Abstract
Cancer is the leading cause of morbidity and mortality in the United States and globally. Owing to improved early diagnosis and advances in oncological therapeutic options, the number of cancer survivors has steadily increased. Such efficient cancer therapies have also lead to alarming increase in cardiovascular complications in a significant proportion of cancer survivors, due to adverse cardiovascular effects such as cardiotoxicity, cardiac atrophy, and myocarditis. This has emerged as a notable concern in healthcare and given rise to the new field of cardioncology, which aims at understanding the processes that occur in the two distinct disorders and how they interact to influence the progression of each other. A key player in both cancer and heart failure is the genome, which is predominantly transcribed to noncoding RNAs (ncRNAs). Since the emergence of ncRNAs as master regulators of gene expression, several reports have shown the relevance of ncRNAs in cancer and cardiovascular disorders. However, the knowledge is quite limited regarding the relevance of ncRNAs in cardioncology. The objective of this review is to summarize the current knowledge of ncRNAs in the context of cardioncology. Furthermore, the therapeutic strategies as well as the prospective translational applications of these ncRNA molecules to the clinics are also discussed.
Keywords: biomarker, cardiotoxicity, doxorubicin, heart failure, noncoding RNA
INTRODUCTION
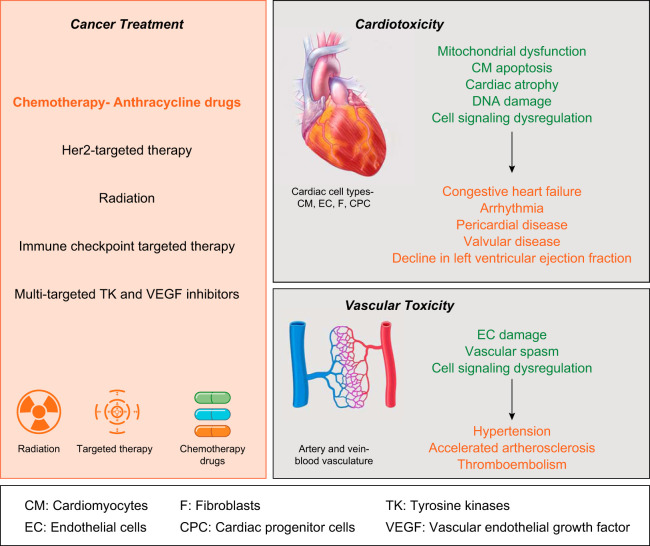
Cardiovascular disorder and cancer are the leading causes of mortality and account for nearly 50% of deaths worldwide (36a). Despite very distinct and independent disease etiology, both these health ailments seem to be interlinked and can be grouped together under the umbrella of cardioncology. Cardioncology is an emerging field and requires combined efforts from clinicians and researchers with expertise in cancer and heart disease to mitigate cardiovascular complications in cancer survivors. There has been a noteworthy increase in number of cancer survivors, reducing mortality rates caused by cancer by nearly half a million every year (2012–2016) in the United States alone (35, 45). Recent reports have indicated that a significant number of cancer survivors are at a higher risk for suffering cardiovascular ailments (49, 54). In a study on 4,122 childhood cancer survivors compared with an age-matched population, a fivefold increased risk of cardiovascular death was observed (54). Similar to childhood survivors, a study by Thavendiranathan et al. (49) on 18,540 breast cancer survivors found a 3% incidence of cardiovascular deaths compared with <1% in age-matched control subjects. The alarming incidence of cardiovascular deaths in cancer survivors is principally due to cardiotoxic side effects of the cancer treatment (14). Cancer treatment strategies include surgery, chemotherapy, radiotherapy, hormone therapy, immunotherapy, bisphosphonates, and stem cell/bone marrow transplants (46, 47, 52, 56, 68). Surgery is one of the most common strategies that is used as a first-line treatment for many different solid tumors that are contained in one area. Depending on the type of cancer and its advanced stage, a combination of cancer treatments is given. Cancer treatments with strong cardiovascular toxic effects include anthracycline chemotherapy, radiation therapy, treatment with monoclonal antibody drugs such as Trastuzumab, and newly identified immune checkpoint inhibitors (Fig. 1) (14, 32). Cardiotoxicity can be attributed to defects at the molecular, structural, and/or functional level of the heart. Antitumor agents that interfere with DNA replication and DNA repair mechanisms ultimately produce dysfunctional endothelial cells that impair the cardiovascular system and cardiac function. Cardiotoxicity can occur as a consequence of nonspecific immune response induction or increased production of reactive oxygen species (ROS) (33). Furthermore, anticancer treatments can inhibit ion channels, which cause electrophysiological perturbations leading to heart failure. Hypertension, heart failure, prolonged QT interval, left ventricular dysfunction, and heart failure with preserved left ventricular ejection fraction are frequently reported cardiovascular complications arising from anticancer therapeutics (23, 36). Of note, there are numerous other risk factors that also influence the occurrence of the deleterious cardiovascular effects such as age, sex, preexisting health conditions, lifestyle, etc. These facts indicate a pressing demand for advanced reliable anticancer medication with minimal cardiovascular risk.
Fig. 1.
Overview of cardiotoxicity induced by cancer treatment. There are several therapeutic strategies used for treatment of cancer (left). In particular, chemotherapy treatment with anthracyclines can be accompanied by detrimental toxic effects on the cardiovascular system of patients leading to cardiac dysfunction (right). Cellular and pathological consequences are highlighted in green and yellow, respectively.
