CP/CVVH/CRRT or XBJ and Ulinastatin) had less mortality compared to those received only HP. With respect to heterogeneity in this result, I2 was 45 showing no heterogeneity. Two studies showed different results compared with other studies. The mortality of patients received HP was not significantly different from those received HP in combination with CVVH.[2,44] With respect to mortality, in three studies, CVVH was the additional treatment besides HP, without any significant effect on mortality compared to those received only HP.[2,38,44] CVVH increased the time to death in the patients. Recent meta-analysis about evaluation of the efficacy of CVVH in the treatment of PQ poisoning supports this issue.[19]
In three studies, CP/MP was the combination treatments with HP, with good effect on survival.[43,46,47] In the study of Ghorbani et al., the mortality rate of patients received HP/CP/MP was lower than those underwent HP only (25% vs. 60.9%).[47] Wu et al. reported that patients received HP and MP/CP had lower mortality compared to HP alone (70.7% vs. 75.7%).[46] However, Tsai et al. in a case–control study showed that mortality rate was not significantly different between patients received HP and those received HP and MP.[43] In the Tsai study, CP was not administered for the patients which may be the reason. Wu et al. had investigated different treatment regimen of immunosuppressive therapy, which showed that the MP alone had the least efficacy compared to other compounds.[46] Of course, the time to performing HP and HP duration has not been mentioned in these two studies which may be the limitation of their studies.[43,46] HP duration and early HP may have effects on more removing PQ from the body. The efficacy of MP/CP in reducing mortality has been reported in two previous meta-analyses.[16,18] The mortality rate had been reported 60.4% and 56.79% in the previous meta-analysis that was performed on the effect of MP and CP on PQ-poisoned patients which is high.[16,18] It may be due to the fact that patients in the included studies did not receive HP except in one study.[16,18]
Finally, XBJ/Ulinastatin in combination with HP had protective effects as well. Shi et al. reported that patient received HP/XBJ or Ulinastatin had lower mortality compared to HP alone (46.34% vs. 70.27%).[45] Therapeutic potential of intravenous traditional Chinese has been reported in previous studies.[48,49]
CONCLUSION
Patients received HP in combination with other treatments (MP/CP/XBJ/Ulinastatin, XBJ) had better prognosis than those received HP alone. When the other treatments are not available, the HP alone also can reduce mortality compared to conventional therapy. As the mortality is still high, an education on harmful effects of PQ ingestion for the population is necessary to reduce the PQ poisoning.
Limitations of our study are as follows:
Data regarding the time from ingestion to admission, amount, and concentration of ingested PQ, blood PQ level, and poisoning severity were not available in all included articles to be able analyze them in the meta-analysis
The other potential heterogeneity sources such as time to performing HP and duration of HP therapy (short or long courses) had not been determined in all articles. Due to lack of sufficient data, we could not analyze the outcome with respect to these which can be another limitation of our study.
We may suggest performing future RCT studies considering the severity of poisoning based on blood PQ level to be able determine whether HP alone or HP with other intervention or medications reduces the mortality.
Financial support and sponsorship
This work was supported by Isfahan University of Medical Sciences (grant number: 395012).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the staffs of Khorshid Hospital Library for their great cooperation.
REFERENCES
- 1.Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: A systematic review. QJM. 2003;96:809–24. doi: 10.1093/qjmed/hcg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Y, Zhang X, Yang Y, Li W. Early haemoperfusion with continuous venovenous haemofiltration improves survival of acute paraquat-poisoned patients. J Int Med Res. 2015;43:26–32. doi: 10.1177/0300060514549782. [DOI] [PubMed] [Google Scholar]
