Abstract
The current study was carried out to evaluate the antioxidant and anti-caspase 3 activity of chitosan-Pinus merkusii nanoparticle in against lead acetate-induced nephrotoxicity in rats. chitosan-P. merkusii nanoparticle was characterized by dynamic light scattering (DLS) and scanning electron microscope (SEM). The male rats were divided into control group (rats were given with distilled water), lead acetate group (rats were injected with lead acetate 15 mg/kg BW i. p), and the treatment group (rats were given the chitosan-P. merkusii nanoparticle 150 mg, 300 mg, 600 mg/kg BW orally and were injected with lead acetate 15 mg/kg BW). The rats blood samples were measured levels of blood urea nitrogen (BUN) and creatinine. The kidney tissues were collected to evaluate the malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx). Histological to evaluate renal damage, and immunohistochemical to analyze the expression of caspase 3. The results showed that DLS showed the size of chitosan-P. merkusii nanoparticle was 165.9 ± 24.18 nm. SEM images of the chitosan-P. merkusii nanoparticles showed an irregular shape and its the rough surface. Administration of lead acetate resulted in a significant increase in levels of the BUN, creatinine, MDA level, caspase 3 expression, and a decrease in SOD and GPx were compared with the control group. Treatment with the chitosan-P. merkusii nanoparticle 600 mg/kg BW significantly decreased the elevated BUN, creatinine, MDA levels, caspase 3 expression and also increase in SOD and GPx as compared to lead acetate group. The lead acetate induced loss of the normal structure of renal cells and necrosis, whereas treated with chitosan-P. merkusii nanoparticle improved renal cell necrosis. This study indicates that chitosan-P. merkusii nanoparticles appeared to be a promising agent for protection against lead-induced nephrotoxicity through increasing antioxidant and inhibiting caspase 3 expression.
Key words: Antioxidant, biochemical serum, caspase 3, chitosan-Pinus merkusii nanoparticle, lead acetate
INTRODUCTION
Lead (Pb) is one of the heavy metals which can cause damage to several organs such as the liver,[1] reproductive organs,[2] immune system,[3] and kidneys[4] in both humans and animals.
Oxidative stress has been proposed as a possible mechanism involved in lead-induced nephrotoxicity.[5,6] Oxidative stress develops when there is an imbalance between the reactive oxygen species (ROS) and the antioxidants in the nephron cells.[6,7] It has been reported that overproduction of ROS or free radicals such as superoxide ion (O2-), hydroxyl radical (OH-), and nitric oxide and consequently can enhance lipid peroxidation, the decrease of antioxidant enzymes activities, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx).[7,8] In addition, free radicals are highly reactive to membrane lipids, protein, DNA, and to be the major contributing factors to stress injuries and to cause rapid renal cell damage.[3,8] It was also reported that malondialdehyde (MDA) is a product of lipid peroxidation which may be used as a biomarker of renal cell damage.[4]
In living systems, the kidney is considered to be highly sensitive to toxic agents. The study of lead acetate in biochemical changes in blood such as blood urea nitrogen (BUN) and creatinine has been found to be of great value in experimental kidney damage.[9] Various molecular, cellular, and intracellular mechanisms of lead nephrotoxicity to explain the toxicological profile of lead that includes generation of oxidative stress, necrosis, and apoptosis.[3,7,8]
The herbal antioxidants have been reported to provide protection against lead-induced oxidative stress and emerged as a potential therapeutic to prevent free radical generated damage in the body. It has been reported that Pinus merkusii was one of the herbals, which have a strong antioxidant effect.[10,11,12] Recent research activities have shown that Pinus plant is an important source of pycnogenol that contains proanthocyanidins.[13,14,15] Proanthocyanidins are potent, antioxidant (free radical scavengers), antibacterial agents, anticancer, anti-inflammatory, immune-stimulating, antidiabetes, and anti-atherosclerosis.[16,17,18]
In recent years, synthesis of natural product nanoparticles is an interesting issue of the nanoscience and nanobiotechnology.[19] Chitosan nanoparticles have been studied extensively by researchers for their controlled drug release properties and are used for both in vitro and in vivo applications.[20] It is also nontoxic and has many biological activities such as antibacterial, antioxidant, antihyperlipidemic, antidiabetic, anti-HIV, anti-inflammatory, drug delivery, and immunoenhancing, make it an ideal delivery agent for applications in medicine.[20,21,22] The present study was carried out to evaluate the antioxidant and anti-caspase 3 activity of chitosan-P. merkusii extract nanoparticle in against lead acetate-induced nephrotoxicity.
