Abstract
Halogens are widely used, highly toxic chemicals that pose a potential threat to humans because of their abundance. Halogens such as bromine (Br2) cause severe pulmonary and systemic injuries; however, the mechanisms of their toxicity are largely unknown. Here, we demonstrated that Br2 and reactive brominated species produced in the lung and released in blood reach the heart and cause acute cardiac ultrastructural damage and dysfunction in rats. Br2-induced cardiac damage was demonstrated by acute (3–24 h) increases in circulating troponin I, heart-type fatty acid-binding protein, and NH2-terminal pro-brain natriuretic peptide. Transmission electron microscopy demonstrated acute (3–24 h) cardiac contraction band necrosis, disruption of z-disks, and mitochondrial swelling and disorganization. Echocardiography and hemodynamic analysis revealed left ventricular (LV) systolic and diastolic dysfunction at 7 days. Plasma and LV tissue had increased levels of brominated fatty acids. 2-Bromohexadecanal (Br-HDA) injected into the LV cavity of a normal rat caused acute LV enlargement with extensive disruption of the sarcomeric architecture and mitochondrial damage. There was extensive infiltration of neutrophils and increased myeloperoxidase levels in the hearts of Br2- or Br2 reactant-exposed rats. Increased bromination of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and increased phosphalamban after Br2 inhalation decreased cardiac SERCA activity by 70%. SERCA inactivation was accompanied by increased Ca2+-sensitive LV calpain activity. The calpain-specific inhibitor MDL28170 administered within 1 h after exposure significantly decreased calpain activity and acute mortality. Bromine inhalation and formation of reactive brominated species caused acute cardiac injury and myocardial damage that can lead to heart failure.
NEW & NOTEWORTHY The present study defines left ventricular systolic and diastolic dysfunction due to cardiac injury after bromine (Br2) inhalation. A calpain-dependent mechanism was identified as a potential mediator of cardiac ultrastructure damage. This study not only highlights the importance of monitoring acute cardiac symptoms in victims of Br2 exposure but also defines calpains as a potential target to treat Br2-induced toxicity.
Keywords: calcium, calpains, cardiac, halogen, neutrophil, sarco(endo)plasmic reticulum Ca2+-ATPase
INTRODUCTION
Halogens present a persistent hazardous material threat because of their abundance, as their accidental or intentional release can cause massive human casualties. Bromine (Br2) is a powerful oxidizing agent in the presence of moisture, and its immediately dangerous to life or health concentration is lower than chlorine (Cl2) (43). Industrial demand of Br2 is increasing rapidly and may soon replace the use of Cl2. Several accidental exposures during transport of halogens have occurred, resulting in significant morbidity and mortality (42, 43). In humans, isolated cases of accidental inhalation of high concentrations of Br2 have reported respiratory and myocardial injury, cardiac arrest, and circulatory collapse (40, 43). However, systematic studies on effects of Br2 inhalation are recently emerging (1, 5, 17, 28, 31, 32, 48, 58, 67, 71). The National Research Council has put forth a LD50 for mice at an exposure of 204 parts per million (ppm) Br2 for 30 min, and human studies have shown eye and throat irritation at 1-ppm exposure for up to 8 h (44a). Others have reported the lethal dose of Br2 to be 1,000 ppm (43). Computational modeling of the Cl2 release in the Graniteville, NC, train disaster suggested an exposure of more than 100,000 ppm at the site of release and ~670 ppm within 60 min at a distance of 2 miles from the accident (27). No such study has been made for Br2 accidents. However, it is unclear whether the cardiac injury and cardiomegaly result from primary cardiac damage or a combined cardiopulmonary process.
The high metabolic demand of the myocardium leads to an increased rate of reactive oxygen species production during stress that is compounded by a lower content of antioxidants, such as catalase, compared with other organs, such as the lung, liver, and kidney. The paucity of antioxidant enzymes makes the heart susceptible to oxidative injury (15, 16), particularly in response to reactive gases such as Br2 and Cl2 (68, 69). We have previously shown that highly bioactive chlorinated intermediates (amines and lipids) produced in response to acute Cl2 exposure cause cell death and significantly reduce force of contraction in an ex vivo rat heart model (4, 18, 68). We have also demonstrated that inhaled halogens (Cl2) cause modification (chlorination and oxidation) and inactivation of cardiac sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2), resulting in cytosolic Ca2 overload, depletion of ATP stores, a decrease in the mitochondrial transmembrane gradient, and apoptotic cardiomyocyte death, which was not attenuated by O2 supplementation (68).
Modification of SERCA2 and cytosolic Ca2+ overload is a central pathophysiological mechanism of various cardiac diseases, including cardiac hypertrophy and heart failure (41). Irreversible oxidation of SERCA2 and subsequent loss of activity occurs in the presence of higher levels of reactive oxygen species (ROS) (33). In addition to being essential for excitation-contraction coupling, another important consequence of SERCA2 pump inhibition and subsequent sustained elevation of intracellular Ca2+ levels is the activation of Ca2+-dependent proteases, such as calpains that break down the cytoskeletal and mitochondrial ultrastructure.
In the heart, calpain activation causes direct degradation of the largest cytoskeletal protein titin, which extends from the z-disks of the cardiomyocyte, and its disruption causes cardiac contractile dysfunction (9, 11). Increased calpain proteolytic activity also degrades the major intermediate filament desmin, in addition to connexins and tight junctions, leading to cardiac arrhythmias and adverse cardiac remodeling (22). Interestingly, cardiac SERCA2 is also a target of degradation by the calpains (19). Based on our previous findings with another halogen (Cl2), we hypothesized that inhalation of Br2 will cause bromination and inactivation of cardiac SERCA and activation of Ca2+-sensitive calpains, leading to myocardial damage and dysfunction. Therefore, in the present study, we investigate the effects of Br2 inhalation on cardiac function and evaluate the downstream biochemical effects of decreased SERCA2 function, including calpain activation and cardiac ultrastructure damage, as they relate to survivability after Br2 exposure.
METHODS
In vivo exposures to Br2.
All animal procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Unanesthetized male Sprague-Dawley rats (200–250 g, Envigo, Indianapolis, IN) were exposed (whole body) to 600 ppm Br2 for 30 or 45 min, as previously described (1, 35). Rats were then returned to room air and monitored continuously up to 8 h and every 12 h thereafter for the specified duration. Clinical scoring was performed during this period, which is a composite measure of activity and respiratory quality, as previously described (61). Briefly, a score of 0 is given to an animal with normal respiratory quality and no stridor or loss of activity. Mild (score of 1), moderate (score of 2), and severe (score of 3) conditions are assessed and added. For example, an animal demonstrating mild respiratory quality, mild stridor, and moderate activity will have a clinical score of 4 (1 + 1 + 2). Oxygen saturation and heart rates were monitored using the MouseOX small animal oximeter (Starr Life Sciences, Oakmont, PA) as previously described in our laboratory (68). Arterial blood gas (from the descending aorta) at necropsy was also estimated using the EPOC automated blood gas analysis reader (Ottawa, ON) (68). Plasma was analyzed for cardiac injury markers heart-type fatty acid-binding protein (hFABP), NH2-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin I by ELISA (68).
Tissue analysis.
Transmission electron microscopy (TEM) was performed as previously described in our laboratory (EMLabs, Birmingham, AL) (6, 21). Immunoblots on cardiac tissues were performed according to previously described methods using previously validated antibodies (4). Picrosirius red (PSR) staining and hydroxyl proline analysis (Sigma, St. Louis, MO) were performed on cardiac tissue as previously described in our laboratory (13). SERCA activity in cardiac tissues was also determined as described previously by us (4).
Hemodynamics and transthoracic M-mode and two-dimensional echocardiography/Doppler.
All experiments were performed under 2% isoflurane anesthesia in compressed room air as previously performed in our laboratory (38). The body temperature was maintained at 37°C during measurements. A 1.4-Fr high-fidelity catheter (SPR 671, Millar Institute, Houston, TX) was inserted into the left ventricular (LV) and right ventricular (RV) chambers via the right carotid artery and right external jugular vein, respectively. LV and RV high-fidelity pressures were measured using the Biopac MP100 data acquisition system with AcqKnowledge III software (ACQ 3.2; Biopac Sytems, Galeto, CA). Transthoracic two-dimensional (2-D) echocardiography/Doppler (echo/Doppler) was performed using a Vevo 2100 high-resolution ultrasound system with the 21-MHz MS250 MicroScanTransducer (VisualSonics, Toronto, ON, Canada), as previously described in our laboratory (68). Parasternal long- and short-axis two-chamber M-mode and B-mode images were obtained at midpapillary level. LV dimensions were measured and averaged at end systole and end diastole from which LV volumes, cardiac output, ejection fraction, and fractional shortening were calculated using the Visualsonics system software. The volume determinations made by the software used B-Mode area measurement in a rotational volume calculation around the long axis of the chamber. A substernal four-chamber view with pulsed wave Doppler was used to determine transmitral early (E) and atrial (A) wave peak velocities (52). Operators blinded to exposure performed image collection and analyses.