ANTHRACYCLINE-INDUCED HEART FAILURE
Anthracyclines are a class of cytotoxic drugs that are widely used to treat various types of cancers. Doxorubicin belongs to the genre of anthracycline drugs and is the most extensively applied and studied chemotherapeutic agent (14). Doxorubicin is derived from Streptomyces peucetius var. caesius and was identified as antitumor agent already in 1969 (2). However, clinical application of doxorubicin is limited due to its severe cardiotoxic effects (48). In a cancer patient cohort of 2,625 subjects, therapy comprising anthracyclines caused heart failure in 9% of patients (7). Out of these, only 11% of patients achieved full recovery by conventional heart failure therapies (7). Such statistics indicate that further detailed investigation is required in the field of cardioncology with special focus on identifying new druggable targets.
Even 5 decades after the discovery of doxorubicin, the precise mechanism behind doxorubicin-induced cardiotoxicity remains elusive. Current literature suggests that inhibition of topoisomerase-IIβ in cardiomyocytes and an accumulation of iron in mitochondria leading to ROS formation are the two major mechanisms for doxorubicin cardiotoxicity (25, 69). Some reports have recently shown that noncoding RNA (ncRNA) is involved in doxorubicin-induced cardiotoxicity.
ncRNAs
Historically, it was believed that the genetic information encoded in our DNA is transcribed into mRNA molecules, which are further translated into proteins that orchestrate major cellular functions. Breakthrough in sequencing and bioinformatics technologies soon revealed that the vast majority of the human genome is predominantly transcribed into functional ncRNA molecules, which do not undergo further translation into protein. ncRNAs comprises of several different types of RNA molecules such as well-known tRNAs and rRNAs as well as more recently discovered microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs), and their role has been recently reviewed in Refs. 3, 5, and 17. Remarkably, this enormous plethora of ncRNA molecules controls epigenetic, posttranscriptional, and translational coordination of gene expression in developmental and disease processes and thus ncRNAs have emerged as key players in various developmental and pathophysiological conditions. miRNAs and lncRNAs are historically defined based on their length. miRNAs are 18–22 nt long, whereas lncRNAs are composed of >200 nt. circRNAs, however, originate through alternative splicing processes of pre-mRNAs. They are formed by back splicing of 3′-exon ends to upstream 5′-exon junctions to form a covalently closed circRNA molecule (4). Several studies have highlighted the relevance of these ncRNAs in cardiovascular disease and disorders (11, 13, 42, 50, 57), some of which are also regulated in cancerous tissue (Table 1) (1, 44). Consequently, miRNAs and lncRNAs were also discovered to be involved in doxorubicin-induced cardiotoxicity (Table 2). Since ncRNAs represent potential therapeutic targets and can be detected in the circulation, they may hold promise for future cardioncology-related therapy, diagnosis, and prognosis. Here, we summarize the current knowledge of ncRNAs in cardioncology and provide a viewpoint of their prospective and consequential influence on the field.
Table 1.
ncRNAs associated with cancer and cardiovascular disorders
| Disorder/ncRNA Type | ncRNAs | References |
|---|---|---|
| Oncology | ||
| miRNA | miR-1*, miR-21*, miR-29b*, miR-30 family*, miR-34a, miR-34c, miR-133a*, miR-133b, miR-140-5p, miR-146a*, miR-206, miR-208a*, miR-308b, miR-215, miR-216b, miR-221*, miR-222, miR-320a, miR-367, miR-499*, miR-let-7 g | Anastasiadou et al. (1) Schmitt and Chang (44) |
| lncRNA | AA174084, ANRIL*, BANCR, CCAT2, FAL1, GAS5, H19*, HOTAIR*, HOTTIP, HULC, MALAT1*, MEG3*, MIR31HG, NBAT-1, NKILA, PCA3, PCAT-1, PTCSC3, PVT1, SCHLAP1, SeCATs, SPRY4-IT1, and TERC | |
| circRNA | ciRS-7, circ-ITCH, circ-Foxo3*, f-circM9, circ-HIPK3, cZNF292* | |
| Heart failure | ||
| miRNA | miR-1*, miR-18, miR-21*, miR-23a, miR-24, miR-26a, miR-26b-5p, miR-27a-3p, miR-29a-3p, miR-29b*, miR-30c*, miR-30d, miR-30e-5p, miR-33, miR-37, miR-92a-3p, miR-101, miR-103, miR-106a-5p, miR-122, miR-125a, miR-126, miR-133a*, miR-132/212, miR-142-3p, miR-145-3p, miR-146a*, miR-150, miR-181b, miR-199a-3p, miR-208*, miR-210, miR-221*, miR-223-3p, miR-301a-3p, miR-328, miR-423-5p, miR-483-5p, miR-486-3p, miR-499*, miR-652-3p, miR-1254, miR-1306-5p | Thum and Condorelli (50) Bär et al. (3) Beermann et al. (5) Vegter et al. (57) Dangwal et al. (11) Devaux et al. (13) Ruggeri et al. (42) Oatmen et al. (37) |
| lncRNA | ANRIL*, APF, BACE-1AS, Carl, CARMEN, Chaer, Chast, Chrf, H19*, HOTAIR*, HoxD-as1, HypERLnc, KCNQ1OT, LIPCAR, LINC00281, Lnc-362, lncRNA-ROR, LOC100507463, Malat1*, Meg3*, Miat, MIRT1, Myheart, UCA1, TINCR, Wisper, ZNF192P1 | |
| circRNA | CDR1as, circ-Foxo3*, Hrcr, Mfacr, cZNF292* |
Noncoding RNAs (ncRNAs) overlapping between cancer and cardiovascular disorders. miRNA, microRNA; lncRNA, long noncoding RNA; circRNA, circulating RNA.
