Abstract
Sex differences in the presentation, outcome, and responses to treatment of systolic heart failure (HF) have been reported. In the present study, we examined the effect of sex on central neural mechanisms contributing to neurohumoral excitation and its peripheral manifestations in rats with HF. Male and female Sprague-Dawley rats underwent coronary artery ligation (CL) to induce HF. Age-matched rats served as controls. Ischemic zone and left ventricular function were similar 24 h and 4 wk after CL. Female rats with HF had a lower mortality rate and less hemodynamic compromise, pulmonary congestion, and right ventricular remodeling 4 wk after CL. Plasma angiotensin II (ANG II), arginine vasopressin (AVP), and norepinephrine levels were increased in HF rats in both sexes, but AVP and norepinephrine levels increased less in female rats. In the hypothalamic paraventricular nucleus, a key cardiovascular-related nucleus contributing to neurohumoral excitation in HF, mRNA levels for the proinflammatory cytokines tumor necrosis factor-α and interleukin-1β as well as cyclooxygenase-2 and the ANG II type 1a receptor were increased in HF rats of both sexes, but less so in female rats. Angiotensin-converting enzyme 2 protein levels increased in female HF rats but decreased in male HF rats. mRNA levels of AVP were lower in female rats in both control and HF groups compared with the respective male groups. Activation of extracellular signal-regulated protein kinases 1 and 2 increased similarly in both sexes in HF. The results suggest that female HF rats have less central neural excitation and less associated hemodynamic compromise than male HF rats with the same degree of initial ischemic cardiac injury.
NEW & NOTEWORTHY Sex differences in the presentation and responses to treatment of heart failure (HF) are widely recognized, but the underlying mechanisms are poorly understood. The present study describes sex differences in the central nervous system mechanisms that drive neurohumoral excitation in ischemia-induced HF. Female rats had a less intense central neurochemical response to HF and experienced less hemodynamic compromise. Sex hormones may contribute to these differences in the central and peripheral adaptations to HF.
Keywords: gender differences, hypothalamic paraventricular nucleus, proinflammatory cytokines, renin-angiotensin system, sympathetic excitation
INTRODUCTION
Ischemia-induced heart failure (HF) is a leading cause of morbidity and mortality for both men and women worldwide. Recent evidence has revealed distinct sex differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart disease and HF (16, 41). For example, women with acute myocardial infarction are relatively protected from apoptosis, exhibit less adverse cardiac remodeling, and often have preservation of left ventricular (LV) size and LV ejection fraction (LVEF) compared with men (16). Additionally, despite the higher risk of developing HF after acute myocardial infarction in women than in men, women have been consistently shown to have an independent survival benefit, including a reduced risk of sudden death (16, 41). However, the mechanisms responsible for these sex differences in ischemia-induced HF remain largely unexplored.
Activation of the sympathetic nervous system, a hallmark of HF, has long been recognized to correlate with the pathophysiology of cardiac remodeling, disease progression, and poor outcomes and survival (36, 55). We and others have demonstrated that increased neuroinflammation and renin-angiotensin system (RAS) activity in cardiovascular-related regions of the central nervous system contribute to increased sympathetic nerve activity (SNA) in HF (24, 27, 68–70, 72). Central interventions targeting these neurochemical abnormalities can significantly reduce SNA and ameliorate the peripheral manifestations of HF (24, 28, 29, 66, 68, 69, 72). However, most studies that have examined the effects of the altered neurochemistry of the brain in HF have been performed in male animals.
The goal of the present study was to determine whether the central neurochemical changes that drive neurohumoral excitation and its peripheral consequences in HF differ between male and female rats. The focus of study was the hypothalamic paraventricular nucleus (PVN), a key cardiovascular-related center in which changes in the brain neurochemistry that regulates SNA and extracellular fluid volume have been implicated in the pathophysiology of HF. The study was performed in young adult rats to allow the influence of sex hormones.
METHODS
Animals.
Adult male and female Sprague-Dawley rats (12 wk of age) were obtained from Envigo/Harlan (Indianapolis, IN). Animals were housed in temperature-controlled (23 ± 2°C) and humidity-controlled light-cycled rooms (with a 12:12-h light-dark cycle), and standard rat chow and water were provided ad libitum. All experiments and procedures in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Experimental protocol.
At 13 wk of age, male (n = 19) and female (n = 14) rats underwent left coronary artery ligation (CL) to induce HF. Within 24 h of CL, and again 4 wk after CL, an echocardiogram was performed to evaluate cardiac function. Animals were then anesthetized for invasive assessment of cardiac hemodynamics and subsequently euthanized to collect brain tissue for molecular experiments and blood and heart and lung tissues to assess peripheral indicators of HF. Age-matched male (n = 8) and female (n = 8) rats that did not undergo any surgical procedure served as controls for the echocardiographic, molecular, hemodynamic, and anatomic measurements.
HF induction and echocardiography.
Rats were anesthetized with ketamine plus xylazine (100 mg/kg ip plus 10 mg/kg ip, respectively) and underwent CL to induce HF, as previously described (18). Echocardiography was performed under ketamine sedation (60 mg/kg ip). The LV end-diastolic volume (LVEDV), LV volume-to-mass ratio (LV vol/mass), LVEF, and ischemic zone as a percent of LV circumference (%IZ) were measured as previously described (69, 71).
Four male HF rats and one female HF rat died within 24 h of CL, and another male HF rat died between 24 h and the end of the experiment. One male HF rat and two female HF rats with small myocardial infarction (%IZ < 30%) were excluded from the study. All remaining male HF rats and female HF rats were used for statistical analysis. There were no deaths among control rats.
Anatomic measurements.
Wet lung weight, LV weight, and right ventricular (RV) weight were measured and corrected by tibial length to assess pulmonary congestion and cardiac remodeling, two indicators of the severity of HF.
Cardiac hemodynamic measurements.
HF rats and age-matched control rats were anesthetized with urethane (1.5 g/kg ip). Systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), LV peak systolic pressure (LVPSP), LV end-diastolic pressure (LVEDP), and maximum rate of rise of LV pressure (LV dP/dtmax) were measured by a Millar catheter that was inserted into the LV via the right carotid artery, as previously described (71).
Real-time PCR and Western blot analysis.
Brains were quickly removed, and the PVN regions, including small amounts of surrounding tissues, were punched with a 15-gauge needle stub (inner diameter: 1.5 mm). Total RNA and protein were extracted from PVN punches with TRIzol Reagent (ThermoFisher Scientific, Waltham, MA).
mRNA expression for the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β; cyclooxygenase (COX)-2; the RAS components angiotensin II (ANG II) type 1a receptor (AT1aR), angiotensin-converting enzyme (ACE), and ACE2, and arginine vasopressin (AVP) was analyzed with SYBR Green real-time PCR after reverse transcription of total RNA, as previously described (68). Sequences for each primer pair are shown in Table 1. The ABI Prism 7000 sequence detection system (Applied Biosystems, Carlsbad, CA) was used to perform real-time PCR. mRNA data were corrected by GAPDH and expressed as fold changes relative to the male control group.
