Abstract
Myocardial hypertrophy is an independent risk factor for heart failure (HF), yet the mechanisms underlying pathological cardiomyocyte growth are incompletely understood. The c-Jun NH2-terminal kinase (JNK) signaling cascade modulates cardiac hypertrophic remodeling, but the upstream factors regulating myocardial JNK activity remain unclear. In this study, we sought to identify JNK-activating molecules as novel regulators of cardiac remodeling in HF. We investigated mixed lineage kinase-3 (MLK3), a master regulator of upstream JNK-activating kinases, whose role in the remodeling process had not previously been studied. We observed increased MLK3 protein expression in myocardium from patients with nonischemic and hypertrophic cardiomyopathy and in hearts of mice subjected to transverse aortic constriction (TAC). Mice with genetic deletion of MLK3 (MLK3−/−) exhibited baseline cardiac hypertrophy with preserved cardiac function. MLK3−/− mice subjected to chronic left ventricular (LV) pressure overload (TAC, 4 wk) developed worsened cardiac dysfunction and increased LV chamber size compared with MLK3+/+ littermates (n = 8). LV mass, pathological markers of hypertrophy (Nppa, Nppb), and cardiomyocyte size were elevated in MLK3−/− TAC hearts. Phosphorylation of JNK, but not other MAPK pathways, was selectively impaired in MLK3−/− TAC hearts. In adult rat cardiomyocytes, pharmacological MLK3 kinase inhibition using URMC-099 blocked JNK phosphorylation induced by neurohormonal agents and oxidants. Sustained URMC-099 exposure induced cardiomyocyte hypertrophy. These data demonstrate that MLK3 prevents adverse cardiac remodeling in the setting of pressure overload. Mechanistically, MLK3 activates JNK, which in turn opposes cardiomyocyte hypertrophy. These results support modulation of MLK3 as a potential therapeutic approach in HF.
NEW & NOTEWORTHY Here, we identified a role for mixed lineage kinase-3 (MLK3) as a novel antihypertrophic and antiremodeling molecule in response to cardiac pressure overload. MLK3 regulates phosphorylation of the stress-responsive JNK kinase in response to pressure overload and in cultured cardiomyocytes stimulated with hypertrophic agonists and oxidants. This study reveals MLK3-JNK signaling as a novel cardioprotective signaling axis in the setting of pressure overload.
Keywords: c-Jun NH2-terminal kinase, cardiac hypertrophy, cardiomyocyte hypertrophy, heart failure, mixed lineage kinase
INTRODUCTION
In response to pathologic stimuli to the left ventricle (LV), ventricular cardiomyocytes (CM) undergo cellular hypertrophy, which serves to normalize wall stress and preserve cardiac output yet ultimately increases the risk of developing heart failure (HF) (19). Most established pharmacological HF therapies attenuate existing CM and LV hypertrophy and remodeling via antagonism of neurohormones such as angiotensin II, aldosterone, and norepinephrine (9, 30, 40). Although significant progress has been made in characterizing signaling components that promote hypertrophic signaling, the elucidation of intracellular signaling molecules that oppose pressure overload-induced dysfunction and remodeling remains incomplete and, if better understood, may provide a novel therapeutic avenue to target this process.
The stress-responsive c-Jun NH2-terminal kinase (JNK) prevents early systolic dysfunction and cardiac hypertrophy in animal models of experimental pressure overload (21). Furthermore, upstream JNK-activating kinases including mitogen-activated protein kinase kinase-4 (MKK4) and MKK7 have each been shown to oppose cardiac hypertrophy (22, 23) through specific functions in the CM. We (1) previously observed, in hearts of mice with mutation of the cGMP-dependent protein kinase-1α, which develop markedly accelerated cardiac hypertrophy and dysfunction after pressure overload, early and selective impairment of activation of both MKK4 and JNK, which correlated with LV dysfunction and preceded adverse cardiac hypertrophic and functional remodeling. Whereas these data suggest that JNK prevents cardiac hypertrophy, other studies have shown that JNK activation can promote adverse cardiac growth and dysfunction in mouse models of HF (7, 20, 39). Since the role of JNK and upstream JNK-activating molecules in regulating myocardial remodeling in vivo remains unclear, a better understanding of the upstream factors that modulate MAPK signaling in the heart may identify novel anti-remodeling signaling mechanisms, and could clarify how JNK regulates beneficial vs. maladaptive remodeling in the setting of HF. To address these questions, we designed the present study to identify and investigate upstream activators of JNK signaling that modulate the cardiac remodeling response.
The mixed lineage kinase (MLK) family proteins are JNK-activating MAPKKKs whose function in cardiac biology remains poorly understood. MLK family proteins include four isoforms, MLK1–4, expressed in a diverse range of tissues, and function as master regulators of the p38, extracellular signal-regulated kinae (ERK), and JNK signaling cascades (5, 12). The MLK3 isoform phosphorylates MKK4 and MKK7 to stimulate JNK activation (32), and although MLK3 is expressed in myocardial tissue (16), its potential function in the regulation of cardiac physiology or hypertrophy has not been investigated. In cell culture models, MLK3 becomes activated upon binding to the small GTPase protein Cdc42 (3, 10), which itself normally inhibits pressure overload-induced cardiac remodeling through JNK-dependent mechanisms in the CM (24). On the basis of prior observations of Cdc42, MKK4, and MKK7 preventing adverse cardiac remodeling (22, 23), we tested the hypotheses that 1) MLK3 deficiency exacerbates cardiac structural and functional remodeling in response to chronic pressure overload, and 2) MLK3 stimulates myocardial JNK signaling. To test these hypotheses, we examined the expression of MLK3 in the normal and failing myocardium, and studied the effect of deletion of MLK3 on myocardial remodeling and JNK activation in the setting of LV pressure overload. We further examined the effects of MLK3 enzymatic inhibition on JNK signaling and cellular hypertrophy in adult cardiac myocytes.
METHODS
Human heart samples.
Nonfailing (NF) donor hearts were obtained from the National Disease Research Interchange. Tissues from patients with nonischemic cardiomyopathy (NICM) were obtained from two sources. First, in patients with reduced LV ejection fraction undergoing surgical implantation of LV assist device for end-stage HF, apical tissue removed for insertion of the inflow cannula was placed immediately on ice, partitioned into segments, and then snap-frozen and stored at −80 C. Additional LV tissue was obtained in the same manner from explanted hearts in patients with NICM undergoing heart transplantation. Tissue from symptomatic patients with hypertrophic cardiomyopathy (HCM) was obtained during LV septal reduction surgery. Tissue banking protocols were approved by the Tufts Medical Center Internal Review Board (IRB no. 9487).
Study approval.
All rodent care and procedures were in accordance with and approved by the Institutional Animal Care and Use Committee of Tufts University School of Medicine and Tufts Medical Center.
Experimental animals.
Whole body MLK3 knockout mice (2) (denoted MLK3−/−) and MLK3 WT littermates (denoted MLK3+/+) maintained on a C57BL/6 background were obtained from breeding MLK3 heterzygote mice. Only male 10- to 12-wk-old mice were used for these studies. Investigators were blinded to animal genotype before the animal surgeries and through the subsequent data analysis.
Transthoracic echocardiography.
Mice were initially anesthetized with 2.5% gaseous isoflurane, and M-Mode images were acquired from the midpapillary short-axis view under 1.0% gaseous isoflurane, as described previously (1). Data were acquired and analyzed using the Vevo 2100 (FUJIFILM VisualSonics). Mice initially underwent echocardiographic analysis preoperatively (baseline) and then every 2 wk until the conclusion of the study.
Transverse aortic constriction.
Body weight- and age-matched littermate mice were randomly assigned to either sham or transverse aortic constriction (TAC) surgery, and aortic constriction was performed as previously described (1, 33). For all pressure overload studies, a 25-gauge (25G) needle was used to constrict the aorta.
LV in vivo hemodynamic measurements.
Mice were initially anesthetized with 2.5% gaseous isoflurane, and hemodynamic analyses were performed using a pressure-volume transducing catheter at 2.0% gaseous isoflurane, as described (1). Hemodynamic data were recorded and analyzed using IOX software (EMKA v. 2.1.10).
Western blotting.
Cells were treated as described in the figure legends, rinsed once in cold PBS, and lysed with tissue lysis buffer (TLB; 20 mmol/l HEPES, 50 mmol/l β-glycerol phosphate, 2 mmol/l EGTA, 1 mmol/l DTT, 10 mmol/l NaF, 1 mmol/l NaVO4, 1% Triton X-100, and 10% glycerol) supplemented with 1 mmol/l PMSF. LV tissues were pulverized on dry ice and lysed in TLB supplemented with 1 mmol/l PMSF and protease inhibitors (EMD Millipore no. 539134). Lysates were cleared by centrifugation, protein content was quantified by BCA assay, and lysates were then diluted in 2× Laemmli sample buffer containing SDS (Sigma S-3401). Protein samples (20–100 µg) and protein marker (Bio-Rad Precision Plus Dual Color Standards no. 161-0374) were loaded into 8% polyacrylamide gels and transferred to supported 0.45-µm nitrocellulose membranes (Bio-Rad, no. 162-0094). Membranes were reversibly stained with Ponceau S to evaluate protein transfer efficiency and to control for protein loading before incubation with primary antibodies. We used the following antibodies in this study: MLK3 (Abcam, ab51068), GAPDH (EMD Millipore, MAB374), and the following antibodies from Cell Signaling Technology: phosphorylated (p-)JNK Thr183/Tyr185 (no. 4668), JNK (no. 9252), p-ERK Thr202/Tyr204 (no. 9101), ERK (no. 9102), p-p38 Thr180/Tyr182 (no. 4511), and p38 (no. 2371). Membranes were incubated with primary antibodies per the manufacturer’s recommendations and incubated with horseradish peroxidase-linked secondary anti-mouse or anti-rabbit antibodies (GE Healthcare, NA931 and NA934). Protein standards were annotated using a chemiluminescent sensitive marker (Li-Cor WesternSure Pen, no. 926-91000) before incubation with ECL substrate (Li-Cor, no. 926-95000). Membranes were visualized using the ProteinSimple FluorChem E system, and images were quantified using Alpha Innotech Imager software. For quantification of total protein as a loading control, Ponceau S staining of the membrane was performed, and the entire protein lane of each sample was quantified.