Table 2.
ncRNA molecules with significant roles in doxorubicin-mediated cardiotoxicity
| ncRNA | Cell type | Species | Levels | Mechanism | Therapeutic Approach | Reference |
|---|---|---|---|---|---|---|
| miR-208a | Myocytes | Mouse | Upregulated | Targets Gata4 | Silencing | Tony et al. (53) |
| miR-532-3p | CM | Mouse in vivo; human, rat, mouse in vitro | Upregulated | Targets ARC | Silencing | Wang et al. (58) |
| miR-30e | CM | Rat | Downregulated | Targets β1AR, β2AR, Gia-2, and BNIP3L | Induction | Roca-Alonso et al. (41) |
| miR-320a | EC | Mouse in vivo; human in vitro | Upregulated | VEGF-A | Silencing | Yin et al. (66) |
| miR-34a | EC, CPC | Rat | Upregulated | Targets Bcl2 and Sirt1 | Silencing | De Angelis et al. (12) |
| miR-146a | CM | Mouse | Upregulated | Regulates ErbB4 | Silencing | Horie et al. (24) |
| Mhrt | CM | Rat | Downregulated | Regulates Nrf2 | Induction | Li et al. (27) |
| Chrf | CM | Mouse | Upregulated | Targets TGF-β/Smads; TGF-β/p38 | Silencing | Chen et al. (8) |
| H19; miR-675 | CM | Rat | Upregulated | Regulates miR-675; Ebp1 | Silencing | Zhang et al. (68) |
| Ttn105-111 | CM | Mouse | Upregulated | Regulated by Qki5 | Silencing | Gupta et al. (20) |
ncRNA, noncoding RNA; CM, cardiomyocytes; EC, endothelial cell; CPC, cardiac progenitor cells ARC, apoptosis repressor with caspase recruitment domain; β1AR, β2AR, β-adrenergic receptor 1 and 2; BNIP3L, BCL2 interacting protein 3 like; VEGF-A, vascular endothelial growth factor A; SIRT1, sitrtuin1; ErbB4, Erb-B2-receptor tyrosine kinase 4; Mhrt, myosin heavy chain associated RNA transcript; Nrf2, nuclear factor erythroid 2 related factor 2; Chrf, cardiac hypertrophy related factor; TGF-β, tumor growth factor-β; Ebp1, ErbB3-binding protein 1; Ttn, Titin; Qki, Quaking.
miRNAs REGULATING DOXORUBICIN-INDUCED CARDIOTOXICITY
miRNAs are short ncRNA molecules that function by downregulating their target gene through sequence-specific binding to target mRNAs (predominantly in the 3′-untranslated region) and subsequent translational suppression or degradation of the RNA-DNA hybrid mediated by the RNA-induced silencing complex (16). Several miRNAs are involved in heart failure, and their modulation has demonstrated therapeutic potential in preclinical models (20, 51, 55). Recent reports have also suggested miRNA-mediated regulation of doxorubicin-induced cardiotoxicity. The cardiac-specific miRNA miR-208a was found to be upregulated in an acute model of doxorubicin-induced cardiotoxicity (53). Antagomir (a class of chemically engineered synthetic antisense RNA molecule that is used to block miRNAs)-mediated inhibition of miR-208a prevented decline in fractional shortening and induction of apoptosis caused by doxorubicin (53). Inhibition of miR-208a derepresses its target Gata4 and further leads to increased expression of the antiapoptotic gene Bcl2 (53). However, the experimental data from this study were insufficient to prove whether miR-208a antagomir affected doxorubicin-induced cardiomyocyte structural damage and cardiac atrophy. Additional experiments with miR-208a inhibition in a chronic model of doxorubicin-induced cardiotoxicity would be clinically more relevant. Another miRNA, miR-532-3p, was also found to be increased in cardiomyocytes treated with doxorubicin (58). miR-532-3p increases cardiomyocyte susceptibility to doxorubicin by promoting mitochondrial fission (58). miR-532-3p directly targets apoptosis repressor with caspase recruitment domain leading to increased cardiomyocyte death in response to doxorubicin (58). Mice overexpressing apoptosis repressor with caspase recruitment domain show preserved cardiac function, inhibited mitochondrial fission, and apoptosis in response to doxorubicin (58). Strikingly, inhibition of miR-532-3p in cancer cells had no effect on doxorubicin-mediated apoptosis (58). Thus, downregulation of miR-532-3p ameliorates doxorubicin-induced cardiomyocyte death and mitochondrial fission without altering its antitumor efficacy (58). However, its application as a preventive therapeutic approach necessitates further tests in animal models. A study from Roca-Alonso et al. (41) reported miR-30 family members to be downregulated in vitro and in vivo after doxorubicin treatment in rats. Overexpression of miR-30e in cultured cardiomyocytes was found to reduce caspase activity, the Bax-to-Bcl-2 ratio, and ROS production by targeting the β-adrenergic pathway (41).
Doxorubicin induces cardiac atrophy in patients as well as preclinical mouse models characterized by decline in ventricular wall thickness (21, 30). A novel strategy to combat adverse effects of doxorubicin may be to inhibit doxorubicin-induced atrophy and promote physiological cardiomyocyte hypertrophy. Cardiac hypertrophy can be physiological or pathological depending on the stimuli; while physiological hypertrophy maintains cardiac structure and function, the latter is maladaptive. Therefore, boosting physiological hypertrophy in doxorubicin-induced cardiotoxicity might be therapeutic strategy. Inhibition of the miR-199 (miR-199a-5p or miR-199b-5p) family induces physiological hypertrophy characterized by increased left ventricular mass and increased cardiomyocyte size with preserved cardiac function (29). Similarly, overexpression of miR-223 in the heart induced physiological hypertrophy with no maladaptive remodeling (64). Hence, it is warranted to investigate whether modulation of miR-199 and miR-223 can protect the heart against doxorubicin-induced atrophy and preserve cardiac function.