- 3.Kang MS, Gil HW, Yang JO, Lee EY, Hong SY. Comparison between kidney and hemoperfusion for paraquat elimination. J Korean Med Sci. 2009;24(Suppl l):S156–60. doi: 10.3346/jkms.2009.24.S1.S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243–51. doi: 10.2165/00002018-199005040-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lee CJ, Hsu HW, Chang YL. Performance characteristics of combined haemodialysis/haemoperfusion system for removal of blood toxins. Med Eng Phys. 1997;19:658–67. doi: 10.1016/s1350-4533(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Bai Y, Zou Y, Cai B, Liu F, Fu P, et al. The value of plasma paraquat concentration in predicting therapeutic effects of haemoperfusion in patients with acute paraquat poisoning. PLoS One. 2012;7:e40911. doi: 10.1371/journal.pone.0040911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou DC, Zhang H, Luo ZM, Zhu QX, Zhou CF. Prognostic value of hematological parameters in patients with paraquat poisoning. Sci Rep. 2016;6:36235. doi: 10.1038/srep36235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HR, Pan J, Shang AD, Lu YQ. Time-dependent haemoperfusion after acute paraquat poisoning. Sci Rep. 2017;7:2239. doi: 10.1038/s41598-017-02527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Lee S, Park S, Gil H, Lee E, Yang J, et al. Concurrent hemoperfusion and hemodialysis in patients with acute pesticide intoxication. Blood Purif. 2016;42:329–36. doi: 10.1159/000451051. [DOI] [PubMed] [Google Scholar]
- 10.Yeo CD, Kim JW, Kim YO, Yoon SA, Kim KH, Kim YS, et al. The role of pentraxin-3 as a prognostic biomarker in paraquat poisoning. Toxicol Lett. 2012;212:157–60. doi: 10.1016/j.toxlet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Chen Y, Mao L, Zhao G, Hong G, Li M, et al. Effects of hemoperfusion and continuous renal replacement therapy on patient survival following paraquat poisoning. PLoS One. 2017;12:e0181207. doi: 10.1371/journal.pone.0181207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37:1006–13. doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 13.Weng CH, Chen HH, Hu CC, Huang WH, Hsu CW, Fu JF, et al. Predictors of acute kidney injury after paraquat intoxication. Oncotarget. 2017;8:51345–54. doi: 10.18632/oncotarget.17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang M, Gao Y, Yang W, Xu Q, Eddleston M, et al. Abnormal pancreatic enzymes and their prognostic role after acute paraquat poisoning. Sci Rep. 2015;5:17299. doi: 10.1038/srep17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal R, Srinivas R, Aggarwal AN, Gupta D. Immunosuppressive therapy in lung injury due to paraquat poisoning: A meta-analysis. Singapore Med J. 2007;48:1000–5. [PubMed] [Google Scholar]
- 16.Li LR, Sydenham E, Chaudhary B, You C. Glucocorticoid with cyclophosphamide for paraquat-induced lung fibrosis. Cochrane Database Syst Rev. 2010:CD008084. doi: 10.1002/14651858.CD008084.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Song L, Jain X. Efficacy of haemoperfusion therapy in patients with paraquat poisoning: A meta-analysis. Int J Clin Exp Med. 2017;10:11371–81. [Google Scholar]
- 18.He F, Xu P, Zhang J, Zhang Q, Gu S, Liu Y, et al. Efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide in patients with paraquat poisoning: A meta-analysis. Int Immunopharmacol. 2015;27:1–7. doi: 10.1016/j.intimp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Lin G, Long J, Luo Y, Wang Y, Zewu Q. Continuous venovenous hemofiltration in the management of paraquat poisoning: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6875. doi: 10.1097/MD.0000000000006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eizadi Mood N, Sabzghabaee AM, Ghodousi A, Yaraghi A, Mousavi A, Massoum G, et al. Histo-pathological findings and their relationship with age, gender and toxin amounts in paraquat intoxication. Pak J Med Sci. 2013;29:403–8. [Google Scholar]
- 21.Eizadi-Mood N, Sabzghabaee AM, Badri S. Paraquat poisoning: What the acute care physician needs to know? Review article. JIMS. 2011;29:997–1006. [Google Scholar]
- 22.Eizadi-Mood N, Sabzghabaee AM, Yaraghi A, Montazeri K, Golabi M, Sharifian A, et al. Effect of antioxidants on the outcome of therapy in paraquat intoxicated patients. TJPR. 2011;10:27–31. [Google Scholar]
- 23.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51:496–500. [PubMed] [Google Scholar]
- 24.Maasoumi G, Eizadi-Mood N, Akabri M, Sohrabi A, Khalili Y. Pattern of poisoning in poisoning referral center. JIMS. 2012;29:1317–24. [Google Scholar]
- 25.Masoumi G, Ganjei Z, Teymoori E, Akabri M, Eizadi-Mood N. Evaluating the prevalence of intentional and unintentional poisoning in vulnerable patients admitted to a referral hospital. JIMS. 2013;31:1452–60. [Google Scholar]