Table 1.
Sequences for primers
| Gene (Primers) | Sequences |
|---|---|
| TNF-α | |
| Forward primer | 5′-CCTTATCTACTCCCAGGTTCTC-3′ |
| Reverse primer | 5′-TTTCTCCTGGTATGAATGGC-3′ |
| IL-1β | |
| Forward primer | 5′-CGACAGAATCTAGTTGTCC-3′ |
| Reverse primer | 5′-TCATAAACACTCTCATCCACAC-3′ |
| COX-2 | |
| Forward primer | 5′-GGCACAAATATGATGTTCGCA-3′ |
| Reverse primer | 5′-CCTCGCTTCTGATCTGTCTTGA-3′ |
| AT1aR | |
| Forward primer | 5′-ACTCACAGCAACCCTCCAAG-3′ |
| Reverse primer | 5′-ATCACCACCAAGCTGTTTCC-3′ |
| ACE | |
| Forward primer | 5′-GTGTTGTGGAACGAATACGC-3′ |
| Reverse primer | 5′-CCTTCTTTATGATCCGCTTGA-3′ |
| ACE2 | |
| Forward primer | 5′-GGCTCCTTCTCAGCCTTG-3′ |
| Reverse primer | 5′-TTCATAAAAGGCAGACCATTTG-3′ |
| AVP | |
| Forward primer | 5′-TGCCTGCTACTTCCAGAACTGC-3′ |
| Reverse primer | 5′-AGGGGAGACACTGTCTCAGCTC-3′ |
| GAPDH | |
| Forward primer | 5′-AAGGTCATCCCAGAGCTGAA-3′ |
| Reverse primer | 5′-ATGTAGGCCATGAGGTCCAC-3′ |
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; COX-2, cyclooxygenase-2; AT1aR, angiotensin II type 1a receptor; ACE, angiotensin-converting enzyme; AVP, arginine vasopressin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Protein levels for ACE2 and phosphorylated (p-) and total extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) in the PVN were determined by Western blot analysis, as previously described (69). Briefly, concentrations of the protein from PVN punches were measured using the BCA Protein Assay Kit (Pierce, Rockford, IL). Protein samples were separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes. The membrane was incubated with primary antibodies to ACE2 (Abcam, Cambridge, MA), p-ERK1/2, total ERK1/2, and β-actin (Cell Signaling Technology, Danvers, MA) followed by horseradish peroxidase secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The density of the Western bands was detected and quantified with Image Lab analysis software (Bio-Rad, Hercules, CA).
Biochemical assays.
Trunk blood was collected at the time of euthanasia for biochemical assays. Plasma levels of ANG II (Cloud Clone, Houston, TX), TNF-α (R&D Systems, Minneapolis, MN), AVP (Enzo Life Sciences, Plymouth Meeting, PA), and norepinephrine (NE; Labor Diagnostika Nord, Nordhorn, Germany) were measured with commercial ELISA kits according to the manufacturers’ instructions.
Statistical analysis.
Data were analyzed using GraphPad Prism 7.0 software (La Jolla, CA). All statistical comparisons were made with a two-way ANOVA followed by post hoc multiple-comparison tests. P < 0.05 was considered statistically significant.
RESULTS
Sex differences in echocardiographic variables.
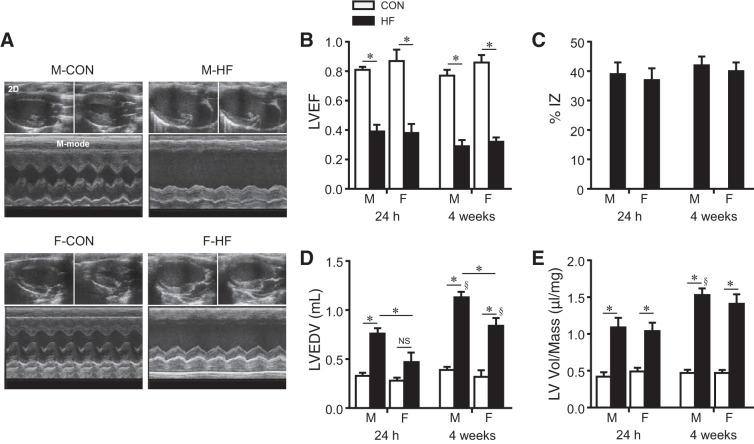
Analysis of echocardiographic images (Fig. 1A) revealed similar degrees of cardiac injury in male and female rats after CL. LVEF was decreased in male and female HF rats at 24 h and 4 wk after CL compared with their age-matched normal control rats (Fig. 1B). However, there was no difference in LVEF (Fig. 1B) or %IZ (Fig. 1C) between male HF and female HF rats at 24 h or 4 wk after CL.
Fig. 1.
A: representative two-dimensional [2-D; top (diastole on the left and systole on the right)] and M-mode (bottom) echocardiographic images from control (CON) and heart failure (HF) rats from each sex at 4 wk after coronary artery ligation. B−E: quantitative comparison of echocardiographic parameters including left ventricular (LV) ejection fraction (LVEF; B), ischemic zone as a percentage of LV circumference (%IZ; C), LV end-diastolic volume (LVEDV; D), and LV volume-to-mass ratio (LV vol/mass; E) from CON and HF rats 24 h and 4 wk after coronary artery ligation. Data are means ± SE; n = 6–13 for each group. F, female; M, male; NS, not significant. *P < 0.05; §P < 0.05 vs. same group at 24 h.
Small differences in indexes of cardiac remodeling were observed. In male HF rats, LVEDV (Fig. 1D) was significantly increased 24 h after CL compared with male control rats and increased further 4 wk after CL. In female HF rats, LVEDV increased significantly only at 4 wk after CL compared with female control rats, and the increase in LVEDV was less in female HF rats than in male HF rats at both time points. LV vol/mass ratio (Fig. 1E) was increased in male and female HF rats at both 24 h and 4 wk after CL compared with their respective control rats. Although the increase in LV vol/mass in male HF rats was greater at 4 wk than at 24 h, there were no significant differences in LV vol/mass ratio between male HF and female HF rats at either time point.
Echocardiographic assessment showed no significant differences in LVEF (Fig. 1B), LVEDV (Fig. 1D), and LV vol/mass ratio (Fig. 1E) between male and female control rats throughout the experimental protocol.