MATERIALS AND METHODS
Preparation of chitosan-Pinus merkusii extract nanoparticles
The chitosan-P. merkusii extract nanoparticle was prepared according to the procedure first reported by based on the ionic gelation of chitosan-P. merkusii extract with sodium tripolyphosphate (TPP) anions.[22,23] Briefly, concentrations of chitosan solutions (0.2% w/v) were prepared in 0.1% v/v glacial acetic acid and filtered. The TPP solution (0.1% w/v) was prepared in deionized water. P. merkusii extract 0.4% w/v in ethanol 70% was added to chitosan solution (0.2% w/v) under constant stirring. The mixture was then sonicated for 5 min and TPP solution was added dropwise under constant stirring. The ratio of chitosan: P. merkusii: TPP solution was maintained at 2:4:1 throughout the experiment. The supernatant obtained was subjected to ultracentrifugation at 25000 rpm for 20 min to sediment the chitosan-P. merkusii conjugated nanoparticles, which were then subjected to further characterization.
Characterization of nanoparticles by scanning electron microscopy and dynamic light scattering
The surface morphological features such as particle size, shape, and topography of the chitosan-P. merkusii extract nanoparticle were observed using scanning electron microscope (SEM).
The particle size of the chitosan-P. merkusii extract nanoparticle was determined using dynamic light scattering (DLS) (Horiba LA 900, Japan).
Experimental animal
Male Wistar rat weighing approximately 200–250 g (2.5–3 months) were obtained from the Gadjah Mada University, Yogyakarta, Indonesia, for experimental purpose. They were housed in plastic cages in an air-conditioned room with a temperature maintained at 26 ± 2°C and 12 h alternate light and dark cycles. The rats were given ad libitum with tap water and fed with a standard commercial rat.
Experimental design
The sample used 50 male rats were divided into five groups: Control group (rats were given daily with distilled water), lead acetate group (rats were injected with lead acetate solution, i.p., at a dose of 15 mg/kg BW for the 7 consecutive days), and the treatment group (rats were given the chitosan-P. merkusii extract nanoparticle 150 mg, 300 mg, and 600 mg/kg BW orally once in a day for 11 days, and on the 4th day, were injected with lead acetate solution, i.p., at a dose of 15 mg/kg BW 1 h after the chitosan-P. merkusii extract nanoparticle). On day 11, the rats blood samples were taken by cardiac puncture to be measured levels of BUN and creatinine. Measurement of BUN was conducted by the diacetyl monoxime method[24] and measurement of creatinine was conducted by the alkaline picrate method.[25] Furthermore, rats were sacrificed and kidney tissues were used for the analyzes of MDA and antioxidant enzymes (SOD and GPx). MDA was determined in the supernatant of homogenate kidney tissue by the thiobarbituric acid method.[4] MDA is expressed as nanomoles MDA/g tissue. The activity of SOD was measured with SOD detection kit according to the manufacturer's instructions. The level of SOD was measured at 505 nm and through a standard curve and expressed as U/mg protein.[2] The activity of GPx was measured with GPx detection kit according to the manufacturer's instructions. The GPx was evaluated spectrophotometrically against blank at 340 nm. The GPx activity was expressed as U/mg protein.[2]
The kidney was also fixed in a 10% neutral-buffered formalin solution for immunohistochemical evaluation of the expression of caspase 3[8] and histopathological evaluation of the kidney damage.[1]
Statistical analysis
Data were presented as means ± standard deviation. One-way ANOVA has carried post hoc test and the statistical comparisons among the groups were performed with an LSD test using a statistical package program SPSS version 17.0 (SPSS Inc, Chicago, USA).
RESULTS
Characterization of chitosan-Pinus merkusii extract nanoparticles by scanning electron microscopy
SEM images of the chitosan-P. merkusii nanoparticles prepared using ionic gelation revealed that the nanoparticle surface showed the rough surface morphology and an irregular shape [Figure 1].
Figure 1.

Scanning electron microscope images of chitosan-Pinus merkusii extract nanoparticles
Characterization of chitosan-Pinus merkusii nanoparticles by dynamic light scattering
The average particle size of the chitosan-P. merkusii extract nanoparticles by DLS was 165.9 ± 24.18 nm as shown in Figure 2.
Figure 2.
