Autophagy is a complex intracellular mechanism for dismantling and recycling amino acid components of damaged or unneeded cellular proteins and organelles. Physiological and pathological roles of autophagy have been connected to numerous diseases. In the cardiovascular system, the role of autophagy has been centered around cardiac tissue (e.g., cardiomyocytes) as a mechanism responding to injury (e.g., ischemia-reperfusion) and heart failure (8). However, autophagy also holds an integral role within the endothelium, responding to chemical (e.g., oxLDL) and physical stimuli (e.g., shear stress) implicated in the development and progression of atherosclerosis. Autophagy is generally considered a prosurvival pathway and is prominently involved in preventing inflammation and atherosclerosis (4). Accumulating evidence suggests that disruption of endothelial autophagy results in endothelial dysfunction (4) and accelerated atherosclerosis (8). Shear stress has emerged as a critical modulator of autophagy. In this context, low (<10–12 dyn/cm2) and oscillatory shear stress reduce autophagy and promote inflammatory pathways within endothelial cells. Conversely, laminar (15–30 dyn/cm2) and high shear stress (>30 dyn/cm2) promote protective intracellular signaling and protect against endothelial dysfunction (4). Importantly, although a basal amount of autophagy is necessary to maintain vascular homeostasis, excessive autophagic flux disrupts vascular homeostasis to a similar degree as loss of autophagy and contributes to development of inflammation and atherosclerosis (8). Because of the critical role of autophagic flux in “detoxifying” during cellular stress and heightened metabolism (8), a connection to free radical production is a logical connection. By breaking down damaged proteins and other cellular constituents, autophagy maintains reactive oxygen species (ROS) production within a physiological range. It is generally accepted that elevated ROS formation reduces nitric oxide (NO) bioavailability, namely through inactivation of NO by superoxide to form peroxynitrite. Data from cultured endothelial cells exposed to laminar shear stress demonstrate a mechanosensitive activation of the autophagy signaling cascade in parallel with enhanced NO signaling and reduced ROS formation. As such, autophagic flux represents a critical mechanism for maintaining vascular homeostasis implicated by release of NO and inhibition of autophagy eliciting shear-induced increases in ROS formation, endothelial NO synthase (eNOS) phosphorylation, and NO production (2). It should be noted that wall shear stress shares an inverse relationship with the internal diameter of arteries and that eNOS protein expression/content is greater in smaller vessels (e.g., resistance arteries) relative to large arteries (e.g., conduit arteries); however, when normalized to wall shear stress, there do not appear to be differences in eNOS content between artery regions (5).
In a recent article published in the American Journal of Physiology-Heart and Circulatory Physiology, Park et. al. (8a) examined for the first time the physiological relevance of shear-induced induction of autophagy in human endothelial cells after in vivo stimulation using a novel approach. Primary endothelial cells were collected from the radial artery before and immediately after rhythmic handgrip exercise. Exercise-induced elevations in shear rate increased NO signaling and reduced generation of ROS concomitant to elevations in markers of autophagy. These findings provide translational evidence in humans on the effects of exercise-induced elevated shear rate and autophagy-mediated NO generation. Previous data from this group have demonstrated that impaired autophagy (via siRNA for Atg3) prevents shear-induced increases in NO bioavailability and that shear-induced endothelial autophagy is maintained via purinergic signaling (1, 2).
Within a broader context, the findings from Park et. al. (8a) add to the existing literature on the mechanotransduction of signaling within the vasculature. In this regard, these results are particularly exciting, as they provide the first in vivo evidence that the mechanosensitive process of autophagy is initiated in response to functional hyperemia within humans and, furthermore, that this initiation of autophagic signaling is associated with elevations in NO bioavailability and reduced ROS generation. Mechanotransduction shear rate into the endothelium has only previously been examined within endothelial cell culture. In this context, laminar shear is also associated with inhibition of the lysophosphatidic acid (LPA) signaling cascade via stimulation of the membrane-bound phosphatase lipid phosphate phosphohydrolase 3 (LPP3), eliciting reductions in ROS and increases in NO (3, 9). The parallels between the shear-mediated mechanotransduction of autophagy and LPP3/LPA are apparent within the context of how each of these processes critically affects endothelium-dependent health. Interestingly, Wu et al. (9) have identified two genetic variants in LPP3 that are associated with an elevated risk for developing coronary artery disease (CAD). Stunningly, the “risk” allele has been shown to be associated with development of CAD independent of any traditional risk factors in >80% of the population (9). Because ∼30% of all patients with CAD have no evidence of traditional risk factors such as hypertension, high cholesterol, or tobacco use, these single-nucleotide point mutations may represent the single most relevant risk factor for developing coronary disease and put autophagy right in the center of interest.