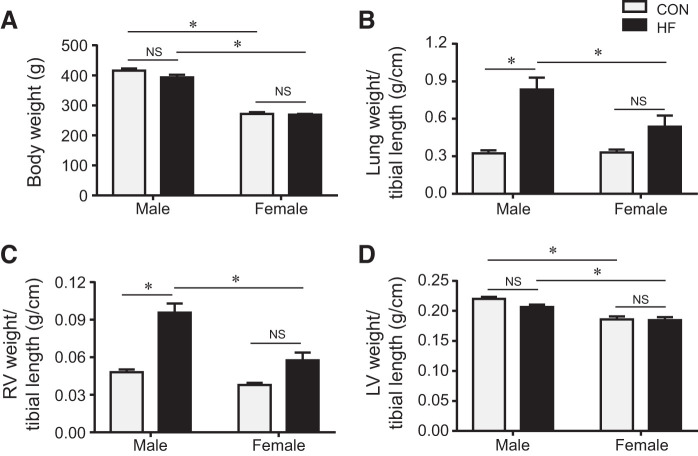
Sex differences in anatomic and hemodynamic parameters.
At the end of the study protocol, female rats had lower body weight than male rats, but there was no difference in body weight between HF and control rats in either sex (Fig. 2A). The ratios of lung weight (Fig. 2B) and RV weight (Fig. 2C) to tibial length were similar, but the ratio of LV weight (Fig. 2D) to tibial length was smaller in female control than male control rats. Compared with their respective controls, male HF but not female HF rats had significant increases in the ratios of lung weight and RV weight to tibial length. No difference in the ratio of LV weight to tibial length was observed between HF and control rats in either sex.
Fig. 2.
Quantitative comparison of anatomic parameters including body weight (A) and the ratios of lung weight (B), right ventricular (RV) weight (C), and left ventricular (LV) weight (D) to tibial length in control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. Data are means ± SE; n = 8–13 for each group. NS, not significant. *P < 0.05.
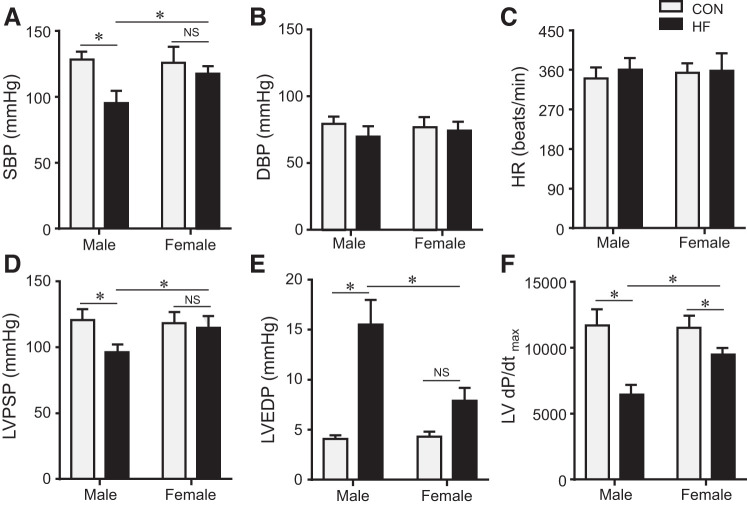
All hemodynamic parameters were comparable between male and female control rats (Fig. 3). Compared with their respective controls, male but not female HF rats exhibited significantly decreased SBP (Fig. 3A) and LVPSP (Fig. 3D) and increased LVEDP (Fig. 3E). There was a tendency for an increase in LVEDP in female HF rats that did not achieve significance. LV dP/dtmax (Fig. 3F) was decreased in both male HF rats and female HF rats, but female HF rats had a higher dP/dtmax compared with male HF rats. DBP (Fig. 3B) and HR (Fig. 3C) were similar in HF rats and control rats of either sex.
Fig. 3.
Quantitative comparison of hemodynamic parameters including systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B), heart rate (HR; C), left ventricular (LV) peak systolic pressure (LVPSP; D), LV end-diastolic pressure (LVEDP; E) and maximum rate of rise of LV pressure (LV dP/dtmax; F) from control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. Data are means ± SE; n = 6–13 for each group. NS, not significant. *P < 0.05.
Sex differences in humoral measures of HF.
Plasma levels of ANG II (Fig. 4A), TNF-α (Fig. 4B), AVP (Fig. 4C), and NE (Fig. 4D) were similar in male and female control rats. Plasma levels of these humoral factors were markedly higher in HF rats in either sex. Notably, compared with male HF rats, plasma levels of AVP and NE were significantly lower in female HF rats, and there was a nonsignificant tendency toward lower levels of ANG II and TNF-α.
Fig. 4.
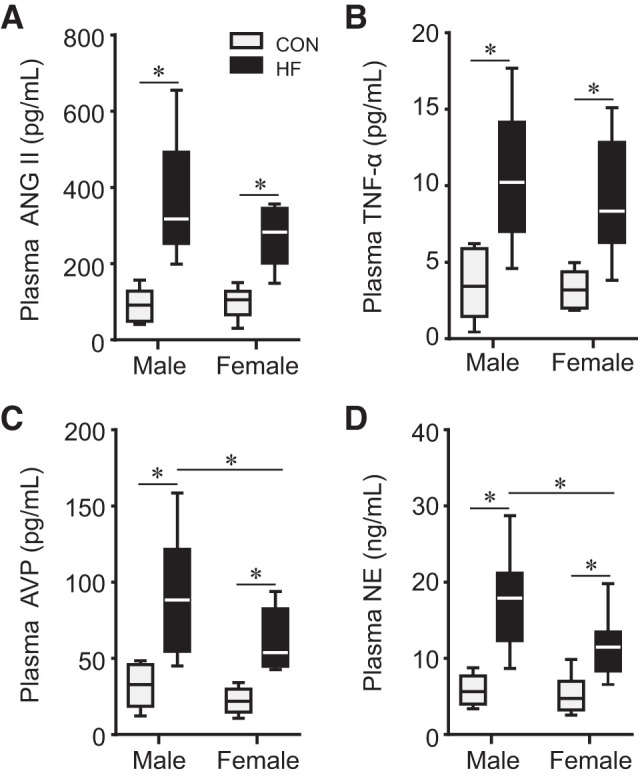
Plasma levels of angiotensin II (ANG II; A), tumor necrosis factor-α (TNF-α; B), arginine vasopressin (AVP; C), and norepinephrine (NE; D) in control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. n = 6–13 for each group. The horizontal lines in each bar represent the median value in each group. *P < 0.05.