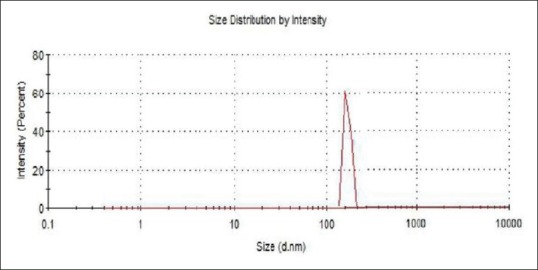
Size distribution of chitosan-Pinus merkusii extract nanoparticles by dynamic light scattering
Effects of chitosan-Pinus merkusii extract nanoparticles to lead acetate-induced changes in the renal serum enzymes
The lead acetate group showed a statistically significant (P < 0.05) increase in renal serum enzymes (BUN and creatinine) compared with the control group. In contrast, the chitosan-P. merkusii extract nanoparticles at dose 600 mg/kg BW showed significantly (P < 0.05) decreased renal serum enzymes (BUN and creatinine) levels were compare with the lead acetate group [Table 1].
Table 1.
Effects of Chitosan-Pinus merkusii extract nanoparticle on lead acetate induced changes in BUN and Creatinin
| Groups | Means±Standard deviation | |
|---|---|---|
| BUN (mmol/L) |
Creatinin (mmol/L) |
|
| Control | 8.37a±2.23 | 35.12a±3.18 |
| Lead Acetate | 25.33b±3.72 | 63.33b±6.24 |
| Chitosan-P. merkusii 150 mg/kg | 26.51b±3.64 | 65.27b±5.99 |
| Chitosan-P. merkusii 300 mg/kg | 19.43b±3.91 | 56.93b±4.82 |
| Chitosan-P. merkusii 600 mg/kg | 13.09c±1.87 | 42.16c±3.71 |
a-cDifferent superscript within each column indicate significant difference between the means (P<0.05)
Effects of chitosan-Pinus merkusii extract nanoparticle on lead acetate-induced changes in malondialdehyde, superoxide dismutase, and glutathione peroxidase of kidney tissue
Table 2 showed in the lead acetate group, the level of MDA of kidney tissue was significantly increased compared to the control group (P < 0.05). Treatment with chitosan-P. merkusii extract nanoparticle at dose 600 mg/Kg BW markedly reduced kidney tissue MDA which was significantly different from the lead acetate group (P < 0.05). Table 2 also showed the results of the lead acetate group, the level of SOD, and GPx of kidney tissue were significantly decreased compared to the control group (P < 0.05). Treatment with chitosan-P. merkusii extract nanoparticle at dose 600 mg/Kg BW markedly enhanced kidney SOD and GPx which was significantly different from the lead acetate group (P < 0.05).
Table 2.
Effects of Chitosan-Pinus merkusii extract nanoparticle on lead acetate induced changes in MDA, SOD and GPx
| Groups | Means±Standard Deviation | ||
|---|---|---|---|
| MDA (nmol/mg) | SOD (u/mg) | GPx (u/mg) | |
| Control | 52.81a±6.15 | 41.18a±4.26 | 53.42a±5.23 |
| Lead Acetate | 73.45b±8.92 | 23.22b±3.91 | 33.63b±3.97 |
| Chitosan-P. merkusii 150 mg/kg | 76.32b±7.24 | 21.31b±2.85 | 35.63b±4.09 |
| Chitosan-P. merkusii 300 mg/kg | 69.52b±5.72 | 26.13b±3.79 | 38.63b±3.42 |
| Chitosan-P. merkusii 600 mg/kg | 61.28c±4.62 | 33.24c±3.27 | 44.63c±4.31 |
a-cDifferent superscript within each column indicate significant difference between the means ( P<0.05)
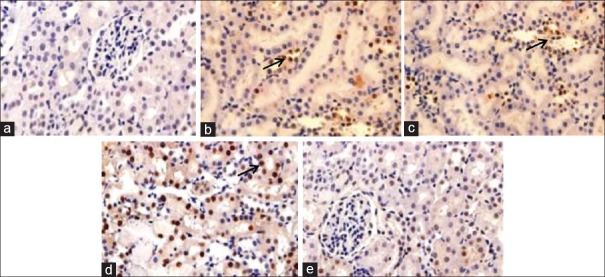
Effects of chitosan-Pinus merkusii extract nanoparticle on the expression of caspase 3 of lead acetate
Figure 3 showed that in the lead acetate group, the expression of caspase 3 of the kidney tissue was significantly increased compared to the control group (P < 0.05). Treatment with chitosan-P. merkusii extract nanoparticle at dose 600 mg/Kg BW markedly reduced kidney tissue caspase 3 expression which was significantly different from the lead acetate group (P < 0.05).