Catecholamine measurement.
Plasma and cardiac (LV) tissue catecholamines (epinephrine and norepinephrine) were measured by ELISA using a commercial kit (Mybiosource, San Diego, CA) according to the manufacturer’s instructions.
Extraction and quantification of brominated species.
Brominated reactants were identified as previously described in the Ford laboratory (17). Rats were euthanized at various time intervals after Br2 exposure. Blood was collected from the descending aorta and centrifuged at 2,000 rpm for 10 min to obtain plasma fraction. LV tissue was collected, flash frozen, and stored at −80°C before being shipped overnight to Dr. Ford’s laboratory on dry ice. Free and total (free + esterified) Br-fatty acids were measured by liquid chromatography mass spectrometry after Dole extraction, as previously described (63). Total fatty acids were measured after base hydrolysis and esterified Br-fatty acids calculated by subtracting free fatty acids from total fatty acids. Samples were spiked with known amounts of 2-chloro fatty acids to use as internal standard.
Calpain expression and activity.
Calpain expression was measured by immunoblot analysis, and activity was determined as previously described using calpain Glo protease assay (Promega) according to the manufacturer’s instructions (36). The assay measures the luminescence intensity of 7-amino-4-methylcoumarin (AMC) cleaved from the labeled peptide substrate. Briefly, cardiac tissue lysates were prepared and centrifuged, and the supernatant was used for calpain activity measurement. Samples were incubated in reaction buffer [63 mM imidazole-HCl (pH 7.3), 10 mM β-mercaptoethanol, 1 mM EDTA, and 10 mM EGTA] with or without calcium chloride (5 μM) at 37°C for 1 h. In some reaction samples, a calpain-selective inhibitor (MDL28170; 10 μM) was added to determine specific calpain activity. The luminescence intensity of cleaved AMC was quantified with a luminescence plate reader, and calpain activity was determined as the difference between Ca2+-dependent and Ca2+-independent luminescence in the absence or presence of the specific inhibitor (MDL28170) by 1 mg cardiac protein.
Closed-chest echocardiographic delivery of brominated fatty aldehydes.
Rats were anesthetized, their chests were shaved, and they were secured supine on a warmed platform. The LV was visualized in a long axis B-Mode with the Vevo 2100 system. A syringe with a 28-gauge needle was loaded with 200 μl of 100 μM 2-bromohexadecanal (Br-HDA) in saline. The needle was manually inserted through the body wall just below the sternum and advanced in a transdiaphragmatic direction toward the LV apex at an approximate 30° angle. The needle was advanced under ultrasound guidance until the tip was positioned in the LV chamber. Br-HDA (100 μl) was administered, and the needle was removed. LV function was monitored by echocardiography throughout the entire procedure. LV echo/Doppler data were compared with preinjection with postinjection images and compared with nonbrominated fatty aldehyde controls.
Isolation of primary rat cardiomyocytes.
Cardiomyocytes were isolated from Sprague-Dawley rats, as previously described by our laboratory (70). Briefly, rat hearts were perfused with perfusion buffer [containing (in mmol/l) 120 NaCl, 15 KCl, 0.5 KH2PO4, 5 NaHCO3, 10 HEPES, and 5 glucose at pH 7.0] for 5 min and digested with perfusion buffer containing 2% collagenase type II (Invitrogen, Carlsbad, CA) for 30 min at 37°C. The RV, atria, and apex were removed, and the perfused heart was minced. The digest was filtered and washed, and cells were pelleted. Only samples with viability (rod-shaped cells) of >80% were used. Cells (10,000 cells/well) were allowed to adhere to laminin-coated 96-well plates in DMEM containing 10% FBS, 2 nM glutamine, 10 U/ml penicillin, and 100 mg/ml streptomycin for 2 h before use.
Statistical analysis.
Data are expressed as means ± SE and were analyzed by one-way ANOVA with a Student’s paired t-test. P values of <0.05 were considered significant. Analysis was conducted using Graphpad Prism version 7 software (LaJolla, CA). The overall survival rates were determined using the Kaplan-Meier method and compared using a log rank test (Mantel-Cox test). All echocardiography analysis and calculations were performed using SPSS (version 19.0, SPSS, Chicago, IL). Two observers with expertise in echocardiography assessed the experiments for intra- and interobserver reproducibility.
RESULTS
Br2 inhalation induced mortality and cardiopulmonary injury.
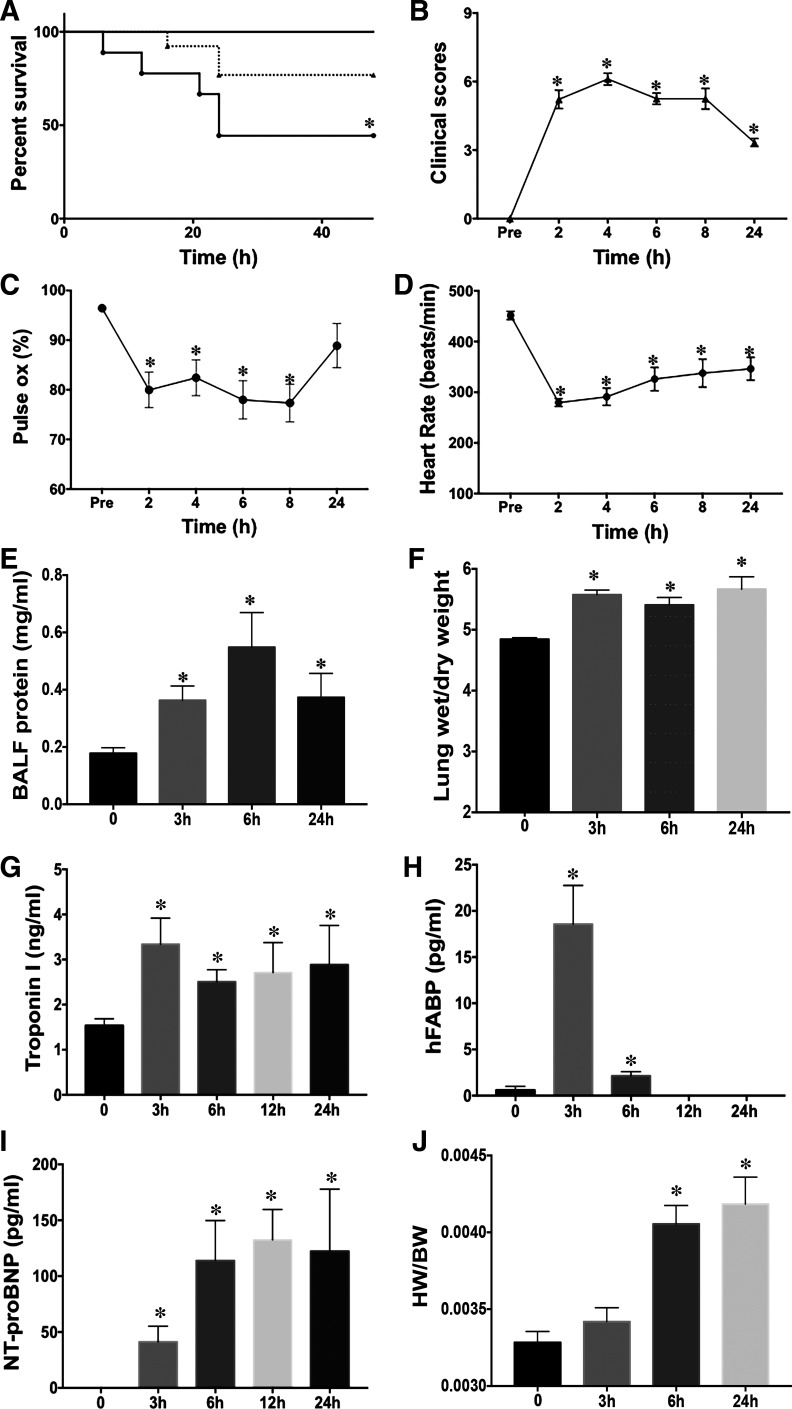
To determine the effects of Br2 inhalation on the cardiopulmonary system, we exposed rats to Br2 gas (600 ppm for 30 min and 600 ppm for 45 min) and transferred them to room air. These concentrations of Br2 and timing were chosen according to previous published reports in murine and rat models of Br2 inhalation, in which there is a consistent incidence of mortality and morbidity (1, 10, 17). Br2 inhalation at 600 ppm for 45 min resulted in 50% mortality within 48 h after exposure (Fig. 1A). Thus, all subsequent experiments in this study used 600 ppm and 45 min exposure. Survivors had increased morbidity measured by increased clinical scores that are a composite evaluation of activity and respiratory distress (Fig. 1B) (61). Oxygen saturation from pulse oximetry was significantly decreased within 2 h and up to 8 h of observation (Fig. 1C) and returned to normal at 24 h, whereas heart rate measured by pulseoximetry was significantly decreased up to 24 h after exposure (Fig. 1D). There was significant lung injury determined by increased bronchoalveolar lavage fluid protein content (Fig. 1E) and pulmonary edema reflected by increase in the lung wet-to-dry weight ratio at 3, 6, and 24 h (Fig. 1F).