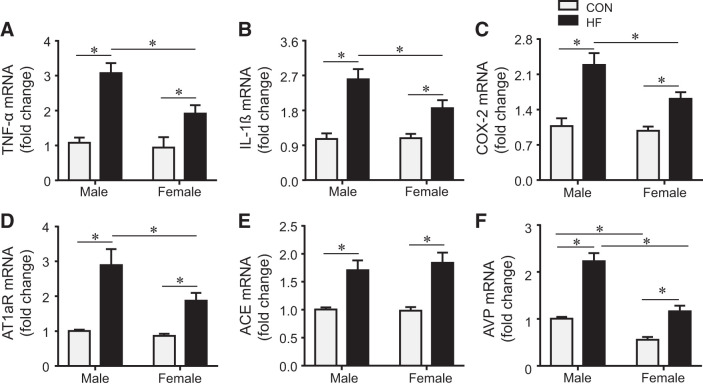
Sex differences in the expression of neurohormonal mediators in the PVN.
mRNA expression for TNF-α (Fig. 5A), IL-1β (Fig. 5B), COX-2 (Fig. 5C), and the RAS components AT1aR (Fig. 5D) and ACE (Fig. 5E) in the PVN was similar in male and female control rats. Both male HF and female HF rats had increases in TNF-α, IL-1β, COX-2, AT1aR, ACE, and AVP (Fig. 5F) mRNA expression compared with their respective control rats, but the expression of TNF-α, IL-1β, COX-2, AT1aR, and AVP mRNA was lower in female HF rats compared with male HF rats. Notably, AVP mRNA expression was also lower in the PVN in female control rats than in male control rats.
Fig. 5.
mRNA expression of the inflammatory mediators tumor necrosis factor-α (TNF-α; A), interleukin-1β (IL-1β; B), and cyclooxygenase-2 (COX-2; C) and the renin-angiotensin system elements angiotensin II type 1a receptor (AT1aR; D) and angiotensin-converting enzyme (ACE; E) and arginine vasopressin (AVP; F) in the hypothalamic paraventricular nucleus in control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. Data are means ± SE; n = 8 for each group. *P < 0.05.
Of note, ACE2 mRNA (Fig. 6A) expression in the PVN, which was similar in male and female control rats, was significantly reduced in male HF rats but was not changed in female HF rats compared with their respective controls. Notably, however, the ACE2 protein (Fig. 6B) level was lower than control in male HF rats but higher than control in female HF rats.
Fig. 6.
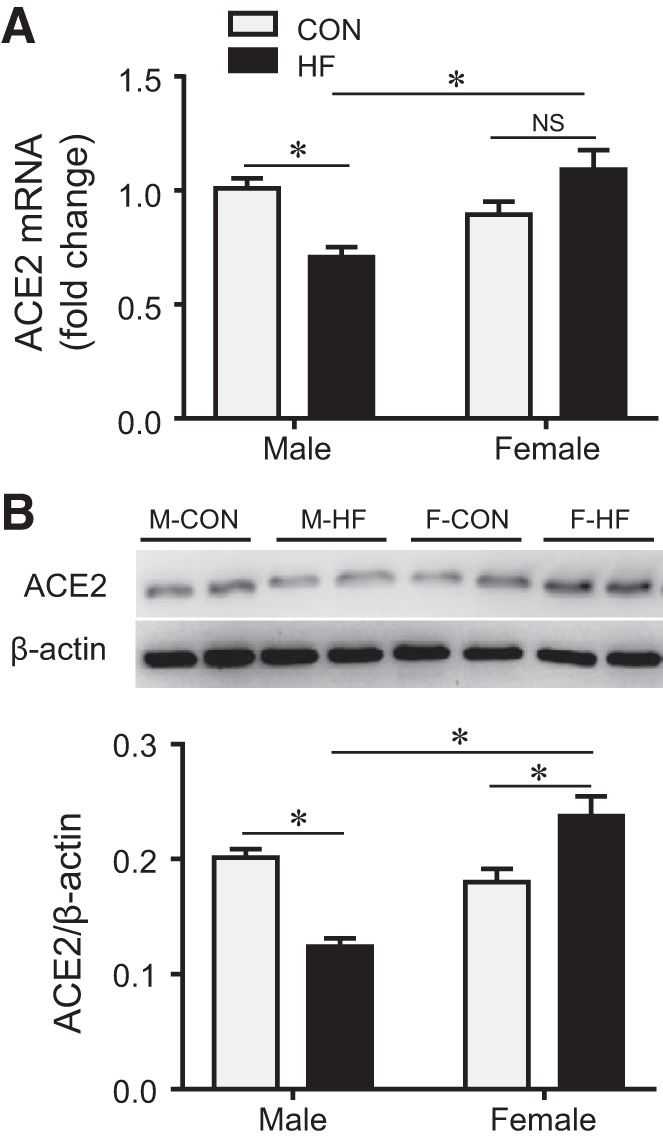
mRNA (A) and protein (B) expression of angiotensin-converting enzyme 2 (ACE2) in the hypothalamic paraventricular nucleus in control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. Data are means ± SE; n = 5–8 for each group. F, female; M, male; NS, not significant. *P < 0.05.
There were no differences between male and female control rats in protein levels of p-ERK1/2 (Fig. 7, A and B) and total ERK1/2 (Fig. 7, A and C) in the PVN. Compared with their respective controls, protein levels of p-ERK1/2 were similarly and significantly increased in both male HF and female HF rats, whereas protein levels of total ERK1/2 were unchanged in HF.
Fig. 7.
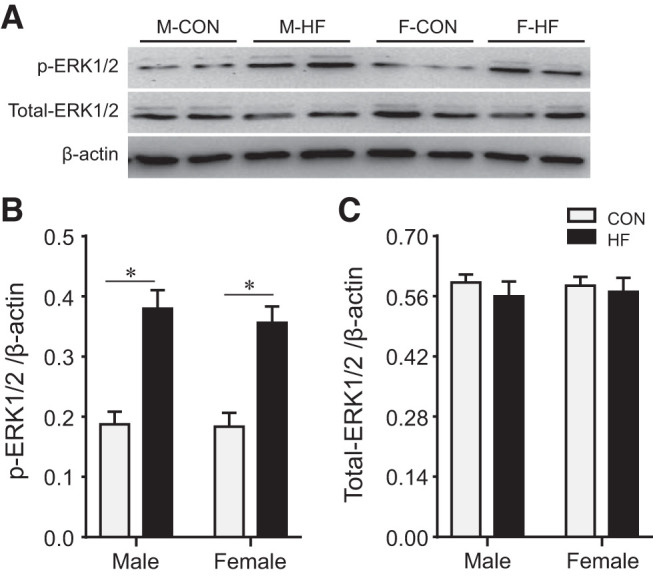
Representative Western blots (A) and quantitative comparison of protein expression for phosphorylated (p-) and total ERK1/2 (B and C) in the hypothalamic paraventricular nucleus in control (CON) and heart failure (HF) rats of both sexes at 4 wk after coronary artery ligation. Data are means ± SE; n = 5–6 for each group. F, female; M, male. *P < 0.05.