Quantitative RT-PCR analysis.
Mouse LV total RNA was extracted using TRIzol (Invitrogen), and 1 µg of RNA was reverse transcribed to cDNA using the QuantiTect Reverse Transcriptase Kit (Qiagen). Target primers and cDNA samples were incubated in a 384-well plate in triplicate and amplified by quantitative real-time PCR using Ssofast Evagreen Supermix (Bio-Rad). Primers for mouse qRT-PCR analysis were as follows: NPPA Fwd TCG TCT TGG CCT TTT GGC T, Rev TCC AGG TGG TCT AGC AGG TTC T; NPPB Fwd AAG TCC TAG CCA GTC TCC AGA, Rev GAG CTG TCT CTG GGC CAT TTC; Ctgf Fwd GGG CCT CTG CGA TTT C, Rev ATC CAG GCA AGT GCA TTG GTA; Col1a2 Fwd AAG GGT CCC TCT GGA GAA CC, Rev TCT AGA GCC AGG GAG ACC CA; Gapdh Fwd AGG TCG GTG TGA ACG GAT TTG, Rev TGT AGA CCA TGT AGT TGA GGT CA. To evaluate Map3k11 (MLK3) expression in cultured adult mouse cardiomyocytes, 6-carboxyfluorescein (FAM)-labeled primers for Map3k11 (Mm.PT.58.6037096) and Gapdh (Mm.PT.39a.1) were purchased from IDT DNA and amplified using PrimeTime Gene Expression Master Mix (IDT DNA) according to the manufacturer’s recommendations.
All samples were amplified for 40 cycles performed at 95°C for 15 s and 60°C for 1 min using an ABI Prism 7900 sequence detection system (Applied Biosystems). qPCR data were analyzed using the ΔΔCT method with Gapdh as the reference control, and values were normalized to represent fold change.
Histological analysis.
The LV was arrested in diastole with a KCl injection, fixed in 10% formalin, embedded in paraffin, and cut into 4-µm sections. For CM size measurements, LV tissue sections were stained with wheat germ agglutinin conjugated to Alexa fluor 488 (ThermoFisher Scientific, W11261) and counterstained with DAPI to visualize nuclei. CMs in the transverse plane with central nuclei were visualized with fluorescent microscopy and traced using Image-Pro Premier Software (MediaCybernetics). For CM size measurements, a minimum of 50 cells per sample were counted. To evaluate fibrosis, LV tissue sections were stained with Masson’s trichrome and visualized under white light. For interstitial fibrosis measurements, trichrome-positive staining was calculated as a percentage of the total tissue area per image. For perivascular fibrosis measurements, the percentage of trichrome-positive staining was calculated and normalized to vessel area.
Adult rat ventricular myocyte culture.
Adult rat ventricular myocytes (ARVMs) were isolated as previously described (4, 36) from 175- to 200-g adult male Sprague-Dawley rats (Envigo). For signaling experiments, ARVMs were plated at a nonconfluent density of 50 cells/mm2 on p60 dishes coated with mouse laminin (Invitrogen). After 1 h of plating, the medium was replaced, and the cells were used the following morning. For CM size measurements, ARVMs were plated on laminin-coated glass coverslips, and after 1 h the medium was replaced with fresh medium containing either DMSO vehicle (diluted 1:10,000 in medium) or the MLK3 inhibitor URMC-099 (Selleckchem) diluted in DMSO to a final concentration of 100 nmol/l. After 48 h of incubation, the cells were fixed and stained with Alexa fluor 488 phalloidin (Life Technologies) according to the manufacturer’s recommendations. Coverslips were mounted on slides with solution containing DAPI (Life Technologies) and visualized by fluorescent microscopy. CM size was measured from fluorescent images by using Image-Pro Premier Software. For each experiment, 20–50 cells were measured per treatment condition.
Adult mouse ventricular cardiomyocyte isolate and culture.
Mouse CMs were isolated and cultured as described by O’Connell et al. (28) from 10- to 12-wk-old male C57BL/6 mice (Jackson Laboratories). Mouse CMs were plated at a nonconfluent density of 50 cells/mm2 on p60 dishes coated with mouse laminin and cultured in minimal essential medium (MEM; GIBCO no. 11575-032) supplemented with penicillin, 2 mM l-glutamine, BSA (0.1 mg/ml). All cells were incubated with propranolol (2 µM) and ascorbic acid (10 µM) and treated with phenylephrine (PE; 10 µM) or endothelin-1 (ET-1; 100 nM) for 18 h. Total RNA was isolated from cells by use of TRIzol according to the manufacturer’s recommendations.
Statistical analysis.
Statistics were performed with a two-tailed unpaired Student’s t-test, one-way ANOVA, or two-way ANOVA for multiple comparisons, where appropriate, as indicated in the figure legends. Bonferroni correction was used as a posttest in cases of multiple comparisons. All data shown represent the results obtained from independent experiments. Instances of multiple replicates of the same biological sample are noted in the figure legends. Data are represented as means ± SE. Values of P < 0.05 were considered statistically significant. In the figures, *P < 0.05, **P < 0.01, and ***P < 0.001; and other symbols depicting statistical testing are noted in the figure legends.
RESULTS
Increased MLK3 protein expression in human HF tissue samples and in cardiomyocytes with hypertrophic stimulation.
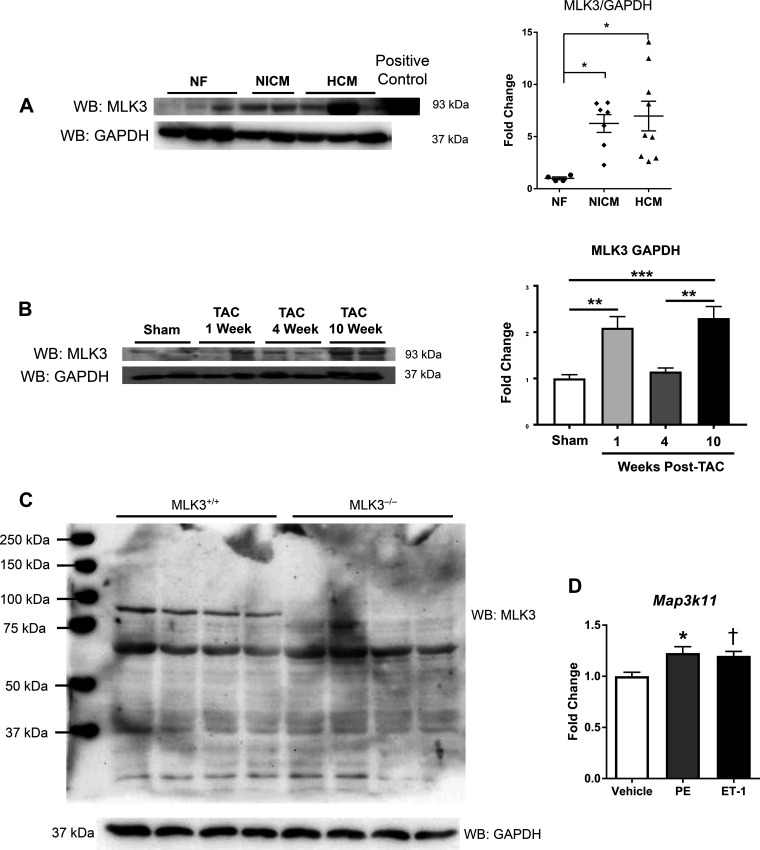
We first assessed the relevance of MLK3 to human cardiovascular disease by quantitating MLK3 protein expression in myocardial tissue samples from patients with two different forms of cardiomyopathy: nonischemic cardiomyopathy (NICM) and hypertrophic cardiomyopathy (HCM). Western blot analysis of MLK3 revealed detectable levels of MLK3 protein expression in NF control heart tissues (Fig. 1A). Furthermore, compared with NF control tissue, total normalized expression of MLK3 protein increased over sixfold in NICM and HCM samples [NICM: 6.26 ± 0.85 arbitrary densitometry units (ADUs) vs. HCM: 6.97 ± 1.42 ADU; Fig. 1A]. We next tested whether MLK3 expression was altered in an experimental model of HF. We used TAC, a standard surgical model of pressure overload in mice, to induce LV hypertrophy and cardiac dysfunction, and measured MLK3 expression at the following times post-TAC: 1 wk (corresponding to compensated phase), 4 wk (corresponding to pathological cardiac remodeling), and 10 wk (overt HF). As shown in Fig. 1B, we detected increased myocardial MLK3 protein expression at 1 wk post-TAC (1.0 ± 0.08 vs. 2.09 ± 0.24 ADU 1 wk TAC, P < 0.01), which returned toward sham levels by 4 wk [1.15 ± 0.08 ADUs in 4 wk TAC, P = not significant (ns) vs. sham]. At 10 wk post-TAC, expression again increased (2.30 ± 0.25 ADUs, P < 0.001 vs. sham). We verified the specificity of the MLK3 antibody used for Western blotting and observed deletion of the 93-kDa MLK3 protein in cardiac tissue lysates from MLK3 knockout mice (Fig. 1C). To test whether CMs exhibit differential MLK3 expression in response to hypertrophic agonists, we isolated adult mouse CMs and subjected them to hypertrophic stimulation for 18 h. In response to PE or ET-1 treatment, we observed increased MLK3 mRNA expression (PE: +122.7 ± 6.22%, ET-1: +119.9 ± 4.35%) in cultured primary CMs (Fig. 1D).
Fig. 1.