Fig. 1.
Bromine (Br2) inhalation increases mortality and causes respiratory distress and acute cardiopulmonary injury in survivors. Rats were exposed to Br2 (600 ppm, 30 or 45 min) and returned to room air. Rats were observed for clinical symptoms for 48 h, and Kaplan-Meier curves were generated for survival data (dotted line, 600 ppm for 30 min; solid line, 600 ppm for 45 min dose) (A). B−D: deterioration in clinical scores (B), decrease in O2 saturation (C) and heart rate (D). Lung injury was determined by increased bronchoalveolar lavage fluid (BALF) protein (E) and lung wet-to-dry weight ratios (F). Cardiac injury was indicated by increased troponin (G) and heart-type fatty acid-binding protein (hFABP) (H). Increased NH2-terminal pro-brain natriuretic peptide (NT-proBNP; I) in plasma and increases in heart-to-body weight ratios (HW/BW; J) also indicated heart damage. Data are means ± SE; n = 8 for each group. *P < 0.05 vs. 0 ppm.
Arterial Po2 did not differ from controls at 3 h after Br2 inhalation but decreased 24 h after exposure (90 ± 4 mmHg in control rats vs. 70 ± 5 mmHg in Br2-exposed rats, P = 0.013). Br2-exposed rats had a significant increase in plasma lactate levels at 3 h (1.53 ± 0.052 in 0 ppm vs. 4.02 ± 0.04 mmol/l, P < 0.0005) as well as elevated plasma troponin I and hFABP at 3 and 6 h after exposure (Fig. 1, G and H). Plasma troponin I remained elevated after Br2 exposure. NT-proBNP and the heart-to-body weight ratio were also increased in Br2-exposed animals (Fig. 1, I and J).
Inhaled Br2 and myocardial ultrastructural damage.
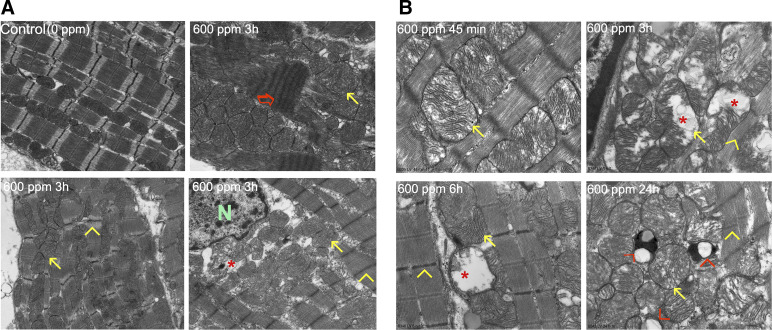
Figure 2A shows TEM (×3,200) evidence of extensive myofibrillar breakdown and contraction band necrosis (red arrow) that occurred at 3 h after Br2 inhalation, consistent with the early and sustained elevation of troponin. Compared with the normal linear array of sarcomeres and mitochondria (Fig. 2A, top left), there was disruption and breakdown of z-disks (yellow arrowheads) and loss of I bands (yellow arrowheads) with disarray and swelling (yellow arrows) of mitochondria (red asterisk). Figure 2B shows TEM images at higher magnification (×17,000) at different time intervals after Br2 exposure to demonstrate the diffuse mitochondrial swelling (yellow arrows) and cristae lysis (red asterisk), as opposed to the densely packed mitochondrial matrix with its normal alignment along the sarcomere in unexposed control (Fig. 2A, top left). Mitochondrial swelling and cristae lysis started at 45 min and persisted for 24 h (red asterisk), where there was an increase in vacuolization and lipid granule deposition (red arrowhead) in areas of myofibrillar breakdown and contraction band necrosis (Fig. 2B).
Fig. 2.
Bromine (Br2) inhalation causes disruption of the cardiac cytoskeleton and loss of the normal highly organized linear mitochondrial sarcomere integrity. A: representative transmission electron microscopy (TEM; ×3,200) images demonstrating a normal control heart (top left). The remaining three images demonstrate myofibrillar loss, contraction band necrosis (red arrow), loss of I bands, and disruption of z-disks (yellow arrowheads) in the left ventricle 3 h after Br2 exposure in addition to mitochondrial swelling (yellow arrows) and cristae lysis (red asterisk) [nucleus (N)]. B: representative TEM image at higher magnification (×17,000) demonstrating the time course of inhaled Br2-induced mitochondrial swelling and cristae lysis. At longer time intervals there were lipid granules (small black dots) and extensive mitochondrial vacuolization (red arrowhead).
Br2 inhalation causes acute cardiac dysfunction in survivors.
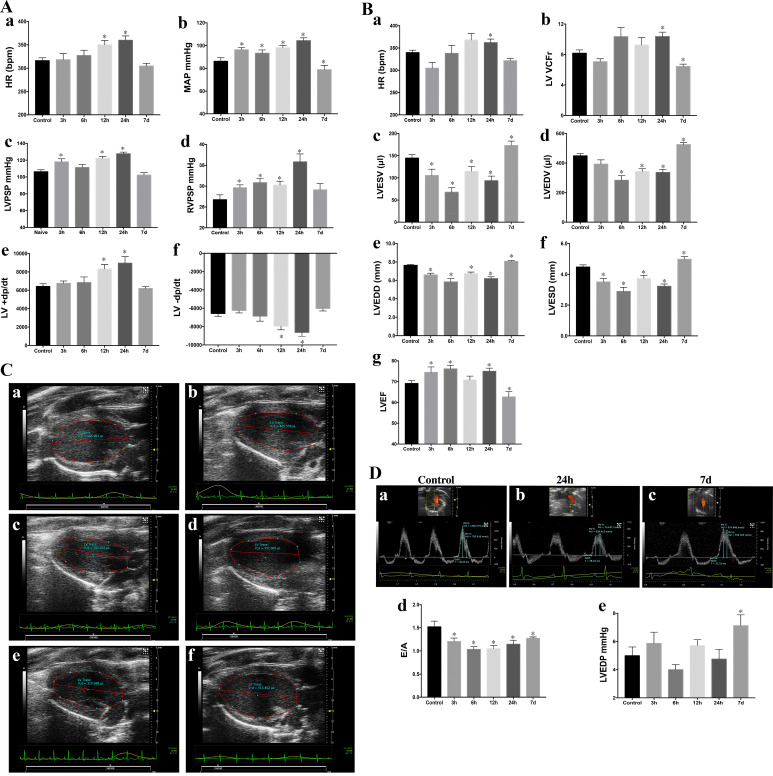
Heart rates after pressure catheter insertion under isofluorane anesthesia were increased 12 and 24 h compared with control (Fig. 3Aa). Mean arterial pressure was increased acutely and remained elevated until 24 h after Br2 inhalation but decreased at 7 days in the survivors of Br2 exposure (Fig. 3Ab). There was an increase in LV peak systolic pressure at the 3- to 24-h time point (Fig. 3Ac). RV peak systolic pressure was significantly increased, peaking at 24 h and returning to normal at the 7-day time point in Br2-exposed rats (Fig. 3Ad). As with mean arterial pressure, +dP/dt and −dP/dt were increased up to 24 h and decreased at the 7-day time point (Fig. 3A, e and f).
Fig. 3.