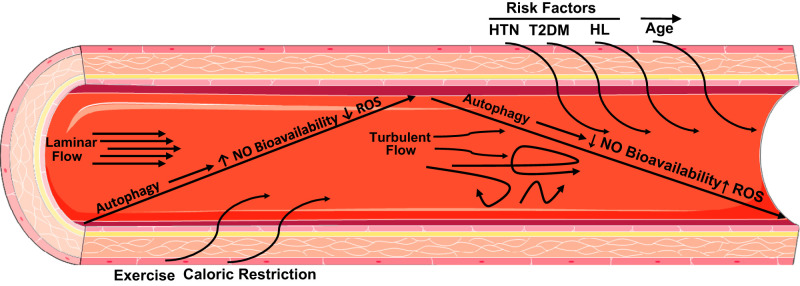
The microcirculation is increasingly being recognized to play a critical role in the development and consequences of a broad range of cardiovascular diseases. Whereas large artery pathologies such as aortic stiffness and atherosclerosis are associated with reductions in autophagy, little to no work has examined small resistance vessels. The microcirculation is a major contributor to organ perfusion and regulation of systemic blood pressure. As such, defects of microvascular autophagy would have an impact on numerous disease phenotypes, including CAD and hypertension, among others (8). In this context, previous in vivo evidence for the role of autophagy within cardiovascular health stems from studies demonstrating that advancing age is associated with reductions in markers of autophagy (∼50%) as well as reductions in NO bioavailability and enhanced ROS formation. Conversely, supplementation with trehalose, a natural disaccharide, has been demonstrated to activate autophagy and increase NO bioavailability concomitant to restored endothelium-dependent vasodilation within the human vasculature (6). Autophagy may be stimulated in a number of different ways, for example, via lifestyle factors such as exercise or caloric restriction that act on the proximal end of the autophagy signaling cascade by inhibiting mammalian target of rapamycin and activating/phosphorylating AMP-activated protein kinase. Figure 1 shows a simplified overview of how autophagy is modulated within the vasculature and its impact on vascular signaling molecules.
Fig. 1.
Conceptual overview of autophagy and endothelial function. HL, hyperlipidemia; HTN, hypertension; NO, nitric oxide; ROS, reactive oxygen species; T2DM, type 2 diabetes mellitus.
Although activation of autophagy may be considered beneficial, it has a dichotomous nature, particularly within the context of cardiovascular disease as well as other chronic diseases. This dual nature is particularly exemplified within heart failure and placement of left ventricular assist devices. Markers of autophagy in cardiomyocytes are elevated before left ventricular assist device placement, whereas after placement markers of autophagy decrease (7). This modulation of autophagy may be a compensatory response within the failing heart. On the other hand, aging is associated with increases in protein misfolding but also reductions in autophagy. Together, these results demonstrate the dual nature of autophagy and highlight the requirement to assess autophagy in the appropriate context. Future explorations of how to effectually modulate autophagy to the “just right” degree may pay off as treatment options for a large spectrum of human disease.
GRANTS
This work was supported by National Institutes of Health Grants T32-GM-089586-09, R01-HL-133029-02, and R01-HL-135901-02.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.H. prepared figures; W.H. and A.M.B. drafted manuscript; W.H. and A.M.B. edited and revised manuscript; W.H. and A.M.B. approved final version of manuscript.
References
- 1.Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD. Endothelial cell autophagy maintains shear stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 37: 1646–1656, 2017. doi: 10.1161/ATVBAHA.117.309510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 92: 605–612, 2014. doi: 10.1139/cjpp-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabowski DS, Kadlec AO, Ait-Aissa K, Hockenberry JC, Pearson PJ, Beyer AM, Gutterman DD. Lysophosphatidic acid acts on LPA1 receptor to increase H2 O2 during flow-induced dilation in human adipose arterioles. Br J Pharmacol 175: 4266–4280, 2018. doi: 10.1111/bph.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng 42: 1978–1988, 2014. doi: 10.1007/s10439-014-1033-5. [DOI] [PubMed] [Google Scholar]
- 5.Guo X, Kassab GS. Role of shear stress on nitrite and NOS protein content in different size conduit arteries of swine. Acta Physiol (Oxf) 197: 99–106, 2009. doi: 10.1111/j.1748-1716.2009.01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, Seals DR. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging (Albany NY) 8: 1167–1183, 2016. doi: 10.18632/aging.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 120, Suppl: S191–S197, 2009. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest 125: 55–64, 2015. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Park SK, La Salle DT, Cerbie J, Cho JM, Bledsoe AD, Nelson AD, Morgan DE, Richardson RS, Shiu YT, Boudina S, Trinity JD, Symons JD. Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00561.2018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Huang RT, Kuo CH, Kumar S, Kim CW, Lin YC, Chen YJ, Birukova A, Birukov KG, Dulin NO, Civelek M, Lusis AJ, Loyer X, Tedgui A, Dai G, Jo H, Fang Y. Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ Res 117: e41–e53, 2015. doi: 10.1161/CIRCRESAHA.117.306457. [DOI] [PMC free article] [PubMed] [Google Scholar]