Mixed lineage kinase-3 (MLK3) expression is increased in human and murine failing hearts. A: Western blot (WB) and quantitation of MLK3 in human left ventricle (LV) tissue lysates from nonfailing (NF) hearts, nonischemic cardiomyopathy (NICM), and hypertrophic cardiomyopathy (HCM). Samples were blotted for MLK3 or GAPDH and quantitated by densitometry relative to GAPDH (n = 4–9 per group). Data were analyzed by one-way ANOVA with Bonferroni’s posttest. B: Western blot and quantitation of MLK3 in LV tissue lysates from mice subjected to sham or transverse aortic constriction (TAC) for 1, 4, and 10 wk. Protein expression of MLK3 was normalized to GAPDH (n = 3–4 per surgical group). C: 100 μg of LV tissue lysates from wild-type (MLK3+/+) and MLK3−/− mice were analyzed by Western blot for MLK3 expression. The membrane was stripped and reprobed for GAPDH as a loading control. D: mRNA expression of Map3k11 (MLK3) in cultured adult mouse cardiomyocytes subjected to vehicle, phenylephrine (PE, 20 µM), or endothelin-1 (ET-1, 100 nM) treatment for 18 h; n = 5–6 per group. *P < 0.05 vs. vehicle; †P < 0.05 vs. vehicle. Data were analyzed by one-way ANOVA with Bonferroni’s posttest.
MLK3 knockout mice exhibit basal cardiac hypertrophy with no change in LV contractile function.
Having observed a correlation between increased MLK3 expression and cardiac remodeling in both human cardiomyopathy samples and in mouse hearts subjected to experimental pressure overload, we next tested the degree to which MLK3 modulates cardiac remodeling and function by studying mice with whole body genetic deletion of MLK3 (denoted MLK3−/−). These mice have a normal lifespan, although the cardiac phenotype has not previously been tested (2). Baseline organ masses of male MLK3−/− and wild-type (WT) MLK3 littermates (denoted MLK3+/+) demonstrated increased LV mass in MLK3−/− mice at 12 and 24 wk of age (Table 1). Cardiac structure and function analysis by echocardiography revealed no basal differences in LV chamber dimensions or systolic function (Table 2). Baseline cardiac function was further evaluated by invasive hemodynamics of the LV by using a pressure-volume-sensing catheter, and we detected no abnormalities in LV function in MLK3−/− mice (Table 3). Systolic (SBP) and diastolic blood pressure (DBP) measured in the aorta were both elevated in 12-wk-old MLK3−/− mice (SBP: MLK3+/+ 94.3 ± 2.1 vs. MLK3−/− 109.3 ± 2.5, P < 0.001; DBP: MLK3+/+ 62.9 ± 2.1 vs. MLK3−/− 70.3 ± 2.1, P < 0.05).
Table 1.
Baseline organ masses in MLK3+/+ and MLK3−/− mice
| MLK3+/+ | MLK3−/− | P Value | |
|---|---|---|---|
| 12 Weeks | |||
| n | 11 | 14 | |
| LV, mg | 83.5 ± 2.6 | 99.4 ± 3.1 | <0.001 |
| HW, mg | 114.1 ± 3.2 | 127.9 ± 3.7 | 0.012 |
| Lung, mg | 145.5 ± 2.3 | 150.3 ± 6.8 | 0.48 |
| LV/TL, mg/cm | 47.1 ± 1.5 | 57.2 ± 1.8 | <0.001 |
| HW/TL, mg/cm | 64.4 ± 1.9 | 73.6 ± 2.1 | 0.004 |
| Lung/TL, mg/cm | 82.0 ± 1.3 | 86.1 ± 0.5 | 0.39 |
| BW, g | 29.3 ± 1.2 | 28.4 ± 0.8 | 0.564 |
| 24 Weeks | |||
| n | 4 | 9 | |
| LV, mg | 91.85 ± 5.32 | 106.57 ± 3.43 | 0.053 |
| HW, mg | 120.15 ± 7.88 | 138.63 ± 3.24 | 0.037 |
| Lung, mg | 182.58 ± 12.04 | 172 ± 4.85 | 0.391 |
| LV/TL, mg/cm | 50.68 ± 2.67 | 59.66 ± 2.13 | 0.045 |
| HW/TL, mg/cm | 66.29 ± 4.04 | 77.60 ± 2.08 | 0.029 |
| Lung/TL, mg/cm | 100.71 ± 6.15 | 96.27 ± 2.84 | 0.503 |
| BW, g | 39.38 ± 1.64 | 37.18 ± 1.06 | 0.316 |
Data are expressed as means ± SE. Organs from mice at 12 wk and at 24 wk of age were weighed and normalized relative to tibia length. MLK3, mixed lineage kinase-3; LV, left ventricle; HW, heart weight; TL, tibia length; BW, body weight. Data were analyzed by ANOVA.
Table 2.
Baseline cardiac structure and function in MLK3+/+ and MLK3−/− mice
| MLK3+/+ (n = 8) | MLK3−/− (n = 8) | P Value | |
|---|---|---|---|
| Ejection fraction, % | 68.4 ± 2.26 | 63.9 ± 3.01 | 0.318 |
| Fractional shortening, % | 37.8 ± 1.72 | 34.5 ± 2.17 | 0.248 |
| End-diastolic diameter, mm | 3.62 ± 0.07 | 3.73 ± 0.11 | 0.478 |
| End-systolic diameter, mm | 2.25 ± 0.08 | 2.46 ± 0.14 | 0.299 |
| Anterior wall thickness, mm | 1.06 ± 0.04 | 1.07 ± 0.04 | 0.887 |
| Posterior wall thickness, mm | 0.91 ± 0.06 | 1.03 ± 0.06 | 0.179 |
| Heart rate, beats/min | 435 ± 11.5 | 417 ± 15.0 | 0.389 |
Data are expressed as means ± SE. Echocardiographic analysis of mice was conducted before randomization for (TAC) surgery. MLK3, mixed lineage kinase-3. Data were analyzed by ANOVA.
Table 3.
Baseline hemodynamic analysis in MLK3+/+ and MLK3−/− mice
| MLK3+/+ | MLK3−/− | P Value | |
|---|---|---|---|
| 12 Weeks | |||
| n | 11 | 14 | |
| SBP, mmHg | 94.3 ± 2.1 | 109.3 ± 2.5 | <0.001 |
| DBP, mmHg | 62.9 ± 2.1 | 70.3 ± 2.1 | 0.03 |
| MAP, mmHg | 73.4 ± 2.1 | 83.3 ± 2.2 | 0.01 |
| LV EDP, mmHg | 5.0 ± 1.4 | 3.6 ± 0.9 | 0.39 |
| LV dP/dtmax, mmHg/s | 7,815 ± 279 | 8,207 ± 421 | 0.49 |
| LV dP/dtmin, mmHg/s | −7,859 ± 290 | −8,167 ± 333 | 0.52 |
| Contractile index, s−1 | 188 ± 8.9 | 180.4 ± 7.1 | 0.48 |
| Stroke volume, µl | 16.6 ± 1.6 | 16.6 ± 1.6 | 0.998 |
| Cardiac output, µl/min | 7,918 ± 681 | 7,636 ± 794 | 0.80 |
| LV ejection fraction, % | 48.1 ± 2.9 | 53.1 ± 3.4 | 0.29 |
| 24 Weeks | |||
| n | 4 | 4 | 0.83 |
| SBP, mmHg | 92.8 ± 1.5 | 94.3 ± 5.3 | 0.99 |
| DBP, mmHg | 59.7 ± 2.8 | 59.8 ± 3.6 | 0.92 |
| MAP, mmHg | 70.7 ± 2.0 | 71.3 ± 4.2 | 0.67 |
| LV EDP, mmHg | 9.1 ± 2.5 | 7.1 ± 2.8 | 0.72 |
| LV dP/dtmax, mmHg/s | 7,463 ± 801 | 6,950 ± 852 | 0.64 |
| LV dP/dtmin, mmHg/s | −7,697 ± 694 | −7,116 ± 729 | 0.42 |
| Contractile index, s−1 | 221.3 ± 16.4 | 202.3 ± 10.3 | 0.07 |
| Stroke volume, µl | 19.8 ± 1.2 | 13.3 ± 2.2 | 0.10 |
| Cardiac output, µl/min | 8,829 ± 427 | 6,380 ± 1,006 | 0.49 |
| LV ejection fraction, % | 54.5 ± 3.6 | 48.4 ± 6.1 | 0.83 |
Data are expressed as means ± SE. Pressure-volume loop hemodynamic analysis of mice at 12 and 24 wk of age is shown. MLK3, mixed lineage kinase-3; SBP, systolic blood pressure; DBP, diastolic blood pressure; LV, left vntricle; EDP, end-diastolic pressure; dP/dtmax/min, impaired rate of ventricular contractility/relaxation; MAP, mean arterial pressure. Data were analyzed by ANOVA.
MLK3 deficiency promotes maladaptive cardiac structural and functional abnormalities after pressure overload.