Acute (3–24 h) and delayed (7-day) effects of Bromine (Br2) inhalation (600 ppm, 45 min) on hemodynamics and cardiac function (n = 8 per group). A: heart rate (HR) during hemodynamic measurements (a), mean arterial pressure (MAP; b), left ventricular (LV) peak systolic pressure (LVPSP; c), right ventricular peak systolic pressure (RVPSP; d), maximal rate of LV pressure rise (+dP/dt; e), and maximal rate of LV pressure fall (−dP/dt; f). Data are means ± SE. *P < 0.05 vs. 0 ppm. B: HR during echo/Doppler measurements (a), LV velocity of circumferential shortening (VCFr; b), LV end-systolic volume (LVESV; c), LV end-diastolic volume (LVEDV; d), LV end-systolic diameter (LVESD; e), LV end-diastolic diameter (LVEDD; f), and LV ejection fraction (LVEF; e). Data are means ± SE; n = 8 for each group. *P < 0.05 vs. 0 ppm. C: representative parasternal long-axis images of the LV of a control rat (a) and rats 3 h (b), 6 h (c), 12 h (d), 24 h (e), and 7 days (f) after Br2 exposure. D: representative image of LV diastolic indexes measured by transmitral pulse Doppler of a control rat (a) and rats 24 h (b) and 7 days (c) after Br2 exposure. MV, mitral valve; E, early filling flow velocity; A, late filling flow velocity, IVRT, isovolumic relaxation time. The ratio of peak velocities of early to late transmitral waves (E/A; d) and LV end-diastolic pressure (LVEDP; e) are shown. Data are means ± SE; n = 8 for each group. *P < 0.05 vs. 0 ppm.
Heart rate during echocardiography was significantly increased only at the 24-h time point (Fig. 3Ba). There was a significant increase in the LV velocity of circumferential shortening (VCFr) at 24 h (Fig. 3Bb). However, LV VCFr was significantly decreased below controls 7 days after Br2 exposure (Fig. 3Bb). LV end-systolic and end-diastolic volumes were significantly decreased at all acute time points and increased above control at 7 days after Br2 exposure (Fig. 3B, c and d), as demonstrated by the representative long-axis 2-D echocardiography images shown in Figure 3C. Similarly, LV end-diastolic and LV end-systolic diameters significantly decreased acutely at all time points but were increased after 7 days in Br2-exposed rats (Fig. 3B, e and f). LV ejection fraction increased at variable acute time points (3–24 h; Fig. 3Bg) but decreased below control at 7 days (Fig. 3Bg). LV echo/Doppler demonstrated a reduction in the ratio of the peak velocities of early to late transmitral waves (E/A ratio) at all acute and 7-day postexposure time points (Fig. 3Dd; representative echo/Doppler images in Fig. 3D, a–c); however, LV end-diastolic pressure increased above control only 7 days after Br2 exposure (Fig. 3De) along with the decrease in LV ejection fraction and LV VCFr in surviving Br2-exposed rats.
In an attempt to attribute hyperadrenergic drive to acute cardiac damage and the increase in LV shortening in the first 24 h, we measured circulating and LV tissue catecholamines. There was an insignificant mean decrease in plasma catecholamines at 3 h after Br2 exposure compared with controls (73 ± 17 in 0 ppm vs. 39 ± 16 pg/ml in 600 ppm, n = 9, P = 0.17) along with LV tissue catecholamines levels at 3 h (33 ± 16 in 0 ppm vs. 17 ± 12 pg/ml in 600 ppm, n = 4, P = 0.35). However, plasma catecholamines decreased significantly from 73 ± 17 pg/ml (at 0 ppm) to 1.6 ± 1.4 pg/ml after 15 min of 600 ppm Br2 inhalation (n = 9, P = 0.0019).
Cardiac histology in 7-day Br2-exposed rats.
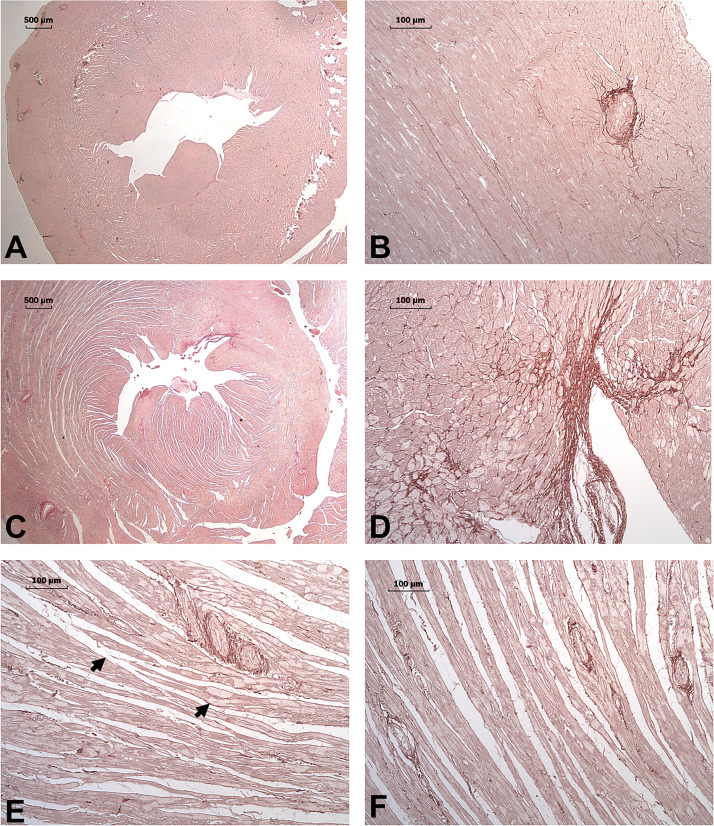
Br2-exposed rats at 7 days demonstrated evidence of LV systolic and diastolic dysfunction. PSR staining was performed to evaluate the potential presence of fibrosis in these 7-day Br2 hearts. In normal rats, PSR demonstrated a very compact tissue structure without evidence of increased collagen deposition (Fig. 4, A and B). However, in Br2-exposed rats, there was marked fracturing and increased interstitial space between myofibers throughout the mid- and endomyocardial layers. Additionally, there was decreased staining of the cytoplasm consistent with myofibrillar breakdown. There was some evidence of a patchy increase in collagen deposition along the endocardial layer; however, there was no evidence of increased collagen in interstitial or perivascular regions (Fig. 4, C–F). The marked increase in interstitial space/edema precluded collagen quantification by PSR. Hydroxyproline content was not increased in control versus 7-day Br2-exposed rats (0.035 ± 0.001 vs. 0.039 ± 0.002 μg/μl, n = 6, P = 0.189), consistent with the absence of diffuse fibrosis. However, at this 7-day time point, continued myocardial damage and failure was also reflected by a persistent increase in troponin I (1.53 ± 0.15 vs. 5.28 ± 0.67 ng/ml, P = 0.001) and NT-proBNP (0 ± 0 vs. 43.33 ± 12.36 pg/ml, P = 0.01) in control versus Br2-exposed rats.
Fig. 4.
Picrosirius red (PSR) staining for collagen in rats 7 days after bromine (Br2) exposure. PSR-stained images of ×1 (A) and ×10 (B) unexposed control demonstrate minimal interstitial and endocardial collagen with homogeneous staining and compact myocardium. In contrast, the Br2-exposed heart at 7 days demonstrates a patchy endocardial fibrosis at ×1 (C) and ×10 (D). E and F: ×10 longitudinal images of the midmyocardium demonstrate the diffuse fracturing and increased interstitial space. Arrows demonstrate the areas of decreased staining in Br2-exposed rats and represent myofibrillar loss compared with the homogeneous staining of normal rats (A and B).
Hypoxia alone does not induce cardiac injury.
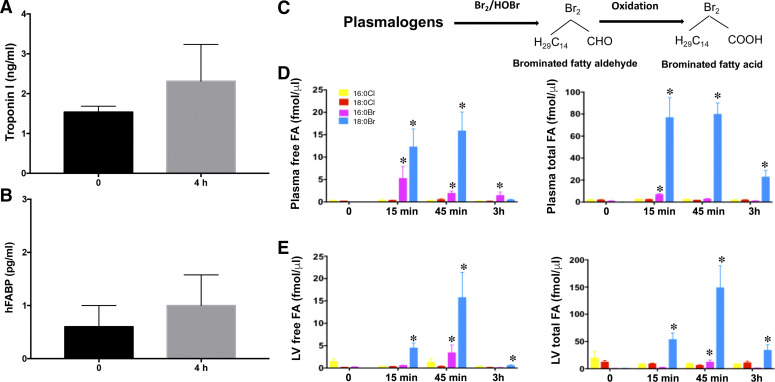
In the present study, Br2 gas was mixed in room air (600 ppm was equivalent to 0.06% Br2 in air); therefore, no hypoxia occurred during exposure. However, respiratory injury may cause decreased oxygenation of blood and contribute to cardiac injury. Also, the pulse oximetry data (Fig. 1C) and arterial blood Po2 of 90 ± 4 mmHg did not reflect severe hypoxic conditions at 3 h after Br2 exposure. Nevertheless, to exclude the possibility of hypoxic damage and evaluate the role of hypoxia, we exposed rats to 4 h (duration in exposure chamber plus the 3-h time point postexposure) of hypoxia (10% O2) and measured cardiac injury markers in plasma. There was an insignificant increase in plasma troponin or hFABP levels (Fig. 5, A and B) at 3 h. Thus, hypoxia may be partially responsible for some myocardial injury but clearly not all effects because the severity observed with Br2 exposure was greater than that of hypoxia alone.