Apart from cardiomyocytes, effects on smooth muscle cells, fibroblasts, cardiac progenitor cells, and endothelial cells might also contribute to the pathology of doxorubicin-induced cardiotoxicity (15). Acute doxorubicin treatment in mice led to declined microvessel density and VEGF-A expression with an accompanying increase in miR-320a (66). Inhibition of miR-320a improved cardiac function, decreased apoptosis, and increased microvessel density in doxorubicin-treated mice, whereas overexpression of miR-320a aggravated doxorubicin cardiotoxicity (66). miR-320a was found to decrease proliferation, increase apoptosis, and reduce tube length formation in human umbilical vein endothelial cells (66). Overexpression of the miR-320a target VEGF-A prevented detrimental effects of miR-320a in doxorubicin-induced cardiotoxicity model confirming VEGF as a direct downstream target molecule (66). By analogy to VEGF-A, another member of the VEGF family, VEGF-B, has been recently shown to exert beneficial effects in a chronic model of doxorubicin-induced cardiotoxicity by protecting endothelial cells. Based on this knowledge, it will be crucial to test whether the miR-320a-VEGF-A axis also shows beneficial effects in chronic models. Another cell type, namely, cardiac progenitor cells (CPCs), is known to be affected by doxorubicin-induced cardiotoxicity (12). CPCs are multipotent cells with the ability to differentiate into any cardiac cell type (15). Doxorubicin induces apoptosis of CPCs, arrests their growth, and enhances oxidative damage (12). Exogenous administration of CPCs in the myocardium increases survival, improves cardiac function, and promotes myocardial regeneration (12). This suggests that measures to maintain and promote proliferation of endogenous CPCs population in the doxorubicin-exposed heart could rescue the detrimental effects of doxorubicin. In this context, Piegari et al. (39) have shown that inhibition of miR-34a in CPCs promotes their proliferation and reduces senescence induced by doxorubicin via a p53-miR-34a-sirtuin 1 positive feedback loop. Hence, in vivo inhibition of miR-34a may inhibit the decline of CPCs induced by doxorubicin and counter its pathological effects.
Although few reports have demonstrated that modulation of miRNAs in vivo has a beneficial effect in combating doxorubicin-induced cardiotoxicity, none of them are done in a clinically relevant chronic model of doxorubicin cardiotoxicity. This highlights the need for new studies to evaluate therapeutic efficacy of miRNAs in chronic models of doxorubicin cardiotoxicity.
miRNAs AS BIOMARKERS TO DETECT DOXORUBICIN-INDUCED CARDIOTOXICITY
The frequency of doxorubicin-induced heart failure has been reported to be ~5% at a dose of 400 mg/m2 (48). However, an improved echocardiography analysis performed by Lipshultz et al. (30) revealed elevated cardiac afterload (pressure that the heart needs to overcome to eject blood from the left ventricle during systole) in 50% of patients even at low dose of 228–360 mg/m2 doxorubicin, demonstrating an urgent need for the development of sensitive techniques and biomarkers to detect cardiotoxicity earlier and start preventive therapies. miRNAs are detectable in body fluids, and several of them have been identified as biomarkers for cardiovascular diseases and could also serve as interesting biomarker candidates to detect cancer therapy-mediated cardiotoxicity (19). In this context, a study on breast cancer cohort of 56 patients reported miR-1 levels in plasma to be a superior biomarker than cardiac troponin I (cTnI) in predicting cardiotoxicity (40). Circulating miR-1 levels were significantly increased after two cycles of doxorubicin in patients who later developed heart failure compared with those who did not (40). In contrast, cTnI did not show any significant difference even at the end of doxorubicin treatment (40). miR-1 levels significantly associated with changes in left ventricular ejection fraction and receiver-operating characteristic curve to predict cardiotoxicity have shown an area under the curve of 0.85 compared with 0.54 for cTnI, demonstrating circulating miR-1 as an indicator of early doxorubicin-induced cardiotoxicity (40). However, cTnI is not very sensitive, and thus studies comparing miR-1 with highly sensitive troponin T (hsTnT), a marker for acute cardiac injury, are needed. Interestingly, a study from Oerlemans et al. (38) found miR-1 levels to be increased in hsTnT negative acute coronary syndrome patients showing higher sensitivity of miR-1 compared with hsTnT. Therefore, miR-1 may emerge as an early biomarker of doxorubicin-induced cardiotoxicity, and further studies into this miRNA are warranted. Another study in 33 children with different forms of cancer found increased levels of circulating miR-29b and miR-499 postchemotherapy treatment (26). Additionally, miR-29b and miR-499 levels were higher in patients with increased hsTnT (26). Thus, it would be interesting to check whether circulating miR-29b and miR-499 levels can predict future cardiotoxicity-related heart failure. Oatmen et al. (37) performed miRNA profiling in patients with pediatric cancer and validated several miRNAs (miR-486-3p, miR-103-3p, miR-142-3p, and miR-92a-3p) as potential biomarkers for acute as well as chronic anthracycline cardiotoxicity. Although these studies have shown promising data for circulating miRNAs as diagnostic tools for cardiotoxicity, larger patient cohort studies are needed to validate the findings and to assess the prognostic potential of circulating miRNAs.
lncRNAs INVOLVED IN DOXORUBICIN-INDUCED CARDIOTOXICITY
lncRNAs are ncRNA molecules defined based on their size of ≥200 nt. lncRNAs have been recently reported to play essential roles in cardiovascular development and disease by acting as regulator of gene expression (3). Some recent reports have demonstrated lncRNAs to be notable players also in the context of doxorubicin-induced cardiotoxicity. lncRNA myosin heavy chain-associated RNA transcripts (Mhrt) derived from Myh7 gene loci have been found to be downregulated in doxorubicin-treated hearts (27). Lowered Mhrt can be rescued by treatment with obestatin, a ghrelin-associated protein (27). Previously, Mhrt overexpression has been shown to prevent pressure overload-induced heart failure (22). Li et al. (27) have shown that overexpression of Mhrt in cardiomyocytes inhibits doxorubicin-induced apoptosis, whereas inhibition exacerbates the phenotype. Moreover, inhibition of Mhrt in vivo impedes the beneficial effects of obestatin seen in the doxorubicin cardiotoxicity model (27). Hence, Mhrt overexpression might be an interesting therapeutic target to prevent doxorubicin-induced heart failure, provided experiments in available Mhrt transgenic mice are further performed.