DISCUSSION
The present study revealed distinct sex differences in the manifestations of ischemia-induced HF in rats. Female rats tolerated ischemia-induced HF better than male rats that had the same degree of initial cardiac injury (%IZ) and the same reduction in LVEF. The survival rate immediately after CL appeared to be higher in female rats, although the numbers were too small for statistical analysis. LVEDV by echocardiography was less in female HF rats at 24 h and 4 wk, and hemodynamic measurements at 4 wk revealed that female HF rats had lower LVEDP, less reduction in dP/dtmax, and normal levels of LVPSP and SBP. Consistent with these echocardiographic and hemodynamic findings, pulmonary congestion and RV remodeling, corrected by tibial length, were significantly less in female rats. Similar sex differences in the peripheral manifestations of HF have been reported by others (51).
In the PVN, the findings in male HF rats were consistent with previous studies (57, 68, 71, 75): mRNA expression for the inflammatory mediators TNF-α, IL-1β, and COX-2 and the RAS components ACE and AT1aR was increased, whereas mRNA and protein expression for ACE2 was reduced. In female HF rats, mRNA expression for markers of inflammation also increased, but to a lesser extent compared with male HF rats. With regard to RAS components, ACE mRNA expression increased similarly in HF in both sexes, and AT1aR mRNA expression increased in both sexes but to a lesser degree in female HF rats. The mRNA expression of ACE2, which cleaves ANG II to produce ANG-(1–7), decreased in the PVN of male HF rats but not female HF rats. ACE2 protein levels, which decreased in male HF rats, actually increased in female HF rats, suggesting that differences in this protective arm of the brain RAS (49, 60) might play an important role in modulating the central response to HF in female rats. mRNA levels of AVP were lower in the PVN of female than male rats, both in age-matched control and HF rats. Female HF rats had correspondingly lower plasma AVP levels than male HF rats.
These findings suggest that at least two of the major central neurochemical systems that drive neurohumoral excitation in HF, the proinflammatory cytokines and the RAS, are less active in the PVN in female than male HF rats. Given these differences in the expression of excitatory neurochemical mediators, it is somewhat surprising that the phosphorylation of ERK1/2, an intracellular signaling mechanism that has been implicated as a mediator of sympathetic excitation by inflammation and RAS activity in HF (69), was similar in the male and female HF rats. The lower level of central neurohumoral excitation in the female HF rats is reflected in the lower plasma levels of NE and AVP.
Previous studies have established a critical role for inflammation and RAS activity in the brain (24, 27–29, 68, 70, 72), particularly within the PVN (28, 66), in the pathogenesis of ischemia-induced HF in male rats. To our knowledge, only one previous study (42) has directly compared the neurochemical milieu of the PVN in age-matched hormonally intact male and female HF rats. That study reported similar hemodynamic and echocardiographic findings in male HF and female HF rats studied 10 wk after myocardial infarction. Sham-operated female rats had “similar or somewhat lower” inflammatory cytokine levels (i.e., IL-1β, IL-6, and TNF-α) in the PVN, but cytokine levels had increased in both sexes 10 wk after myocardial infarction; RAS components were not assessed. The reasons for these discordant findings from the same HF model are not readily apparent, although differences in methodology, analysis techniques, and protocol duration may all have contributed.
The present findings suggest that the excitatory neurochemical milieu in the PVN is more intense in male than female rats after the induction of HF but beg the question of whether these sex-related central differences contribute to the improved outcomes in female rats or, conversely, simply reflect a less severe degree of cardiovascular compromise in female rats.
Sex hormones have well-established cardiac, renal, and vascular effects (5, 6, 30) that might influence the severity of HF. Testosterone stimulates RAS activity and promotes vasoconstriction and cardiac hypertrophy, whereas estrogen modulates RAS activity, promotes vasodilation, and is associated with a more benign course of cardiac remodeling in various pathophysiological states including ischemia-induced HF (6, 16, 17, 32). The sex steroids also have contrasting effects on renal sodium handling, with estrogen stimulating nitric oxide activity and testosterone stimulating local RAS activity (6). These and other peripheral effects of sex steroids may help determine the HF phenotype and thus, via altered neural and humoral afferent signals, may affect the expression of neurochemical mediators in the brain. For example, circulating levels of ANG II and proinflammatory cytokines generated by peripheral tissues may act on the central nervous system to increase neuroinflammation and brain RAS activity (67, 68). In the present study, no significant differences were observed in plasma levels of ANG II and TNF-α at 4 wk after CL. However, we cannot exclude a role for effects of sex hormones on peripheral tissues as a determinant of the differences in the PVN neurochemistry. Thus, the milder degree of HF in female rats, manifested by lower LV filling pressures, less impairment of LV contractility, and a sustained blood pressure, may have provoked a milder central response. In contrast, the more intense neurochemical response in the PVN of male HF rats with a comparable degree of cardiac injury but higher LV filling pressures and hypotension suggests a greater dependence on central mechanisms to compensate for cardiac dysfunction. One may speculate that male rats required higher plasma levels of NE and AVP to maintain a viable blood pressure, at the expense of increased LVEDV, LVEDP, pulmonary congestion, and RV remodeling.
A strong case can be made for a potential role for sex steroids in the modulation of central inflammation and brain RAS activity in HF. Estrogen and testosterone readily cross the blood-brain barrier (15, 45), and estrogen (8) and androgen (8, 12) receptors are present in the PVN (4) and in other cardiovascular-related nuclei innervating the PVN (58) in male and female rats. Notably, within the PVN, estrogen receptor (ER)β and the androgen receptor (AR) are expressed on preautonomic neurons (4).