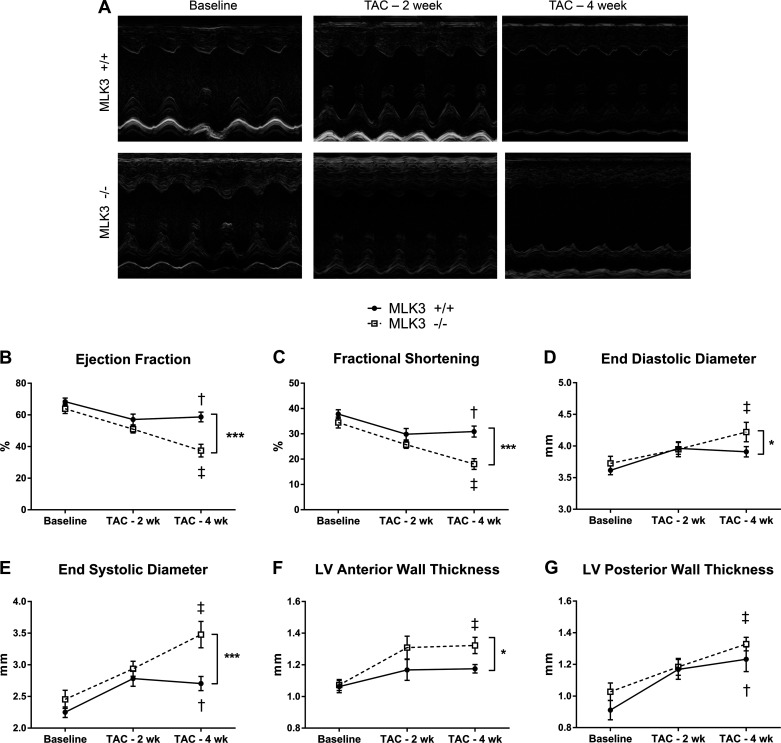
To test the cardiac hypertrophic and remodeling response to extrinsic stress, we subjected MLK3+/+ mice and MLK3−/− littermates to 4 wk of pressure overload via TAC or to sham surgery without aortic constriction. Cardiac structural and functional parameters were evaluated at baseline and thereafter every 2 wk by echocardiography (Fig. 2A). LV contractile function, as evaluated by ejection fraction and fractional shortening, was significantly impaired after 4 wk of TAC in MLK3−/− mice (Fig. 2, B and C) compared with MLK3+/+ littermates. LV chamber dimensions increased selectively in MLK3−/− but not in MLK3+/+ mice after 4 wk of TAC, consistent with LV dilation (Fig. 2, D and E). Additionally, MLK3−/− TAC mice displayed increased anterior wall thickness compared with MLK3+/+ TAC mice (Fig. 2F). Thickening of the LV posterior wall also occurred in both TAC groups after 4 wk of TAC and trended toward increased thickness in the MLK3−/− TAC mice compared with MLK3+/+ (Fig. 2G). Hemodynamic analysis after 4 wk of TAC also demonstrated, in MLK3−/− TAC hearts, trends toward increased LV end-diastolic pressure (LV EDP: MLK3+/+ TAC 13.33 ± 1.95 vs. MLK3−/− TAC 18.43 ± 3.17 mmHg, P = 0.133); impaired rate of ventricular contractility (dP/dtmax: MLK3−/− sham 9,072 ± 306 vs. MLK3−/− TAC 7,893 ± 580 mmHg/s, P = 0.11); and impaired rate of ventricular relaxation (dP/dtmin: MLK3+/+ TAC 8,760 ± 228 vs. MLK3−/− TAC 7576 ± 525 mmHg/s, P = 0.071; Table 4). TAC induced a significant pressure overload of 158.3 ± 4.27 and 156.2 ± 4.87 mmHg in MLK3+/+ and MLK3−/− TAC mice, respectively (P < 0.0001 sham vs. TAC by 2-way ANOVA; Table 4). Together, these data support that deletion of MLK3 leads to increased cardiac functional impairments and structural remodeling after chronic pressure overload.
Fig. 2.
Mixed lineage kinase-3-deficient (MLK3−/−) mice develop increased left ventricular (LV) dilation and systolic dysfunction after 4 wk of pressure overload. Cardiac structure and function were evaluated by serial echocardiography in wild-type (MLK3+/+) and MLK3−/− mice subjected to sham or 25-gauge transverse aortic constriction (TAC) surgery for 4 wk. A: representative M-Mode images of the LV in MLK3+/+ and MLK3−/− mice at baseline and after 2 or 4 wk of TAC. B: ejection fraction calculated from M-Mode images of the LV. C: fractional shortening. D: end-diastolic diameter of the LV. E: end-systolic diameter of the LV. F: anterior LV wall thickness. G: posterior LV wall thickness; n = 8 per genotype. Data were analyzed by two-way ANOVA with repeated measures, and genotypes were compared using a Bonferroni posttest. †P < 0.05 baseline vs. 4-wk TAC for MLK3+/+ mice; ‡P < 0.05 baseline vs. 4-wk TAC for MLK3−/− mice; *P < 0.05 between genotypes at 4-wk TAC time point; ***P < 0.001 between genotypes at 4-wk TAC time point.
Table 4.
Hemodynamic analysis in MLK3+/+ and MLK3−/− mice subjected to TAC for 4 wk
| Sham |
TAC |
||||
|---|---|---|---|---|---|
| MLK3+/+ (n = 6) | MLK3−/− (n = 5) | MLK3+/+ (n = 10) | MLK3−/− (n = 10) | P Value (Sham vs. TAC) | |
| LV EDP, mmHg | 4.88 ± 1.62 | 6.14 ± 2.59 | 13.3 ± 1.95 | 18.4 ± 3.17 | P = 0.0007 |
| LV dP/dtmax, mmHg/s | 8,810 ± 762 | 9,184 ± 667 | 9,072 ± 306 | 7,893 ± 579 | P = 0.384 |
| LV dP/dtmin, mmHg/s | −8,363 ± 590 | −8,534 ± 722 | −8,760 ± 228 | −7,576 ± 525 | P = 0.589 |
| SBP, mmHg | 99.1 ± 5.26 | 118.1 ± 7.98* | 158.3 ± 4.27 | 156.2 ± 4.87 | P < 0.0001 |
Data are expressed as means ± SE. Wild-type (MLK3+/+) and mixed lineage kinase-3-deficient (MLK3−/−) mice subjected to sham or 25-gauge transverse aortic constriction (TAC) surgery for 4 wk were analyzed using invasive hemodynamics. LV, left ventricle; dP/dtmax/min, impaired rate of ventricular contractility/relaxation; EDP, end-diastolic pressure; SBP, systolic blood pressure. Data were analyzed by two-way ANOVA; P values indicate comparison between sham and TAC-operated groups.
P < 0.05 vs. MLK3+/+ sham group.
MLK3 deletion increases pathological LV hypertrophy with pressure overload.
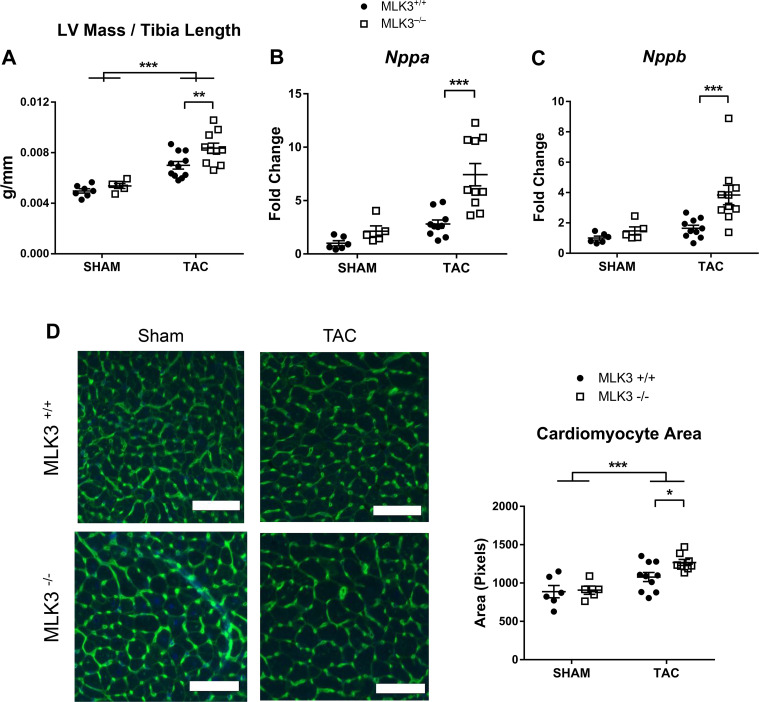
Hearts of MLK3−/− mice subjected to TAC exhibited increased LV mass compared with hearts of MLK3+/+ TAC mice (Fig. 3A). Gene expression of the hypertrophic markers natriuretic peptide A (Nppa) and natriuretic peptide B (Nppb), which increase in expression in pathological cardiac hypertrophy, did not differ in sham-treated MLK3−/− compared with MLK3+/+ mice, suggesting no overt fetal gene reexpression in the basal state in the mutant mice. However, both of these fetal genes increased in hearts of MLK3−/− TAC mice compared with MLK3+/+ TAC mice (Fig. 3, B and C). Histological analysis of CM size demonstrated increased CM cross-sectional area in MLK3−/− TAC hearts compared with MLK3+/+ TAC hearts (Fig. 3, D and E).
Fig. 3.
Mixed lineage kinase-3-deficient (MLK3−/−) mice develop increased pathological left ventricular (LV) hypertrophy after pressure overload. Wild-type (MLK3+/+) and MLK3−/− mice subjected to sham or 25-gauge transverse aortic constriction (TAC) surgery for 4 wk were evaluated for LV mass normalized to tibia length (n = 5–11 per group). A: RNA expression of Nppa (B) and Nppb (C) were evaluated by quantitative PCR in LV tissues (n = 5–10 per group). D: LV tissue sections were stained with wheat germ agglutinin and counterstained with DAPI. Cardiomyocyte area was quantified in the transverse plane (n = 5–10 mice per group, 50 myocytes per mouse). Scale bars, 100 pixels in each image. Data were analyzed by two-way ANOVA. ***P < 0.001.
MLK3 deletion increases pro-fibrotic gene expression changes, but not overt fibrosis, with TAC.
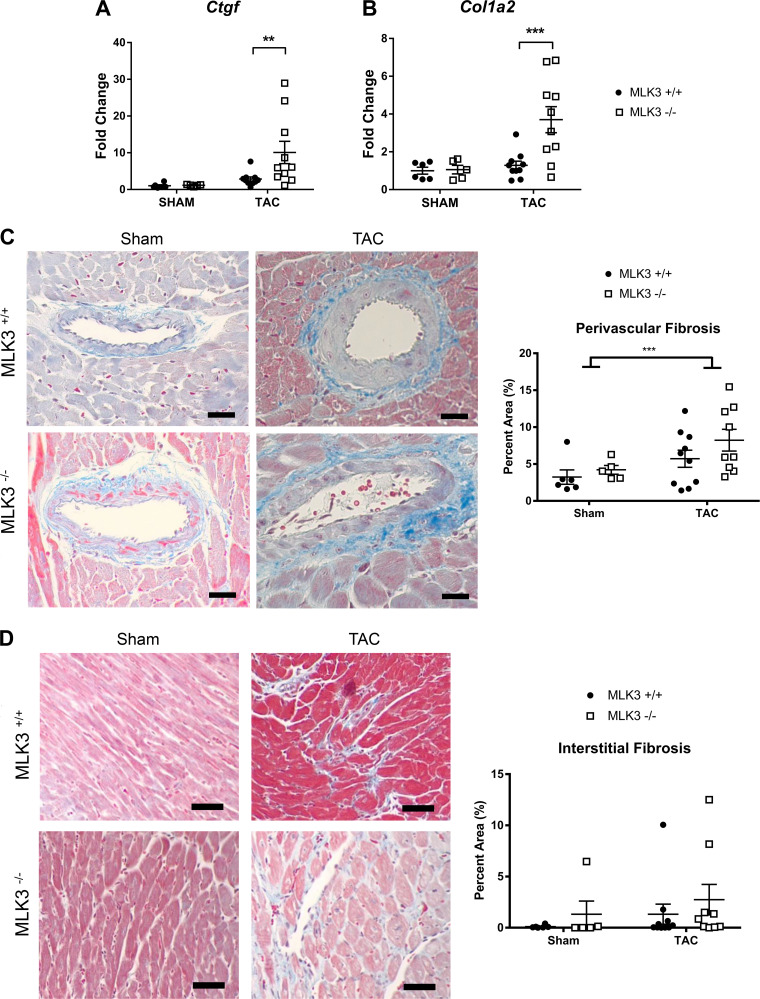
On the basis of the degree of cardiac hypertrophic and functional remodeling in MLK3−/− hearts, we hypothesized that MLK3−/− mice would also exhibit enhanced cardiac fibrosis in response to TAC. Gene expression analysis did demonstrate increased connective tissue growth factor (Ctgf) and collagen 1, α2 (Col1a2) mRNA levels in MLK3−/− TAC hearts compared with MLK3+/+ TAC hearts (Fig. 4, A and B). However, histological analysis of Masson’s trichrome-stained LV tissue sections revealed overall increased perivascular fibrosis in TAC hearts compared with sham, but perivascular fibrosis did not differ by genotype between MLK3+/+ and MLK3−/− mice after TAC (Fig. 4C). Interstitial fibrosis also did not differ between MLK3+/+ and MLK3−/− TAC hearts (Fig. 4D).