Figure 3.
Immunohistochemical study of chitosan-Pinus merkusii extract nanoparticle on caspase 3 expression of lead acetate-induced nephrotoxicity. The lead acetate-treated group showed caspase 3 expression (indicated by red arrows). Negative control Group (a); Positive control Group (b); Rats treated with chitosan-Pinus merkusii extract nanoparticle 150 mg/kg BW; 300 mg/kg BW and 600 mg/kg (c-e)
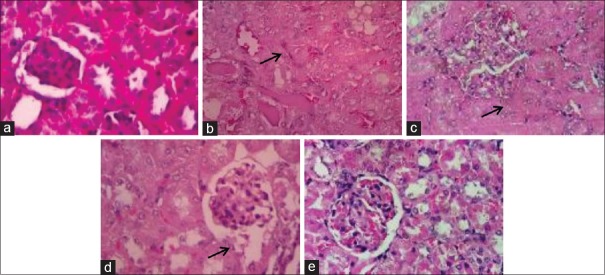
Effects of chitosan-Pinus merkusii extract nanoparticle on lead acetate-induced kidney cell damage
Histological observations on the control group showed that in kidney cell appear normal architecture of the renal cell. In the lead acetate group showed renal cell damage (necrosis). The treated chitosan-P. merkusii extract nanoparticles can inhibit renal cell damage caused by lead acetate [Figure 4].
Figure 4.
Histological study of pretreatment with chitosan-Pinus merkusii extract nanoparticle on lead acetate-induced nephrotoxicity. Normal morphology of liver sections in the negative control group (a). The lead acetate treated group showed necrosis (indicated by black arrows) (b). Rats treated with chitosan-Pinus merkusii extract nanoparticle 150 mg/kg BW and 300 mg/kg BW showed necrotic changes (c and d). Rats treated with chitosan-Pinus merkusii extract nanoparticle 600 mg/kg showed regeneration in hepatic cells damage (e). H and E ×400
DISCUSSION
In this study, we made P. merkusii extract was encapsulated into chitosan nanoparticle with the use of sodium TPP (STPP) as a cross-linking agent on ionotropic gelation method, which has more advantages over P. merkusii extract. This modification can improve biodistribution and increase specificity and sensitivity and reduced pharmacological toxicity.[18,23] The P. merkusii extract is dissolved in chitosan solution and when the STPP solution with negative charge is added to the positively charged chitosan solution, the nanoparticles are formed rapidly and the P. merkusii extract is surrounded by a polymeric network due to interaction between two polymers. The nanoparticles obtained in the present study had small particle size (165.9 ± 24.18 nm), which may increase the antioxidant and anti-caspase 3 activity. The SEM images of the chitosan-P. merkusii extract nanoparticles showed an irregular shape and its the rough surface.
In our study, administration of lead acetate can increase MDA and inducing oxidative damage in the kidney. MDA may be used as an indicator of cell membrane injury. The increase in MDA levels in the kidney suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent the formation of excessive free radicals.[4,5] Treatment of rats with chitosan-P. merkusii extract nanoparticle at a dose of 600 mg/kg BW prevented the levels of MDA to rise when the rats were challenged with lead acetate in the kidney, which might be due to the ability of chitosan-P. merkusii extract nanoparticle to reduce the accumulation of free radicals. It is known that chitosan-P. merkusii extract nanoparticle, which behaves as a powerful antioxidant and free radical scavenger, can decrease the MDA level perturbed by lead acetate in rats kidney. The findings of this study suggest that chitosan-P. merkusii extract nanoparticle could attenuate oxidative stress by decreasing the lipid peroxidation (MDA level) in the lead-treated kidney.
The serum enzyme markers such as BUN and creatinine are recommended for the assessment of renal cell damage in preclinical studies as it is considered a more specific and sensitive indicator of kidney damage. Most increases BUN and creatinine levels are caused by kidney damage.[9] The current work showed an increase in the levels of a BUN and creatinine in lead acetate-treated rat in comparison to the control and this may be due to the degeneration of renal cell by necrosis which causes leakage of these BUN and creatinine into blood circulation. The similar observation has reported that lead acetate treatment induced significant elevation of serum BUN and creatinine activities.[4,5] Our results indicated that the chitosan-P. merkusii nanoparticle 600 mg/kg BW showed an improvement in the BUN and creatinine levels. This might be through its direct action on free radicals of lead acetate to prevent the renal cellular damage by maintaining its membrane integrity. Reduction of the BUN and creatinine levels near-normal levels suggested regeneration of renal cell with a healing of nephrotoxicity.