Perhaps because of the cardiovascular protection enjoyed by female subjects, studies of the role of sex steroids in cardiovascular-related nuclei have focused primarily on estrogen effects. The nuclear receptors ERα and ERβ and the more recently identified membrane receptor G protein-coupled estrogen receptor 1 (GPER) are all expressed in cardiovascular-related regions of the brain (52). ERβ appears to be the predominant ER in the PVN (52) and the one most clearly associated with PVN modulation of sympathetic responses (64), whereas ERα has been reported to modulate AVP release (20). Estrogens have been shown to regulate brain RAS activity (31, 34). Ovariectomy (OVX) increases ACE and AT1R binding in the PVN, and subcutaneous administration of 17β-estradiol (E2) downregulates ACE and AT1R binding in OVX rats (13). Estrogens can also modulate the central inflammatory response via an ERα-mediated inhibition of microglial activation (56) and the production of inflammatory cytokines via NF-κB (47). An important effect of estrogens in hypertension appears to be the modulation of ANG II-induced reactive oxygen species (61, 65), another driver of SNA in HF (37). Interestingly, in the context of cytokine-induced (50) and ANG II-induced (9) production of reactive oxygen species, E2 has recently been reported to activate the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2), which initiates antioxidant activity (10). Although little is yet known of the role of GPER (74) in central cardiovascular and autonomic regulation, these receptors are also well represented in parvocellular and magnocellular divisions of the PVN (21, 52) and so may influence both presympathetic and neuroendocrine neurons. Notably, in other brain regions and in the context of neurodegenerative diseases, GPER has been shown to have important anti-inflammatory effects (23, 40, 73). It has also been suggested that activation of GPER may inhibit ERK1/2 activity in the PVN (21).
ERα and ERβ have both been shown to confer protection against ANG II- and aldosterone-induced hypertension, via reductions in sympathetic drive (63, 64). In these studies, the principal site of action for ERα is the subfornical organ and the principal site of action for ERβ is the PVN. In a rat model of HF similar to the one used here, high-dose E2 replacement in OVX rats seemed to increase ACE and AT1R binding in the PVN (14) compared with female rats that had not undergone OVX, although as expected OVX alone markedly increased and E2 replacement markedly reduced both RAS components. A comparison of ACE and AT1R expression in male rats with HF was not made in that study. In the present study, with ovaries intact, female HF rats had less of an increase in AT1aR mRNA expression than male HF rats did but had no protection with regard to ACE expression.
Much less information is available regarding the central cardiovascular and autonomic effects of testosterone. Testosterone has been reported to have both potentially harmful and potentially beneficial effects on peripheral tissues (54). Multiple studies have shown that testosterone contributes to the development of hypertension, most by demonstrating that castration and systemic administration of the AR antagonist flutamide are effective treatments for several animal models of hypertension (30). Other studies have suggested that testosterone may have a beneficial effect in hypertensive animal models (46) and in humans in certain settings (48).
Little has been done to isolate a contribution of testosterone to central neural mechanisms driving neurohumoral excitation in cardiovascular diseases. Since AR expression in the brain is testosterone dependent (8), studies using castration or flutamide [which increases circulating testosterone (19)] as interventions can offer no insights into the potential role of central versus peripheral AR-mediated mechanisms. To our knowledge, few efforts have been made to directly evaluate the central effects of testosterone. One study showed that testosterone increases catecholamine expression in the posterior hypothalamus, but that effect was not associated with the development of hypertension in spontaneously hypertensive rats (11). An unpublished study (62) has reported that centrally administered flutamide significantly attenuated the blood pressure response to ANG II infusion in male rats, but the central neurochemical mechanisms were not addressed. From what is known of its peripheral effects, one would anticipate that testosterone might increase brain RAS activity. In studies related to central mechanisms in neurodegenerative diseases, testosterone has been found to increase oxidative stress-induced inflammatory signaling via NF-κB and COX-2 (26) and to exacerbate the course of disease (39). If testosterone acts centrally in HF to increase RAS activity and promote inflammation in HF, ARs may play as important a role as ERs in determining sex differences in the peripheral manifestations of HF. On the other hand, testosterone has been reported to regulate the activity of astrocytes and microglia (3), a potential beneficial effect on inflammatory responses in HF. A consideration of the potential central effects of testosterone is complicated by its conversion to estradiol by aromatase (15). Thus, all of testosterone’s central effects may not be attributable to activation of AR.
Several limitations of the present study must be acknowledged. First, we did not undertake an extensive analysis of the more subtle aspects of cardiac function, on the assumption that the clear differences in LVEDV, LVEDP, dP/dtmax, and arterial pressure adequately reflect the sex differences in cardiac performance in response to a comparable degree of cardiac injury. However, these differences may reflect differing influences of sex hormones on peripheral tissues, resulting in differing degrees of neural and humoral afferent signaling to the brain, or the peripheral consequences of more or less intense central nervous system activation determined by central actions of sex hormones, or some combination of these factors. Further studies are needed to address these issues. Second, we used plasma NE as a measure of sympathetic activation. Although plasma NE is clearly not the most sensitive measure of sympathetic activity (22), it has been widely used as a general indicator of overall sympathetic drive in human and animal studies. In the present study, significant differences in plasma NE were observed in both sexes between HF rats and their respective controls and between male HF and female HF rats. A more sensitive measure may have yielded greater differences. Third, we used wet lung weight as a measure of pulmonary congestion. A ratio of wet lung weight to dry lung weight might have provided a more accurate assessment of pulmonary congestion.
Finally, and importantly, our study of the central neurochemistry driving neurohumoral excitation was confined to one key cardiovascular/autonomic region of brain, the PVN. We would anticipate similar sex differences in the neurochemistry of other cardiovascular-related regions that contribute to neurohumoral excitation in HF, likely all leading to similar outcomes though potentially affected by differences in the central distribution of ERs and ARs.
Perspectives
These experiments were conducted in young adult rats to permit the influence of sex steroids, in addition to nonhormonal genetic factors that might also contribute to sex differences. Although the incidence of HF is low in young (premenopausal) women (59), it is not an infrequent occurrence. Etiologies include early myocardial infarction, nonischemic cardiomyopathies of various etiologies, and valvular heart disease (7). Although the known effects of estrogen may reasonably be presumed to be protective in premenopausal women with HF, a beneficial influence of nonhormonal genetic factors cannot be excluded in this setting (2). Interestingly, many studies suggesting a better survival for women with HF have included female subjects at or well beyond the age of menopause (1, 25, 38, 44). In the postmenopausal setting, in which estrogen levels are minimal (43), nonhormonal sex-dependent factors may well be contributing to sex differences.
The present study in young rats revealed major sex differences in the central and peripheral manifestations of HF. The results raise the obvious question of whether the differences in the neurochemistry of the PVN of male and female HF rats are causative or secondary, with respect to the major differences in peripheral manifestations of HF. On the one hand, a lesser degree of central inflammation and RAS activity might account for lower levels of plasma NE and AVP with less volume accumulation and cardiac remodeling in female HF rats. On the other hand, a less severe female HF phenotype mediated by differences in the peripheral effects of sex hormones (or other sex differences) might account for a less intense central neurochemical response. This conundrum will only be resolved by future studies examining the effects of central manipulations of sex hormone receptors on the neurochemical systems known to drive sympathetic activity in HF. To fairly assess the effects of sex hormones and their receptors in the HF setting, such studies should be conducted in animals with intact gonads, since the circulating levels of sex steroids can affect both the integrity of the blood-brain barrier (33, 35) and the expression of ERs (53) and ARs (8) in brain tissue.