Fig. 4.
Analysis of profibrotic gene expression and cardiac fibrosis in mixed lineage kinase-3-deficient (MLK3−/−) left ventricles (LVs) after 4 wk of transverse aortic constriction (TAC). Wild-type (MLK3+/+) and MLK3−/− mice subjected to sham or 25-gauge TAC surgery for 4 wk were evaluated for RNA expression by quantitative PCR of Ctgf (A) and Col1a2 (B) in LV tissues (n = 5–10 per group). C: Masson’s trichrome staining of perivascular collagen in the LV (n = 5–10 per group). D: Masson’s trichrome staining of interstitial collagen in LV tissue sections (n = 5–10 per group). Scale bars, 100 pixels in each image. Data were analyzed by two-way ANOVA. **P < 0.01, ***P < 0.001.
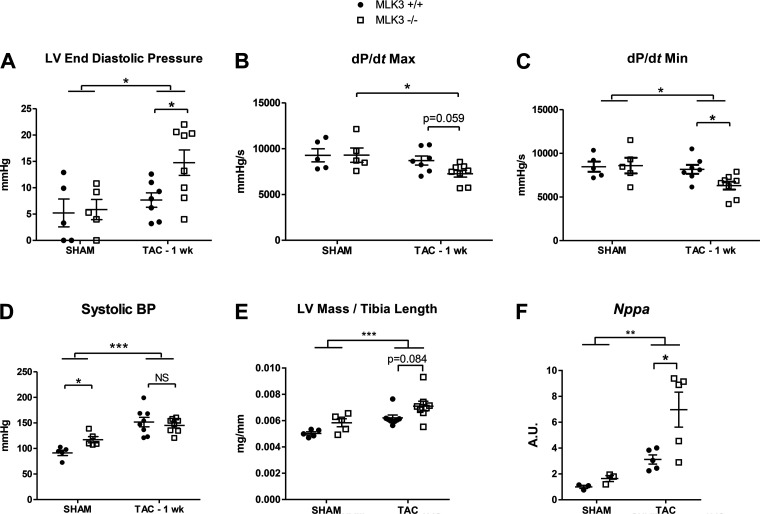
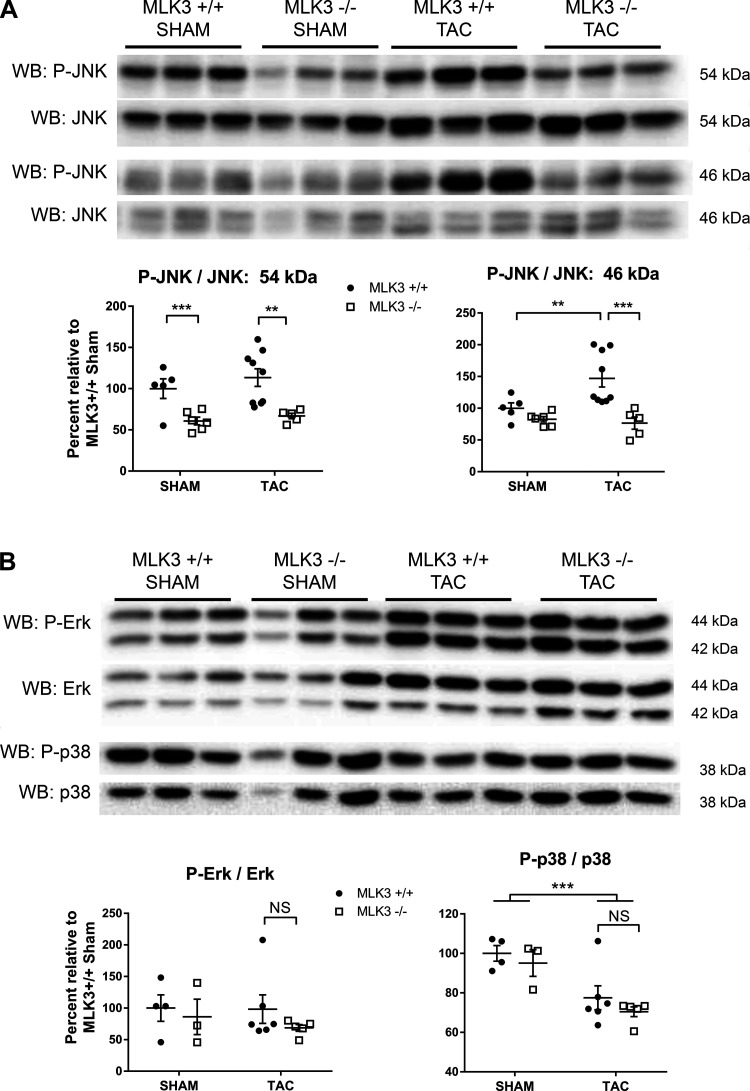
Deletion of MLK3 promotes LV dysfunction after short-term TAC and prevents myocardial JNK phosphorylation.
Given the established role of MLK3 in regulating JNK signaling in noncardiac tissues (32), we hypothesized that disrupted JNK activation contributes to the increased LV dysfunction and remodeling observed after 4 wk of TAC in MLK3−/− mice. Furthermore, JNK becomes rapidly activated after pressure overload (1, 21, 34), and deletion of JNK leads to increased LV contractile dysfunction detectable within the first 7 days after TAC (35). We therefore performed a short-term (1 wk) TAC experiment on a separate cohort of MLK3+/+ and MLK3−/− mice to examine the LV adaptive response to pressure overload and to explore early stress-responsive signaling. Evaluation of LV function by invasive hemodynamics demonstrated increased LV EDP, impaired rate of ventricular contractility (dP/dtmax), and impaired rate of ventricular relaxation (dP/dtmin) in MLK3−/− TAC mice compared with MLK+/+ TAC mice (Fig. 5, A–C), consistent with early LV dysfunction at a time point preceding significant cardiac structural changes with pressure overload. TAC induced a significant pressure overload of 151.8 ± 9.27 and 145.1 ± 4.94 mmHg in MLK3+/+ and MLK3−/− mice, respectively, after 1 wk of TAC (P < 0.0001 sham vs. TAC by 2-way ANOVA; Fig. 5D). LV mass normalized to tibia length trended toward an increase in the MLK3−/− TAC mice compared with MLK3+/+ TAC mice (Fig. 5E). Nppa expression, however, increased significantly in in the MLK3−/− TAC compared with MLK3+/+ TAC LVs (Fig. 5F), indicating an early presence of pathological hypertrophy in MLK3−/− pressure-overloaded hearts. We next assayed downstream JNK activation in MLK3−/− hearts. In sham-treated mice, JNK activation, assayed by Western blot of phosphorylated/total JNK, was significantly decreased in MLK3−/− sham compared with MLK3+/+ sham hearts, suggesting that MLK3 normally promotes basal myocardial JNK activation in vivo (Fig. 6A; JNK 54 kDa). After TAC, myocardial JNK phosphorylation increased compared with sham (Fig. 6A; JNK 46 kDa). However, compared with MLK3+/+ TAC hearts, MLK3−/− TAC hearts again displayed significantly impaired JNK phosphorylation (Fig. 6A; JNK 54 and 46 kDa). We also assayed activation of other MAPK signaling cascades, including p38 and ERK, which are regulated by MLK3 in other tissues (5). We observed no significant difference in ERK phosphorylation between MLK3+/+ or MLK3−/− TAC hearts. Phosphorylation of p38 was significantly impaired after TAC to a similar degree in both genotypes (Fig. 6B). These findings provide further support that in the myocardium MLK3 selectively promotes activation of the JNK signaling cascade as a potential mechanism to prevent adverse cardiac remodeling.
Fig. 5.
Acute pressure overload induces cardiac functional changes in mixed lineage kinase-3-deficient (MLK3−/−) mice in the absence of overt structural abnormalities. Left ventricular (LV) function was evaluated by in vivo hemodynamics in wild-type (MLK3+/+) and MLK3−/− subjected to sham or 25-gauge transverse aortic constriction (TAC) surgery for 1 wk. A: LV end-diastolic pressure (LVEDP; n = 6–8 per group). B: maximal rate of LV contractility. C: maximal rate of LV relaxation. D: systolic blood pressure (BP) measured proximal to the TAC ligature. E: LV mass normalized to tibia length. F: RNA expression of Nppa evaluated by quantitative PCR in LV tissues. Data were analyzed by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 6.