SOD and GPx are important antioxidant enzymes. They constitute a mutually supportive defense mechanism against ROS. Many studies have shown that lead has the high affinity for SH groups in several enzymes such as SOD and GPx, thus it can alter antioxidant activities by inhibiting functional SH groups in these enzymes.[5,6] In the present study, the activity of SOD and GPx in rat kidney was decreased by lead acetate treatment. Lead acetate is known to cause free radical damage in tissues by increased ROS and by causing depletion of antioxidant reserves. This suggested that lead acetate exposure induced oxidative stress by inhibiting the activity of this antioxidant enzyme. Interestingly, the administration of chitosan-P. merkusii extract nanoparticle increased the activities of SOD and GPx in the kidney of lead-treated rats, which might be due to the ability of chitosan-P. merkusii extract nanoparticle to reduce the accumulation of free radicals. Chitosan-P. merkusii extract nanoparticle acts as a scavenger for the oxygen-derived free radicals, thus protecting from kidney damage. The decrease in lipid peroxidation due to chitosan-P. merkusii extract nanoparticle has been attributed to alterations in the antioxidant defense system which includes enzymes such as SOD and GPx, which normally protects against free radical toxicity.
It has been reported that the lead toxicity condition can cause excessive production of ROS, there is an imbalance between the production of oxidants and the defense systems of antioxidant which may promote the induction of lipid peroxidation, proteins and DNA damage, leading to renal cell death via apoptosis or necrosis.[7,8,9] Expression of the caspase-3 is a hallmark of apoptosis and can be used in cellular assays to quantify activators and inhibitors of the “death cascade.”[8] The results of this study showed that the expression of caspase 3 of kidney tissue was significantly increased on the lead acetate group. Dose-dependent manner of chitosan-P. merkusii extract nanoparticle decreased kidney tissue caspase 3 expression in lead acetate treatment. It has been also reported that tissue cell apoptosis induced by lead toxicity was associated with mitochondrial injury and changes in levels of apoptogenic proteins including Bcl-2, Bax, and caspase-3.[7] In lead toxicity, the expression levels of caspase-3 and Bax significantly increased, while the levels of Bcl-2 significantly decreased. The renal apoptotic cell death is highly associated with lead loading and changes in caspase-3 expression may play an important role in this process.
The histopathological results demonstrating structural changes in kidney tissue of heavy metal toxicity such as lead acetate were reported by some researchers. In the present study, histopathological view of kidney sections in the lead acetate group showed the renal cell damage (necrosis) as compared to the control group. The kidney damage (necrosis) was considered mild in the groups treated with chitosan-P. merkusii extract nanoparticle.
CONCLUSION
In conclusion, the present study indicates that the administration of chitosan-P. merkusii extract nanoparticle inhibits the effects of lead acetate-induced nephrotoxicity might be related with both antioxidant and anti-caspase 3. The administration of chitosan-P. merkusii extract nanoparticle lessened the effects of lead acetate-induced nephrotoxicity possibly by increasing antioxidant and inhibiting caspase 3 expression.