GRANTS
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, and by National Institutes of Health Grants R01-HL-073986 (to R. B. Felder), R01-HL-136149 (to R. B. Felder), and S10-OD-019941 (to R. M. Weiss).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B.F., Y.Y. and S.-G.W. conceived and designed research; Y.Y. and S.-G.W. performed experiments; Y.Y. and R.M.W. analyzed data; Y.Y., S.-G.W., R.M.W., and R.B.F. interpreted results of experiments; Y.Y. prepared figures; Y.Y. drafted manuscript; R.B.F., S.-G.W. and R.M.W. edited and revised manuscript; Y.Y.,S.-G.W., R.M.W. and R.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Kathy Zimmerman for diligent and expert assistance in the performance of the echocardiograms.
REFERENCES
- 1.Adams KF Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol 28: 1781–1788, 1996. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 2.Arain FA, Kuniyoshi FH, Abdalrhim AD, Miller VM. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ J 73: 1774–1782, 2009. doi: 10.1253/circj.CJ-09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arevalo MA, Santos-Galindo M, Acaz-Fonseca E, Azcoitia I, Garcia-Segura LM. Gonadal hormones and the control of reactive gliosis. Horm Behav 63: 216–221, 2013. doi: 10.1016/j.yhbeh.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-β distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol 499: 911–923, 2006. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- 5.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res 118: 1294–1312, 2016. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boese AC, Kim SC, Yin KJ, Lee JP, Hamblin MH. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 313: H524–H545, 2017. doi: 10.1152/ajpheart.00217.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B, Khalaf S. Heart failure in women. Methodist Debakey Cardiovasc J 13: 216–223, 2017. doi: 10.14797/mdcj-13-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol 27: 264–276, 2015. doi: 10.1111/jne.12258. [DOI] [PubMed] [Google Scholar]
- 9.Chan SH, Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid Redox Signal 19: 1074–1084, 2013. doi: 10.1089/ars.2012.4585. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Tseng YT, Hsu YY, Lo YC. Nrf2-Keap1 antioxidant defense and cell survival signaling are upregulated by 17β-estradiol in homocysteine-treated dopaminergic SH-SY5Y cells. Neuroendocrinology 97: 232–241, 2013. doi: 10.1159/000342692. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci 48: 85–96, 1991. doi: 10.1016/0024-3205(91)90428-E. [DOI] [PubMed] [Google Scholar]
- 12.Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One 8: e71694, 2013. doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean SA, Tan J, O’Brien ER, Leenen FH. 17β-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 288: R759–R766, 2005. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- 14.Dean SA, Tan J, White R, O’Brien ER, Leenen FH. Regulation of components of the brain and cardiac renin-angiotensin systems by 17β-estradiol after myocardial infarction in female rats. Am J Physiol Regul Integr Comp Physiol 291: R155–R162, 2006. doi: 10.1152/ajpregu.00497.2005. [DOI] [PubMed] [Google Scholar]
- 15.Diotel N, Charlier TD, Lefebvre d’Hellencourt C, Couret D, Trudeau VL, Nicolau JC, Meilhac O, Kah O, Pellegrini E. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci 12: 84, 2018. doi: 10.3389/fnins.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlay SM, Roger VL. Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr Heart Fail Rep 9: 267–276, 2012. doi: 10.1007/s11897-012-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298: R1597–R1606, 2010. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 18.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1734–R1745, 2001. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- 19.Ganten U, Schröder G, Witt M, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens 7: 721–726, 1989. doi: 10.1097/00004872-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Grassi D, Amorim MA, Garcia-Segura LM, Panzica G. Estrogen receptor α is involved in the estrogenic regulation of arginine vasopressin immunoreactivity in the supraoptic and paraventricular nuclei of ovariectomized rats. Neurosci Lett 474: 135–139, 2010. doi: 10.1016/j.neulet.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Grassi D, Lagunas N, Pinos H, Panzica G, Garcia-Segura LM, Collado P. NADPH-diaphorase colocalizes with GPER and is modulated by the GPER agonist G1 in the supraoptic and paraventricular nuclei of ovariectomized female rats. Neuroendocrinology 104: 94–104, 2017. doi: 10.1159/000445190. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 116: 976–990, 2015. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan J, Yang B, Fan Y, Zhang J. GPER agonist G1 attenuates neuroinflammation and dopaminergic neurodegeneration in Parkinson disease. Neuroimmunomodulation 24: 60–66, 2017. doi: 10.1159/000478908. [DOI] [PubMed] [Google Scholar]
- 24.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail 10: 625–634, 2008. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88: 107–115, 1993. doi: 10.1161/01.CIR.88.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Holmes S, Singh M, Su C, Cunningham RL. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology 157: 2824–2835, 2016. doi: 10.1210/en.2015-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang BS, Chen A, Ahmad M, Wang HW, Leenen FH. Mineralocorticoid and AT1 receptors in the paraventricular nucleus contribute to sympathetic hyperactivity and cardiac dysfunction in rats post myocardial infarct. J Physiol 592: 3273–3286, 2014. doi: 10.1113/jphysiol.2014.276584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, Zhao XF, Zhang J, Zhang LH, Yang ZM, Francis J. TNF-α in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med 222: 251–263, 2010. doi: 10.1620/tjem.222.251. [DOI] [PubMed] [Google Scholar]
- 29.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79, 2008. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 31.Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res 844: 34–42, 1999. doi: 10.1016/S0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- 32.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 24: 687–698, 2010. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 33.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 101: 1252–1261, 2006. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 34.Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K. Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5′ leader sequence of the receptor mRNA. Endocrinology 140: 5435–5438, 1999. doi: 10.1210/endo.140.11.7242. [DOI] [PubMed] [Google Scholar]
- 35.Kuruca SE, Karadenizli S, Akgun-Dar K, Kapucu A, Kaptan Z, Uzum G. The effects of 17β-estradiol on blood brain barrier integrity in the absence of the estrogen receptor alpha; an in-vitro model. Acta Histochem 119: 638–647, 2017. doi: 10.1016/j.acthis.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Lairez O, Legallois D, Agostini D. Sympathetic nervous system, systolic heart failure, and central sleep apnea: are we about to find the missing link? J Nucl Cardiol 24: 1938–1940, 2017. doi: 10.1007/s12350-016-0584-2. [DOI] [PubMed] [Google Scholar]