Fig. 5.
Role of hypoxia alone in cardiac injury and production of reactive brominated lipids on bromine (Br2) inhalation. Plasma troponin (A) and heart-type fatty acid binding protein (hFABP) (B) did not increase significantly in rats exposed to normoxia (21% oxygen) or hypoxia (10% oxygen) for 4 h. Data are means ± SE; n = 4 for each group. Plasma and heart tissues of Br2-exposed animals were also analyzed for the production of brominated fatty acids (FAs) by mass spectrometry, demonstrating the formation of 2-bromopalmitic acid (pink bars) and 2-bromostearic acid (blue bars). The yellow and red bars are the internal standards. Data are means ± SE; n = 6 for each group. *P < 0.05 vs. 0 ppm (C–E).
Circulating Br2 reactants induce cardiac injury.
It has been previously reported that Br2 ions and brominated compounds formed on the pulmonary epithelial surface are stable and may persist in the circulation for days after exposure (25, 57). We have also shown that highly bioactive halogenated intermediates (amines and lipids) produced in the pulmonary vascular bed with direct drainage to the left atrium provide a “first pass effect” into the coronary arteries, resulting in severe cardiac dysfunction (4, 59, 68). Br2 reacts with pulmonary plasmalogens to form highly reactive brominated fatty aldehydes that are quickly oxidized to brominated fatty acids (Fig. 5C) (17). In the present study, concentrations of both free and esterified brominated fatty acids were significantly increased within 15 min of exposure (Fig. 5, D and E) in the plasma and LV of Br2-exposed rats, peaking at the end of exposure duration (45 min) and remaining elevated until 3 h postexposure.
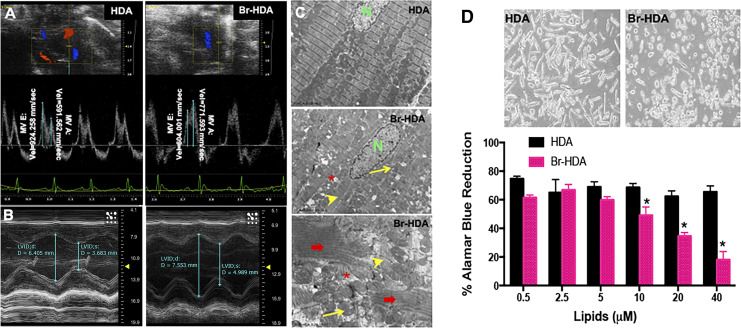
To further strengthen the concept of a first pass effect, we also directly injected brominated fatty aldehydes (i.e., Br-HDA) into the LV cavity under echocardiography guidance. Direct injection of Br-HDA resulted in a decrease in transmitral E/A (Fig. 6A) and LV fractional shortening along with LV dilatation 4 h after injection (Fig. 6B). Br-HDA also induced severe cardiac ultrastructural damage as shown by TEM (Fig. 6C) similar to that with Br2 inhalation. To further determine the effect of Br-HDA on cardiomyocytes, Br-HDA added to adult rat cardiomyocyte cultures resulted in a dose-dependent decrease in cell viability estimated by Alamar blue (Fig. 6D). The Alamar blue reduction also reflects mitochondrial damage and dysfunction, consistent with ultrastructural mitochondrial damage after Br2 inhalation and Br-HDA injection into the LV cavity in vivo. Thus, brominated reactants may mediate the cardiac toxicity after Br2 inhalation.
Fig. 6.
Cardiac dysfunction and cardiomyocyte death with 2-bromohexadecanal (Br-HDA). A: left ventricular (LV) diastolic dysfunction was reflected by the decrease in mitral valve (MV) early and late wave velocities 4 h after injection of Br-HDA into the LV cavity. B: Br-HDA caused an increase in LV end-diastolic dimension and end-systolic dimension, resulting in a decrease in fractional shortening. Image demonstrates M-mode echocardiography of the LV before and 4 h after intracardiac injection of Br-HDA. C: Br-HDA caused extensive disruption of the cardiac cytoskeleton and loss of the normal highly organized linear mitochondrial sarcomere integrity. Transmission electron microscopy (×3,200 in the top images and ×8,000 in the bottom image) demonstrated contraction band necrosis (red arrows), loss of I bands, and disruption of z-disk (yellow arrowheads) in the LV of rats administered Br-HDA as well as mitochondrial swelling (yellow arrows) and cristae lysis (red asterisk). D: Br-HDA-induced cardiac cardiomyocyte death in primary adult rat cardiomyocytes with viability by alamar blue dye reduction. n = 8. *P < 0.05 vs. HDA. Top: representative phase-contrast microscope image of cardiomyocytes treated with 40 μM HDA or Br-HDA.
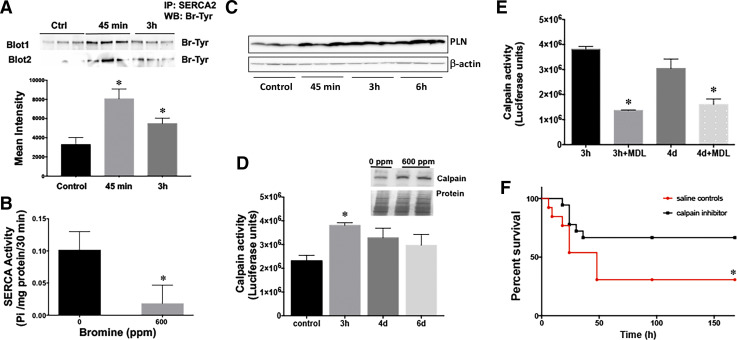
Br2 inhalation inactivates cardiac SERCA and activates calpains.
Abnormal Ca2+ handling caused by the defect in SERCA is key to heart failure (39). Br2 inhalation caused significant chemical modification of SERCA protein via bromination of its tyrosine residues (Fig. 7A) and led to a significant reduction in LV SERCA activity (Fig. 7B). In previous studies of Cl2 inhalation, we demonstrated halogenation of several critical cardiac proteins (4) and Cl2-induced accumulation of cytosolic Ca2+ in both cardiac and pulmonary cells (4, 34). Increases in cytosolic Ca2+ resulting from SERCA inactivation may activate a variety of highly destructive intracellular proteases, such as calpains. SERCA inactivation by binding to phosphalamban (PLN) also increases cytosolic Ca2+ and causes Ca2+ overload and calpain activation in a myocardial ischemia-reperfusion model (54). In the present study, PLN levels were significantly increased within 45 min of Br2 exposure and remained elevated at 6 h after exposure (Fig. 7C). Calpains digest a number of cytoskeletal intermediate filaments, such as desmin, and also degrade myofibrillar proteins, such as titin and troponin (9). To further reinforce the concept that Br2 causes increased Ca2+-dependent calpain activity, calpain protein expression and activity were significantly increased in the LV 3 h after Br2 inhalation (Fig. 7D).
Fig. 7.
Bromine (Br2) exposure causes bromination and inactivation of cardiac sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) and activation of cardiac calpain. A: Br2 inhalation induced chemical modification of SERCA protein via bromination of its tyrosine residues. Bottom, quantitation of the immunoblots for bromotyrosine. Data are means ± SE values; n = 6 for each group. *P < 0.05 vs. 0 ppm. B: left ventricular (LV) SERCA protein and activity were decreased in rats exposed to Br2 (600 ppm for 45 min). *P < 0.05 vs. 0 ppm. C: SERCA regulator phosphalamban (PLN) was also increased in the hearts of rats inhaling Br2. D: Br2-induced LV calpain activity. Inset, Western blot image of calpain 2 protein and the Ponceu-stained gel to show protein loading. Data are means ± SE values; n = 6 for each group. *P < 0.05 vs. 0 ppm. E: inhibition of Br2-induced LV calpain activity by the specific calpain inhibitor MDL28170 (1 mg/kg). Data are means ± SE values; n = 6 for each group. *P < 0.05 vs. saline control. F: Kaplan-Meier curves demonstrating that calpain inhibition improves survival after Br2 inhalation 7 days posttreatment. *P < 0.05 vs. saline control.