- 26.Zhang Q, Wu WZ, Lu YQ, Wang JZ, Shang AD, Yao F, et al. Successful treatment of patients with paraquat intoxication: Three case reports and review of the literature. J Zhejiang Univ Sci B. 2012;13:413–8. doi: 10.1631/jzus.B1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CW, Lin JL, Lin-Tan DT, Chen KH, Yen TH, Wu MS, et al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One. 2012;7:e48397. doi: 10.1371/journal.pone.0048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang X, Hu DY, Li CB, Li XH, Fan SL, Liu Y, et al. The volume ratio of ground glass opacity in early lung CT predicts mortality in acute paraquat poisoning. PLoS One. 2015;10:e0121691. doi: 10.1371/journal.pone.0121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao LK, Fang TC. Dialysis catheter-related pulmonary embolism in a patient with paraquat intoxication. Ci Ji Yi Xue Za Zhi. 2016;28:166–9. doi: 10.1016/j.tcmj.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29:1441–9. doi: 10.3346/jkms.2014.29.11.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh YW, Lin JL, Lee SY, Weng CH, Yang HY, Liu SH, et al. Paraquat poisoning in pediatric patients. Pediatr Emerg Care. 2013;29:487–91. doi: 10.1097/PEC.0b013e31828a347e. [DOI] [PubMed] [Google Scholar]
- 33.Tsai TY, Weng CH, Lin JL, Yen TH. Suicide victim of paraquat poisoning make suitable corneal donor. Hum Exp Toxicol. 2011;30:71–3. doi: 10.1177/0960327110368419. [DOI] [PubMed] [Google Scholar]
- 34.Chen HW, Tseng TK, Ding LW. Intravenous paraquat poisoning. J Chin Med Assoc. 2009;72:547–50. doi: 10.1016/S1726-4901(09)70426-5. [DOI] [PubMed] [Google Scholar]
- 35.Critical Appraisal Skills Program. Critical Appraisal Skills Program. CASP (Randomized Controlled Trial) Checklist. 2018 [Google Scholar]
- 36.Critical Appraisal Skills Program. CASP (Case Control Study) Checklist. 2018 [Google Scholar]
- 37.Critical Appraisal Skills Program. CASP (Cohort Study) Checklist. 2018 [Google Scholar]
- 38.Li A, Li W, Hao F, Wang H. Early stage blood purification for paraquat poisoning: A multicenter retrospective study. Blood Purif. 2016;42:93–9. doi: 10.1159/000445991. [DOI] [PubMed] [Google Scholar]
- 39.Rao RR, Bhat R, Pathadka S, ChenjI SK, Dsouza S. Golden hours in severe paraquat poisoning-the role of early haemoperfusion therapy. J Clin Diagn Res. 2017;11:OC06–8. doi: 10.7860/JCDR/2017/24764.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagadeesan M, Nithyananthan P, Banupriya M, Mahendrakumar K, Prasanna KS, Kannan R. A study on clinical profile of paraquat poisoning in a tertiary care hospital. Int J Adv Med. 2017;4:1088–91. [Google Scholar]
- 41.Sun IO, Shin SH, Yoon HJ, Lee KY. Predicting the probability of survival in acute paraquat poisoning. Kidney Res Clin Pract. 2016;35:102–6. doi: 10.1016/j.krcp.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y, Lee JH, Seong AJ, Hong CK, Lee HJ, Shin DH, et al. Arterial lactate as a predictor of mortality in emergency department patients with paraquat intoxication. Clin Toxicol (Phila) 2012;50:52–6. doi: 10.3109/15563650.2011.639716. [DOI] [PubMed] [Google Scholar]
- 43.Tsai JP, Lee RP, Wang CH, Fang TC, Hsu BG. Clinical study of prognosis and glucocorticoid pulse treatment in patients with acute paraquat intoxication. Tzu Chin Med J. 2009;21:156–60. [Google Scholar]
- 44.Koo JR, Kim JC, Yoon JW, Kim GH, Jeon RW, Kim HJ, et al. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. Am J Kidney Dis. 2002;39:55–9. doi: 10.1053/ajkd.2002.29880. [DOI] [PubMed] [Google Scholar]
- 45.Shi X, Zhang Y, Wang Y. Impact of xuebijing and ulinastatin as assistance for hemoperfusion in treating acute paraquat poisoning. Int J Clin Exp Med. 2015;8:14018–23. [PMC free article] [PubMed] [Google Scholar]
- 46.Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ, et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: A nationwide study. PLoS One. 2014;9:e87568. doi: 10.1371/journal.pone.0087568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghorbani A, Masoumi K, Forouzan A, Rahmani AH, Rahim F, Taherinezhad TB, et al. Effect of pulse therapy with glucocorticoids and cyclophosphamide in patients with paraquat poisoning. Hong Kong J Emerg Med. 2015;22:235–340. [Google Scholar]
- 48.Gong P, Lu Z, Xing J, Wang N, Zhang Y. Traditional Chinese medicine xuebijing treatment is associated with decreased mortality risk of patients with moderate paraquat poisoning. PLoS One. 2015;10:e0123504. doi: 10.1371/journal.pone.0123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng X, Sun X, Ma P, Liu Z, Jiang N. Therapeutic potential of intravenous xuebijing on transforming growth factor beta1 and procollagen type III peptide in patients with acute paraquat poisoning. J Tradit Chin Med. 2012;32:584–9. doi: 10.1016/s0254-6272(13)60075-8. [DOI] [PubMed] [Google Scholar]