- 37.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Sellés M, Domínguez M, Martínez E, García Fernández MA, García E. Women with left ventricular ejection fraction ≤20% have better prognosis than men. Int J Cardiol 120: 276–278, 2007. doi: 10.1016/j.ijcard.2006.07.195. [DOI] [PubMed] [Google Scholar]
- 39.Massa MG, David C, Jörg S, Berg J, Gisevius B, Hirschberg S, Linker RA, Gold R, Haghikia A. Testosterone differentially affects T cells and neurons in murine and human models of neuroinflammation and neurodegeneration. Am J Pathol 187: 1613–1622, 2017. doi: 10.1016/j.ajpath.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Mendes-Oliveira J, Lopes Campos F, Videira RA, Baltazar G. GPER activation is effective in protecting against inflammation-induced nigral dopaminergic loss and motor function impairment. Brain Behav Immun 64: 296–307, 2017. doi: 10.1016/j.bbi.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MH, Hillege HL, Voors AA. Neurohormonal and clinical sex differences in heart failure. Eur Heart J 34: 2538–2547, 2013. doi: 10.1093/eurheartj/eht152. [DOI] [PubMed] [Google Scholar]
- 42.Najjar F, Ahmad M, Lagace D, Leenen FH. Sex differences in depression-like behavior and neuroinflammation in rats post-MI: role of estrogens. Am J Physiol Heart Circ Physiol 315: H1159–H1173, 2018. doi: 10.1152/ajpheart.00615.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Overlie I, Moen MH, Morkrid L, Skjaeraasen JS, Holte A. The endocrine transition around menopause: a five years prospective study with profiles of gonadotropines, estrogens, androgens and SHBG among healthy women. Acta Obstet Gynecol Scand 78: 642–647, 1999. doi: 10.1080/j.1600-0412.1999.780714.x. [DOI] [PubMed] [Google Scholar]
- 44.Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, Gottdiener JS. Race, gender, and mortality in adults ≥65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol 103: 1120–1127, 2009. doi: 10.1016/j.amjcard.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest 64: 145–154, 1979. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perusquía M, Herrera N, Ferrer M, Stallone JN. Antihypertensive effects of androgens in conscious, spontaneously hypertensive rats. J Steroid Biochem Mol Biol 167: 106–114, 2017. doi: 10.1016/j.jsbmb.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci 1089: 302–323, 2006. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 48. Rosano GM, Spoletini I, Vitale C. Cardiovascular disease in women, is it different to men? The role of sex hormones. Climacteric 20: 125–128, 2017. [Erratum in Climacteric 21: 92, 2017. 10.1080/13697137.2018.1417070.] doi: 10.1080/13697137.2017.1291780. [DOI] [PubMed] [Google Scholar]
- 49.Santos RA, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 98: 505–553, 2018. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J 20: 1589–1598, 2006. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 51.Souza NS, Dos-Santos RC, Silveira AL, Sonoda‐Côrtes R, Gantus MA, Fortes FS, Olivares EL. Effects of autonomic balance and fluid and electrolyte changes on cardiac function in infarcted rats: a serial study of sexual dimorphism. Clin Exp Pharmacol Physiol 43: 476–483, 2016. doi: 10.1111/1440-1681.12543. [DOI] [PubMed] [Google Scholar]
- 52.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38: 185–196, 2009. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Handa RJ. Regulation of estrogen receptor-β expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology 145: 3658–3670, 2004. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 54.Tostes RC, Carneiro FS, Carvalho MH, Reckelhoff JF. Reactive oxygen species: players in the cardiovascular effects of testosterone. Am J Physiol Regul Integr Comp Physiol 310: R1–R14, 2016. doi: 10.1152/ajpregu.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747–1762, 2009. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100: 9614–9619, 2003. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei SG, Yu Y, Weiss RM, Felder RB. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am J Physiol Heart Circ Physiol 311: H871–H880, 2016. doi: 10.1152/ajpheart.00362.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol 503: 717–740, 2007. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- 59.Wong CM, Hawkins NM, Ezekowitz JA, Jhund PS, Savu A, MacDonald MR, Kristensen SL, Petrie MC, McMurray JJ, McAlister FA, Kaul P. Heart failure in young adults is associated with high mortality: a contemporary population-level analysis. Can J Cardiol 33: 1472–1477, 2017. doi: 10.1016/j.cjca.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol Heart Circ Physiol 297: H1638–H1646, 2009. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue B, Skala K, Pamidimukkala J, Hay M. Androgens modulate angiotensin II-induced hypertension in conscious male mice (Abstract). FASEB J 18: A649, 2004. [Google Scholar]
- 63.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Genetic knockdown of estrogen receptor-α in the subfornical organ augments ANG II-induced hypertension in female mice. Am J Physiol Regul Integr Comp Physiol 308: R507–R516, 2015. doi: 10.1152/ajpregu.00406.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61: 1255–1262, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, Yi QY, Zhang J, Zhu GQ, Zhu Z, Kang YM. Interaction between AT1 receptor and NF-κB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol 13: 381–390, 2013. doi: 10.1007/s12012-013-9219-x. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, Wei SG, Weiss RM, Felder RB. Angiotensin II type 1a receptors in the subfornical organ modulate neuroinflammation in the hypothalamic paraventricular nucleus in heart failure rats. Neuroscience 381: 46–58, 2018. doi: 10.1016/j.neuroscience.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Y, Wei SG, Weiss RM, Felder RB. TNF-α receptor 1 knockdown in the subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am J Physiol Heart Circ Physiol 313: H744–H756, 2017. doi: 10.1152/ajpheart.00280.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol 310: H732–H739, 2016. doi: 10.1152/ajpheart.00703.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 101: 304–312, 2007. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 71.Yu Y, Zhang ZH, Wei SG, Weiss RM, Felder RB. Peroxisome proliferator-activated receptor-γ regulates inflammation and renin-angiotensin system activity in the hypothalamic paraventricular nucleus and ameliorates peripheral manifestations of heart failure. Hypertension 59: 477–484, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Huang BS, Leenen FH. Brain renin-angiotensin system and sympathetic hyperactivity in rats after myocardial infarction. Am J Physiol Heart Circ Physiol 276: H1608–H1615, 1999. doi: 10.1152/ajpheart.1999.276.5.H1608. [DOI] [PubMed] [Google Scholar]
- 73.Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav 6: e00449, 2016. doi: 10.1002/brb3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmerman MA, Budish RA, Kashyap S, Lindsey SH. GPER-novel membrane oestrogen receptor. Clin Sci (Lond) 130: 1005–1016, 2016. doi: 10.1042/CS20160114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706, 2014. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]