SERCA2 inhibition and subsequent calpain activation can be reversed by calpain inhibitor MDL28170 (19). Cardiac calpain activation was reversed by intraperitoneal administration of specific calpain inhibitor MDL28170 (1 mg/kg) 60 min after Br2 exposure (Fig. 7E), which was sufficient to inhibit the Br2-induced increase in cardiac calpain up to 4 days after exposure. MDL28170 administration also improved survival in Br2-exposed rats compared with vehicle (saline)-treated control rats (Fig. 7F). MDL28170-treated rats also had improved O2 saturation compared with vehicle-treated rats (59 ± 5% vs. 65 ± 4%, P = 0.05) at 4 h postexposure, suggesting potential attenuation in lung pathology that translated to improved clinical symptoms.
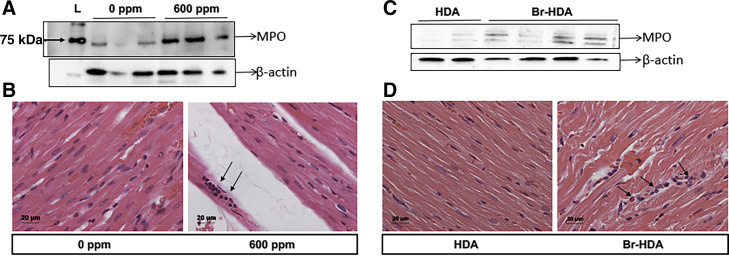
Cardiac neutrophil accumulation after Br2 inhalation.
Neutrophils can contribute to the production of halogenated derivatives/fatty aldehydes that can cause cardiac damage (59). Cl2 inhalation caused extensive neutrophil accumulation at 24 h after exposure in the lungs (64). Neutrophils also localize rapidly to the regions of reperfusion after myocardial ischemia (62) and myeloperoxidase (MPO) has been implicated after ischemic arrhythmogenic ventricular remodeling (44). Here, we demonstrate that Br2 inhalation resulted in increased neutrophil accumulation by hematoxylin and eosin staining and increased MPO content in the cardiac tissues (Fig. 8, A and B). Br-HDA injected into the LV cavity also resulted in an increase in LV neutrophils compared with HDA-injected rats (Fig. 8, C and D).
Fig. 8.
Bromine (Br2)- and 2-bromohexadecaneal (Br-HDA)-induced neutrophil accumulation in the heart. Left ventricles (LVs) were collected 20 h after Br2 inhalation (A and B). A: Western blot for myeloperoxidase (MPO). B: hematoxylin and eosin (H&E) staining in a representative images (×20) of Br2 (0 or 600 ppm)-exposed LV sections, where arrows show neutrophils. C and D: LV Western blots and H&E staining 4 h after Br-HDA injection. C: Western blots for MPO demonstrating a similar increase in MPO and neutrophils (H&E staining; D) in Br-HDA compared with HDA.
DISCUSSION
In this report, we build on our previous work with Cl2-induced SERCA dysfunction and attempt to understand the cardiotoxic effects of Br2 inhalation exposure on heart ultrastructure and function. Indeed, we now provide in vivo and in vitro evidence showing that Br2 and Br2 reactants inactivate SERCA and lead to activation of Ca2+-sensitive calpains and cardiomyocyte cytoskeletal ultrastructural and mitochondrial damage that result in LV diastolic and systolic dysfunction at 7 days after exposure.
The acute increase in circulating troponin in Br2-exposed rats is corroborated by the striking TEM findings of contraction band necrosis, mitochondrial damage and disorganization, and disruption of myofibrils and z-disks. This is associated with calpain activation and infiltration of polymorphonuclear cells, all of which are similar to the findings in ischemia reperfusion injury of the heart. Calpain activity increases as a result of increased levels of free intracellular Ca2+ achieved during ischemia-reperfusion but can also increase with excessive oxidative stress (20, 26, 30, 46). Calpain also causes release of ROS from mitochondria, leading to apoptotic/necrotic cell death (53, 56), whereas Cl2 exposure to airway epithelial cells causes ROS release from mitochondria, creating a feedforward cycle of mitochondrial damage and ROS production (29). Calpain activation and calpain-induced cardiac ultrastructure damage can occur as early as 1 h after toxic exposure, such as that observed with doxorubicin treatment (37).
Consistent with our previous work with inhaled Cl2 gas, Br2 inhalation causes significant bromination of SERCA tyrosine residues and a profound reduction in LV SERCA activity. LV PLN levels are also significantly increased within 45 min of exposure to Br2 and remained elevated at 6 h after exposure (Fig. 7C), further contributing to increased cytosolic Ca2+ and calpain activation. Previously, we have shown that exposure of the isolated rat heart to Cl2 reactant taurochloramine 30–100 µmol/l resulted in >90% loss of force of contraction. However, despite SERCA2 inactivation and severe ultrastructural damage in response to Br2 inhalation in vivo, LV systolic function was hyperdynamic at 3 h in Br2-exposed rats. These changes along with a 20% decrease in LV volume most likely result from third spacing of fluid as manifested by increased lung and heart weight.
Another possible explanation for the combined paradoxical findings of hyperdynamic function despite severe ultrastructural damage is acute adrenergic discharge. However, the profound decrease in plasma catecholamine levels at 3 h of Br2 exposure is unexpected in the face of such a severe stress. It is of interest that previous studies of the rat brain after Br2 (methyl bromide gas) exposure also demonstrated subsequent decreases in tissue dopamine and norepinephrine levels and inhibition of tyrosine hydroxylase, which is the rate-limiting enzyme for catecholamine synthesis (23, 24). These changes persist in a dose-dependent fashion and are achieved with levels of exposure of methyl bromide gas of <250 ppm for 8 h, much less than the Br2 gas levels used in the present study (23). Of great interest, unanesthetized Br2-exposed rats have a significant decrease in heart rate within the first 24 h consistent with Cl2 inhalation. This finding of bradycardia and respiratory depression in halogen exposure suggests a direct or indirect effect of halogen exposure on the brain regulatory centers and/or sympathetic ganglia, resulting in a reduced ventilatory drive, hypoventilation, and bradycardia (47). Indeed, cardiac ultrastructural damage is consistent with acute adrenergic discharge that, although not manifest acutely, certainly is evident at 7 days when there is unequivocal evidence of LV dilatation and LV systolic and diastolic dysfunction. Future studies will evaluate the effects of acute Br2 exposure on cardiac sympathetic ganglia damage and the long-term effects on cardiac function after Br2 exposure.
Although highly reactive locally in the lung, other direct tissue effects of Br-HDA and 2-chlorohexadecanal (Cl-HDA) circulating byproducts may contribute to myocardial damage. To further strengthen the concept of a first pass effect, direct injection into the LV cavity resulted in decreases in transmitral E/A, LV fractional shortening, and LV dilatation 4 h after administration, which were accompanied by severe mitochondrial and myofibrillar damage by TEM similar to the Br2 inhalation. As in Br2-exposed rats, Br-HDA was associated with neutrophil infiltration that can result in locally generated Br/Cl-HDA (7, 12) and cause LV contractile dysfunction (59). Although the mechanisms responsible for this dysfunction remain to be elucidated, it is known that Cl-HDA and Br-HDA are neutrophil chemoattractants, and Cl-HDA has been shown to cause endothelial cell dysfunction through the NF-κB pathway (65). Finally, exogenous Br-HDA added to adult rat cardiomyocytes in vitro showed a direct toxic effect on mitochondria. Taken together, Br-HDA may additionally target mitochondria, thereby initiating a perpetual cycle of mitochondrial ROS production, SERCA inhibition, and calpain activation (33). Moreover, SERCA inactivation, mitochondrial ROS production, and neutrophil infiltration can produce a feedforward cycle that results in calpain activation and cardiac damage.
Previous work has demonstrated the effectiveness of calpain inhibitors in cardiac ischemia-reperfusion injury, where calpain inhibitors, when infused in the initial period of reperfusion, reduce infarct size (19, 45, 60). Here, cardiac calpain activation is reversed up to 4 days after exposure with intraperitoneal administration of specific calpain inhibitor MDL28170 (1 mg/kg). MDL28170 administration also improves O2 saturation and survival in Br2-exposed rats compared with vehicle (saline)-treated control rats. In addition to calpain digestion of myofibrillar and cytoskeletal proteins, acute calpain activation has been shown to play a major role in the cleavage of SERCA2 and ryanodine receptor 2 (49, 55). Calpain-mediated cleavage of ryanodine receptor 2 renders the channel unable to close in the presence of elevated cytosolic Ca2+ levels and can also contribute to permanent endoplasmic reticulum Ca2+ depletion and loss of endoplasmic reticulum ability to buffer elevations in cytosolic Ca2+ (49, 51). Leupeptin, a calpain inhibitor with antioxidant properties, has been shown to prevent calpain activation in a rat model of ischemia-reperfusion injury, suggesting that a combined therapy may be of benefit (14, 50).