Another lncRNA derived from the intron of Dcc gene named cardiac hypertrophy-related factor (Chrf) was found to be upregulated in the heart in response to doxorubicin (8). Chrf inhibition prevents doxorubicin-induced apoptosis and transforming growth factor-β1 expression. Chrf induction was prevented in the presence of valsartan, an angiotensin II receptor blocker (8). Adenovirus-mediated overexpression of Chrf in the heart counteracts the protective effect of valsartan in a murine model of doxorubicin-induced cardiotoxicity (8). Chrf has been previously shown to function as sponge for miR-489 (60); whether a similar sponge mechanism is involved in mediating protection from doxorubicin remains unclear.
A highly abundant and conserved lncRNA, H19, has been reported to be upregulated in the doxorubicin cardiotoxicity model (70). siRNA-mediated inhibition of H19 lncRNA blocks doxorubicin-induced apoptosis and maintains cardiac function (70). miR-675 is located within the H19 gene and has been shown to be one of the downstream mediators of H19 functions (31). Similar to H19, miR-675 was also upregulated in doxorubicin-exposed hearts (70). Overexpression of miR-675 induces cardiomyocyte apoptosis by targeting ErbB3-binding protein 1 and can reverse the protective effect of H19 (70). In contrast to a proapoptotic effect in response to doxorubicin, H19 depicted an antiapoptotic effect in streptozotocin-induced diabetic rat model (28). Thus the apoptotic function of H19 is a complex and context-dependent phenomenon.
Similar to doxorubicin, ischemia-reperfusion injury also leads to mitochondrial fission (61). Cardiac apoptosis-related lncRNA (Carl) inhibits mitochondrial fission and cardiomyocyte apoptosis in response to ischemia-reperfusion (61). Therefore, it is tempting to speculate if overexpression of Carl lncRNA could inhibit doxorubicin-induced apoptosis and mitochondrial fission and thus improve cardiac function.
circRNAs AS POTENTIAL REGULATORS OF DOXORUBICIN-INDUCED CARDIOTOXICITY
circRNAs are yet another class of ncRNA formed by back splicing of mRNA molecules (63). Dcc was the first gene reported to form endogenous eukaryotic circular RNA in 1990, which was considered a splicing error initially. Recent studies from Salzman et al. (43) and Memczak et al. (34) have verified the presence of thousands of circRNAs and shown them as an integral feature of gene expression. The heart has also been reported to express numerous circRNAs whose expression are regulated during physiological and pathological changes (62). Wang et al. (59) identified a circRNA named mitochondrial fission and apoptosis-related circRNA (Mfacr) that regulates mitochondrial fission. Mfacr was found to be upregulated with anoxia/reoxygenation, and its inhibition decreases myocardial apoptosis and mitochondrial fission in the heart (59). Mfacr functions as an endogenous sponge for miR-652-3p and, in turn, derepresses its target Mtp18 (59). As Mfacr can regulate mitochondrial fission, inhibition of Mfacr may inhibit doxorubicin-induced mitochondrial fission and apoptosis.
circRNA formation is dependent on the presence of inverted repeats and RNA-binding proteins (63). Quaking, a RNA-binding protein, was found to promote circRNA formation during epithelial-to-mesenchymal transition (10). Recently, our group reported Quaking to be repressed in the myocardium of doxorubicin-treated mice (21). Quaking was found to maintain the expression levels of circRNA, which were downregulated after doxorubicin treatment (21). CircRNAs derived from titin gene (Ttn105-111) were shown to be regulated by Qki5 to mediate cardioprotective effects. Adeno-associated virus serotype 9-mediated overexpression of Quaking isoform Qki5 prevented doxorubicin-induced cardiotoxicity highlighting Quaking as an interesting therapeutic target (21). This study also highlights the need for further studies on the potential involvement of circRNAs in doxorubicin-induced cardiotoxicity and their therapeutic application.
OTHER CANCER THERAPIES AND HEART FAILURE
Trastuzumab is a monoclonal antibody against ErbB2 used for the treatment of breast cancer. It exerts high cardiotoxicity when used in combination with doxorubicin (49). ErbB2 heterodimerizes with Nrg-1-bound ErbB4 and activates the downstream Nrg-1/ErbB survival pathway (65). Horie et al. (24) found that doxorubicin downregulates the expression of ErbB4 via upregulation of miR-146a. Thus, a simultaneous doxorubicin-mediated decrease in ErbB4 and inhibition of ErbB2 activity by Trastuzumab might completely block the Nrg-1/ErbB survival pathway and contribute to higher apoptosis. Inhibition of miR-146a in cardiomyocytes was found to increase ErbB4 expression and decrease doxorubicin-induced apoptosis (24). Hence, it needs to be tested in a preclinical animal model whether inhibition of miR-146a via antagomir can reduce the cardiotoxicity induced by the combined administration of doxorubicin and trastuzumab.