Left ventricles (LVs) of mixed lineage kinase-3-deficient (MLK3−/−) mice display selective impaired c-Jun NH2 kinase (JNK) phosphorylation after pressure overload. A: Western blot (WB) for phosphorylated (P-) and total JNK in LV tissue lysates from wild-type (MLK3+/+) and MLK3−/− mice subjected to sham or 1-wk 25-gauge transverse aortic constriction (TAC) surgery. The 54- and 46-kDa band sizes from the same membrane are shown separately (n = 5–9). B: Western blotting for phosphorylated and total protein for extracellular signal-regulated kinase (ERK) and p38 (n = 3–6). In A and B summary data, the ratio of phosphorylated to total signal was calculated by densitometry and expressed as a percentage relative to MLK3+/+ sham samples for each protein. Data were analyzed by two-way ANOVA. **P < 0.01, ***P < 0.001.
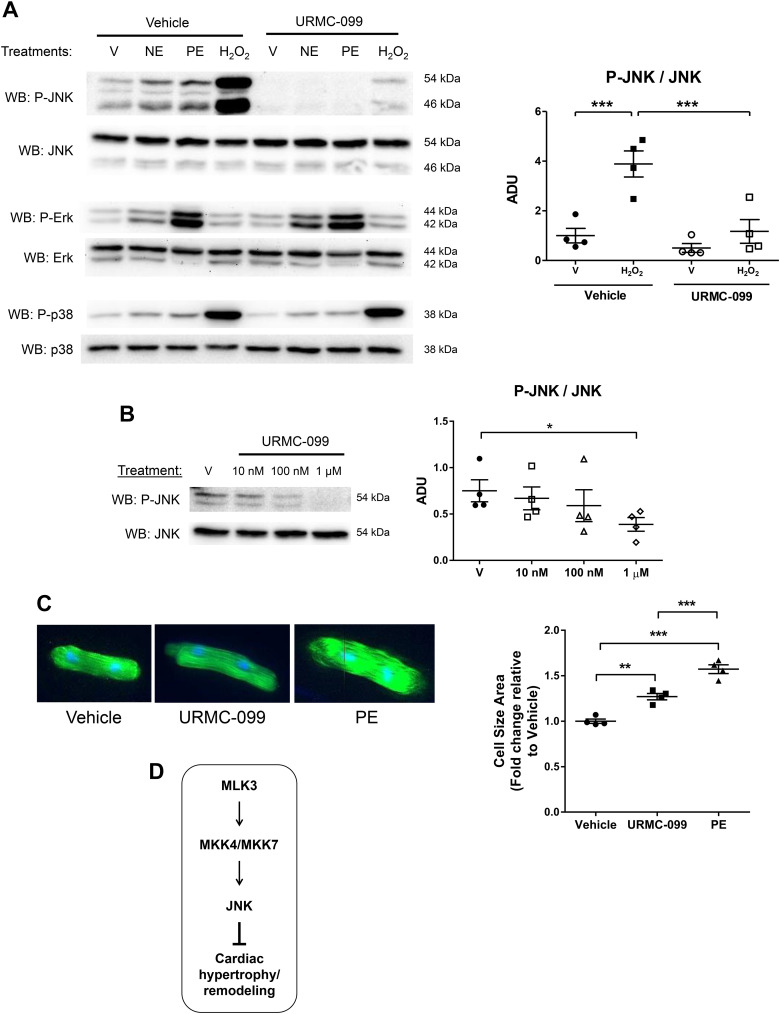
MLK3 inhibition selectively modulates JNK phosphorylation and promotes cardiomyocyte hypertrophy.
The reduced JNK activation in MLK3−/− sham and TAC hearts led us to investigate MLK3-dependent regulation of JNK specifically in the CM. Since MLK3 promotes JNK activation through kinase signaling, we tested the effect of administration of the MLK3 inhibitor URMC-099 on JNK activation (phosphorylation) in isolated CMs. Treatment of ARVMs with NE, PE, or H2O2 each stimulated JNK phosphorylation. Pretreatment with the MLK3 inhibitor URMC-099 prevented agonist-induced JNK phosphorylation (Fig. 7A). Whereas NE, PE, and H2O2 each stimulated phosphorylation of ERK, and H2O2 robustly increased p38 phosphorylation, pretreatment with URMC-099 had no effect on these MAPK pathways, supporting selective inhibition of the JNK signaling cascade with this inhibitor (Fig. 7A). Short-duration treatment (1 h) of ARVMs with increasing doses of URMC-099 reduced basal JNK phosphorylation, with the most pronounced effect observed at the 1 µM dose (Fig. 7B). Because of the previously reported role of JNK signaling in opposing cardiac hypertrophy and cardiac remodeling (21), we next tested the effects of longer-term (48 h) URMC-099 administration on CM hypertrophy. ARVMs treated with either PE, an adrenergic receptor agonist known to produce hypertrophy in ARVMs (15), or URMC-099 developed increased ARVM cell size (URMC-099: 27.0 ± 3.49% increase vs. vehicle; PE: 57.3% ± 4.82% increase vs. vehicle; Fig. 7C). These data support that CM MLK3 normally represses cellular hypertrophy via activation of JNK phosphorylation.
Fig. 7.
Administration of mixed lineage kinase-3 (MLK3) inhibitor selectively impairs c-Jun NH2 kinase (JNK) phosphorylation and promotes hypertrophy in cardiomyocytes. A: adult rat ventricular cardiomyocytes (ARVMs) were pretreated with vehicle (DMSO) or MLK3 kinase inhibitor URMC-099 (1 μM) for 60 min before stimulation with vehicle (H2O, labeled V), norepinephrine (NE, 1 μM, 20 min), phenylephrine (PE, 20 µM, 20 min), or hydrogen peroxide (H2O2, 100 µM, 60 min). Levels of phosphorylated (P-) and total forms of JNK, extracellular signal-regulated kinase (ERK), and p38 were evaluated by Western blotting. Representative blots are shown of n = 3–4 separate experiments. Densitometry of phosphorylated/total JNK is also shown. B: ARVMs were pretreated with DMSO vehicle (V) or URMC-099 (10 nM, 100 nM, or 1 μM) for 60 min. JNK phosphorylation was quantified and expressed relative to total JNK (n = 4, 54 kDa). C: ARVMs were treated with vehicle (DMSO), URMC-099 (100 nM), or DMSO + PE (20 μM) for 48 h. Cells were then fixed and stained with phalloidin and DAPI. Cardiomyocyte (CM) size was quantified and expressed as fold change relative to vehicle-treated cells (n = 4). For these experiments, each replicate represents an independent experiment from separate CM preparations; All data were analyzed by one-way ANOVA with Bonferroni’s posttest. D: proposed model of antiremodeling MLK3-MAP kinase kinase-4 (MKK4)/MKK7-JNK signaling cascade in the CM.
DISCUSSION
In the present study we observed 1) elevated MLK3 protein expression in human cardiomyopathy and failing mouse LV tissue, 2) increased LV dysfunction and pathological hypertrophic remodeling after TAC in MLK3−/− mice, 3) impaired JNK activation in MLK3−/− TAC hearts, 4) abrogation of JNK phosphorylation in CMs exposed to MLK3 inhibition, and 5) induction of CM hypertrophy in response to MLK3 inhibition. Taking all these together, we interpret these findings to support the model in Fig. 7D, in which MLK3 opposes pressure overload-induced cardiac hypertrophy, dysfunction, and remodeling and promotes antihypertrophic JNK signaling through a kinase-dependent mechanism.
Increased MLK3 expression in human cardiomyopathy.
Here, we present the first evidence, to our knowledge, of MLK3 expression in the human heart. Furthermore, we demonstrate that MLK3 expression increases in cardiac tissues from human patients with two distinct forms of cardiomyopathy. In mice subjected to an experimental model of HF, we observed early increased LV MLK3 expression at 1wk post-TAC, followed by normalization of expression at 4 wk after TAC. This suggests that lack of sustained MLK3 elevation in the LV after pressure overload may contribute to the pathological remodeling observed normally in WT mice at the 4-wk time point and may explain the more advanced pathological remodeling response at 4 wk with complete deletion in MLK3−/− mice (Figs. 2 and 3). It is interesting that MLK3 expression again becomes elevated late after TAC (10 wk), which appears to correlate with the increased MLK3 expression observed in human end-stage HF LVs. We do note, however, that MLK3 expression increased in our human HF samples to a higher degree (~6-fold) than that observed in our late-stage experimental models (2-fold), which may reflect that the human samples originated from patients with end-stage HF, whereas we obtained the mouse samples from an intermediate time point during the remodeling process. The correlation of MLK3 expression with end-stage HF also does not allow us to conclude whether MLK3 normally promotes adverse remodeling or rather whether its increased expression might represent a protective mechanism that ultimately becomes overwhelmed by pro-remodeling processes. These questions led us to test the effects of MLK3 deletion in the MLK3−/− mouse model.
In cultured CMs, MLK3 mRNA expression increased in response to hypertrophic treatment, suggesting that CM-derived MLK3 contributes to the elevated MLK3 protein levels observed in HF. It is conceivable that endothelial cells (31) and/or vascular smooth muscle cells (11) within the myocardium increase synthesis of MLK3 protein in response to TAC. An alternative, or additional, explanation could be that circulating inflammatory cells such as T cells (18, 27) and/or macrophages (17), both of which express MLK3, become recruited to the remodeled myocardium and might also contribute to the increased MLK3 protein detected in failing hearts.
Basal LV hypertrophy observed in MLK3-deficient mice.
Our observation of increased LV mass in MLK3−/− mice supports a novel role of MLK3 in the regulation of LV hypertrophy in vivo. The initial characterization of whole body MLK3−/− mice reported expected Mendelian birth ratios and normal lifespans and detected no overt pathologies (2). MLK3−/− mice develop increased carotid artery injury-induced neointimal formation and associated vascular smooth muscle cell proliferation compared with MLK3+/+ mice (11). However, the effects of MLK3 specifically on cardiac structure and function in vivo had not, to our knowledge, been investigated previously. In MLK3−/− mice, we observed baseline LV hypertrophy but no change in cardiac function, LV structure, or basal pathological gene expression. We do note that the baseline LV hypertrophy in MLK3−/− mice must be interpreted in the setting of the modestly increased SBP and DBP that we detected via hemodynamic analysis. Although these measurements were obtained from anesthetized mice and therefore may not reflect the BP difference between genotypes in the conscious state, they raise the possibility that increased blood pressure may contribute to the basal LV hypertrophy in the MLK3−/− mice. Given prior reports that MLK3 functions in the vascular smooth muscle cell to oppose proliferation, we speculate that MLK3 could regulate blood pressure as well through a smooth muscle cell-specific mechanism. Addressing these questions remains beyond the scope of the present study. Importantly in our TAC studies, LV afterload was increased to the same degree between genotypes; additionally, MLK3 kinase inhibition induced hypertrophy of isolated CMs in vitro (Fig. 7). These two observations support that at least some component of the basal LV hypertrophy in MLK3−/− mice results directly from loss of MLK3 in CMs.