In the present study, we provide evidence of severe cardiomyocyte ultrastructural damage that results in LV systolic and diastolic dysfunction 7 days after Br2 exposure. There are multiple potential causes of the acute cardiomyocyte ultrastructural damage that require further investigation, including local hypoxia, decreases in SERCA2 activity, neutrophil accumulation, and calpain activation. Besides calpain inhibition, other potential therapeutic targets in acute halogen exposure, including adrenergic discharge and mitochondrial damage and ROS production, may also be effective in preventing lung and heart damage. Thus, future studies will explore a combined targeted approach that results in improvement in both acute survival and long-term cardiac function.
GRANTS
This work was supported by intramural funds from the Department of Anesthesiology and Perioperative Medicine at the University of Alabama at Birmingham (UAB), a Bridge fund from the Dean’s office, Department of Medicine (UAB) and CounterACT Program, and National Institutes of Health Office of the Director (to S. Ahmad); National Institute of Environmental Health Sciences Grant U01-ES028182 and supplemental Grant U01-ES028182-02S1 (to S. Ahmad and L. J. Dell’Italia); and the CounterACT Program, National Institutes of Health Office of the Director, NIEHS Grant U01-ES025069, and National Heart Lung and Blood Institute Grant R01-HL-114933 (to A. Ahmad).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A., A.A, and L.J.D. conceived and designed research; S.A., J.X.M.J., A.A., A.Z., C.-C.W., W.B., I.Z., P.P., N.M., N.V., and S.D. performed experiments; S.A., J.X.M.J., A.A., A.Z., C.-C.W., W.B., I.Z., P.P., N.M., N.V., and L.J.D. analyzed data; S.A., J.X.M.J., A.A., A.Z., C.-C.W., W.B., I.Z., P.P., N.M., and L.J.D. interpreted results of experiments; S.A., A.A., W.B., and N.V. prepared figures; S.A., A.A., D.A.F., and L.J.D. drafted manuscript; S.A., A.A., A.Z., W.E.L., D.A.F., S.M., and L.J.D. edited and revised manuscript; S.A., J.X.M.J., A.A., A.Z., C.-C.W., W.B., I.Z., P.P., N.M., W.E.L., D.A.F., S.D., S.M., and L.J.D. approved final version of manuscript.
REFERENCES
- 1.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid Redox Signal 24: 99–112, 2016. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schöneich C, Reisdorph N, Powell RL, Chandler JD, Day BJ, Veress LA, White CW. Sarcoendoplasmic reticulum Ca2+ ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol 52: 492–502, 2015. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed KA, Nichols AL, Honavar J, Dransfield MT, Matalon S, Patel RP. Measuring nitrate reductase activity from human and rodent tongues. Nitric Oxide 66: 62–70, 2017. doi: 10.1016/j.niox.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed MI, Guichard JL, Soorappan RN, Ahmad S, Mariappan N, Litovsky S, Gupta H, Lloyd SG, Denney TS, Powell PC, Aban I, Collawn J, Davies JE, McGiffin DC, Dell’Italia LJ. Disruption of desmin-mitochondrial architecture in patients with regurgitant mitral valves and preserved ventricular function. J Thorac Cardiovasc Surg 152: 1059–1070.e2, 2016. doi: 10.1016/j.jtcvs.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes. J Biol Chem 277: 4694–4703, 2002. doi: 10.1074/jbc.M110875200. [DOI] [PubMed] [Google Scholar]
- 9.Barta J, Tóth A, Edes I, Vaszily M, Papp JG, Varró A, Papp Z. Calpain-1-sensitive myofibrillar proteins of the human myocardium. Mol Cell Biochem 278: 1–8, 2005. doi: 10.1007/s11010-005-1370-7. [DOI] [PubMed] [Google Scholar]
- 10.Bitron MD, Aharonson EF. Delayed mortality of mice following inhalation of acute doses of CH2O, SO2Cl2, and Br2. Am Ind Hyg Assoc J 39: 129–138, 1978. doi: 10.1080/0002889778507726. [DOI] [PubMed] [Google Scholar]
- 11.Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol 61: 340–351, 2007. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 12.Chapman AL, Skaff O, Senthilmohan R, Kettle AJ, Davies MJ. Hypobromous acid and bromamine production by neutrophils and modulation by superoxide. Biochem J 417: 773–781, 2009. doi: 10.1042/BJ20071563. [DOI] [PubMed] [Google Scholar]
- 13.Chen YW, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, Cui X, Husain A, Dell’italia LJ. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol 300: H2251–H2260, 2011. doi: 10.1152/ajpheart.01104.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chohan PK, Singh RB, Dhalla NS, Netticadan T. L-arginine administration recovers sarcoplasmic reticulum function in ischemic reperfused hearts by preventing calpain activation. Cardiovasc Res 69: 152–163, 2006. doi: 10.1016/j.cardiores.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Costa VM, Carvalho F, Duarte JA, Bastos ML, Remião F. The heart as a target for xenobiotic toxicity: the cardiac susceptibility to oxidative stress. Chem Res Toxicol 26: 1285–1311, 2013. doi: 10.1021/tx400130v. [DOI] [PubMed] [Google Scholar]
- 16.Damiani RM, Piva MO, Petry MR, Saldiva PH, Tavares Duarte de Oliveira A, Rhoden CR. Is cardiac tissue more susceptible than lung to oxidative effects induced by chronic nasotropic instillation of residual oil fly ash (ROFA)? Toxicol Mech Methods 22: 533–539, 2012. doi: 10.3109/15376516.2012.692109. [DOI] [PubMed] [Google Scholar]
- 17.Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J Lipid Res 59: 696–705, 2018. doi: 10.1194/jlr.M083279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res 57: 1529–1540, 2016. doi: 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol 290: H128–H136, 2006. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Dorado D, Rodríguez-Sinovas A, Ruiz-Meana M, Inserte J. Protection against myocardial ischemia-reperfusion injury in clinical practice. Rev Esp Cardiol (Engl Ed) 67: 394–404, 2014. doi: 10.1016/j.recesp.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Guichard JL, Rogowski M, Agnetti G, Fu L, Powell P, Wei CC, Collawn J, Dell’Italia LJ. Desmin loss and mitochondrial damage precede left ventricular systolic failure in volume overload heart failure. Am J Physiol Heart Circ Physiol 313: H32–H45, 2017. doi: 10.1152/ajpheart.00027.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo A, Hall D, Zhang C, Peng T, Miller JD, Kutschke W, Grueter CE, Johnson FL, Lin RZ, Song LS. Molecular determinants of calpain-dependent cleavage of junctophilin-2 protein in cardiomyocytes. J Biol Chem 290: 17946–17955, 2015. doi: 10.1074/jbc.M115.652396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honma T. Alteration of catecholamine metabolism in rat brain produced by inhalation exposure to methyl bromide. Sangyo Igaku 29: 218–219, 1987. doi: 10.1539/joh1959.29.218. [DOI] [PubMed] [Google Scholar]
- 24.Honma T, Miyagawa M, Sato M. Inhibition of tyrosine hydroxylase activity by methyl bromide exposure. Neurotoxicol Teratol 13: 1–4, 1991. doi: 10.1016/0892-0362(91)90020-W. [DOI] [PubMed] [Google Scholar]
- 25.Hustinx WN, van de Laar RT, van Huffelen AC, Verwey JC, Meulenbelt J, Savelkoul TJ. Systemic effects of inhalational methyl bromide poisoning: a study of nine cases occupationally exposed due to inadvertent spread during fumigation. Br J Ind Med 50: 155–159, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iizuka K, Kawaguchi H, Yasuda H. Calpain is activated by beta-adrenergic receptor stimulation under hypoxic myocardial cell injury. Jpn Circ J 55: 1086–1093, 1991. doi: 10.1253/jcj.55.1086. [DOI] [PubMed] [Google Scholar]