Financial support and sponsorship
The authors would like to acknowledge the support of Airlangga University, Surabaya, Indonesia, in conducting this research work.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wardani G, Farida N, Andayani R, Kuntoro M, Sudjarwo SA. The potency of red seaweed (Eucheuma cottonii) extracts as hepatoprotector on lead acetate-induced hepatotoxicity in mice. Pharmacognosy Res. 2017;9:282–6. doi: 10.4103/pr.pr_69_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudjarwo SA, Sudjarwo GW, Koerniasari Protective effect of curcumin on lead acetate-induced testicular toxicity in wistar rats. Res Pharm Sci. 2017;12:381–90. doi: 10.4103/1735-5362.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimaa AA. Ameliorative potential of Spirulina platensis against lead acetate induced immuno-suppression and kidney apoptosis in rats. Ann Clin Pathol. 2017;5:1120. [Google Scholar]
- 4.Sudjarwo SA, Eraiko K, Sudjarwo GW, Koerniasari Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran J Basic Med Sci. 2017;20:1227–31. doi: 10.22038/IJBMS.2017.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Q, Ha X, Yang Z, Hui L, Yang X. Oxidative stress: A possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol Mech Methods. 2012;22:705–10. doi: 10.3109/15376516.2012.718811. [DOI] [PubMed] [Google Scholar]
- 6.Lamidi IY, Akefe IO. Mitigate effects of antioxidants in lead toxicity. Res Rep Toxicol. 2017;1:1–9. [Google Scholar]
- 7.Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH, et al. Lead induces oxidative stress, DNA damage and alteration of p53, bax and bcl-2 expressions in mice. Food Chem Toxicol. 2008;46:1488–94. doi: 10.1016/j.fct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol. 2014;7:2905–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Okediran BS, Kasali OB, Omotainse SO, Akinloye OA. Haemato-biochemical alteration as biomamarkers of lead induced toxicity in male wistar rat. Bangladesh J Vet Med. 2016;14:227–32. [Google Scholar]
- 10.Tillah M, Batubara I, Sari RK. Antimicrobial and antioxidant activities of resins and essential oil from pine (Pinus merkusii, Pinuso ocarpa, Pinus insularis) and Agathis (Agathis loranthifolia) Biosaintifika. 2017;9:134–9. [Google Scholar]
- 11.Qadir M, Shah WA. GC-MS Analysis, antibacterial, antioxidant and anticancer activity of essential oil of Pinus roxburghii from Kashmir India. Int J Res Pharm Chem. 2014;4:228–32. [Google Scholar]
- 12.Li YY, Feng J, Zhang XL, Cui YY. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J Pharmacol Exp Ther. 2015;353:9–16. doi: 10.1124/jpet.114.220277. [DOI] [PubMed] [Google Scholar]
- 13.Park IJ, Cha SY, Kang M, So YS, Go HG, Mun SP, et al. Effect of proanthocyanidin-rich extract from pinus radiata bark on immune response of specific-pathogen-free white leghorn chickens. Poult Sci. 2011;90:977–82. doi: 10.3382/ps.2010-01160. [DOI] [PubMed] [Google Scholar]
- 14.Kim NY, Jang MK, Lee DG, Yu KH, Jang H, Kim M, et al. Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts. Nutr Res Pract. 2010;4:16–22. doi: 10.4162/nrp.2010.4.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku CS, Mun SP. Characterization of proanthocyanidin in hot water extracts isolated from Pinus radiata bark. Wood Sci Technol. 2007;41:235–47. [Google Scholar]
- 16.Yang L, Xian D, Xiong X, Lai R, Song J, Zhong J, et al. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. Biomed Res Int. 2018;2018:8584136. doi: 10.1155/2018/8584136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ince I, Yesil-Celiktas O, Karabay-Yavasoglu NU, Elgin G. Effects of pinus brutia bark extract and pycnogenol in a rat model of carrageenan induced inflammation. Phytomedicine. 2009;16:1101–4. doi: 10.1016/j.phymed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Sudjarwo SA, Wardani G, Eraiko K, Koerniasari The potency of nanoparticle of Pinus merkusii as immunostimulatory on male wistar albino rat. Int J Nutr Pharmacol Neurol Dis. 2018;8:10–5. [Google Scholar]
- 19.Kumari A, Kumar V. Nanotechnology: A tool to enhance therapeutic values of natural plant products. Trends Med Res. 2012;7:34–42. [Google Scholar]
- 20.Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman M, Juneja VK. Review of antimicrobial and antioxidative activities of chitosans in food. J Food Prot. 2010;73:1737–61. doi: 10.4315/0362-028x-73.9.1737. [DOI] [PubMed] [Google Scholar]
- 22.Wardani G, Mahmiah , Sudjarwo SA. In vitro antibacterial activity of chitosan nanoparticles against Mycobacterium tuberculosis. Pharmacogn J. 2018;10:162–6. [Google Scholar]
- 23.Vimal S, Abdul Majeed S, Taju G, Nambi KS, Sundar Raj N, Madan N, et al. Chitosan tripolyphosphate (CS/TPP) nanoparticles: Preparation, characterization and application for gene delivery in shrimp. Acta Trop. 2013;128:486–93. doi: 10.1016/j.actatropica.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Patke VG, Kansara GS. A comparative analytical quality evaluation study between two methods for blood urea nitrogen estimation. IOSR J Dent Med Sci. 2018;17:48–56. [Google Scholar]
- 25.Nade VS, Shendye NV, Kawale IA. Ameliorative effect of resveratrol on HPA axis modulated chronic restraint stress in rats. Int J Exp Pharmacol. 2014;4:132–9. [Google Scholar]