LV dysfunction and pathological hypertrophic remodeling in MLK3-deficient TAC hearts.
Our 4-wk TAC study reveals that deletion of MLK3 promotes increased systolic dysfunction and pathological hypertrophic remodeling in the setting of pressure overload. Serial echocardiography of TAC mice demonstrated a progressive decline of LV contractile function in MLK3−/− mice and increased LV chamber dimensions compared with MLK3+/+ littermates. Combined with higher levels of hypertrophic gene markers (Nppa, Nppb), we interpret these data to identify for the first time that MLK3 normally attenuates pathological LV hypertrophy with chronic pressure overload.
It is interesting to note that, although MLK3−/− TAC hearts exhibited increased profibrotic gene expression (Ctgf and Col1a2) compared with MLK3+/+ TAC hearts, the degree of interstitial and perivascular fibrotic collagen deposition did not differ between genotypes after TAC. Given the increased profibrotic gene expression in hearts of MLK3−/− TAC mice at 4 wk, it is conceivable that a longer duration of chronic pressure overload could result in detection of increased overt cardiac fibrosis in MLK3−/− hearts. However, an alternative possibility is that MLK3 may selectively attenuate CM hypertrophy, dysfunction, and remodeling after pressure overload but may have minimal or even opposing effects on mechanisms of cardiac fibrosis. This interpretation is supported by our observation that robust structural and functional abnormalities in MLK3−/− TAC LVs occurred at a time point at which we observed no difference in perivascular or interstitial fibrosis between genotypes. Delineating these separate effects will require temporal and tissue-specific modulation of MLK3 in vivo and remains beyond the scope of this study. At the least, these observations support that the TAC-induced functional abnormalities in MLK3−/− TAC LVs do not result as a consequence of enhanced fibrosis. Taking these together, we interpret our findings to reveal for the first time that MLK3 functions as a cardioprotective molecule to oppose cardiac dysfunction, hypertrophy, and remodeling with chronic pressure overload independently of direct effects on fibrosis.
Significance of impaired JNK phosphorylation in MLK3-deficient TAC hearts.
Previously, we and others (1, 20, 21, 26, 34) have observed activation of the JNK signaling cascade in the mouse heart in response to pressure overload. Myocardial signaling mechanisms promoting this rapid JNK activation remain incompletely understood. In noncardiac cells, MLK3 phosphorylates and activates the MKK4/MKK7 kinases to stimulate JNK activation (32), which led us to evaluate JNK phosphorylation in MLK3−/− hearts. Our in vivo signaling studies identified selective ablation of JNK phosphorylation in MLK3−/− LVs at baseline and after pressure overload. These findings support a requirement of MLK3 for both the maintenance of basal myocardial JNK activation and the activation of JNK in response to LV pressure overload.
Genetic deletion of the upstream activators MKK4/MKK7 or of JNK increases TAC-induced cardiac hypertrophy (21–23), supporting that JNK activation normally attenuates cardiac hypertrophy and remodeling in vivo. Similarly, CM-specific deletion of Cdc42, a potent upstream JNK activator and allosteric activator of MLK3, results in increased cardiac hypertrophy and reduced JNK activation after TAC, which could be attenuated by overexpressing MKK7 in the myocardium (24). On the basis of our current findings that MLK3 is required for myocardial JNK phosphorylation, we posit that the cardioprotective effects of MLK3 are mediated through downstream JNK signaling. This is supported by our findings that MLK3−/− mice displayed reduced JNK activation both at baseline and after TAC yet did not display abnormalities in activation of other MAPK pathways (ERK, p38) after TAC compared with MLK3+/+ controls.
Our short-term (1 wk) TAC study in MLK3−/− mice provides further support for the role of JNK in mediating the protective actions of MLK3 in the heart. First, the blunted JNK activation in 1-wk MLK3−/− TAC LVs temporally precedes the later increased hypertrophy and remodeling observed in the 4-wk TAC study, suggesting an early disruption of JNK signaling contributing to the later phenotype. Second, the more severe LV dysfunction in MLK3−/− TAC mice at 1 wk post-TAC corresponds to rapid LV functional decompensation observed in JNK knockout mice subjected to TAC (35), in which TAC-induced LV dysfunction occurs early after pressure overload in the absence of hypertrophic or structural remodeling. These observations therefore provide further support that MLK3 normally promotes LV compensation to pressure overload and opposes pathological remodeling through activation of JNK in the myocardium.
In contrast to our data and the studies described above, others have reported that opposing JNK phosphorylation by expressing dominant-negative MKK4 in the heart can promote adverse cardiac growth and dysfunction with TAC (7). Additionally, decreasing JNK phosphorylation via increased dual specific phosphatase-12 activity was associated with improved cardiac function and less cardiac hypertrophy with aortic banding (20). Moreover, mice with genetic deletion of the upstream MAPK protein apoptosis signal-regulating kinase-1 (ASK1) are protected from TAC-induced cardiac remodeling and concomitantly exhibit reduced cardiac JNK activation with TAC (39). It is conceivable that the phenotypic differences observed between ASK1- and MLK3-deficient mice in the context of cardiac remodeling are dependent on downstream JNK cellular targeting/localization and warrants further investigation. Thus, although the role of JNK and its upstream regulators in modulating CM hypertrophy and function remains controversial and is highly complex, our current findings support the notion that stimulation of the MLK3-MKK4/MKK7-JNK signaling cascade represents a mechanism that preserves normal cardiac structure and function with pressure overload.
Role of MLK3 in regulating CM JNK signaling and cellular hypertrophy.
Our findings also identify a novel role of MLK3 specifically in the CM in stimulating JNK signaling and opposing CM hypertrophy. Although JNK signaling modulates the cardiac remodeling response (21), the upstream mechanisms governing JNK regulation specifically in the CM remain incompletely understood. In neonatal CM, kinase inhibition of MLK family members 1–3 with CEP-11004 blunts JNK activation in response to hypertrophic agonists (29). In brain tissue and in immortalized microglial cell lines, the MLK3 kinase inhibitor URMC-099 suppressed JNK phosphorylation (14, 25). On the basis of these reports, we tested the conditions under which MLK3 regulates JNK phosphorylation in the adult CM, and we have demonstrated that MLK3 inhibition prevents both neurohormonal and oxidant-stimulated JNK phosphorylation. Furthermore, acute treatment with the MLK3 inhibitor URMC-099 reduced basal JNK phosphorylation, and sustained treatment with URMC-099 for 48 h caused CM hypertrophy (Fig. 7, B and C), supporting that MLK3 normally promotes JNK activation and opposes hypertrophy in CMs.
In contrast with our in vitro findings, previous reports observed that MKK7 overexpression in neonatal rat CMs stimulates JNK signaling and causes CM hypertrophy (38). Similarly, transduction of neonatal rat CMs with dominant-negative SEK-1 (also known as MKK4) prevents CM hypertrophy induced by ET-1 (8), suggesting that the JNK pathway promotes CM growth at least in response to select membrane receptor agonists. The difference between our data and other published results may reflect a difference in model systems (neonatal vs. adult CMs) and/or may represent potential selective actions of JNK activation by MLK3 compared with other JNK regulators. Furthermore, it remains possible that additional MLK3 kinase-dependent signaling pathways also contribute to this observed regulation of CM growth. However, our MLK3 kinase inhibitor findings of selective disruption of JNK signaling in CMs, taken with our in vivo studies discussed above, support the idea that MLK3 opposes myocardial hypertrophy primarily via JNK signaling. Taking all that together, we therefore interpret our results to support first, that basal, neurohormonal, and oxidant-induced phosphorylation/activation of JNK in the CM requires MLK3 catalytic function, and second, that MLK3 kinase activity represents a novel antihypertrophic mechanism in the CM.
Clinical significance of MLK3 as antiremodeling JNK activator.
In addition to the regulation of MLK3 expression in the human heart described above, our dual observations, that deletion of MLK3 worsens TAC-induced cardiac remodeling and that MLK3 inhibition promotes CM hypertrophy, are of specific interest, because inhibition of MLK3 kinase activity with URMC-099 is under preclinical investigation for a number of noncardiac conditions (25, 37). Therefore, understanding the potential adverse myocardial hypertrophic effects of MLK3 inhibition in humans may be of particular importance. Conversely, our findings support that augmentation of MLK3 expression or potentially MLK3 activity may represent a novel strategy to oppose adverse cardiac remodeling and should be explored in future studies.
Limitations.
One potential limitation of the current study is that the degree of cardiac hypertrophy, fetal gene induction, and fibrosis in our TAC experiments was relatively modest in the MLK3+/+ littermates. We intentionally used a moderate degree of aortic constriction, so as to best detect potential increases in the chronic remodeling response of MLK3−/− mice. Whereas MLK3+/+ mice subjected to this moderate constriction for 4 wk exhibited indexes of compensatory cardiac remodeling, i.e., increased LV hypertrophy, modestly impaired LV contractile function, and no induction of prohypertrophic/fibrotic gene expression changes, MLK3−/− mice developed a dilated cardiomyopathy phenotype with pathological gene expression changes. Thus, our experimental model did permit detection of increased remodeling and the subsequent interpretations of the role of MLK3 in normally opposing this process in vivo.