- 27.Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res 26: 58–74, 2016. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jilling T, Ren C, Yee A, Aggarwal S, Halloran B, Ambalavanan N, Matalon S. Exposure of neonatal mice to bromine impairs their alveolar development and lung function. Am J Physiol Lung Cell Mol Physiol 314: L137–L143, 2018. doi: 10.1152/ajplung.00315.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurkuvenaite A, Benavides GA, Komarova S, Doran SF, Johnson M, Aggarwal S, Zhang J, Darley-Usmar VM, Matalon S. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation. Free Radic Biol Med 85: 83–94, 2015. doi: 10.1016/j.freeradbiomed.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Kain V, Sitasawad SL. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochim Biophys Acta 1820: 907–920, 2012. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Lam A, Vetal N, Matalon S, Aggarwal S. Role of heme in bromine-induced lung injury. Ann NY Acad Sci 1374: 105–110, 2016. doi: 10.1111/nyas.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert JA, Carlisle MA, Lam A, Aggarwal S, Doran S, Ren C, Bradley WE, Dell’Italia L, Ambalavanan N, Ford DA, Patel RP, Jilling T, Matalon S. Mechanisms and treatment of halogen inhalation-induced pulmonary and systemic injuries in pregnant mice. Hypertension 70: 390–400, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Short communication: oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res 107: 228–232, 2010. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 46: 51–61, 2009. doi: 10.1016/j.freeradbiomed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 38.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca2+ ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther 10: 29–41, 2010. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liubchenko PN, Alekseeva GA. Acute poisoning with bromine vapors of a pharmaceutical plant operator [in Russian]. Gig Tr Prof Zabol 9: 32–34, 1991. [PubMed] [Google Scholar]
- 41.Luo M, Anderson ME. Mechanisms of altered Ca2+ handling in heart failure. Circ Res 113: 690–708, 2013. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackie E, Svendsen E, Grant S, Michels JE, Richardson WH. Management of chlorine gas-related injuries from the Graniteville, South Carolina, train derailment. Disaster Med Public Health Prep 8: 411–416, 2014. doi: 10.1017/dmp.2014.81. [DOI] [PubMed] [Google Scholar]
- 43.Makarovsky I, Markel G, Hoffman A, Schein O, Brosh-Nissimov TM, Finkelstien A, Tashma Z, Dushnitsky T, Eisenkraft A. Bromine—the red cloud approaching. Isr Med Assoc J 9: 677–679, 2007. [PubMed] [Google Scholar]
- 44.Mollenhauer M, Friedrichs K, Lange M, Gesenberg J, Remane L, Kerkenpaß C, Krause J, Schneider J, Ravekes T, Maass M, Halbach M, Peinkofer G, Saric T, Mehrkens D, Adam M, Deuschl FG, Lau D, Geertz B, Manchanda K, Eschenhagen T, Kubala L, Rudolph TK, Wu Y, Tang WHW, Hazen SL, Baldus S, Klinke A, Rudolph V. Myeloperoxidase mediates postischemic arrhythmogenic ventricular remodeling. Circ Res 121: 56–70, 2017. doi: 10.1161/CIRCRESAHA.117.310870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.National Research Council; Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies . Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 9. Washington, DC: National Academies, 2010. [Google Scholar]
- 45.Neuhof C, Götte O, Trumbeckaite S, Attenberger M, Kuzkaya N, Gellerich F, Möller A, Lubisch W, Speth M, Tillmanns H, Neuhof H. A novel water-soluble and cell-permeable calpain inhibitor protects myocardial and mitochondrial function in postischemic reperfusion. Biol Chem 384: 1597–1603, 2003. doi: 10.1515/BC.2003.177. [DOI] [PubMed] [Google Scholar]
- 46.Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol 6: 638–652, 2014. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okponyia OC, McGraw MD, Dysart MM, Garlick RB, Rioux JS, Murphy AL, Roe GB, White CW, Veress LA. Oxygen administration improves survival but worsens cardiopulmonary functions in chlorine exposed rats. Am J Respir Cell Mol Biol 58: 107–116, 2018. doi: 10.1165/rcmb.2016-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavićević L, Frković A, Vukelić M. Changes in the anterior segment of the eye in workers in the coke manufacturing industry [in Croatian]. Arh Hig Rada Toksikol 40: 405–408, 1989. [PubMed] [Google Scholar]
- 49.Pedrozo Z, Sánchez G, Torrealba N, Valenzuela R, Fernández C, Hidalgo C, Lavandero S, Donoso P. Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta 1802: 356–362, 2010. doi: 10.1016/j.bbadis.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Perrin C, Vergely C, Zeller M, Rochette L. In vitro antioxidant properties of calpain inhibitors: leupeptin and calpain inhibitor-1. Cell Mol Biol (Noisy-le-grand) 48 Online Pub: OL267–OL270, 2002. [PubMed] [Google Scholar]
- 51.Rardon DP, Cefali DC, Mitchell RD, Seiler SM, Hathaway DR, Jones LR. Digestion of cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles with calpain II. Effects on the Ca2+ release channel. Circ Res 67: 84–96, 1990. doi: 10.1161/01.RES.67.1.84. [DOI] [PubMed] [Google Scholar]
- 52.Rogers DF, Williams DA, Jeffery PK. Nicotine does not cause ‘bronchitis’ in the rat. Clin Sci (Lond) 70: 427–433, 1986. doi: 10.1042/cs0700427. [DOI] [PubMed] [Google Scholar]
- 53.Shi M, Zhang T, Sun L, Luo Y, Liu DH, Xie ST, Song XY, Wang GF, Chen XL, Zhou BC, Zhang YZ. Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis 18: 435–451, 2013. doi: 10.1007/s10495-012-0786-2. [DOI] [PubMed] [Google Scholar]
- 54.Shintani-Ishida K, Yoshida K. Ischemia induces phospholamban dephosphorylation via activation of calcineurin, PKC-α, and protein phosphatase 1, thereby inducing calcium overload in reperfusion. Biochim Biophys Acta 1812: 743–751, 2011. doi: 10.1016/j.bbadis.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemic-reperfused heart. J Mol Cell Cardiol 37: 101–110, 2004. doi: 10.1016/j.yjmcc.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Sobhan PK, Seervi M, Deb L, Varghese S, Soman A, Joseph J, Mathew KA, Raghu G, Thomas G, Sreekumar E, Manjula S, Santosh KT. Calpain and reactive oxygen species targets Bax for mitochondrial permeabilisation and caspase activation in zerumbone induced apoptosis. PLoS One 8: e59350, 2013. doi: 10.1371/journal.pone.0059350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soremark R. Distribution and kinetics of bromide ions in the mallalian body: some experimental investigations using Br80m and Br82. Acta Radiol Suppl 190: 1–114, 1960. [PubMed] [Google Scholar]
- 58.Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA; ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly . An Official American Thoracic Society workshop report: chemical inhalational disasters. Biology of lung injury, development of novel therapeutics, and medical preparedness. Ann Am Thorac Soc 14: 1060–1072, 2017. doi: 10.1513/AnnalsATS.201704-297WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am J Physiol Heart Circ Physiol 288: H2955–H2964, 2005. doi: 10.1152/ajpheart.00834.2004. [DOI] [PubMed] [Google Scholar]
- 60.Trumbeckaite S, Neuhof C, Zierz S, Gellerich FN. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochem Pharmacol 65: 911–916, 2003. doi: 10.1016/S0006-2952(02)01610-6. [DOI] [PubMed] [Google Scholar]
- 61.Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. Am J Respir Cell Mol Biol 48: 439–447, 2013. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61: 481–497, 2004. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Wacker BK, Albert CJ, Ford BA, Ford DA. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic Biol Med 59: 92–99, 2013. doi: 10.1016/j.freeradbiomed.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wigenstam E, Elfsmark L, Koch B, Bucht A, Jonasson S. Acute respiratory changes and pulmonary inflammation involving a pathway of TGF-β1 induction in a rat model of chlorine-induced lung injury. Toxicol Appl Pharmacol 309: 44–54, 2016. doi: 10.1016/j.taap.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem 281: 16849–16860, 2006. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 66.Wu HB. Release of cardiac troponin from healthy and damaged myocardium. Frontiers in Laboratory Medicine 1: 144–150, 2017. doi: 10.1016/j.flm.2017.09.003. [DOI] [Google Scholar]
- 67.Zaky A, Ahmad A, Dell’Italia LJ, Jahromi L, Reisenberg LA, Matalon S, Ahmad S. Inhaled matters of the heart. Cardiovasc Regen Med 2: e997, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell’Italia LJ, Matalon S, Ahmad S. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep 3: e12439, 2015. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng J, Wei CC, Hase N, Shi K, Killingsworth CR, Litovsky SH, Powell PC, Kobayashi T, Ferrario CM, Rab A, Aban I, Collawn JF, Dell’Italia LJ. Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog. PLoS One 9: e94732, 2014. doi: 10.1371/journal.pone.0094732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou T, Song WF, Shang Y, Yao SL, Matalon S. Halogen Inhalation-Induced Lung Injury and Acute Respiratory Distress Syndrome. Chin Med J (Engl) 131: 1214–1219, 2018. doi: 10.4103/0366-6999.231515. [DOI] [PMC free article] [PubMed] [Google Scholar]