Other cancer treatment regimens like immune checkpoint inhibitors and chimeric antigen receptor (CAR)-modified T cell-mediated therapy also have cardiovascular side effects (6, 52). Immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA4 have revolutionary effects on cancer treatment but cause autoimmune myocarditis as PD-L1 is also expressed on cardiomyocytes (52). Newly highlighted CAR-modified T cell therapy was also found to cause new systolic dysfunction in ~10% of patients (6). With new measures of combining immune checkpoint inhibitors with CAR-modified T cell therapy, the likelihood for cardiovascular events will further increase (67). Efforts are being made to use ncRNAs in the field of cancer immunotherapeutic, which would allow specific delivery of the treatment to the tumor and conceivably circumvent any adverse cardiotoxic outcomes. ncRNAs might come to the rescue for the cardiotoxicity thread arising from immune-targeted therapy as ncRNAs are involved in gene regulation of the immune system (9).
CONCLUSIONS AND FUTURE PERSPECTIVES
The advances in the treatment options for cancer have significantly improved the life expectancy of patients with cancer but unfortunately have overlooked the effects of these therapeutic strategies on the cardiovascular system. There are four distinct clinical stages of cardiotoxicity, namely, acute, subacute, chronic, and late, defined based on the time elapsed between the administration of treatment and onset of toxicity. Another challenge in the health industry is to cure patients with cancer who have a history of cardiovascular disorders. Presumably, there will be continual cases of diverse cancer malignancies even in the near future. Hence, the current research in the field of tumor therapy should focus to elucidate the mechanism of action of anticancer drugs in both tumorous as well as cardiac cell types to refrain from any possible cardiotoxic effects on the patients. Additionally, there is an unmet need to cure patients who are already suffering from cancer therapy-induced cardiovascular disorders. Such circumstances emphasize the need to initiate multi- as well as interdisciplinary studies in the field of cardioncology. In this review, we describe ncRNA molecules as novel and promising targets for the prognosis and treatment of cardiotoxicity (shown in Table 2). Circulating ncRNAs available in serum/plasma can be used for risk prediction for cardiotoxicity (shown in Fig. 2). The majority of studies have been performed in small cohorts; hence, large-scale randomized controlled clinical studies will aid in determining the most reliable ncRNAs as biomarkers for cardiotoxicity. Furthermore, the ncRNA candidates that are dysregulated during cardiovascular disorders can also be targeted with application of various antisense oligonucleotide chemistries such as locked nucleic acid, GapmeR, and antagomiR for inhibition and activation via other gene therapy tools such as adeno-associated viruses and nanoparticles for targeted delivery (Fig. 2). It should be taken into consideration that therapeutic strategies based on ncRNA would be more lucrative options for patients suffering from chronic cardiotoxicity compared with acute cardiotoxicity.
Fig. 2.
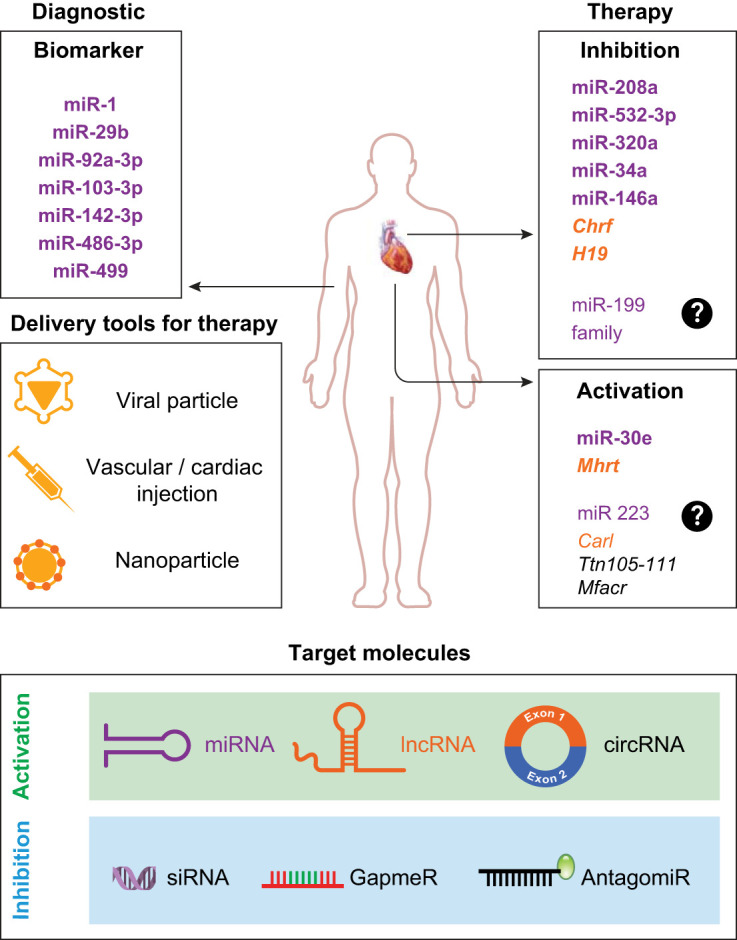
Noncoding RNA (ncRNA)-based diagnostic and therapeutic approaches for cardiotoxicty. ncRNAs [microRNAs (miRNAs; purple), long ncRNAs (lncRNAs; yellow), and circular RNAs (circRNAs; gray)] can be used as novel biomarkers of cancer-induced cardiotoxicity. A few examples of miRNA and lncRNA molecules with reported biomarker and therapeutic potential for cardiotoxicity have been highlighted (in bold), whereas the predicted therapeutic molecules (not in bold) are symbolized with a question mark. Furthermore, ncRNAs are potential therapeutic targets. Unique antisense chemistries or the noncoding sequence itself may be delivered via viral particles, via nanoparticles, or through injection of the free molecule into the cardiomyocytes of vascular system. The strategy is to silence or activate the endogenous ncRNAs.