Furthermore, although we observed that MLK3 kinase activity opposes CM hypertrophy in cell culture, our in vivo findings of increased TAC-induced remodeling were in a whole body deletion model and thus do not identify the specific cardiovascular cell type(s) through which MLK3 normally opposes remodeling in vivo. For example, MLK3 resides in the cardiac fibroblast and vascular smooth muscle cell (11), and our results do not delineate the degree to which the MLK3−/− cardiac response to pressure overload may be due to deletion of MLK3 in these and other cells. Similarly, the whole body deletion of MLK3 in our in vivo model raises the possibility that the accentuated adverse remodeling and dysfunction observed in pressure-overloaded MLK3−/− mice reflect the baseline hypertrophy in these animals rather than specific effects of MLK3. Future investigations in conditional deletion and gain-of-function models will be required to determine the specific cell types contributing to the cardiac dysfunction with pressure overload. Finally, we investigated the role of MLK3 in promoting myocardial hypertrophy by using a chemical inhibitor of MLK3. While the chemical inhibitor URMC-099 inhibits the MLK3 isoform (IC50 = 14 nM), it was also noted to target other MLK family isoforms (MLK1 and MLK2) as well as the unrelated MAPK protein leucine-rich repeat kinase-2 (LRRK2) (14). Interestingly, LRRK2 also stimulates the MKK4-JNK signaling cascade (6, 13). Therefore, we cannot rule out the possibility that regulation of JNK by other MLK family members or LRRK2 may contribute to this effect. Despite these limitations, we suggest that the combined cell culture and in vivo approaches of our study demonstrate a novel role of MLK3 as a cardioprotective molecule in the setting of chronic pressure overload.
In summary, this work provides the first evidence that MLK3 opposes cardiac hypertrophy, prevents adverse remodeling with LV pressure overload-induced HF, mediates CM JNK activation, and opposes CM hypertrophy. These findings suggest that activation of MLK3 could represent a novel approach to oppose cardiac remodeling in the setting of HF. More broadly, our findings support the general concept that investigating upstream regulators of myocardial JNK activation, such as MLK3, can reveal novel mechanisms governing pathological cardiac remodeling.
GRANTS
This study was supported by NIH Grants R03-AG-042367, KL2-TR-001063-01, and R01-HL-131831 (to R. M. Blanton). T. D. Calamaras and R. A. Baumgartner were supported by an institutional training grant (T32-HL-069770).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.B., R.H.K., and T.D.C. conceived and designed the study conceived and designed study; T.D.C., R.A.B., M.J.A., A.L.M., K.T., D.A.R., C.W.C., N.L., W.E.B., X.Q., G.-R.W., and R.M.B. performed experiments; T.D.C., R.A.B., A.L.M., K.T., D.A.R., C.W.C., G.-R.W., and R.M.B. analyzed data; T.D.C., R.A.B., M.J.A., A.L.M., K.T., D.A.R., R.H.K., and R.M.B. interpreted results of experiments; T.D.C., R.A.B., K.T., D.A.R., and R.M.B. prepared figures; T.D.C. and R.M.B. drafted manuscript; T.D.C., R.J.D., and R.M.B. edited and revised manuscript; T.D.C., R.A.B., M.J.A., A.L.M., K.T., D.A.R., C.W.C., N.L., W.E.B., X.Q., G.-R.W., R.J.D., N.K.K., R.H.K., and R.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Tufts Animal Histology Core and members of the Molecular Cardiology Research Institute.
Present addresses: R. A. Baumgartner, Division of Cardiology, University of Pittsburgh Medical Center, Pittsburgh, PA; A. L. McLaughlin:, Department of Medicine, Brown University School of Medicine, Providence, RI.
REFERENCES
- 1.Blanton RM, Takimoto E, Lane AM, Aronovitz M, Piotrowski R, Karas RH, Kass DA, Mendelsohn ME. Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc 1: e003731, 2012. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol 25: 3670–3681, 2005. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem 270: 29071–29074, 1995. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 4.Calamaras TD, Lee C, Lan F, Ido Y, Siwik DA, Colucci WS. The lipid peroxidation product 4-hydroxy-trans-2-nonenal causes protein synthesis in cardiac myocytes via activated mTORC1-p70S6K-RPS6 signaling. Free Radic Biol Med 82: 137–146, 2015. doi: 10.1016/j.freeradbiomed.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol 6: 770–776, 2004. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, Weng YH, Chien KY, Lin KJ, Yeh TH, Cheng YP, Lu CS, Wang HL. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ 19: 1623–1633, 2012. doi: 10.1038/cdd.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero JL, Picard M, Rosenzweig A, Force T. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH2-terminal kinases. J Clin Invest 104: 391–398, 1999. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J Clin Invest 102: 1311–1320, 1998. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 345: 1667–1675, 2001. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 10.Du Y, Böck BC, Schachter KA, Chao M, Gallo KA. Cdc42 induces activation loop phosphorylation and membrane targeting of mixed lineage kinase 3. J Biol Chem 280: 42984–42993, 2005. doi: 10.1074/jbc.M502671200. [DOI] [PubMed] [Google Scholar]
- 11.Gadang V, Konaniah E, Hui DY, Jaeschke A. Mixed-lineage kinase 3 deficiency promotes neointima formation through increased activation of the RhoA pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 34: 1429–1436, 2014. doi: 10.1161/ATVBAHA.114.303439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3: 663–672, 2002. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- 13.Gloeckner CJ, Schumacher A, Boldt K, Ueffing M. The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J Neurochem 109: 959–968, 2009. doi: 10.1111/j.1471-4159.2009.06024.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodfellow VS, Loweth CJ, Ravula SB, Wiemann T, Nguyen T, Xu Y, Todd DE, Sheppard D, Pollack S, Polesskaya O, Marker DF, Dewhurst S, Gelbard HA. Discovery, synthesis, and characterization of an orally bioavailable, brain penetrant inhibitor of mixed lineage kinase 3. J Med Chem 56: 8032–8048, 2013. doi: 10.1021/jm401094t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong MY, Walker JS, Brown RD, Moore RL, Vinson CS, Colucci WS, Long CS. AFos inhibits phenylephrine-mediated contractile dysfunction by altering phospholamban phosphorylation. Am J Physiol Heart Circ Physiol 298: H1719–H1726, 2010. doi: 10.1152/ajpheart.00937.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SO, Irwin P, Katz S, Pelech SL. Expression of mitogen-activated protein kinase pathways during postnatal development of rat heart. J Cell Biochem 71: 286–301, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Kuwahara F, Kai H, Tokuda K, Niiyama H, Tahara N, Kusaba K, Takemiya K, Jalalidin A, Koga M, Nagata T, Shibata R, Imaizumi T. Roles of intercellular adhesion molecule-1 in hypertensive cardiac remodeling. Hypertension 41: 819–823, 2003. doi: 10.1161/01.HYP.0000056108.73219.0A. [DOI] [PubMed] [Google Scholar]
- 18.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129: 2111–2124, 2014. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 20.Li WM, Zhao YF, Zhu GF, Peng WH, Zhu MY, Yu XJ, Chen W, Xu DC, Xu YW. Dual specific phosphatase 12 ameliorates cardiac hypertrophy in response to pressure overload. Clin Sci (Lond) 131: 141–154, 2017. doi: 10.1042/CS20160664. [DOI] [PubMed] [Google Scholar]
- 21.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J 22: 5079–5089, 2003. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Zi M, Chi H, Jin J, Prehar S, Neyses L, Cartwright EJ, Flavell RA, Davis RJ, Wang X. Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload. J Mol Cell Cardiol 50: 702–711, 2011. doi: 10.1016/j.yjmcc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Zi M, Jin J, Prehar S, Oceandy D, Kimura TE, Lei M, Neyses L, Weston AH, Cartwright EJ, Wang X. Cardiac-specific deletion of mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ Res 104: 905–914, 2009. doi: 10.1161/CIRCRESAHA.108.188292. [DOI] [PubMed] [Google Scholar]
- 24.Maillet M, Lynch JM, Sanna B, York AJ, Zheng Y, Molkentin JD. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J Clin Invest 119: 3079–3088, 2009. doi: 10.1172/JCI37694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marker DF, Tremblay ME, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, Gelbard HA. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 33: 9998–10010, 2013. doi: 10.1523/JNEUROSCI.0598-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadruz W Jr, Kobarg CB, Kobarg J, Franchini KG. c-Jun is regulated by combination of enhanced expression and phosphorylation in acute-overloaded rat heart. Am J Physiol Heart Circ Physiol 286: H760–H767, 2004. doi: 10.1152/ajpheart.00430.2003. [DOI] [PubMed] [Google Scholar]
- 27.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velázquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 8: 776–787, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 29.Ola A, Kerkelä R, Tokola H, Pikkarainen S, Skoumal R, Vuolteenaho O, Ruskoaho H. The mixed-lineage kinase 1-3 signalling pathway regulates stress response in cardiac myocytes via GATA-4 and AP-1 transcription factors. Br J Pharmacol 159: 717–725, 2010. doi: 10.1111/j.1476-5381.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651–1658, 2001. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 31.Ramo K, Sugamura K, Craige S, Keaney JF Jr, Davis RJ. Suppression of ischemia in arterial occlusive disease by JNK-promoted native collateral artery development. eLife 5: e18414, 2016. doi: 10.7554/eLife.18414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis JM, Avruch J. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem 271: 19025–19028, 1996. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- 33.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A 88: 8277–8281, 1991. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatner SF. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest 110: 271–279, 2002. doi: 10.1172/JCI0214938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tachibana H, Perrino C, Takaoka H, Davis RJ, Naga Prasad SV, Rockman HA. JNK1 is required to preserve cardiac function in the early response to pressure overload. Biochem Biophys Res Commun 343: 1060–1066, 2006. doi: 10.1016/j.bbrc.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 36.Thoonen R, Giovanni S, Govindan S, Lee DI, Wang GR, Calamaras TD, Takimoto E, Kass DA, Sadayappan S, Blanton RM. Molecular screen identifies cardiac myosin-binding protein-C as a protein kinase G-Iα substrate. Circ Heart Fail 8: 1115–1122, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomita K, Kohli R, MacLaurin BL, Hirsova P, Guo Q, Sanchez LHG, Gelbard HA, Blaxall BC, Ibrahim SH. Mixed-lineage kinase 3 pharmacological inhibition attenuates murine nonalcoholic steatohepatitis. JCI Insight 2: e94488, 2017. doi: 10.1172/jci.insight.94488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem 273: 5423–5426, 1998. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A 100: 15883–15888, 2003. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]