It is pivotal to establish a firm association between researchers and clinicians to aid in the decision-making process and select a suitable treatment option for the patient to eliminate cancer without invoking any adverse symptoms in the heart. Innovative and cutting edge research will pave the way for a deeper and better understanding of the deterioration of cardiac function and physiology in response to cancer treatment. Access to such information will be crucial to expand the range of safe therapeutic options for patients with cancer with the least possible burden on the heart. More sensitive biomarkers that can detect subclinical cardiotoxicity should be used before medication is prescribed and drug combinations are administered to patients with cancer. To summarize, the health care system requires a strong collaboration among the internists, cardiologists, and oncologists to deal with the unconventional effects of cancer treatments on cardiac function. Considering that ncRNAs have evolved as crucial regulators of numerous physiological and pathological pathways, they can also be very convincing therapeutic options in the context of cardioncology.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grants BA5631/2-1 (to C. Bär), TH903/20-1 (to T. Thum), and the ERC Consolidator Grant Longheart (to T. Thum).
DISCLOSURES
T. Thum and S. K. Gupta have filed patents about the diagnostic and therapeutic use of several cardiovascular ncRNAs. T. Thum is founder and holds shares in Cardior Pharmaceuticals. The other authors do not have any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.C. and S.K.G. prepared figures; S.C. and S.K.G. drafted manuscript; C.B. and T.T. edited and revised manuscript; C.B. and T.T. approved final version of manuscript.
REFERENCES
- 1.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer 18: 5–18, 2018. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng 11: 1101–1110, 1969. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- 3.Bär C, Chatterjee S, Thum T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 134: 1484–1499, 2016. doi: 10.1161/CIRCULATIONAHA.116.023686. [DOI] [PubMed] [Google Scholar]
- 4.Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development 143: 1838–1847, 2016. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96: 1297–1325, 2016. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 6.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor t cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant 24: 1590–1595, 2018. doi: 10.1016/j.bbmt.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131: 1981–1988, 2015. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Yan KP, Liu XC, Wang W, Li C, Li M, Qiu CG. Valsartan regulates TGF-β/Smads and TGF-β/p38 pathways through lncRNA CHRF to improve doxorubicin-induced heart failure. Arch Pharm Res 41: 101–109, 2018. doi: 10.1007/s12272-017-0980-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nat Immunol 18: 962–972, 2017. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell 160: 1125–1134, 2015. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Dangwal S, Schimmel K, Foinquinos A, Xiao K, Thum T. Noncoding RNAs in heart failure. Handb Exp Pharmacol 243: 423–445, 2017. doi: 10.1007/164_2016_99. [DOI] [PubMed] [Google Scholar]
- 12.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121: 276–292, 2010. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I; Cardiolinc network . Circular RNAs in heart failure. Eur J Heart Fail 19: 701–709, 2017. doi: 10.1002/ejhf.801. [DOI] [PubMed] [Google Scholar]
- 14.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 12: 547–558, 2015. [Erratum in Nat Rev Cardiol 12: 620, 2015]. doi: 10.1038/nrcardio.2015.65. [DOI] [PubMed] [Google Scholar]
- 15.Fiedler J, Gupta SK, Thum T. Identification of cardiovascular microRNA targetomes. J Mol Cell Cardiol 51: 674–681, 2011. doi: 10.1016/j.yjmcc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114, 2008. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Gupta SK, Thum T. Long non-coding RNAs−a crucial part of the vasculature puzzle. Vascul Pharmacol pii: S1537-1891(18)30010-7, 2018. doi: 10.1016/j.vph.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet 3: 484–488, 2010. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T. Preclinical development of a microrna-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol 68: 1557–1571, 2016. doi: 10.1016/j.jacc.2016.07.739. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, Garg A, Bar C, Chatterjee S, Foinquinos A, Milting H, Streckfuss-Bomeke K, Fiedler J, Thum T. Quaking inhibits doxorubicin-mediated cardiotoxicity through regulation of cardiac circular RNA expression. Circ Res 122: 246–254, 2018. doi: 10.1161/CIRCRESAHA.117.311335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien H, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HV, Quertermous T, Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514: 102–106, 2014. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Grines CL, Marmagkiolis K. Vascular toxicities of cancer therapies: the old and the new-an evolving avenue. Circulation 133: 1272–1289, 2016. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horie T, Ono K, Nishi H, Nagao K, Kinoshita M, Watanabe S, Kuwabara Y, Nakashima Y, Takanabe-Mori R, Nishi E, Hasegawa K, Kita T, Kimura T. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res 87: 656–664, 2010. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124: 617–630, 2014. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leger KJ, Leonard D, Nielson D, de Lemos JA, Mammen PP, Winick NJ. Circulating microRNAs: potential markers of cardiotoxicity in children and young adults treated with anthracycline chemotherapy. J Am Heart Assoc 6: e004653, 2017. doi: 10.1161/JAHA.116.004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li HQ, Wu YB, Yin CS, Chen L, Zhang Q, Hu LQ. Obestatin attenuated doxorubicin-induced cardiomyopathy via enhancing long noncoding Mhrt RNA expression. Biomed Pharmacother 81: 474–481, 2016. doi: 10.1016/j.biopha.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Wang H, Yao B, Xu W, Chen J, Zhou X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep 6: 36340, 2016. doi: 10.1038/srep36340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Liu L, Hou N, Song Y, An X, Zhang Y, Yang X, Wang J. miR-199-sponge transgenic mice develop physiological cardiac hypertrophy. Cardiovasc Res 110: 258–267, 2016. doi: 10.1093/cvr/cvw052. [DOI] [PubMed] [Google Scholar]
- 30.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324: 808–815, 1991. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, An X, Li Z, Song Y, Li L, Zuo S, Liu N, Yang G, Wang H, Cheng X, Zhang Y, Yang X, Wang J. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res 111: 56–65, 2016. doi: 10.1093/cvr/cvw078. [DOI] [PubMed] [Google Scholar]
- 32.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZ, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71: 1755–1764, 2018. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markman TM, Markman M. Cardio-oncology: mechanisms of cardiovascular toxicity. F1000 Res 7: 113, 2018. doi: 10.12688/f1000research.12598.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495: 333–338, 2013. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 35.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66: 271–289, 2016. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 36.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375: 1457–1467, 2016. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 36a.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, Agrawal A, et al.; GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980−2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390: 1151–1210, 2017. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oatmen KE, Toro-Salazar OH, Hauser K, Zellars KN, Mason KC, Hor K, Gillan E, Zeiss CJ, Gatti DM, Spinale FG. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am J Physiol Heart Circ Physiol 315: H1443–H1452, 2018. doi: 10.1152/ajpheart.00252.2018. [DOI] [PubMed] [Google Scholar]
- 38.Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med 4: 1176–1185, 2012. doi: 10.1002/emmm.201201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piegari E, Russo R, Cappetta D, Esposito G, Urbanek K, Dell’Aversana C, Altucci L, Berrino L, Rossi F, De Angelis A. MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget 7: 62312–62326, 2016. doi: 10.18632/oncotarget.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Ávila MS, Brandão SM, Cruz FD, Santos MH, Cruz CB, Alves MS, Issa VS, Guimarães GV, Cunha-Neto E, Bocchi EA. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget 8: 6994–7002, 2017. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roca-Alonso L, Castellano L, Mills A, Dabrowska AF, Sikkel MB, Pellegrino L, Jacob J, Frampton AE, Krell J, Coombes RC, Harding SE, Lyon AR, Stebbing J. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in β-adrenergic signaling and enhances apoptosis. Cell Death Dis 6: e1754, 2015. doi: 10.1038/cddis.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggeri C, Gioffré S, Achilli F, Colombo GI, D’Alessandra Y. Role of microRNAs in doxorubicin-induced cardiotoxicity: an overview of preclinical models and cancer patients. Heart Fail Rev 23: 109–122, 2018. doi: 10.1007/s10741-017-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7: e30733, 2012. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell 29: 452–463, 2016. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62: 220–241, 2012. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 46.Sonnenburg DW, Morgans AK. Emerging therapies in metastatic prostate cancer. Curr Oncol Rep 20: 46, 2018. doi: 10.1007/s11912-018-0692-z. [DOI] [PubMed] [Google Scholar]
- 47.Sparreboom A, Verweij J. Advances in cancer therapeutics. Clin Pharmacol Ther 85: 113–117, 2009. doi: 10.1038/clpt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97: 2869–2879, 2003. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 49.Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, Lee DS. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol 34: 2239–2246, 2016. doi: 10.1200/JCO.2015.65.1505. [DOI] [PubMed] [Google Scholar]
- 50.Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res 116: 751–762, 2015. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 51.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 52.Tocchetti CG, Galdiero MR, Varricchi G. Cardiac toxicity in patients treated with immune checkpoint inhibitors: it is now time for cardio-immuno-oncology. J Am Coll Cardiol 71: 1765–1767, 2018. doi: 10.1016/j.jacc.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 53.Tony H, Yu K, Qiutang Z. MicroRNA-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction. Oxid Med Cell Longev 2015: 597032, 2015. doi: 10.1155/2015/597032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, Guérin S, Pacquement H, Aouba A, Hawkins M, Winter D, Bourhis J, Lefkopoulos D, Diallo I, de Vathaire F. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28: 1308–1315, 2010. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 55.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 3: 1078, 2012. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Acker HH, Anguille S, Willemen Y, Smits EL, Van Tendeloo VF. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol Ther 158: 24–40, 2016. doi: 10.1016/j.pharmthera.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 18: 457–468, 2016. doi: 10.1002/ejhf.495. [DOI] [PubMed] [Google Scholar]
- 58.Wang JX, Zhang XJ, Feng C, Sun T, Wang K, Wang Y, Zhou LY, Li PF. MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis 6: e1677, 2015. doi: 10.1038/cddis.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K, Gan TY, Li N, Liu CY, Zhou LY, Gao JN, Chen C, Yan KW, Ponnusamy M, Zhang YH, Li PF. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ 24: 1111–1120, 2017. doi: 10.1038/cdd.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114: 1377–1388, 2014. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 61.Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 5: 3596, 2014. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 62.Werfel S, Nothjunge S, Schwarzmayr T, Strom TM, Meitinger T, Engelhardt S. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol 98: 103–107, 2016. doi: 10.1016/j.yjmcc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Wilusz JE. A 360° view of circular RNAs: from biogenesis to functions. Wiley Interdiscip Rev RNA 9: e1478, 2018. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, Li Y, Wang X, Mu X, Qin D, Huang W, Alshahrani S, Nieman M, Peng J, Essandoh K, Peng T, Wang Y, Lorenz J, Soleimani M, Zhao ZQ, Fan GC. Overexpression of miR-223 tips the balance of pro- and anti-hypertrophic signaling cascades toward physiologic cardiac hypertrophy. J Biol Chem 291: 15700–15713, 2016. doi: 10.1074/jbc.M116.715805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 66.Yin Z, Zhao Y, Li H, Yan M, Zhou L, Chen C, Wang DW. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging (Albany NY) 8: 192–207, 2016. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-In CAR-T. Int J Mol Sci 19: 340, 2018. doi: 10.3390/ijms19020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang CL, Huang T, Wu BL, He WX, Liu D. Stem cells in cancer therapy: opportunities and challenges. Oncotarget 8: 75756–75766, 2017. doi: 10.18632/oncotarget.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18: 1639–1642, 2012. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhang M, Xu W, Chen J, Zhou X. The long non-coding RNA H19 promotes cardiomyocyte apoptosis in dilated cardiomyopathy. Oncotarget 8: 28588–28594, 2017. doi: 10.18632/oncotarget.15544. [DOI] [PMC free article] [PubMed] [Google Scholar]